Summary
Chemokine ligand/receptor interactions affect melanoma cell growth, stimulate or inhibit angiogenesis, recruit leukocytes, promote metastasis, and alter the gene expression profile of the melanoma associated fibroblasts. Chemokine/chemokine receptor interactions can protect against tumor development/growth or can stimulate melanoma tumor progression, tumor growth and metastasis. Metastatic melanoma cells express chemokine receptors that play a major role in the specifying the organ site for metastasis, based upon receptor detection of the chemokine gradient elaborated by a specific organ/tissue. A therapeutic approach that utilizes the protective benefit of chemokines involves delivery of angiostatic chemokines or chemokines that stimulate the infiltration of cytotoxic T cells and natural killer T cells into the tumor microenvironment. An alternative approach that tackles the tumorigenic property of chemokines uses chemokine antibodies or chemokine receptor antagonists to target the growth and metastatic properties of these interactions. Based upon our current understanding of the role of chemokine-mediated inflammation in cancer, it is important that we learn to appropriately regulate the chemokine contribution to the tumorigenic `cytokine/chemokine storm', and to metastasis.
Keywords: melanoma, chemokine, chemokine receptor, metastasis, angiogenesis, tumor microenvironment, inflammation
Introduction
Chemokines are secreted, low molecular weight proteins (~8–30 kDa) that bind to seven transmembrane G protein-coupled receptors. There are approximately 50 chemokines characterized to date and over 20 chemokine receptors (Zlotnik et al., 2006) (Figure 1). Chemokines have conserved cysteine residues that play an important role in their structural conformation and function. Depending upon whether there are intervening amino acids between the first two conserved cysteines, chemokines are classified into subfamilies denoted as CXC, CC, CX3C and C, where the CXC chemokines have one amino acid between the first two cysteine residues, CC chemokines lack this intervening amino acid, CX3C have three intervening amino acids between the first two cysteines, and the C subfamily lacks one cysteine in the first pair of cysteine residues. Figure 1 shows the chemokines that bind to each chemokine receptor. For example CXCR1 binds CXCL6 and CXCL8, while CXCR2 binds CXCL1–3, 5–8. CXCR4 only binds CXCL12, CX3CR1 binds only CX3CL1, and CCR9 only binds CCL25. CCR7 binds CCL19 and 21, while CCR2 binds CCL2, 7, 8, 13 and 16.
Figure 1.
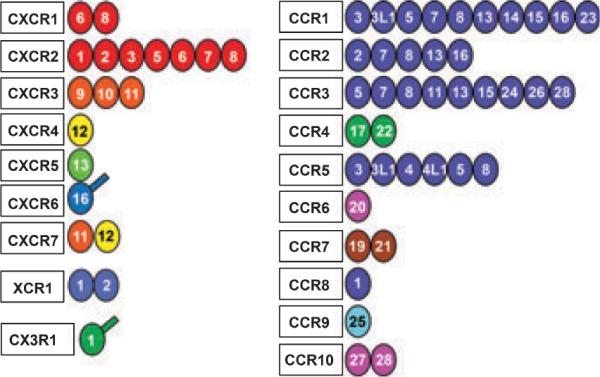
Diagramic representation of the family of human chemokines and their receptors. The chemokines are represented by the ligand number and the receptors to which they bind are indicated according to their classification of CXC, C, CX3C or CC. Accordingly the ligand number beside the receptor indicates CC or CXC chemokine. For example, the `8' adjacent to `CXCR1' represents CXCL8. The colors are an indication of different chromosomal localization of the genes for that ligand such that if the color for all the ligands for one receptor is the same, then they are all found on the same chromosome. Two chemokines, CXCL16 and CX3CL1, are transmembrane proteins and the extra line on the ligand circle indicates this. [This figure is reprinted with permission from Zlotnik et al. (2006), Biomed Central.]
