Abstract
Human leucine zipper transcription factor like 1 (LZTFL1) is a novel gene with unknown biological functions. It is located in the chromosome region 3p21.3, a hot spot for tumor suppressor genes. To understand the biological functions of LZTFL1, we surveyed the expression level of LZTFL1 in tumor and normal samples in tissue microarrays and a clinical archive of 84 gastric cancer specimens using immunohistochemistry. We found that LZTFL1 is expressed highly in the epithelial cells of normal tissues and is significantly down regulated in the corresponding tumor samples. The expression level of LZTFL1 correlated significantly with the survival outcomes of the patients and had significant inverse correlation with tumor metastasis. Overexpression of LZTFL1 in tumor cells inhibited anchorage-independent cell growth and cell migration in vitro, and repressed tumor growth in vivo. Furthermore, we show that LZTFL1 expression is upregulated upon epithelial cell differentiation and is graded along the crypt-villus axis of the intestine with weakest expression level in the proliferative zone of the crypt and highest expression level at the apex of the differentiation zone in the villus. Expression of LZTFL1 overlaps with that of E-cadherin at the plasma membrane. Our results indicate that LZTFL1 is a tumor suppressor and that loss of LZTFL1 expression has significant clinical outcomes. LZTFL1 expression may serve as an independent prognostic marker for survival outcome of gastric cancer patients. We propose that LZTFL1 may inhibit tumorigenesis by stabilizing E-cadherin-mediated adherens junction formation and promoting epithelial cell differentiation.
Keywords: tumor suppressor, adherens junction, epithelial cell differentiation, E-cadherin associated protein
Introduction
Chromosomal abnormalities at the 3p21.3 region are frequent and early events in the formation of human tumors of the lung, kidney, head and neck, breast, cervix, and gastrointestinal tract (1, 2). These abnormalities range from homozygous deletions and loss of heterozygosity to loss of protein expression, suggesting the presence of tumor-suppressor gene(s) (TSGs) in this region. As TSGs offer opportunities for molecular cancer therapy, there has been intense efforts during the past decades to identify candidate 3p21.3 TSGs and to characterize their biological functions. A critical region of approximately 120 kb in the 3p21.3 centromeric border (also called LUCA region) has been identified and contains nine candidate TSGs: HYAL2, HYAL1, FUS1, RASSF1, BLU, NPRL1, 101F6, PL6, and CACNA2DA (2). The functions of many of these gene products are being elucidated and they appear to be involved in a wide spectrum of biological processes, including cell proliferation, cell cycle kinetics, signaling transduction, ion exchange and transportation, and cell death. The 3p21.3 region also contains other candidate TSGs outside the LUCA region, but they are less well studied.
In an effort to discover potential TSGs in the 3p21.3 region, Kiss et al. identified and cloned Leucine zipper transcription factor like 1 (LZTFL1) through an elimination test and subsequent genomic sequencing and cDNA cloning (3). LZTFL1 is located approximately 5 Mb from the LUCA region on the telomeric end of the 3p21.3 region. Northern analysis indicates that LZTFL1 mRNA is expressed ubiquitously in both human and mouse. The open reading frame from human and mouse cDNAs revealed a protein of 299 amino acids with molecular weight of 34.6 kDa. The sequence analysis suggested that LZTFL1 shares 90.6% sequence identity between human and mouse. LZTFL1 contains a basic region, a coil-coil domain, and a leucine zipper domain, suggesting that LZTFL1 may be a transcription factor (3, 4). However, the biological and molecular function of LZTFL1 remains to be determined.
The loss of differentiation in cancer cells is often associated with tumor progression, but the underlying causes and mechanisms remain poorly understood. The majority of human solid tumors are carcinomas that originated from various epithelial cell types. Differentiated carcinomas are composed of cohesive polarized epithelial cells connected to one another by intercellular adherens junctions. E-cadherin is the core molecule of adherens junctions (5). The cytoplasmic tail of E-cadherin is indirectly linked to the actin cytoskeleton through catenins, including α and β-catenin and other associated proteins. The attachments of E-cadherin to the cytoskeleton; hence associated proteins in the adherens junction, are essential for maintaining the differentiated state of epithelial cells and the apico-basal polarity of the epithelium. Disruption of the adherens junction can generate invasive mesenchymal cells through a process called epithelial-mesenchymal transition (EMT) that converts polarized, immotile epithelial cells to motile invasive mesenchymal cells. EMT has been proposed to be a potential mechanism for carcinoma metastases (6, 7). Loss of membranous E-cadherin can also increase the cytoplasmic pool of β-catenin, which can then translocate to the nucleus and activate genes that promote cell proliferation and EMT.
In the present study, we sought out to test whether LZTFL1 functions as a tumor suppressor. We asked three experimental questions. First, is LZTFL1 expression downregulated in tumors and whether loss of LZTFL1 expression has any clinical significance? Second, can LZTFL1 gain-of-function inhibit tumor growth? Finally, we examine a potential mechanism(s) by which LZTFL1inhibits tumor cell growth. Our results revealed that LZTFL1 is a tumor suppressor and may inhibit tumor growth and metastases by stabilizing E-cadherin-mediated adhesive function, thereby inhibiting EMT.
