Abstract
The onset of sudden cardiac death and large inter- and intra-familial clinical variability of hypertrophic cardiomyopathy pose an important clinical challenge. Cardiac magnetic resonance imaging is a high-resolution imaging modality that has become increasingly available in the past decade and has the unique possibility to demonstrate the presence of fibrosis or scar using late gadolinium enhancement imaging. As a result, the diagnostic and prognostic potential of cardiac magnetic resonance imaging has been extensively explored in acute and chronic ischaemic cardiomyopathy, as well as in several nonischaemic cardiomyopathies.
This review aims to provide a critical overview of recently published studies on hypertrophic cardiomyopathy and discusses the role of cardiac magnetic resonance imaging in differentiating underlying causes of hypertrophic cardiomyopathy, such as familial hypertrophic cardiomyopathy, cardiac involvement in systemic disease and left ventricular hypertrophy due to endurance sports. Also, it demonstrates the use of cardiac magnetic resonance in risk stratification for the onset of sudden cardiac death, and early identification of asymptomatic family members of hypertrophic cardiomyopathy patients who are at risk for the development of hypertrophic cardiomyopathy. (Neth Heart J 2010;18:135–43.)
Keywords: Cardiomyopathy Hypertrophic ; Hypertrophy, Left Ventricular; Magnetic Resonance Imaging
This review will focus on the clinical applicability of cardiac magnetic resonance (CMR) to determine the underlying cause of different forms of hypertrophic cardiomyopathy (HCM). According to the recent position statement of the European Society of Cardiology, HCM is to be subdivided into familial and nonfamilial forms. Since considerable overlap in aetiology may exist between HCM and restrictive cardiomyopathy (RCM), several causes of RCM will also be briefly discussed.1
Hypertrophic cardiomyopathy
The clinical diagnosis of HCM is based on the presence of left ventricular (LV) hypertrophy in the absence of increased afterload, such as systemic hypertension or aortic stenosis. The prevalence of HCM in the general population is estimated at 1:500, indicating that in the Netherlands, approximately 33,000 people have HCM. An overview of recent CMR studies on HCM is presented in table 1. The nonpathological form of LV hypertrophy, as observed in athletes, can be distinguished from pathological hypertrophy, based on the maximal end-diastolic wall thickness to volume ratio.2 An end-diastolic wall thickness to volume ratio <0.15 mm∙m2∙ml–1 − which was calculated by dividing the maximal end-diastolic wall thickness by indexed LV end-diastolic volume − was found to have a 99% specificity to differentiate an athlete’s heart from pathological hypertrophy.
Table 1 .
Role of cardiac magnetic resonance in hypertrophic cardiomyopathy.
| Cardiomyopathy | Author | Year | Design | n | Description | |
|---|---|---|---|---|---|---|
| HCM, familial | Choudhury18 | 2002 | SC, P, C | 21 | Patchy LGE mainly located at insertion points was observed in 81% (17/21) of familial HCM patients and related to LV wall thickness. This finding may help to differentiate familial HCM from other forms of HCM | |
| Moon20 | 2003 | SC, P, C | 53 | The extent of LGE in HCM patients with ≥2 clinical risk factors for sudden cardiac death was higher than in patients with <2 clinical risk factors, especially when <40 years | ||
| Teroaka19 | 2004 | SC, P, C | 59 | HCM patients with ventricular arrhythmias on 24-hour Holter monitoring (14/59) had more extensive LGE than HCM patients without ventricular arrhythmias (45/59) | ||
| Germans5 | 2006 | SC, P, C | 32 | Crypts were visible at inferior insertion point 80% (13/16) of HCM mutation carriers without hypertrophy and not in healthy volunteers, yielding a 100% PPV and 84% NPV for identifying HCM carriers | ||
| Adabag21 | 2008 | SC, P, C | 177 | In HCM patients with no or mild symptoms, the presence of LGE was associated with an increased likelihood and frequency of ventricular tachyarrhythmias on Holter | ||
| HCM, non-familial | ||||||
| Anderson-Fabry | Moon42 | 2003 | SC, P, C | 26 | Inferolateral ill-defined subendocardial pattern of LGE was present in 50% (13/26) of Anderson-Fabry patients and related to increased LV mass | |
| Amyloidosis | Maceira39 | 2005 | SC, P, C | 46 | Global subendocardial pattern of LGE was found in 67% (20/30) of cardiac amyloidosis patients, as diagnosed with echocardiography. These patients also had higher T1-weighted signal intensity of myocardium compared with hypertensive patients (16). A combination of T1 values and the presence of LGE yields a PPV of 97% and an NPV of at least 88% for diagnosing cardiac involvement in amyloidosis | |
| Maceira37 | 2008 | SC, P, L | 28 | The presence of LGE was not a predictor of mortality after 5 years, but the extent of cardiac amyloid burden was. This was determined by the difference between subendocardial and subepicardial T1-weighted signal intensity on CMR images | ||
| Athlete’s heart | Petersen2 | 2005 | SC, P, C | 120 | Maximum end-diastolic wall to volume ratio >0.15 mm∙m2∙ml–1 has 99% specificity to differentiate athlete’s heart from pathological hypertrophy |
Design=study design, n=patient number, SC=single centre, P=prospective, C=cross-sectional, LGE=late gadolinium enhancement, HCM=hypertrophic cardiomyopathy, LV=left ventricular,, PPV=positive predictive value, NPV=negative predictive value, L=longitudinal, CMR= cardiac magnetic resonance.