Though chemokines were first characterized in reference to their role in leukocyte trafficking, over time we have come to understand that chemokines are produced by most tissues and chemokine receptors are expressed not only on leukocytes, but on most cell types. Chemokine/chemokine receptor interactions are important in development, wound healing, infection, and tissue maintenance. The expression of chemokines and chemokine receptors are often strongly up-regulated during tumorigenesis (Figure 2). This is true in cancers of the breast, lung, prostate, colon, ovary, bladder, pancreas, liver, skin (including melanocytes), head and neck, cervix, kidney, brain, and blood cells including multiple myeloma and leukemia (Raman et al., 2007). Tumor cells take advantage of the expression of chemokines and chemokine receptors to either stimulate the immune response to the tumor or to induce tumor angiogenesis and tumor growth, alter the tumor microenvironment, and facilitate metastasis to specific target organs (Figure 3). A number of reviews have been written previously attesting to the role of chemokines in melanoma tumor growth and metastasis (Dhawan and Richmond, 2002; Dhawan et al., 2008; Kakinuma and Hwang, 2006; Luan et al., 1997; Murakami et al., 2004; Payne and Cornelius, 2002; Raman et al., 2007; Zigler et al., 2008) and signal transduction pathways associated with chemokine receptor activation that modulate cell motility, cell growth, and chemotaxis have been recently reviewed (Thelen and Stein, 2008). Therefore, this review will provide an update on chemokine/chemokine receptors as mediators of melanoma tumor progression/growth, tumor angiogenesis, metastasis, alterations in the tumor microenvironment, or as regulators of the innate immune response to the tumor. Finally, chemokines and chemokine receptors as targets for melanoma therapy and prognosis will be discussed.
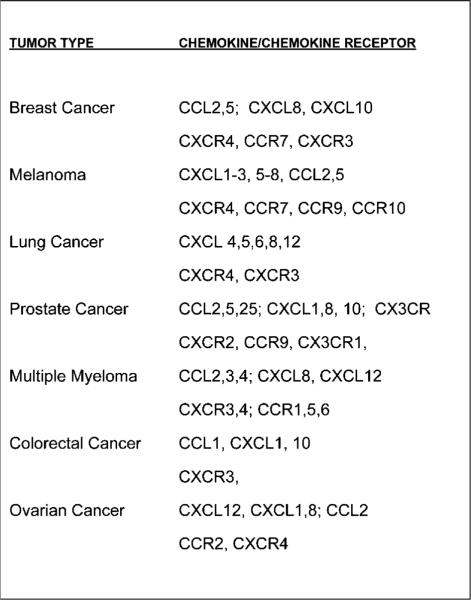
Figure 2.
Cancer cells display a variety of chemokine receptors and produce multiple chemokines. This figure lists the chemokine receptors and ligands reported to be produced in a number of types of cancer.
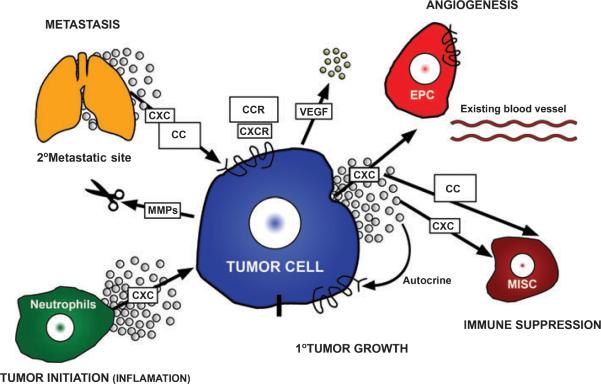
Figure 3.
Melanoma tumor cells elaborate CXC and CC chemokines that affect angiogenesis, inflammation, the tumor microenvironment, immune cell response, recruitment of leukocytes, tumor growth and metastasis. The melanoma cells also express chemokine receptors that direct metastasis to specific target organs that elaborate a chemokine gradient that binds and activates these melanoma cell chemokine receptors. Chemokines also stimulate production of metalloproteinases that degrade the matrix and facilitate melanoma metastasis. CXC, CXC chemokine ligands; CXCR, CXC chemokine ligand receptor; CC, CC chemokine ligands; CCR, CC chemokine ligand receptor; EPC, endothelial progenitor cell; MISC, myeloid immune suppressor cells; MMPs, matrix metalloproteinases; VEGF, vascular endothelial growth factor.