Materials and Methods
Plasmids
The expression vector of LZTFL1 (pcDNA-Flag-LZTFL1) was constructed by subcloning a PCR-amplified insert corresponding to the mouse LZTFL1open-reading-frame (Invitrogen). pTRE2-LZTFL1-ires-EGFP plasmid was constructed by a two-step cloning through PCR and restriction enzyme digestion; the Flag-LZTFL1 fragment from pcDNA-Flag-LZTFL1 was first subcloned into pIres-EGFP vector (Clontech) to yield pLZTFL1-ires-EGFP-ires plasmid. The Flag-LZTFL1-ires-EGFP fragment was then subsequently subcloned into pTRE2 vector (Clontech). GST-LZTFL1 construct (pGEX-kg-LZTFL1) was constructed by subcloning PCR-amplified LZTFL1 fragment into pGEX-kg vector. Construction details are available upon request. The sequences of all cloning products were verified using an automated sequencer.
Generation of LZTFL1 specific antibody
Recombinant Glutathione-S-transferase (GST)-LZTFL1 protein was produced and purified according to a standard protocol (8). After cleaved and separated from the GST protein, full length LZTFL1 protein was used as the antigen to immunize rabbits (Cocalico Biological, PA).
Cell lines, transfection, and siRNA knock-down
Human intestinal epithelial cell line HT-29, and human breast cancer cell line MCF-7 (ATCC) were maintained in complete medium (DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, and penicillin-streptomycin). Cells were transfected with combination of plasmids indicated for each experiment using lipofectamine 2000 according to manufacturer’s protocol (Invitrogen). To induce cell differentiation, HT-29 cells were cultured in complete medium supplemented with 3mM sodium butyrate (NaB) for 3 days. For stable transfection, HT-29 cells were transfected with pLZTFL1-ires-EGFP and pIres-EGFP control vector. Cells were selected with G418 for 3–4 weeks.
Three independent LZTFL1 specific siRNA duplexes in TriFecta kit were purchased from IDT (IDT technology) and transfected into C2C12 cells using lipofectamine 2000 (Invitrogen). FITC-labeled non-silencing duplex siRNA was used as the negative control.
Generation of Hela-tet-on cells with doxycycline (Dox)-inducible expression of LZTFL1
Hela-tet-on cells expressing the rtTA (Invitrogen) were maintained in complete medium containing 0.5 mg/ml G418. The cells were transfected with either pTRE2-LZTFL1-ires-EGFP or control pTRE2-ires-EGFP vector using Lipofectamine 2000. GFP was used here as a reporter for the ease of selection. Transfected cells were double selected with hygromycin (0.1 mg/ml) and G418 (0.5 mg/ml). The resistant clones were screened for the induction of GFP expression upon the addition of 1 μg/ml Dox by live fluorescence imaging.
Tissue microarray, immunohistochemistry (IHC), and data analysis
Tissue microarrays were from IMGENEX (San Diego, CA). Slide IMH-326 and IMH-327 contained total of 118 samples of common tumor/cancers. IMH-336 and IMH-337 contained corresponding matched normal/normal adjacent tissues of IMH-326 and IMH-327, respectively. Each individual tumor array contained 8–10 cases. Clinical and pathological information for individual cancer samples was provided by the array manufacturers. IHC staining was carried out following manufacturer’s protocol using LZTFL1 antibody as the primary antibody followed by biotinylated secondary antibody and Streptavidin-horseradish peroxidase. Diaminobenzidine was used as the substrate chromagen and slides were counterstained with hematoxylin. Staining intensity (0: no staining; 1: weak staining; 2: moderate staining; and 3: intense staining) and the proportion of stained cells (0: no staining; 1: <10% staining; 2: between 11% to 33%; 3: between 34% to 66%; and 4: great than 67%) were semi-quantitatively determined following published protocols (9). All slides were scored by two observers blinded to the pathology and clinical features. Student t-test was used for statistical analysis. p < 0.05 was considered statistically significant.
Tumor samples, IHC, patients’ data, and statistical analysis
Paraffin-embedded tumor and corresponding normal tissue sample slides from 84 gastric cancer patients were stained with LZTFL1 antibody using immunohistochemistry and the slides were scored as described above. Normal tissue structures near the cancerous ones in the same histological section or normal corresponding tissue from the same individual in a separate slide were used as positive control. The clinical data were obtained from Department of Surgical Oncology, Sir Run Run Shaw Hospital, China. The patients were enrolled between July 1995 and March 2007. All patients were underwent a curative gastrectomy and none of the patients received preoperative treatments. Total gastrectomy was performed in 10 patients and subtotal gastrectomy in 74 patients. Post-surgery pathological examination showed various tumor types including papillary, mucinous, signet ring, and mucinous adenocarcinoma. All clinical pathological profiles were evaluated in accordance with the criteria of the World Health Organization. Tumor stage was evaluated according to the TNM classification of the 6th edition criteria of the International Union against Cancer.
The patients were followed up until death or until the date of last follow-up on November 30, 2007 and no patient had been lost to follow-up. Thirty-three out of 84 patients (39.2%) died during the follow-up period, and the median follow-up interval was 50.6 months (range from 2.8 to 119.2 months). Informed consents from patients were obtained for the use of paraffin-embedded tumor and normal tissues. This study was approved by the Ethics committee of Sir Run Run Shaw Hospital.