Familial HCM
Familial HCM is mainly caused by mutations in genes which encode for sarcomeric proteins that have an autosomal dominant pattern of inheritance. Histologically, HCM is characterised by myocyte disarray, hypertrophy and interstitial fibrosis, which predominantly results in diastolic dysfunction, and may serve as a substrate for ventricular arrhythmias.3 HCM accounts for at least 25% of sudden cardiac death (SCD) in young athletes, and is sometimes the first symptom of disease.4 Since CMR has a high spatial and temporal resolution and may determine myocardial tissue characteristics such as lipomatous metaplasia and fibrosis, its role in early identification of HCM mutation carriers without LV hypertrophy, in diagnosing HCM, and in risk stratification for SCD in HCM patients has been extensively explored.
Early identification of HCM mutation carriers
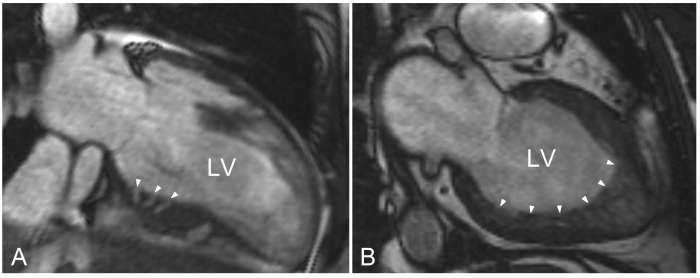
CMR cine imaging can be used to evaluate myocardial structure in HCM mutation carriers. With CMR cine imaging, structural abnormalities described as crypts of the inferoseptum were recently found in 80 to 90% of HCM mutation carriers, in whom no hypertrophy was present.5,6 These crypts were only visible on an end-diastolic dedicated image plane through the inferoseptum (figure 1A). While a single crypt or paired crypts have also occasionally been described in a referral-based population without HCM, it is likely, yet unproven, that crypts are the macroscopic representation of myocyte disarray since mild forms of disarray are known to occur in the inferoseptum in the general population.7 Additionally, very prominent crypts can also be observed with 2D echocardiography.8 Of note, as a sign of probable HCM mutation carriership, these crypts should be carefully discriminated from hypertrabecularisation of the myocardium, which is not typically found in HCM mutation carriers (figure 1B).
Figure 1.
Cardiac magnetic resonance cine images. A) Modified two-chamber end-diastolic cine image through the inferoseptum of a hypertrophic cardiomyopathy mutation carrier. Crypts are present in the basal inferoseptum (denoted by white arrowheads) penetrating compact myocardium and can easily be distinguished from noncompaction cardiomyopathy. B) Two-chamber cine imaging in a patient with noncompaction cardiomyopathy. The noncompacted layer aligning a compact layer is most profound in the apical region, and sometimes extends towards the inferior and/or lateral regions, as illustrated by the white arrowheads. LV= left ventricle.