Melanoma tumor cells have been reported to produce CXCL1–3, CXCL5–8, CXCL10, CCL2, and CCL5. Interestingly, chemokine receptors have also been reported to be expressed by melanoma cells, including CXCR1, CXCR2, CXCR3, CXCR4, CXCR6, CXCR7, CCR1, CCR2, CCR5, CCR7, CCR9, CCR10 (Navarini-Meury and Conrad, 2008; Raman et al., 2007; Richmond, 2008). CCR5 and CCR7 are expressed in primary melanoma lesions and some metastatic lesions, while CXCR4 and CCR1 are expressed in melanocytes, melanoma cell lines, primary and metastatic melanomas (Seidl et al., 2007). Expression of CXCR3 by melanoma tumor cells has been correlated with absence of the tumor infiltrating lymphocytes and a poorer prognosis in melanoma (Monteagudo et al., 2007). The mechanism for this quite interesting observation is unclear.
Chemokines/chemokine receptors in melanoma tumor progression and tumor growth
CXCL8 as well as CXCR1 and CXCR2 are associated with melanoma tumor progression by affecting growth of the tumor cells, angiogenesis and metastasis (Bar-Eli, 1999; Norgauer et al., 1996; Ramjeesingh et al., 2003; Schadendorf et al., 1993; Varney et al., 2006; Wang et al., 1990). During melanoma tumor progression, chemokine expression progressively increases, in part due to the deregulation of the NF-κB family of transcription factors (Richmond, 2002; Yang and Richmond, 2001). In fact, blocking NF-κB inhibits the endogenous production of angiogenic chemokines and inhibits melanoma tumor growth in mice (Yang et al., 2006; Yang et al., 2007).
Gene expression profiling studies show that CXCL1 mRNA is highly upregulated in three dimensional cultures of melanoma cells and in human melanoma tumors (Ghosh et al., 2005; Haqq et al., 2005; Taxman et al., 2003). CXCL1–3 can act as oncogenes in immortalized murine melanocytes. Over-expression of CXCL1, 2 or 3 in spontaneously immortalized melanocytes (Bennett et al., 1987) confers the malignant phenotype on these cells based upon enhanced growth in soft agar and tumor formation in mice (Balentien et al., 1991; Owen et al., 1997). Moreover, antibodies to CXCL1, 2, or 3 inhibit the growth of melanoma tumors expressing each of these respective chemokines in mice (Haghnegahdar et al., 2000; Owen et al., 1997). Additional evidence in support for the transforming properties of CXCL1 comes from genetically modified mice. INK4a/ARF null transgenic mice expressing MIP-2 (murine ortholog of CXCL1) under the transcriptional direction of the tyrosinase promoter/enhancer, exhibited increased melanoma tumor incidence after treatment of the skin with the chemical carcinogen, DMBA (Yang et al., 2001). Altogether, these data point to an important role for this chemokine in the promotion of melanocyte transformation.
Angiogenesis
A number of angiogenic chemokines are produced by malignant melanoma cells. The major ones are CXCL1–3, CXCL5–8. These ligands bind to CXCR2, which is expressed on endothelial cells, and promote the growth and migration of these endothelial cells to facilitate tumor angiogenesis. CXCL8 and CXCL6 also bind CXCR1 which functions much like CXCR2. Varney et al. have shown that CXCR1 is expressed ubiquitously in melanoma tumor specimen but the CXCR1 expression does not correlate with the Clark level (Varney et al., 2003). In contrast, expression of CXCL8 and CXCR2 increased between thin and thick melanoma as well as with metastatic lesions. CXCL8 and CXCR2 expression increase during the transition from radial to vertical growth phase is thought to be important in the transition to increased aggressiveness of tumors and the metastatic properties of the tumor (Varney et al., 2006).
Melanoma cells exhibit the capacity to form tube-like structures that mimic endothelial cells, and this is termed `vasculogenic mimicry' (Maniotis et al., 1999). CXCL8 and several other genes co-expressed with galectin-3 have been linked to melanoma cell vascular mimicry (Mourad-Zeidan et al., 2008). Silencing of galectin-3 reduces the transcription of vascular endothelial-cadherin and CXCL8 and results in a loss of vascular mimicry properties of human melanoma cells. Though the data do not point to a direct link between chemokine expression and vascular mimicry, the association is quite interesting.