For correlation between LZTFL1 IHC score and clinical parameters, a Pearson r correlation coefficient was calculated using GraphPad Prism Software (GraphPad Software Inc, San Diego, CA). Two-tailed parametric analysis was considered significant when p < 0.05. Survival curves were estimated by the Kaplan-Meir method with log-rank test using GraphPad Prism program.
Soft agar assays and tumorigenicity assays in athymic nude mouse
For soft agar assays, cell aliquots in growth medium mixed with 0.35% soft agar were plated in triplicate onto a 12-well plate with a 0.5% semi-solid agar basal layer. For HT-29 and MCF-7 cells, fresh medium were added every 7 days. For Hela-tet-on cells, fresh medium with and without Dox were added every 3 days. After 4 weeks incubation at 37°C, colonies in the soft agar were photographed and scored under an inverted microscope. For tumorigenicity assays in nude mice, we injected a suspension of 1×107 cells in 0.2 ml PBS subcutaneously into the flank of 6 weeks odd female BALB/C athymic nude mice (NCI). Mice were scored for tumor development and tumor size at each site of injection. Mice were sacrificed and the tumors were weighted when the largest tumor reached 2 cm maximal diameter (at the end of 5 weeks after injection). All animal experiments were performed in accordance with institutional guidelines.
Western blot and immunofluorescence analysis and antibodies
Western blot and immunofluorescence were performed according to standard protocols. Antibodies used in this study are anti-Flag (Sigma), anti-cadherin (Cell Signaling), anti-β-catenin and anti-GAPDH (Santa Cruz), and anti-LZTFL1 (this study). Confocal immunofluorescence images were taken on Zeiss LSM510.
In vitro migration assay
Parental, EGFP-, and LZTFL1- expressing Heta-tet-on cells were cultured in the presence and absence of Dox for 48 hrs, washed, trypsinized, and suspended in DMEM plus 0.1% BSA. Approximately 1×105 cells in 100 μl DMEM/0.1%BSA were placed into the upper chamber of a 24-well Transwell cell culture plate (Corning Costar). The lower chamber contained DMEM with 5% FBS as a chemoattractant. After 8 hrs of incubation, migrated cells on the lower surface were stained with hematoxylin and counted under a microscope.
Results
LZTFL1 expression is downregulated in human tumors
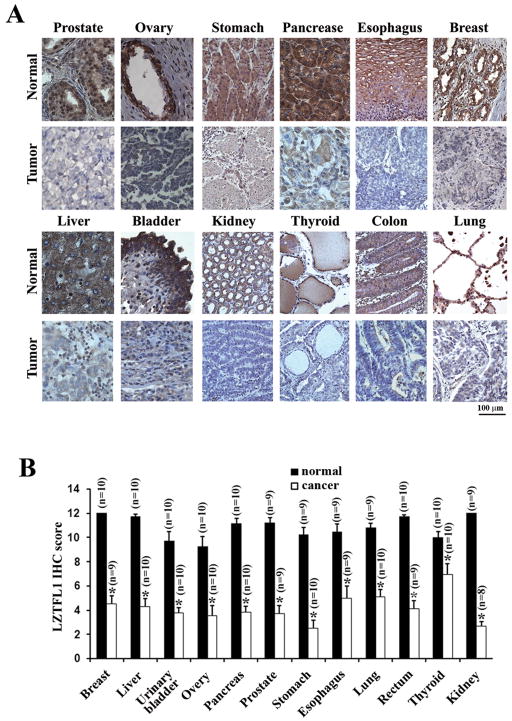
In order to study the biological function of LZTFL1, we first generated and affinity-purified a rabbit polyclonal antibody against LZTFL1 using bacterially-derived full length mouse LZTFL1 as the antigen. This antibody recognized endogenous and overexpressed LZTFL1 specifically in both western blot and immunohistochemistry (Supplemental Figure 1). Using this antibody, we surveyed the expression of LZTFL1 in various normal human tissues and their corresponding cancer samples by immunohistochemical analysis of tissue microarrays. Intense LZTFL1 staining was visible in epithelial cells of normal tissues of breast, esophagus, pancreas, stomach, ovary, prostate, lung, colon, thyroid, kidney, bladder, and liver (Figure 1A). In almost all the corresponding invasive carcinoma samples, only diffused, low levels of LZTFL1 staining were observed. Analysis of multiple cases in each individual type of normal and matched cancer samples in the tissue microarray showed that LZTFL1 was significantly downregulated in the aforementioned human tumors (Figure 1B).
Figure 1. LZTFL1 expression is down-regulated in human tumors.
(A) Representative micrographs of LZTFL1 IHC staining in normal and tumor samples. LZTFL1 is ubiquitously expressed in the epithelial cells of a wide array of tissues and in hepatocytes. (B) Average staining of LZTFL1 from multiple samples (n) of each individual normal and cancerous tissue on the array (n ± SEM, *, p < 0.01, student t-test).