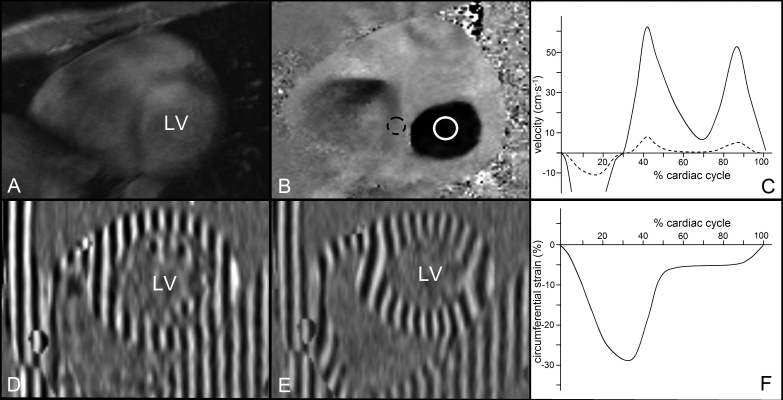
Beside structural abnormalities, HCM mutation carriers also exhibit reduced systolic and early diastolic mitral valve annulus velocities in septal and lateral segments prior to the development of manifest HCM. These characteristics can be measured by both echocardiography (using tissue Doppler imaging) and CMR (using a phase-contrast imaging technique).9-11 With phase contrast imaging, static tissue remains grey and tissue that moves at relatively high velocities appears as very bright or dark, depending on the direction of movement (figure 3). This technique is generally used for blood flow quantification, but can also be accurately employed to measure (diastolic) myocardial velocities.2 Therefore, this CMR technique may serve as an alternative to echocardiographic tissue Doppler imaging.
Measurement of LV wall thickness in hypertrophic cardiomyopathy
Although HCM is generally diagnosed with 2D echocardiography, a direct comparison between CMR and 2D echocardiography revealed that the diagnostic accuracy of CMR to identify HCM patients among a group of patients with suspected HCM was higher than 2D echocardiography.3 CMR identified HCM in 6% of patients who would otherwise remain undetected by 2D echocardiography. With echocardiography, the magnitude of hypertrophy in the basal lateral wall and the presence of extreme hypertrophy (>30 mm) were underestimated in 20 and 10% of patients respectively.
Risk assessment of sudden death in hypertrophic cardiomyopathy
Risk stratification for SCD involves the assessment of five clinical risk factors:
Extreme LV hypertrophy (>30 mm);
Blunted blood pressure response to exercise in patients younger than 40 years;
A family history of SCD;
Unexplained syncope;
Nonsustained ventricular tachycardia on 24-hour Holter monitoring.14
The occurrence of ventricular arrhythmias and subsequent SCD in HCM patients has been reported to be associated with an increased amount of fibrosis per LV segment.3
Fibrosis can be detected in vivo with CMR using late gadolinium enhancement (LGE) imaging. The most widely used CMR contrast agent is gadolinium chelate. This contrast agent is readily washed out of normal myocardium, but resides in areas of myocardium with increased extracellular space, e.g. where large amounts of interstitial fibrosis or scar tissue are present.15
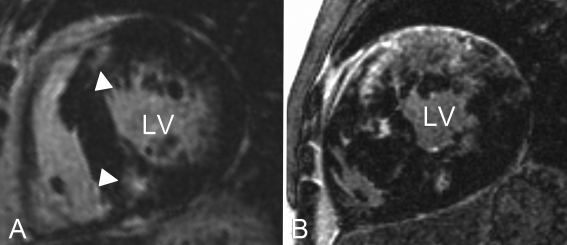
In HCM patients, the areas with contrast enhancement (LGE) are predominantly localised at the insertion points of the right ventricle into the LV and are related to increased amounts of local collagen deposits and oedema.6,17 The pattern of LGE is often described as patchy or confluent and found in approximately 80% of HCM patients (figure 2).8 Indeed, LGE is more often found in HCM patients with documented ventricular arrhythmias on Holter monitoring and is associated with an increased number of contrast enhanced LV segments and lower LV ejection fractions.9-21 Moon and colleagues found that the extent of LGE was higher in patients with two or more clinical risk factors for SCD.20 Interestingly, LGE, as well as clinical risk factors for SCD, are also found in HCM mutation carriers without overt hypertrophy.5,22 Although these data suggest a relation between LGE and the onset of ventricular arrhythmias/SCD, only single-centre, cross-sectional data are available. To further elucidate the prognostic value of LGE in HCM patients, longitudinal, multicentre studies are warranted. At this point, HCM patients with LGE cannot be considered at increased risk for ventricular arrhythmias/SCD per se, but the presence of LGE in HCM may be used as an arbitrator when ambiguity on ICD implantation remains after consideration of clinical risk factors.
Figure 2 .
