Abstract
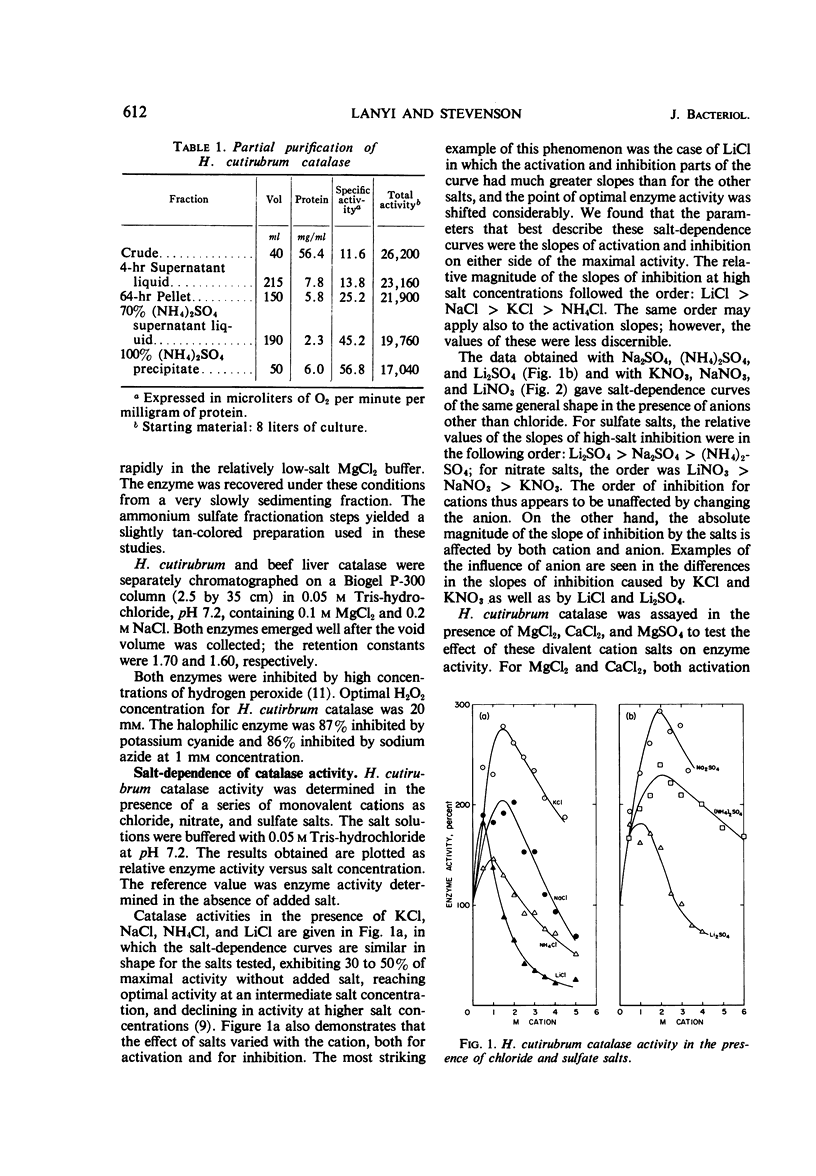
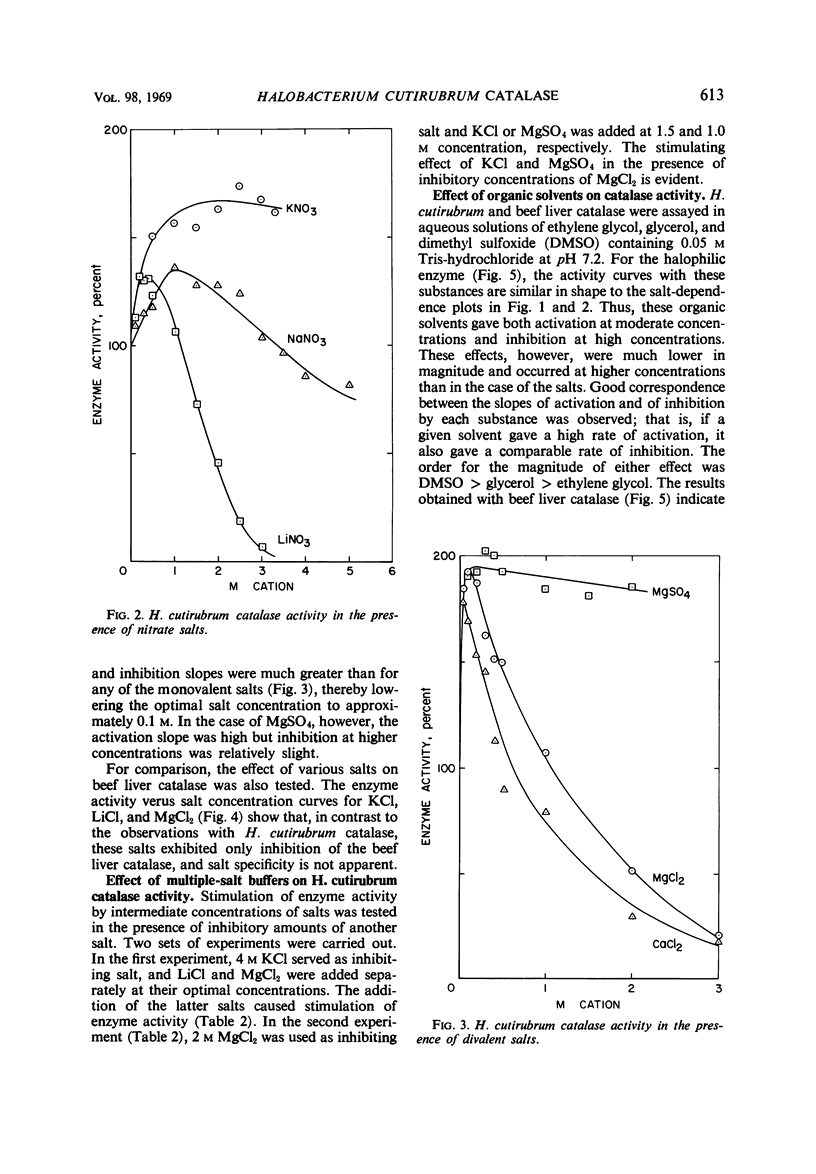
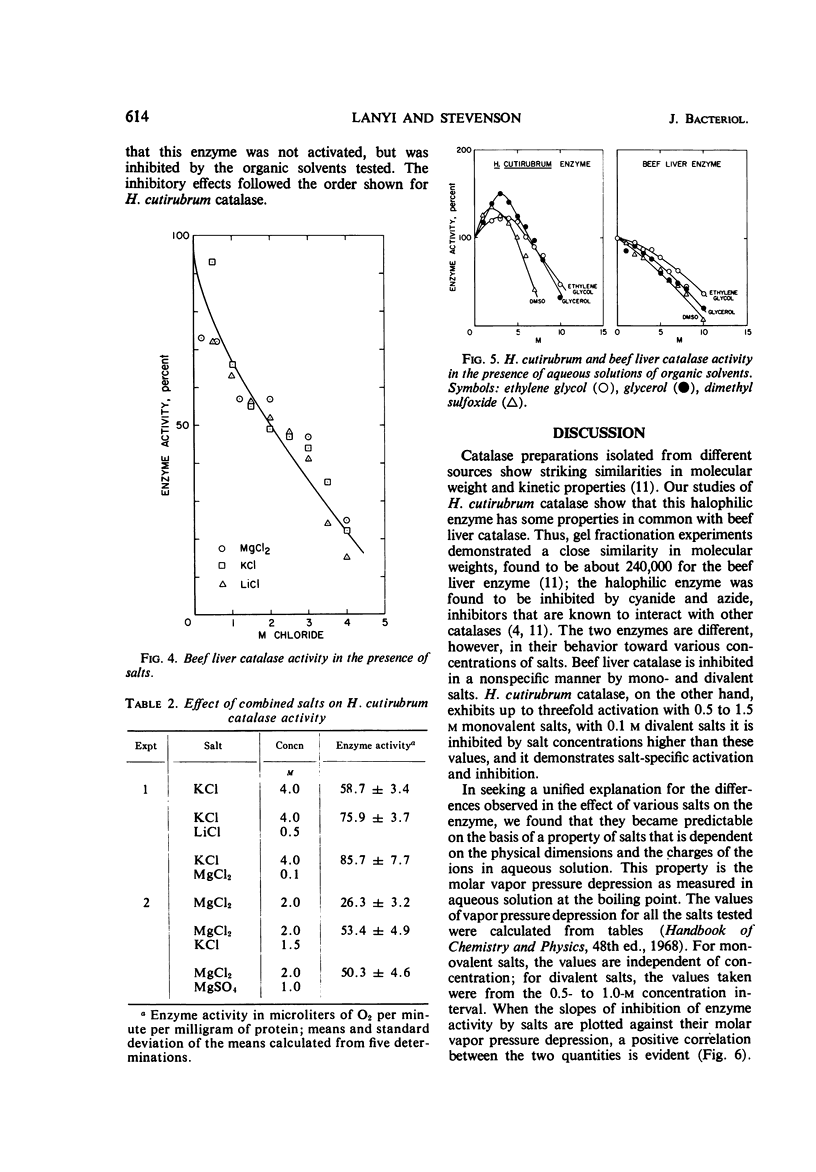
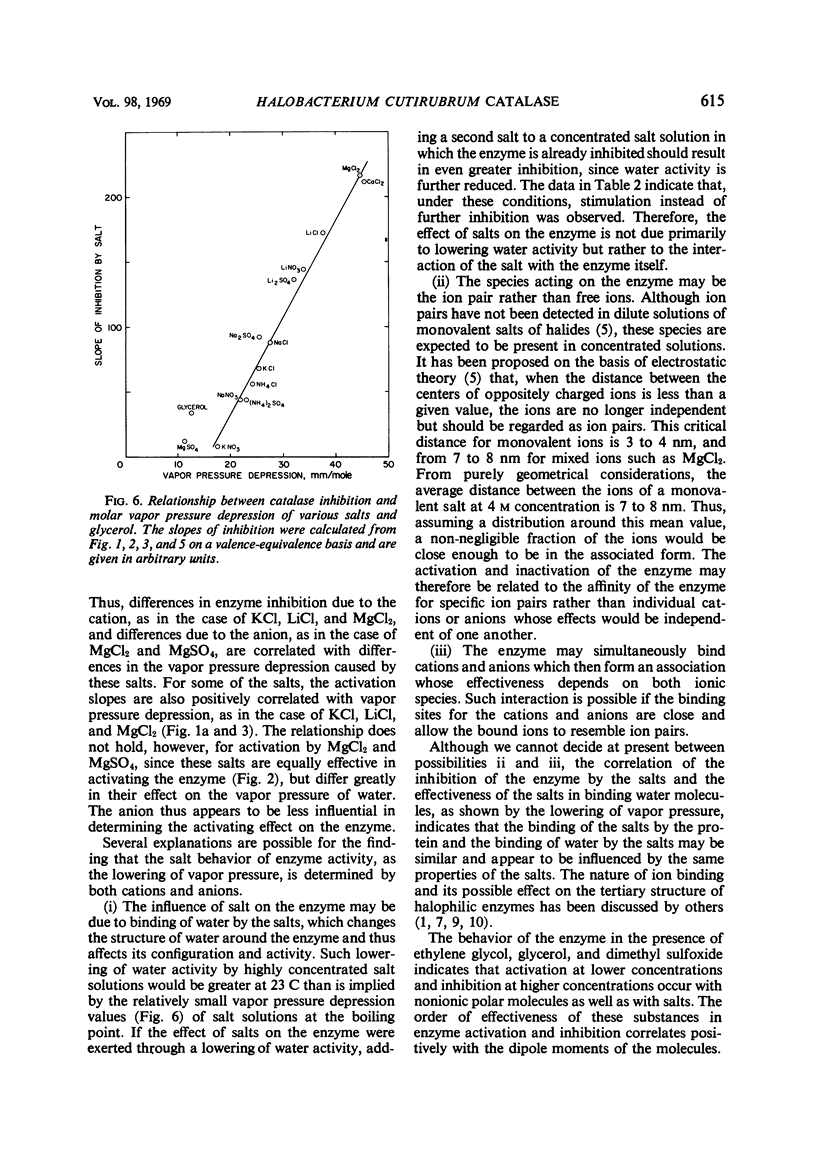
Catalase in extracts of the extreme halophile Halobacterium cutirubrum exhibits up to threefold stimulation by 0.5 to 1.5 m monovalent salts and by 0.1 m divalent salts. Above these concentrations, inhibition of enzyme activity is observed. The inhibitory effect, and to some extent the stimulation, is salt-specific; the effectiveness of a salt in inhibiting enzyme activity depends on both cation and anion. Thus, the order of effectiveness is MgCl2 > LiCl > NaCl > KCl > NH4Cl, and LiCl > LiNO3 > Li2SO4. The magnitude of enzyme