Abstract
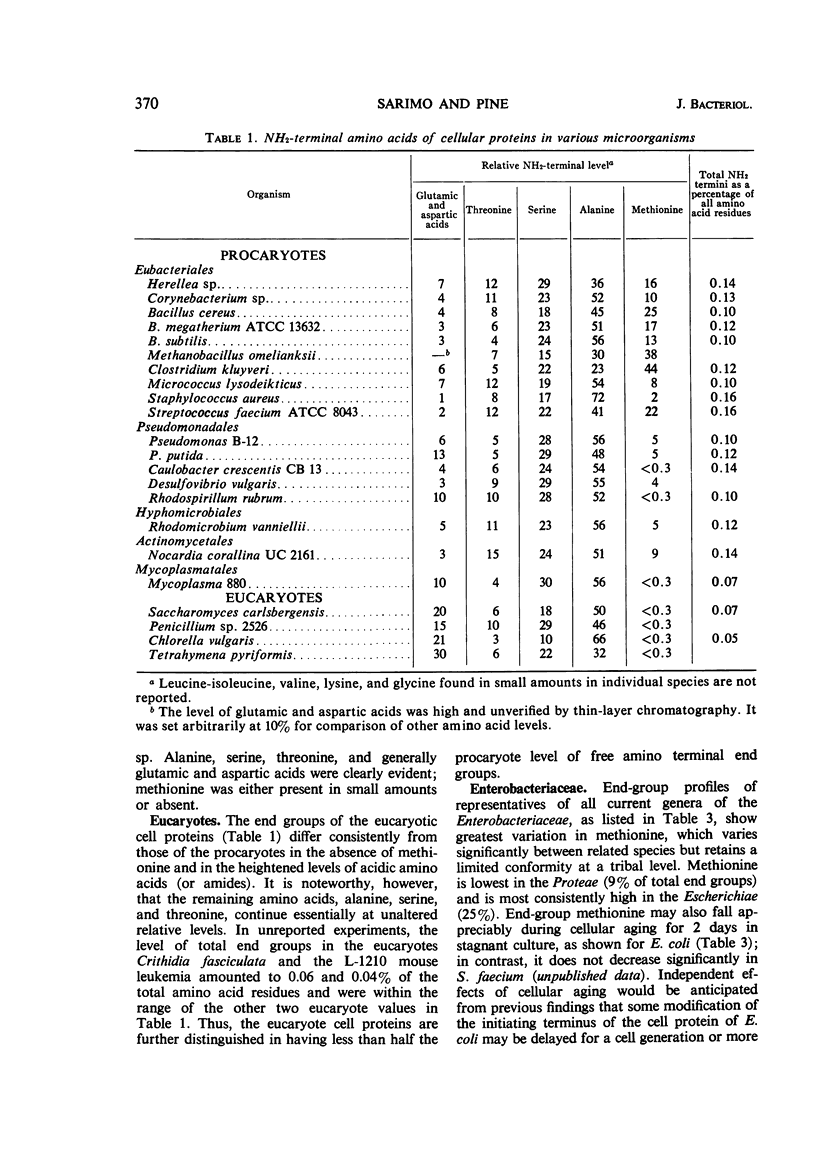
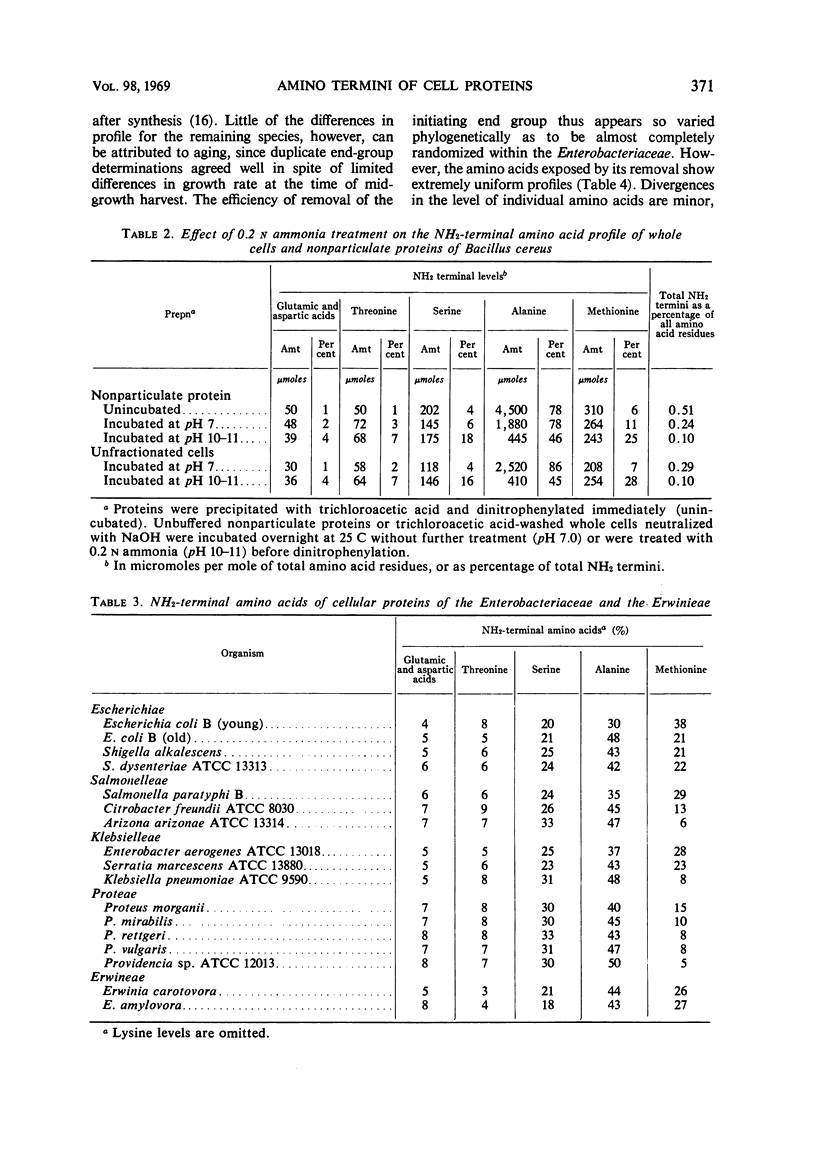
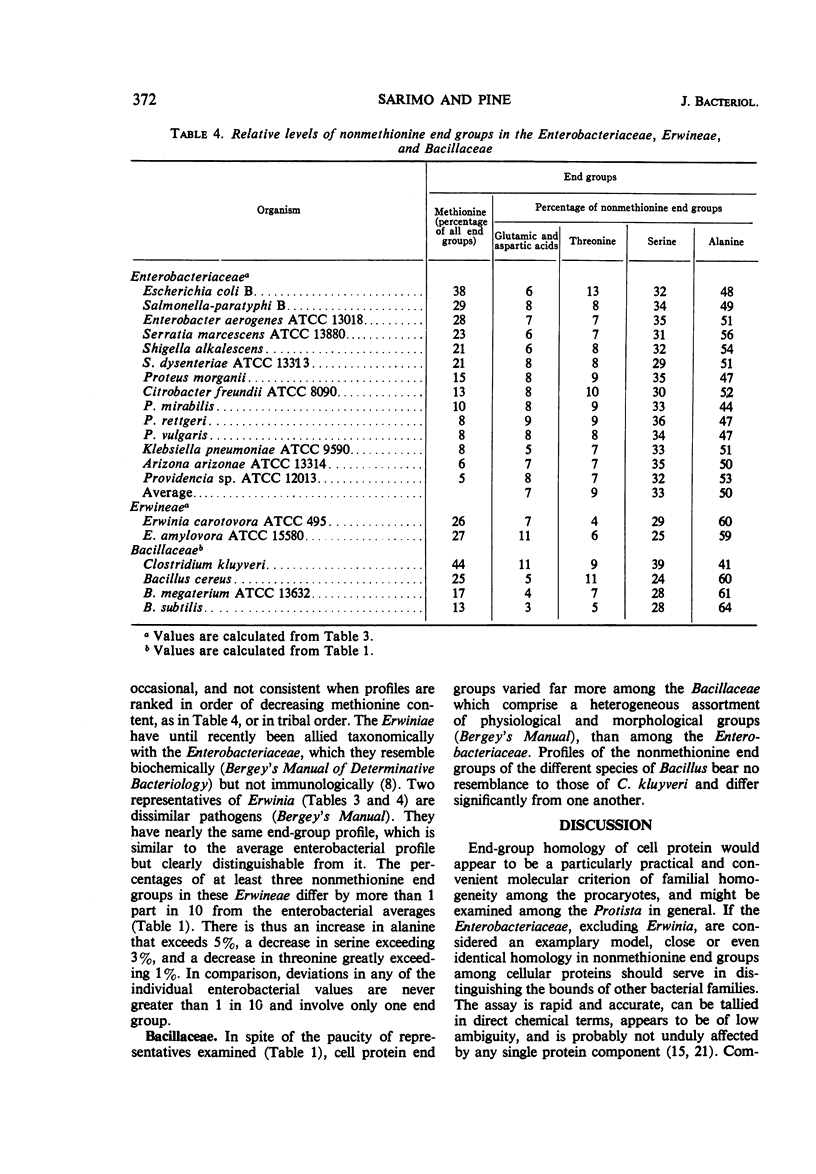
A comparison was made of the distribution of amino terminal end groups in the cellular proteins of a number of microbes. Among the procaryotes, methionine is a highly variable but virtually ubiquitous major protein end group. This is consistent with its possible role as a general amino acid initiator of protein biosynthesis in the procaryotes. Generally, however, alanine is the most abundant of the major end groups, followed in decreasing order by serine, threonine, the acidic amino acids, and occasionally lysine. No other new major end-groups were found. Among 15 representatives of the Enterobacteriaceae, retention of the initiating methionine terminus of the cellular protein varies considerably at a tribal level and is randomized at a familial level. The profiles of the five remaining end groups, however, are strikingly uniform, and are, for example, close to but significantly different from those of the Erwineae. Among the taxonomically more heterogeneous Bacillaceae, end-group profiles vary more and are sometimes unrelated. End-group analysis is thus particularly useful as a molecular criterion of taxonomy in assessing familial homogeneity. Free NH2 termini in eucaryote cell proteins are fewer, and they have increased acidic amino acid components and no methionine; they are otherwise similar to those of the procaryotes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Capecchi M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jan;55(1):147–155. doi: 10.1073/pnas.55.