Short-axis left ventricular late gadolinium enhancement images of two HCM patients. Note that the fibrotic burden in patient B (confluent type LGE) is much more extensive than in patient A (patchy type LGE), which is related to increased risk of ventricular arrhythmias. Typically, late gadolinium enhancement (white arrowheads) is located at the insertion points of the right ventricle into the septum, see patient A. This pattern is observed in approximately 80% of HCM patients. LV=left ventricle.
Nonfamilial HCM
Many systemic disorders are associated with HCM, of which diabetes, end-stage renal disease, amyloidosis and lysosomal storage diseases are most frequently reported as underlying causes. The most important role of CMR in the diagnosis of patients with acquired HCM lies within the differentiation of the underlying systemic diseases, which is important for the choice of treatment and prognosis.
Diabetic cardiomyopathy
Diabetic cardiomyopathy is diagnosed by finding LV hypertrophy and diastolic dysfunction in the absence of obstructive coronary artery or valvular disease in patients with diabetes. The proposed mechanisms through which cardiomyopathy develops in patients with diabetes include a deranged cardiomyocyte metabolism and calcium homeostasis, concomitant systemic hypertension and endothelial dysfunction.23
The findings of recent CMR studies suggest that the development of LV hypertrophy in diabetics is race dependent and coheres with systemic hypertension and obesity.23-25 This may not only result in subclinical diastolic, but also systolic dysfunction in type 1 and type 2 diabetes patients, as demonstrated in CMR studies using a dedicated CMR technique called myocardial tissue tagging.26,27 Myocardial tissue tagging allows visualisation and quantification of the regional deformation of the myocardium as well as global twist/untwist dynamics of the LV, and has proven to be more sensitive in the evaluation of regional function than wall thickening (figure 3).28 In patients with type 1 diabetes, a CMR tagging study evaluating the twist/untwist dynamics found that twisting rate was increased irrespective of heart rate, and may represent the early detrimental effects of diabetes on myocardial function. Thus far, no studies describe typical patterns of LGE in diabetics. Although the capability to detect subclinical myocardial disease in diabetics with CMR may allow timely initiation of therapy, the relation between subclinical LV dysfunction and clinical outcome in diabetic patients is yet to be elucidated.
Figure 3 .
Cardiac magnetic resonance functional images. A) Short-axis, phase-contrast magnitude image, displaying the anatomical information. Magnitude images are used to define the regions of interest for velocity quantification. B) Matching flow encoded, phase-contrast image. Tissue moving at low velocities has an intermediate signal intensity (grey) and tissue moving at high velocities has either a low signal intensity (black) or high signal intensity (white), depending on the direction of movement. Regions of interest can be drawn on the myocardium (dashed circle) and in the mitral valve orifice (solid circle) in every phase of the cardiac cycle. C) From these data, myocardial velocity (dashed line) and mitral valve inflow curves (solid line) can be derived for analysis of systolic and diastolic function. D) Short-axis end-diastolic myocardial tissue tagging image. At this point in the cardiac cycle, a horizontal and vertical tagging pattern is applied on the myocardium. E) This tagging pattern deforms concomitantly with the in plane deformation of the myocardium throughout the entire cardiac cycle. The temporal resolution may reach up to 14 ms. From the deformation in two orthogonal directions, strain and rotation in every direction can be calculated. F) Circumferential strain is most frequently used for analysis of myocardial deformation. LV=left ventricle.