Downregulation of LZTFL1 expression in gastric cancer patients correlates with poorer survival outcome
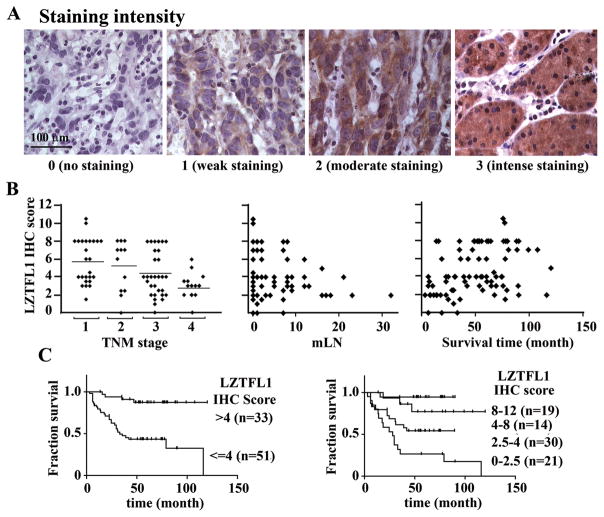
To address the clinical significance of downregulation of LZTFL1 in cancers, tissue samples from a cohort of 84 patients diagnosed with stomach cancer between the ages of 31 and 79 were screened by immunohistochemistry for LZTFL1 expression. Patients’ characteristics are summarized in Table 1. IHC staining was quantitatively scored on a 0–12 scale based on the product of the staining intensity (Figure 2A, 0 to 3) and percentage of stained area (not shown, 0–4) as described in the methods section.
Table 1.
Patients and tumor characteristics stratified by LZTFL1 expression.
| Feature | Number of patients | Pearson r | p-value | ||
|---|---|---|---|---|---|
| LZTFL1 IHC score |
|||||
| ≤4 | >4 | ||||
| age (range) | 0.09307 | 0.3997 | |||
| 36–79 | 83 | 51(median=55) | 32(median=58) | ||
| 31 | 1 | 1 | |||
| Sex | 0.07834 | 0.4787 | |||
| Male | 55 | 35 | 20 | ||
| Female | 29 | 15 | 14 | ||
| Histology | 0.08062 | 0.4660 | |||
| papillary | 1 | 0 | 1 | ||
| signet ring cell | 13 | 8 | 5 | ||
| mucinous | 4 | 2 | 2 | ||
| tubular | 66 | 40 | 26 | ||
| TNM stage | −0.4117 | 0.0001 | |||
| I | 25 | 11(44%) | 14(56%) | ||
| II | 12 | 5(42%) | 7(58%) | ||
| III | 30 | 20(67%) | 10(33%) | ||
| IV | 17 | 15(88%) | 2(12%) | ||
| mLN | −0.3229 | 0.0027 | |||
| ≤ 2 | 48 | 21(44%) | 27(56%) | ||
| >3 | 36 | 30(83%) | 6(17%) | ||
| Survival | 0.3805 | 0.0004 | |||
| live | 49 | 20(41%) | 29(59%) | ||
| death | 35 | 31(89%) | 4(11%) | ||
Figure 2.
(A) Representative micrographs showing typical examples of the four intensity grades of LZTFL1staining in tumor samples of the cohort of 84 gastric cancer patients. (B) Correlations between LZTFL1 IHC score and TNM stage (left panel, Pearson r=−0.4117, p=0.0001, two-tailed. bar, average IHC score for each individual TNM stage), number of mLN (middle panel, Pearson r=−0.3229, p=0.0027, two-tailed; not all the data points are in view since the majority of the patients have mLN less than 10), and survival times (right panel, Pearson r=0.3805, p=0.0004, two-tailed) in the cohort of patients in this study. (C) Kaplan-Meier survival curves according to LZTFL1 IHC scores for negligible and weak (IHC score <=4) versus moderate and strong expression (IHC scores >4) (left panel, p=0.0002, log-rang test), and the individual stratified IHC scores (right panel, p=0.0002, log-rank test). n, number of patients.
LZTFL1 IHC scores and the clinical parameters for the cohort under study were tabulated and their correlations were analyzed for their statistical significance (Table 1). Loss of LZTFL1 expression was found to have significant inverse correlations with TNM stages of the tumor (Pearson r= −0.4117, p=0.0001, two tailed) (Figure 2B, left panel) and with the number of metastasized lymph nodes (mLN) (Pearson r=−0.3229, p=0.0027, two tailed) (Figure 2B, middle panel). No significant differences of LZTFL1 IHC scores were found among age, gender distribution, or tumor classifications (Table 1).
LZTFL1 expression level correlated significantly with the survival time as well (Pearson r=0.3805, p=0.0004) (Figure 2B, right panel). The overall survival was significantly better for patients with tumors demonstrating moderate or strong LZTFL1 expression (IHC score >4) than those whose tumors showed negligible or weak expression (IHC score <=4) (Figure 2C, left panel, p=0.0002, log-rank test). The median survival for patients with IHC score less than or equal to 4 was 32.8 months. While the median survival was not achieved for patients with IHC score greater than 4, patients in this group have a lower risk of death with hazard ratio of 0.22 (95% CI: 0.1052 to 0.4175). When analyzed according to the stratified expression levels, patients with weak LZTFL1 expression (IHC score<=2.5) were found to have the worst median survival (29.3 months, p<0.0001, log-rank test). There is a significant trend toward longer survival times with higher LZTFL1 expression levels (p<0.0001, log-rank test) (Figure 2C, right panel).