Thrombin induction of angiogenesis has been shown to up-regulate CXCL1 expression, which in turn stimulates angiogenesis around melanoma tumors, MMP1, MMP2, VEGF, angiopoietin-2, CD31 and KDR as well as CXCR2 expression in HUVECs. The effects of thrombin on the induction of gene expression and angiogenesis in B16 melanoma cells were blocked with shRNA for CXCL1, strongly supporting a major role for CXCL1 in melanoma tumor angiogenesis (Caunt et al., 2006). In addition, there are reports that activation of CXCR2 leads to an up-regulation of VEGF, which can in turn further promote tumor angiogenesis (Caunt et al., 2006).
Several chemokines outside the CXC group are elaborated by melanoma cells and affect the tumor vasculature, including CCL2 (Graves et al., 1992), CX3CL (Ren et al., 2007) and CCL5 (Mrowietz et al., 1999). CCL2 has been implicated in arteriogenesis (Keeley et al., 2008). Fractalkine (CX3CL) is expressed on the surface of B16 melanoma cells and promotes cell-cell adhesion. When CX3CL1 expression is targeted by sh-RNA vectors, the growth of the B16 melanoma tumors is slowed and there is reduced tumor angiogenesis (Ren et al., 2007). The full mechanism for this reduced angiogenesis has not yet been determined.
In addition to angiogenic chemokines, melanoma cells also produce the angiostatic chemokines CXCL9, CXCL10, and CXCL11. These angiostatic chemokines bind CXCR3 and inhibit angiogenesis (Keeley et al., 2008; Mehrad et al., 2007). In addition, a variant of another angiostatic chemokine, CXCL4, is reported to block angiogenesis and inhibit melanoma tumor growth (Struyf et al., 2007). CXCL11 binds to both CXCR3 and CXCR7, but the functional significance of the CXCR7 interaction remains unclear. CXCR7 is expressed on both endothelial cells and tumor cells. There are reports that CXCR7 competes with CXCR4 for CXCL12 binding and in this way serves as a `decoy' receptor (Boldajipour et al., 2008). Other reports argue that CXCR7 is capable of stimulating the growth of tumors (Meijer et al., 2008; Miao et al., 2007; Wang et al., 2008). CXCR2, CXCR3, CXCR4 and CXCR7 are expressed on melanoma cells and to a lesser extent on endothelial cells (Schutyser et al., 2007). These data argue that each of these chemokine receptors are available in vivo for modulation of melanoma tumor cell growth, regulation of angiogenesis, or migration.
CXCR4 expression on vascular endothelial cells is reported to be pro-angiogenic (Chen et al., 2006; Guleng et al., 2005; Liang et al., 2007; Petit et al., 2007; Salcedo and Oppenheim, 2003; Zagzag et al., 2006), though controversy remains as to whether this is a direct or indirect effect. Hypoxia has been shown to induce the expression of CXCR4 on HMEC-1 microvascular endothelial cells and on SK-Mel5 melanoma cells, but hypoxia does not induce the expression of CXCR2 or CXCR3 (Schutyser et al., 2007). Since hypoxia induces new angiogenesis, perhaps induction of CXCR4 on endothelial cells facilitates this.
Metastasis
Perhaps the most progress in understanding the role of chemokines in melanoma in the last 5 yrs has been made in the area of metastasis. We have come to understand that CXCR4, CCR7 and CCR9 are major determinates of melanoma metastasis to specific target organs. CXCR4 expressing melanomas tend to metastasize to the lung, bone marrow and liver, while CCR7 and CXCR3 expressing melanoma lesions metastasize to the lymph node (Cardones et al., 2003; Kawada et al., 2004; Kim et al., 2006; Murakami et al., 2004; Murakami et al., 2002; Scala et al., 2007). CXCR4 is the most widely expressed chemokine receptor in melanoma based upon microarray studies and qRT (Kim et al., 2006). CXCL12 expression by liver, lung and bone marrow promote metastasis of melanoma tumor cells to these organs (Kim et al., 2006). For uveal melanoma, both CXCR4 and CCR7 are reported to be associated with liver metastasis (Li et al., 2008; Scala et al., 2007). CCR9 expressing melanomas have a very high probability of metastasizing to the small intestine (Amersi et al., 2008). In addition, there is preclinical evidence that CXCR2 is important for melanoma metastasis to the lung (Singh et al., 2009).