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- COWPERTHWAITE J., WEBER M. M., PACKER L., HUTNER S. H. Nutrition of Herpetomonas (Strigomonas) culicidarum. Ann N Y Acad Sci. 1953 Oct 14;56(5):972–981. doi: 10.1111/j.1749-6632.1953.tb30277.x. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Initiation of E. coli proteins. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1517–1524. doi: 10.1073/pnas.55.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland C. F., Briggs W. R. Flowering Responses of the Long-day Plant Lemna gibba G3. Plant Physiol. 1967 Nov;42(11):1553–1561. doi: 10.1104/pp.42.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt J., Lengyel P. Formylmethionyl-tRNA dependence of amino acid incorporation in extracts of trimethoprim-treated Escherichia coli. Science. 1966 Oct 28;154(3748):524–527. [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- HAKALA M. T., HOLLAND J. F., HOROSZEWICZ J. S. Change in pyrimidine deoxyribonucleoside metabolism in cell culture caused by Mycoplasma (PPLO) contamination. Biochem Biophys Res Commun. 1963 Jun 20;11:466–471. doi: 10.1016/0006-291x(63)90094-9. [DOI] [PubMed] [Google Scholar]
- Horikoshi K., Doi R. H. The NH2-terminal residues of Bacillus subtilis proteins. J Biol Chem. 1968 May 10;243(9):2381–2384. [PubMed] [Google Scholar]
- Johnson J. L., Ordal E. J. Deoxyribonucleic acid homology in bacterial taxonomy: effect of incubation temperature on reaction specificity. J Bacteriol. 1968 Mar;95(3):893–900. doi: 10.1128/jb.95.3.893-900.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., FALKOW S., MANDEL M. NEW APPROACHES TO BACTERIAL TAXONOMY. Annu Rev Microbiol. 1963;17:329–372. doi: 10.1146/annurev.mi.17.100163.001553. [DOI] [PubMed] [Google Scholar]