LZTFL1 inhibits tumor cell growth in vitro and in vivo
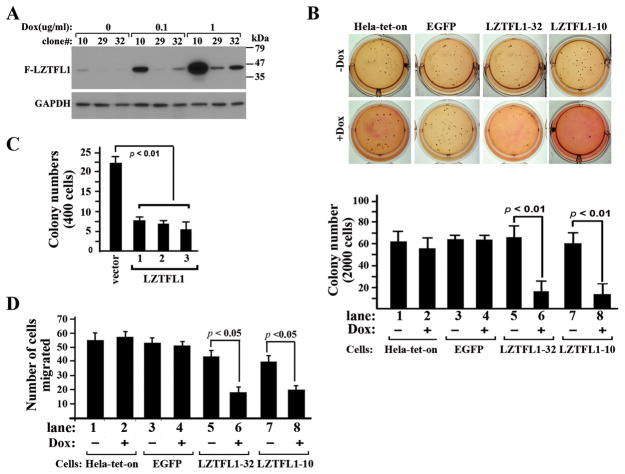
In order to determine whether LZTFL1 plays a direct role in tumorigenesis, we performed gain-of- function studies to test whether increased level of LZTFL1 expression in tumor cells can inhibit tumor cell growth. We used an inducible expression system to induce LZTFL1 expression through addition of doxycycline (Dox) in cultured Hela-tet-on cells that constitutively produce the reverse tetracycline transactivator. Three clonal cell lines were obtained (Figure 3A). Clones LZTFL1-29 and 32 had minimal basal expression of LZTFL1 whereas clone LZTFL1-10 showed weak LZTFL1 expression. Clones LZTFL1-10, 32, and 29 had highest, modest, and weakest inducible LZTFL1 expression upon addition of Dox, respectively. Clones LZTFL1-32 and 10 were chosen for further experiments.
Figure 3. Overexpression of LZTFL1 in Hela cells inhibits anchorage-independent cell growth in vitro.
(A) Dox-regulated production of LZTFL1 in stable Hela-tet-on-LZTFL1 clones. Clones 10, 29, and 32 were generated by transfecting a pTRE2 plasmid containing a Dox-responsive expression cassette that encodes Flag-LZTFL1 and a EGFP reporter into Hela-tet-on cells. Cells were exposed to various amounts of Dox as indicated for 24 hrs, and whole cell extracts were analyzed by immunoblotting using anti-Flag antibody. (B) The colony-forming ability of the parental, EGFP-expressing, and LZTFL1-expressing Hela-tet-on cells in soft agar in the presence and absence of Dox was examined as a measure of anchorage-independent growth. 2000 cells were seeded and colonies were photographed four weeks after plating (upper panel, representative ones) and counted (lower panel, n=3 ± SEM). (C) Soft agar assays for HT-29 cells expressing EGFP or LZTFL1. HT-29 cells were transfected with control vector pEGFP and pEGFP-ires-LZTFL1, respectively. Stable clones (vector, 1, 2, and 3) were selected and expanded in the presence of G418. 400 cells from clones indicated were seeded for soft agar assays. Colony numbers were counted (n=3 ± SEM). (D) Transwell migration assays with Hela-tet-on, EGFP, LZTFL1-32 and LZTFL1-10 cells previously cultured in the absence and presence of Dox (n=3 ± SEM). Induction of LZTFL1 inhibited the migration of Hela cells in both LZTFL1-32 and LZTFL1-10 clones.
We first investigated the effect of LZTFL1 expression on the growth phenotype of Hela-tet-on cells under adherent conditions in monolayer cultures using MTT and flow cytometry assays. No significant differences were observed between Hela-tet-on-LZTFL1 cells in the absence and presence of Dox and between LZTFL1 expressing cells and parental Hela-tet-on cells (data not shown). We next asked whether LZTFL1 has any effect on tumor cell growth under anchorage-independent conditions in soft agar assays. A large number of colonies of uninduced cells were visible within 4 weeks (Figure 3B, upper panel). The LZTFL1-expressing cells in both LZTFL1-32 and 10 clones showed dramatically reduced number of colonies upon addition of Dox (Figure 3B, lower panel). As controls, the numbers of colonies in Hela-tet-on and Hela-tet-on-EGFP cells with and without Dox and in Hela-LZTFL1 cells without Dox were similar, suggesting that LZTFL1 indeed specifically inhibited anchorage-independent growth of tumor cells. We also tested whether overexpression of LZTFL1inhibits the colony formation ability of other tumor cells, including intestinal epithelial carcinoma cells HT-29 and breast carcinoma cells MCF-7. We observed similar inhibitory effects of LZTFL1 in these cells (Figure 3C and data not shown).
As downregulation of LZTFL1 in human gastric tumors correlated with tumor metastases in patients, we next investigated a role for LZTFL1 in cell migration. Upregulation of LZTFL1 in Hela-LZTFL1-10 and Hela-LZTFL1-32 upon Dox induction significantly reduced the migration properties of Hela cells in Transwell assays (Figure 3D, lanes 6 and 8). In negative controls, Dox had no effect on migration of parental or EGFP expressing Hela-tet-on cells. The number of migrated cells migrated are similar among parental or EGFP-expressing Hela-tet-on cells with or without Dox, and Hela-LZTFL1-10 and LZTFL1-32 cells without Dox (Figure 3D, lanes 1–4, 5 and 7).