CXCR4 is important for early steps in melanoma metastasis to the lung. Knocking down CXCR4 in melanoma cells impairs melanoma metastasis to the lung in a murine xenograft model (Bartolome et al., 2009). CXCR4 activation by CXCL12 also induces expression of the MT1 membrane-bound matrix metalloproteinase (MT1-MMP), but this matrix metalloproteinase (MMP) remains intracellular until the cells come in contact with the appropriate basement membrane proteins, then MT1-MMP moves to the plasma membrane in a PI-3K dependent manner (Bartolome et al., 2009). Tumor cell expression of CXCR4, down regulation of E-cadherin, and elevations in activated Cdc42 are associated with poor prognosis in melanoma. Inhibition of the CXCR4/CXCL12 pathway by pretreatment of melanoma cells with CTCE-9908, a small peptide antagonist of CXCR4, reduces pulmonary metastasis in a melanoma mouse tail vein injection model (Kim et al., 2008). This inhibition did not occur unless the melanoma cells were pretreated with CTCE-9908, indicating the antagonist was disrupting the interaction of tumor cell CXCR4 with ligand and was not due to an effect on the tumor infiltrating lymphocytes or endothelial cells.
CCL21, one of the ligands for CCR7, is produced by lymphatic endothelial cells and the release of this chemokine by the lymphatic endothelial cells is associated with the metastatic properties of CCR7 expressing malignant melanoma cells (Shields et al., 2007; Wiley et al., 2001). CCL27 is produced by the skin and when melanoma cells express its receptor, CCR10, this facilitates skin metastasis (Ben-Baruch, 2008; Forster et al., 2001; Simonetti et al., 2006; Takeuchi et al., 2004; Takeuchi et al., 2007). A study of 59 cutaneous melanocytic lesions demonstrated that CCR10 is expressed in both benign and malignant melanoma lesions and the CCR10 expression level correlates with the Breslow depth (Simonetti et al., 2006). Interestingly, in that study CCL27 expression levels correlated with reduced levels of CD3+ and CD8+ lymphocytes in the lesions, suggesting that CCR10/CCL27 may facilitate escape from host immune surveilance and facilitate tumor growth.
CCR9 has been described as a `homing receptor' for melanoma metastasis to the small bowel. CCL25, the ligand for CCR9 is highly expressed in small bowel and thymus. It has been demonstrated that 102 out of 198 melanomas metastasize to the small bowel and 88 of 102 with small bowel metastasis expressed CCR9. Thus CCR9 expression on melanoma carries a greater than 60% chance that there will be small bowel metastasis (Amersi et al., 2008).
Expression of CCL5 by melanoma cells has been linked to tumor progression. Melanomas that express CCL5 are reported to be more aggressive, though receptors for this chemokine are not usually expressed by melanoma cells. However CCR5 expression on stromal cells has been reported to aid lung metastasis for melanoma in a murine model (van Deventer et al., 2005). The mechanism for this is unclear, but probably involves recruitment of macrophages into the tumor microenvironment that subsequently facilitate metastasis. Interestingly, the expression of the mutant form of CCR5, CCR5Δ32, in stage IV melanoma patients was associated with a decreased survival following immunotherapy, suggesting that patients with CCR5Δ32 mutations are less prone to benefit from immunotherapy (Ugurel et al., 2008).
Ultraviolet-B irradiation stimulates the production of CXCL8 which in turn enhances the migration of metastatic melanoma cells in vitro (Gebhardt et al., 2007). One of the receptors for CXCL8, CXCR1, is reported to be important for trans-endothelial migration of melanoma cells (Ramjeesingh et al., 2003). In vivo murine studies show that CXCR2 plays a major role in melanoma metastasis to the lung. In a very elegant series of experiments the CXCR2−/− Balb/C mouse was bred onto a Balb/C nude background. B16 melanoma xenografts exhibited inhibition of melanoma tumor growth and inhibition of lung metastasis on CXCR2−/− nude mice. This was accompanied by reduction in tumor cell proliferation, angiogenesis and reduced inflammation (Singh et al., 2009).
Alterations in the tumor microenvironment
Mantovani has recently reviewed the role that inflammatory cytokines play to facilitate the metastatic capacity of tumor cells. Included are interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNFα) and receptor activator of nuclear factor-kappa B ligand (RANKL). Each of these cytokines is regulated by the nuclear factor-kappa B (NF-κB) family of transcription factors (Mantovani, 2009). Toll-like receptors (TLRs) also can play a role in this NF-κB activation that induces expression of chemokines and chemokine receptors to augment the inflammatory process and produce the `cytokine storm', a never ending cycle of macrophage recruitment, release of cytokines, and activation of signal transduction pathways that can facilitate tumor growth and metastasis. These chemokines, cytokines and growth factors released by macrophages subsequently affect the stromal cells as well and activate expression of a number of genes that can predispose the microenvironment to enhanced tumor growth.