- Marcker K. The formation of N-formyl-methionyl-sRNA. J Mol Biol. 1965 Nov;14(1):63–70. doi: 10.1016/s0022-2836(65)80230-3. [DOI] [PubMed] [Google Scholar]
- PINE M. J. Alcohol-soluble protein of microorganisms. J Bacteriol. 1963 Feb;85:301–305. doi: 10.1128/jb.85.2.301-305.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J., Gordon B., Sarimo S. S. Protein initiation without folate in Streptococcus faecium. Biochim Biophys Acta. 1969 Apr 22;179(2):439–447. doi: 10.1016/0005-2787(69)90052-5. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Kinetics of maturation of the amino termini of the cell proteins of Escherichia coli. Biochim Biophys Acta. 1969 Jan 21;174(1):359–372. doi: 10.1016/0005-2787(69)90261-5. [DOI] [PubMed] [Google Scholar]
- Sueoka N. CORRELATION BETWEEN BASE COMPOSITION OF DEOXYRIBONUCLEIC ACID AND AMINO ACID COMPOSITION OF PROTEIN. Proc Natl Acad Sci U S A. 1961 Aug;47(8):1141–1149. doi: 10.1073/pnas.47.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLER J. P., HARRIS J. I. Studies on the composition of the protein from Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1961 Jan 15;47:18–23. doi: 10.1073/pnas.47.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLER J. P. THE NH2-TERMINAL RESIDUES OF THE PROTEINS FROM CELL-FREE EXTRACTS OF E. COLI. J Mol Biol. 1963 Nov;7:483–496. doi: 10.1016/s0022-2836(63)80096-0. [DOI] [PubMed] [Google Scholar]