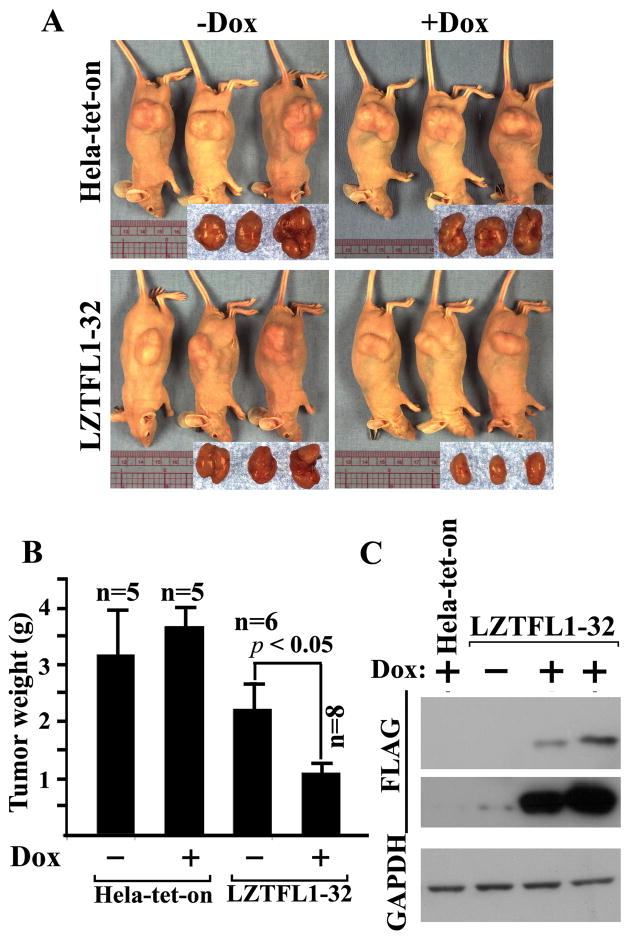
To further test whether overexpression of LZTFL1 results in suppression of tumor growth in vivo, we injected subcutaneously Hela-tet-on and LZTFL1-32 cells into the flank of nude mice. We chose the LZTFL1-32 clone to avoid any artifact that might arise due to the overexpression since the level of induction of LZTFL1 in this clone is similar to the physiological level of LZTFL1 in differentiated HT-29 cells (see Figure 5 for details). As expected, at the end of 5 weeks, mice injected with Hela-tet-on cells developed large tumors (Figure 4A). The tumor size in mice with LZTFL1-32 cells in the presence of Dox in the drinking water for induction of LZTFL1 were significantly reduced compared to those in the absence of Dox (Figure 4B). As Dox in drinking water had little effects on the tumor size of the mice injected with Hela-tet-on cells, our results suggest that LZTFL1 inhibited tumor growth significantly in vivo. It is noted that tumor sizes in mice with un-induced LZTFL1-32 cells are smaller compared to those with Hela-tet-on cells although they are not statistically significant. This is probably due to the leaky expression of LZTFL1 as shown in the Western blot of tumor lysates with long exposure time (Figure 4C, middle panel).
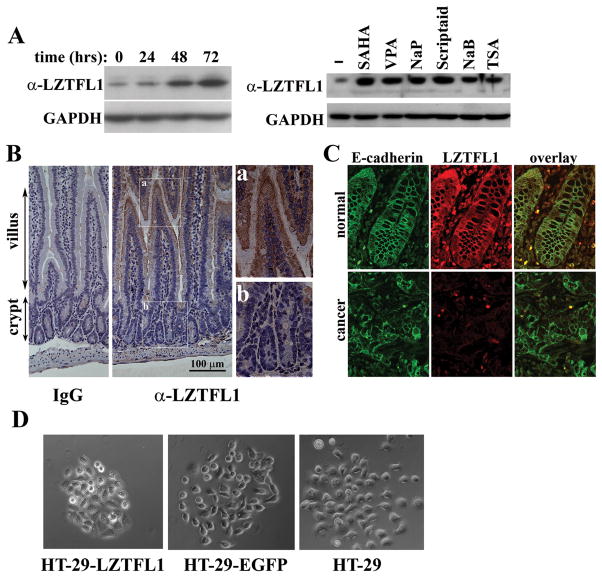
Figure 5. LZTFL1 expression is up-regulated upon cell differentiation.
(A) Left panel, HT-29 cells were cultured in the presence of NaB for the indicated times. The LZTFL1 expression level was measured by Western blot. Right panel, HT-29 cells were cultured for three days in the absence (−) and presence of the HDAC inhibitors, suberoylanilide (SAHA), Valproic acid (VPA), sodium propionate (NaP), scriptaid, NaB, and Trichostatin A (TSA). Cell lysates were subjected to Western blot analysis with anti-LZTFL1 antibody. (B) Immunohistochemical staining of IgG (negative control) and LZTFL1 in the mouse small intestine. There is a graded LZTFL1 staining along the crypt-villus axis (middle panel, 20×). Inset a shows a robust LZTFL1 staining in the apex of villus (40×). Inset b shows absence of LZTFL1 staining in the crypt. (C) Confocal microscopy images of human normal colonic tissue (upper panels) and colorectal carcinoma (lower panels) stained with E-cadherin (green) and LZTFL1 (red). The expression of LZTFL1 is overlapping with that of E-cadherin at the plasma membrane in normal colonic epithelial cells. (D) Parental and pires-EGFP and pLZTFL1-ires-EGFP transfected HT-29 cells were seeded with a low density to allow them to grow as discrete colonies. PMA (0.5 μg/ml) was added to the cell to induce cell scattering. Micrographs were taken 8 hrs after addition of PMA.