Uraemic cardiomyopathy in end-stage renal failure
In patients with end-stage renal failure (ESRF), LV hypertrophy is commonly diagnosed in the presence of conventional risk factors for the development of LV hypertrophy. Uraemic cardiomyopathy is defined by the presence of LV hypertrophy, dilation and systolic dysfunction. Also, increased interstitial fibrosis is found in ESRF patients.29,30 Experimental studies suggest direct cardiotoxicity of uraemia, but the circulatory abnormalities associated with ESRF such as aortic stiffening, coronary atherosclerosis with prominent calcification, and volume overload due to anaemia, may well account for the specific phenotype of uraemic cardiomyopathy. LGE is present in 29% of ESRF patients, and related to an increased number of conventional risk factors for ischaemic heart disease.29
Although these data illustrate that LGE imaging may serve as a valuable adjunct to the diagnostic work-up of ESRF, recent reports show that the use of gadolinium-based contrast agents is associated with increased prevalence of nephrogenic systemic sclerosis in these patients.31 Therefore, LGE imaging should only be performed in ESRF patients when no diagnostic alternatives are available. Then, it may be prudent to institute prompt dialysis after LGE imaging. Of notice, increased LV mass and dimensions are strongly associated with increased risk of cardiovascular death in ESRF patients; therefore, CMR may be used for LV volume and mass measurement in the clinical follow-up of ESRF patients.32
Amyloidosis
Cardiac amyloid protein deposition frequently involves primary (AL-type) and secondary amyloidosis and is a major determinant of prognosis. Approximately 50% of patients with amyloidosis die from heart failure or ventricular arrhythmias.33 In senile amyloidosis, which results from wild-type transthyretin deposition, cardiomyopathy is usually the sole manifestation of disease. When cardiac involvement with heart failure is present, median survival in AL-type amyloidosis is 0.8 years and in senile amyloidosis approximately five years.34,35 The gold standard for diagnosing cardiac involvement of amyloidosis is endomyocardial biopsy, but the procedure exposes the patient to a small but considerable risk. Observing an increased intraventricular wall thickness with echocardiography or CMR in combination with a low voltage electrocardiogram has a sensitivity of 72% and specificity of 91%, and yields a positive predictive value of 79% and negative predictive value of 88% to diagnose cardiac involvement in amyoloidosis.36
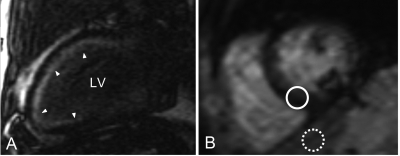
In addition, cardiac involvement of amyloidosis displays a characteristic pattern of LGE on LGE imaging if the images are acquired four minutes after injection of contrast agent (figure 4). LGE is found in approximately 70% of patients.37,38 This ill-defined, generally subendocardial pattern of LGE matches the distribution of the amyloid deposition.39 Although most profound in the subendocardial layer of the myocardium, amyloid deposition occurs throughout the entire myocardium, and therefore has a higher T1 signal than normal myocardium. The typical pattern of late enhancement on LGE imaging, together with the increased T1 signal of myocardium, yield a diagnostic accuracy of 97% in patients with cardiac involvement in biopsy-proven amyloidosis, as determined with echocardiography.39
Figure 4 .
Cardiac magnetic resonance late gadolinium enhancement imaging. A) Two-chamber view of an amyloidosis patient with cardiac involvement. Note the global, subendocardial, ill-defined late gadolinium enhancement that is found in approximately 70% of patients (see arrowheads). B) Short-axis T2* weighted image of the left ventricle. Signal intensity of the septum has rapidly decayed, yielding a dark tone (solid circle) while signal intensity of the liver (dashed circle) is preserved. This demonstrates the poor correlation between T2* values of the liver and the Heart.
Whether CMR is more sensitive than echocardiography in detecting cardiac involvement in amyloidosis remains to be explored. To detect LV hypertrophy in patients suspected with cardiac involvement in amyloidosis, CMR can be considered an alternative to echocardiography. However, to additionally perform LGE imaging in these patients has shown to be clinically relevant, since the presence of LGE helps to confirm the diagnosis and also the difference in T1 signal between epicardial and subendocardium is of prognostic importance in cardiac amyloidosis.37,38 A higher T1 signal in LGE imaging indicates higher amyloid deposition. When amyloid deposition is mainly present subendocardially, than T1 difference between subendocardium and subepicardium is high. When amyloid burden increases, amyloid deposition in the subepicardium occurs, and as a result the difference in T1 signal is lower. Maceira and colleagues found that patients with a T1 difference higher than 23 ms had an improved survival.37
Anderson-Fabry disease
Anderson-Fabry disease is the second most prevalent lysomal storage disease resulting from an X-linked recessive disorder of glycosphingolipid metabolism. Cardiac involvement is common, resulting from glycosphingolipid within the cardiomyocytes, valves and vascular endothelium, ultimately resulting in HCM. Although generally believed to be a rare disorder, recent studies on the prevalence of this treatable cause of HCM showed that Anderson-Fabry disease was the underlying cause of disease in 3 to 6% of middle-aged men in an HCM referral population.40,41
Histological studies on cardiac involvement mainly report intra-cardiomyocytal inclusions within the lysosomes with typical concentric lamellar configuration. However, LGE in Andersen-Fabry disease likely represents replacement scarring and typically occurs in the midventricular and epicardial layers of basal inferolateral wall in 92% of patients.42 Again, the pattern of LGE is more ill defined compared with scarring found after myocardial infarction.