inhibition for the salts tested is positively correlated with their molar vapor pressure depression in aqueous solution. Stimulation of enzyme activity was observed when one salt was added at its optimal concentration in the presence of inhibiting concentrations of another salt, indicating that the effect on the enzyme is not due to changing water activity but probably to enzyme-salt interaction. Aqueous solutions of ethylene glycol, glycerol, and dimethyl sulfoxide containing no ions influence enzyme activity in the same manner as do salts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAXTER R. M. An interpretation of the effects of salts on the lactic dehydrogenase of Halobacterium salinarium. Can J Microbiol. 1959 Feb;5(1):47–57. doi: 10.1139/m59-006. [DOI] [PubMed] [Google Scholar]
- BAXTER R. M., GIBBONS N. E. Effects of sodium and potassium chloride on certain enzymes of Micrococcus halodenitrificans and Pseudomonas salinaria. Can J Microbiol. 1956 Oct;2(6):599–606. doi: 10.1139/m56-072. [DOI] [PubMed] [Google Scholar]
- BAXTER R. M., GIBBONS N. E. The glycerol dehydrogenases of Pseudomonas salinaria, Vibrio costicolus, and Escherichia coli in relation to bacterial halophilism. Can J Biochem Physiol. 1954 May;32(3):206–217. [PubMed] [Google Scholar]
- Brown J. P. Polarographic comparison of some microbial catalases in vivo. Biochim Biophys Acta. 1968 Aug 6;165(1):174–176. doi: 10.1016/0304-4165(68)90204-3. [DOI] [PubMed] [Google Scholar]
- HOLMES P. K., HALVORSON H. O. PROPERTIES OF A PURIFIED HALOPHILIC MALIC DEHYDROGENASE. J Bacteriol. 1965 Aug;90:316–326. doi: 10.1128/jb.90.2.316-326.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein L. I., Dalton B. P. Salt specificity of a reduced nicotinamide adenine dinucleotide oxidase prepared from a halophilic bacterium. J Bacteriol. 1968 Jan;95(1):37–42. doi: 10.1128/jb.95.1.37-42.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. I. Spectrophotometric identification of the cytochromes of Halobacterium cutirubrum. Arch Biochem Biophys. 1968 Dec;128(3):716–724. doi: 10.1016/0003-9861(68)90080-5. [DOI] [PubMed] [Google Scholar]