Figure 4. Upregulation of LZTFL1 in Hela cells inhibits tumor growth in vivo.
(A) Representative mice subcutaneously injected with Hela-tet-on (upper panel), LZTFL1-32 (lower panel) cells and fed without (left panel) and with Dox (right panel) at the end of 5 weeks after injection. Corresponding tumors are pictured in the inset. (B) Average tumor mass resulting from cells injected into the flank of nude mice. (n, number of mice for each clone ± SEM, p < 0.05, student t-test). (C) Western blot analysis of tumor lysates using anti Flag-antibody (upper panel, short exposure; lower panel, long exposure).
LZTFL1 expression is up-regulated upon epithelial cell differentiation and co-localizes with E-cadherin at the plasma membrane
It has been demonstrated that many TSGs are inactivated in cancer by epigenetic silencing induced by aberrant methylation of CpG islands in the promoter region of the TSG or by overexpression of histone deacetylases (HDACs) (10–13). To understand the mechanism of LZTFL1 inactivation in tumor cells, we treated HT-29 cells with 5′-aza-2′-deoxycytidine, a DNA methylation inhibitor, and Sodium butyrate (NaB), a HDAC inhibitor, respectively. No difference of LZTFL1 expression was observed between 5′-aza-2′-deoxycytidine treated and untreated cells (data not shown) whereas NaB treatment increased the level of LZTFL1 expression (Figure 5A, left panel). Other HDAC inhibitors had similar effects on the upregulation of LZTFL1 expression in HT-29 cells (Figure 5A, right panel). These results suggest that LZTFL1 is inactivated in HT-29 cells by alterations in chromatin structure.
NaB is a naturally-occurring compound in the intestine and induces differentiation of epithelial cells in culture (14, 15). Upregulation of LZTFL1 in NaB treated HT-29 cells suggests that the expression level of LZTFL1 may be correlated with the differentiation status of the cell. To test this hypothesis in vivo, we stained the mouse small intestine with anti-LZTFL1 antibody along the crypt-villus axis. The intestinal epithelium undergoes constant self-renewing processes. The stem cells in the crypt give rise to an intermediate cell population that undergoes rapid proliferation and differentiation as they migrate towards the apex of the villus (16). Indeed, a graded expression of LZTFL1 along the crypt-villus axis was observed with a minimal staining of LZTFL1 in the crypt and maximum staining at the apex of the villus (Figure 5B). Next, we performed co-localization studies of LZTFL1 with E-cadherin/β-catenin using confocal immunofluorescence microscopy. Expression of LZTFL1 overlaps with that of E-cadherin at the plasma membrane in differentiated normal colonic epithelial cells (Figure 5C). This co-localization was absent in colorectal carcinomas due to a loss of LZTFL1 protein expression.
Co-localization of LZTFL1 with E-cadherin suggested that LZTFL1 may stabilize E-cadherin-mediated adherens junction. Indeed, we observed that, when treated with phorbol 12-myristate 13-acetate (PMA), a known scatter factor to break down the epithelial tight junction (17), the LZTFL1-expressing HT-29 cells are more resistant to PMA-induced cell scattering than EGFP-expressing or parental HT-29 cells (Figure 5D).
Discussion
LZTFL1 is a novel gene with unknown biological function. Although it was predicted to have a tumor suppressive function based on its genomic location at the 3p21.3 region, there was no experimental evidence for this hypothesis. In this study, we present the first biochemical and functional evidence supporting a function for LZTFL1 in tumor suppression. LZTFL1 is expressed highly in epithelial cells of a wide array of normal tissues. Its expression is downregulated significantly in the corresponding tumor samples (Figure 1). Clinically, we found that downregulation of LZTFL1 correlated significantly with tumor metastases and predicted poorer survival outcome in gastric cancer patients (Figure 2). Restoration of LZTFL1 expression in tumor cells inhibited anchorage-independent cell growth and cell migration in vitro (Figure 3) and tumor growth in vivo (Figure 4). These data define LZTFL1 as a tumor suppressor. How does LZTFL1 inhibit tumorigenesis?
LZTFL1 may inhibit cell proliferation by promoting its differentiation. This is based on the experimental observations in Figure 5; LZTFL1 is upregulated in differentiated epithelial cells and co-localizes with E-cadherin at plasma membrane. The epithelium is composed of epithelial cells that are polarized and cohesively connected through E-cadherin-mediated adherens junctions. E-cadherin is attached to the actin cytoskeleton through a protein complex containing α- and β-catenin and other associated proteins. The stability of the attachment is essential for maintaining the epicol-basal polarities, and therefore the fully differentiated state of epithelial cells. We observed that LZTFL1 can bind actin in vitro (QW and ZPL, unpublished result). Thus, LZTFL1 could serve as a component of the protein complex at the adherens junction and bridge E-cadherin and the actin cytoskeleton. Loss of LZTFL1 could destabilize the E-cadherin-mediated adhesive complex and promote epithelial cell dedifferentiation. In support of this hypothesis, we observed that LZTFL1-overexpressing HT-29 cells are more resistant to PMA-induced cell scattering than HT-29 cells. A detailed biochemical analysis of the interaction between LZTFL1 and E-cadherin-associated protein complex at adherens junction is needed in the future to validate this hypothesis.