Restrictive cardiomyopathy
Cardiac iron overload
In patients with iron overload, caused by increased intestinal iron absorption (haemochromatosis) or by increased erythrocyte destruction and transfusional siderosis in thalassaemia, heart failure is the most common cause of death.43 Cardiomyopathy probably results from the toxic effects of nontransferrin bound iron on the mitochondrial respiratory chain when the cardiac iron storage capacity is exhausted. This form of cardiomyopathy is reversible with chelate therapy, provided therapy is initiated before heart failure develops.44,45
However, the diagnosis of cardiac iron overload is often delayed due the relatively late onset of symptoms, echocardiographic abnormalities and unpredictability of development. Additionally, the extent of iron overload in the heart does not correlate with iron overload in the liver, which is usually employed in clinical practice as an indicator of systemic iron overload.44,45
With the CMR, the concentration of iron within the myocardium can be estimated by measuring the T2* relaxation time. This represents the velocity by which signal decay of tissue occurs on T2* weighted images (figure 4). The more rapid the signal decay, the more iron present. In myocardium, T2* relaxation times <20 ms are found in patients with substantial cardiac iron overload and are strongly related to a decrease in LV ejection fraction.44,46 Therefore, repetitive T2* time measurement in patients at risk of cardiac iron overload allows timely initiation of chelate therapy and may prevent the development of heart failure.
Endomyocardial fibrosis
In hypereosinophilic syndrome, eosinophil-mediated endomyocardial fibrosis and subsequent RCM evolves through three stages: an acute necrotic stage which develops five to six weeks after the onset of illness, an intermediate stage in which thrombus formation along necrotic endocardium occurs in often asymptomatic patients, and a fibrotic stage. In the fibrotic stage, LV wall thickening by endomyocardial replacement scarring and thrombus formation can be observed with CMR, and warrants anticoagulation therapy. Also, this form of LV wall thickening can by discriminated from LV myocardial hypertrophy using LGE imaging, which reveals thickening of the endocardium and increased signal intensity, accompanied by LV cavity thrombus formation, indicated by black spots aligning the endocardium.47
Conclusions
Within the last decade, a large body of research has demonstrated the diagnostic accuracy of CMR imaging, and the development of new sequences and techniques holds promise for an increasing role of CMR in the diagnostic work-up and monitoring of nonischaemic cardiomyopathies. However, the majority of previous studies were single centre, and therefore its diagnostic accuracy in common practice is currently unknown. With the use of CMR, it is possible to noninvasively visualise crypts, fibrosis and oedema in HCM, to determine the cardiac involvement in systemic diseases including amyloidosis, and to measure the severity of iron overload. However, scarcity remains regarding the prognostic value of these findings and warrants further research.
To date, an increasing number of medical centres have embraced CMR as a standard diagnostic tool for diagnosis of both ischaemic and nonischaemic cardiomyopathies. Combined efforts of multiple medical centres to conduct the necessary long-term follow-up studies in patients with nonischaemic cardiomyopathies will ultimately allow us to answer the questions on the accuracy and prognostic value of CMR in patients with overt disease and those at risk to develop disease in day-to-day practice.
Authors note
Tjeerd Germans is supported by a grant from the Netherlands Heart Foundation: grant no. 2006B213. Robin Nijveldt is supported by a grant from the Netherlands Heart Foundation: grant no. 2003B126.
References
- 1.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270-6 [DOI] [PubMed] [Google Scholar]
- 2.Petersen SE, Selvanayagam JB, Francis JM, Myerson SG, Wiesmann F, Robson MD, et al. Differentiation of athlete's heart from pathological forms of cardiac hypertrophy by means of geometric indices derived from cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2005;7:551-8. [DOI] [PubMed] [Google Scholar]
- 3.Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart. 2000;84:476-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maron BJ, Roberts WC, McAllister HA, Rosing DR, Epstein SE. Sudden death in young athletes. Circulation. 1980;62:218-29. [DOI] [PubMed] [Google Scholar]
- 5.Germans T, Wilde AA, Dijkmans PA, Chai W, Kamp O, Pinto YM, et al. Structural abnormalities of the inferoseptal left ventricular wall detected by cardiac magnetic resonance imaging in carriers of hypertrophic cardiomyopathy mutations. J Am Coll Cardiol. 2006;48:2518-23. [DOI] [PubMed] [Google Scholar]
- 6.Germans T, Wilde AA, van Echteld CJ, Kamp O, Pinto YM, van Rossum AC. Structural abnormalities of the left ventricle in hypertrophic cardiomyopathy mutation carriers detectable before the development of hypertrophy. Neth Heart J. 2007;15:161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson B, Maceira AM, Babu-Narayan SV, Moon JC, Pennell DJ, Kilner PJ. Clefts can be seen in the basal inferior wall of the left ventricle and the interventricular septum in healthy volunteers as well as patients by cardiovascular magnetic resonance. J Am Coll Cardiol. 2007;50:1294-5. [DOI] [PubMed] [Google Scholar]
- 8.Germans T, Dijkmans PA, Wilde AA, Kamp O, van Rossum AC. Images in cardiovascular medicine. Prominent crypt formation in the inferoseptum of a hypertrophic cardiomyopathy mutation carrier mimics noncompaction cardiomyopathy. Circulation. 2007;115:e610-e611. [DOI] [PubMed] [Google Scholar]
- 9.Michels M, Soliman OI, Kofflard MJ, Hoedemaekers YM, Dooijes D, Majoor-Krakauer D, et al. Diastolic abnormalities as the first feature of hypertrophic cardiomyopathy in Dutch myosin-binding protein C founder mutations. JACC Cardiovasc Imaging. 2009;2:58-64. [DOI] [PubMed] [Google Scholar]
- 10.Nagueh SF, Bachinski LL, Meyer D, Hill R, Zoghbi WA, Tam JW, et al. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation. 2001;104:128-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paelinck BP, Lamb HJ, Bax JJ, van der Wall EE, de Roos A. Assessment of diastolic function by cardiovascular magnetic resonance. Am Heart J. 2002;144:198-205. [DOI] [PubMed] [Google Scholar]
- 12.Paelinck BP, de Roos A, Bax JJ, Bosmans JM, Der Geest RJ, Dhondt D, et al. Feasibility of tissue magnetic resonance imaging: a pilot study in comparison with tissue Doppler imaging and invasive measurement. J Am Coll Cardiol. 2005;45:1109-16. [DOI] [PubMed] [Google Scholar]
- 13.Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112:855-61. [DOI] [PubMed] [Google Scholar]
- 14.Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212-8. [DOI] [PubMed] [Google Scholar]
- 15.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461-74. [DOI] [PubMed] [Google Scholar]
- 16.Knaapen P, van Dockum WG, Bondarenko O, Kok WE, Gotte MJ, Boellaard R, et al. Delayed contrast enhancement and perfusable tissue index in hypertrophic cardiomyopathy: comparison between cardiac MRI and PET. J Nucl Med. 2005;46:923-9. [PubMed] [Google Scholar]
- 17.Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, et al. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;43:2260-4. [DOI] [PubMed] [Google Scholar]
- 18.Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156-64. [DOI] [PubMed] [Google Scholar]
- 19.Teraoka K, Hirano M, Ookubo H, Sasaki K, Katsuyama H, Amino M, et al. Delayed contrast enhancement of MRI in hypertrophic cardiomyopathy. Magn Reson Imaging. 2004;22:155-61. [DOI] [PubMed] [Google Scholar]
- 20.Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41:1561-7. [DOI] [PubMed] [Google Scholar]
- 21.Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369-74. [DOI] [PubMed] [Google Scholar]
- 22.Michels M, Soliman OI, Phefferkorn J, Hoedemaekers YM, Kofflard MJ, Dooijes D, et al. Disease penetrance and risk stratification for sudden cardiac death in asymptomatic hypertrophic cardiomyopathy mutation carriers. Eur Heart J. 2009;30:2593-8. [DOI] [PubMed] [Google Scholar]
- 23.Bertoni AG, Goff DC, Jr., D'Agostino RB, Jr., Liu K, Hundley WG, Lima JA, et al. Diabetic cardiomyopathy and subclinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2006;29:588-94. [DOI] [PubMed] [Google Scholar]