LZTFL1 may inhibit carcinoma metastases by inhibiting the EMT. In order for carcinoma to metastasize, the tumor epithelial cell has to go through the EMT to break away from its neighbors, lose E-cadherin-mediated cell-cell contacts, and gain migratory properties and other mesenchymal cell traits. Destabilization of E-cadherin-mediated adherens junction due to a loss of LZTFL1 would subject the tumor cells more susceptible to the EMT in response to signals from host stroma, whereas upregulation of LZTFL1 could enable the cells to resist it. Consistent with this idea, we found that downregulation of LZTFL1 in gastric tumors correlated with carcinoma metastases whereas upregulation of LZTFL1in tumor cells inhibited cell migration and anchorage-independent growth (Figure 2 and 3), a hallmark of tumor cell transformation.
Although LZTFL1 has several structural features that are shared by many transcription factors (18), the biochemical evidence remains to be established. It is conceivable that LZTFL1 may translocate into nucleus in response to certain external signals and function as a transcriptional cofactor, the membrane and cytoplasmic localization of LZTFL1favors the hypothesis that LZTFL1 is a cytoplasmic adaptor that participates in cell proliferation/differentiation.
In summary, our studies underscore the importance of LZTFL1 as a human tumor suppressor protein and provide mechanistic insights into the role LZTFL1 in tumor suppression. In the future, it will be worthy to validate its prognostic value in a separate group of patients and to study the effect of the lost-of function of LZTFL1 in tumorigenesis and its interplay with other oncogenic signaling pathways.
Supplementary Material
Acknowledgments
Qun Wei is a graduate student fellow of Chinese Scholarship Council. This study was supported by Texas Advanced Research Program under grant number 010019-0070-2007, and RO1 HL085749 from the National Heart, Lung, and Blood Institute to Z.-P. L.
References
- 1.Ji L, Minna JD, Roth JA. 3p21.3 tumor suppressor cluster: prospects for translational applications. Future Oncol. 2005;1:79–92. doi: 10.1517/14796694.1.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Hesson LB, Cooper WN, Latif F. Evaluation of the 3p21.3 tumor-suppressor gene cluster. Oncogene. 2007;26:7283–301. doi: 10.1038/sj.onc.1210547. [DOI] [PubMed] [Google Scholar]
- 3.Kiss H, Kedra D, Kiss C, Kost-Alimova M, Yang Y, Klein G, Imreh S, Dumanski JP. The LZTFL1 gene is a part of a transcriptional map covering 250 kb within the common eliminated region 1 (C3CER1) in 3p21.3. Genomics. 2001;73:10–9. doi: 10.1006/geno.2000.6498. [DOI] [PubMed] [Google Scholar]
- 4.Vinson C, Acharya A, Taparowsky EJ. Deciphering B-ZIP transcription factor interactions in vitro and in vivo. Biochim Biophys Acta. 2006;1759:4–12. doi: 10.1016/j.bbaexp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–66. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 6.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–9. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–7. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 9.Zhou YQ, He C, Chen YQ, Wang D, Wang MH. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing variants and their oncogenic potential. Oncogene. 2003;22:186–197. doi: 10.1038/sj.onc.1206075. [DOI] [PubMed] [Google Scholar]
- 10.Momparler RL. Epigenetic therapy of cancer with 5-aza-2′-deoxycytidine (decitabine) Semin Oncol. 2005;32:443–51. doi: 10.1053/j.seminoncol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Botrugno OA, Santoro F, Minucci S. Histone deacetylase inhibitors as a new weapon in the arsenal of differentiation therapies of cancer. Cancer Lett. 2009;280:134–44. doi: 10.1016/j.canlet.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Howell PM, Jr, Liu S, Ren S, Behlen C, Fodstad O, Riker AI. Epigenetics in human melanoma. Cancer Control. 2009;16:200–18. doi: 10.1177/107327480901600302. [DOI] [PubMed] [Google Scholar]
- 13.Pfeifer GP, Rauch TA. DNA methylation patterns in lung carcinomas. Semin Cancer Biol. 2009;19:181–7. doi: 10.1016/j.semcancer.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen E, Ophir I, Shaul YB. Induced differentiation in HT29, a human colon adenocarcinoma cell line. J Cell Sci. 1999;112:2657–66. doi: 10.1242/jcs.112.16.2657. [DOI] [PubMed] [Google Scholar]
- 15.Cummings TH, Pomare EW, Branch WJ, Naylor CPE, MacFarlane GT. Short chain fatty acids in human large intestine, portal, hepatic, and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto D, Clevers H. Wnt, stem cells and cancer in the intestine. Biol Cell. 2005;97:185–96. doi: 10.1042/BC20040094. [DOI] [PubMed] [Google Scholar]
- 17.Díaz VM, Hurtado M, Kort EJ, Resnati M, Blasi F, Thomson T, Paciucci R. Requirement of the enzymatic and signaling activities of plasmin for phorbol-ester-induced scattering of colon cancer cells. Exp Cell Res. 2006;312:2203–13. doi: 10.1016/j.yexcr.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Vinson C, Acharya A, Taparowsky EJ. Deciphering B-ZIP transcription factor interactions in vitro and in vivo. Biochim Biophys Acta. 2006;1759:4–12. doi: 10.1016/j.bbaexp.2005.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.