- 24.Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, et al. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003;42:328-35. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues B, Cam MC, McNeill JH. Metabolic disturbances in diabetic cardiomyopathy. Mol Cell Biochem. 1998;180:53-7. [PubMed] [Google Scholar]
- 26.Fonseca CG, Dissanayake AM, Doughty RN, Whalley GA, Gamble GD, Cowan BR, et al. Three-dimensional assessment of left ventricular systolic strain in patients with type 2 diabetes mellitus, diastolic dysfunction, and normal ejection fraction. Am J Cardiol. 2004;94:1391-5. [DOI] [PubMed] [Google Scholar]
- 27.Chung J, Abraszewski P, Yu X, Liu W, Krainik AJ, Ashford M, et al. Paradoxical increase in ventricular torsion and systolic torsion rate in type I diabetic patients under tight glycemic control. J Am Coll Cardiol. 2006;47:384-90. [DOI] [PubMed] [Google Scholar]
- 28.Gotte MJ, Germans T, Russel IK, Zwanenburg JJ, Marcus JT, van Rossum AC, et al. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging: studies in normal and impaired left ventricular function. J Am Coll Cardiol. 2006;48:2002-11. [DOI] [PubMed] [Google Scholar]
- 29.Mark PB, Johnston N, Groenning BA, Foster JE, Blyth KG, Martin TN, et al. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006;69:1839-45. [DOI] [PubMed] [Google Scholar]
- 30.McMahon AC, Naqvi RU, Hurst MJ, Raine AE, MacLeod KT. Diastolic dysfunction and abnormality of the Na+/Ca2+ exchanger in single uremic cardiac myocytes. Kidney Int. 2006;69:846-51. [DOI] [PubMed] [Google Scholar]
- 31.Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647-9. [DOI] [PubMed] [Google Scholar]
- 32.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. 1995;5:2024-31. [DOI] [PubMed] [Google Scholar]
- 33.Kyle RA, Gertz MA, Greipp PR, Witzig TE, Lust JA, Lacy MQ, et al. Long-term survival (10 years or more) in 30 patients with primary amyloidosis. Blood. 1999;93:1062-6. [PubMed] [Google Scholar]
- 34.Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91:141-57. [DOI] [PubMed] [Google Scholar]
- 35.Cacoub P, Axler O, De Zuttere D, Hausfater P, Amoura Z, Walter S, et al. Amyloidosis and cardiac involvement. Ann Med Interne (Paris). 2000;151:611-7. [PubMed] [Google Scholar]
- 36.Rahman JE, Helou EF, Gelzer-Bell R, Thompson RE, Kuo C, Rodriguez ER, et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43:410-5. [DOI] [PubMed] [Google Scholar]
- 37.Maceira AM, Prasad SK, Hawkins PN, Roughton M, Pennell DJ. Cardiovascular magnetic resonance and prognosis in cardiac amyloidosis. J Cardiovasc Magn Reson. 2008;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruberg FL, Appelbaum E, Davidoff R, Ozonoff A, Kissinger KV, Harrigan C, et al. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in light-chain cardiac amyloidosis. Am J Cardiol. 2009;103:544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186-93. [DOI] [PubMed] [Google Scholar]
- 40.Sachdev B, Takenaka T, Teraguchi H, Tei C, Lee P, McKenna WJ, et al. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002;105:1407-11. [DOI] [PubMed] [Google Scholar]
- 41.Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, et al. An atypical variant of Fabry's disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288-93. [DOI] [PubMed] [Google Scholar]
- 42.Moon JC, Sheppard M, Reed E, Lee P, Elliott PM, Pennell DJ. The histological basis of late gadolinium enhancement cardiovascular magnetic resonance in a patient with Anderson-Fabry disease. J Cardiovasc Magn Reson. 2006;8:479-82. [DOI] [PubMed] [Google Scholar]
- 43.Olivieri NF, Brittenham GM, McLaren CE, Templeton DM, Cameron RG, McClelland RA, et al. Long-term safety and effectiveness of iron-chelation therapy with deferiprone for thalassemia major. N Engl J Med. 1998;339:417-23. [DOI] [PubMed] [Google Scholar]
- 44.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171-9. [DOI] [PubMed] [Google Scholar]
- 45.Henry WL, Nienhuis AW, Wiener M, Miller DR, Canale VC, Piomelli S. Echocardiographic abnormalities in patients with transfusion-dependent anemia and secondary myocardial iron deposition. Am J Med. 1978;64:547-55. [DOI] [PubMed] [Google Scholar]
- 46.Mavrogeni SI, Markussis V, Kaklamanis L, Tsiapras D, Paraskevaidis I, Karavolias G, et al. A comparison of magnetic resonance imaging and cardiac biopsy in the evaluation of heart iron overload in patients with beta-thalassemia major. Eur J Haematol. 2005;75:241-7. [DOI] [PubMed] [Google Scholar]
- 47.Syed IS, Martinez MW, Feng DL, Glockner JF. Cardiac magnetic resonance imaging of eosinophilic endomyocardial disease. Int J Cardiol. 2008;126:e50-e52. [DOI] [PubMed] [Google Scholar]