Macrophages recruited into the tumor microenvironment are known as tumor associated macrophages or TAMs. There are two types of TAMs, M1 and M2 (Hagemann et al., 2008). The M1 macrophages are the TAMs classically activated by microbial products or IFN-γ. M1 TAMs are characterized by high levels of production of IL-12, high production of pro-inflammatory cytokines and high levels of MHC molecules. When NF-κB is activated in these macrophages, they convert from the classically activated and cytotoxic M1 to the alternatively activated M2 phenotype. M2 macrophages exhibit a change in cytokine production from IL-12 and IL-10 with high levels of MHC molecules (M1) to high production of TGFβ and IL-10 but not IL-12, and there is low production of MHC. M2 macrophages with their respective cytokine profile promote tumorigenesis, while M1 macrophages are thought to be more anti-tumorigenic. M2 macrophages release CCL2, which can stimulate metalloproteinase production. Inhibition of IKKβ in bone marrow derived macrophages or tumor associated macrophages enhanced M1 macrophage mediated tumoricidal activity in vitro (Hagemann et al., 2008). This enhanced tumoricidal activity was accompanied by increased production of IL-12, decreased arginase-1 expression and elevated iNOS2 expression, all traits of M1 macrophages.
In vivo, melanoma produced chemokines also recruit tumor-specific T cells. Production of CCL2 by melanoma cells is important for recruitment of cytotoxic T lymphocytes (CTLs) to the tumor microenvironment through interaction with lymphocyte CCR4 expression (Ugurel et al., 2008; Zhang et al., 2006a). Depending upon the level of expression of CCL2, this chemokine can either promote or reduce melanoma tumor growth (Bottazzi et al., 1992). In elegant experiments CCL2 was targeted with shRNA in B16 melanoma cells and the transfected melanoma cells were injected into the pleural cavity of syngeneic immunocompetent mice (Stathopoulos et al., 2008). Mice injected with the B16 melanoma cells expressing shRNA against CCL2 exhibited reduced malignant pleural effusions and enhanced survival, compared to control shRNA transfectants. If CCL2 was over-expressed, there was an increase in malignant pleural effusions, enhanced vascular permeability, mononuclear recruitment, angiogenesis and decreased survival. CCL2 also plays a role in recruitment of M2 macrophages which subsequently stimulate massive tumor angiogenesis (Gazzaniga et al., 2007). However, these data are in direct conflict with another report that over-expression of CCL2 in B16 melanoma cells resulted in enhanced production of Th2 cytokines and reduced melanoma tumor growth in syngenic C57Bl/6 mice (Hu et al., 2007). This reduction in tumor growth with over-expression of CCL2 was not observed in the CCL2 expressing B16 melanoma cells xenografted into nude mice. Since nude mice with human melanoma xenografts that do not express CCL2 exhibit reduced T cell homing to the tumor after adoptive transfer of human cytotoxic lymphocytes (CTLs), it appears that CCL2 produced by tumor cells can boost the recruitment of adoptively transferred human CTLs to the tumor microenvironment and suppress tumor growth (Brown et al., 2007). This type of dichotomy in the role of chemokines on the immune system may be tightly linked to the tumor microenvironment. There will be different outcomes depending upon whether the T cells that are recruited are capable of tumor cell killing, or whether they promote tumor metastasis through release of factors that facilitate intravasation of tumor cells into the vascular system.
Recruitment of CD8+ T cells and natural killer T cells (NKT) to the tumor microenvironment is very important for immune surveillance and anti-tumor defense processes. Parovirus delivery of CCL7 (MCP3) has been shown to boost T and NKT cell responses to melanoma tumors (Wetzel et al., 2007). Interestingly, expression of CXCR3 by CD8+ CD45RO+ T cells is associated with enhanced survival of stage III, but not stage IV, melanoma patients. This parameter may prove to be important for prognosis (Mullins et al., 2004). When melanoma cells were transfected to express CCL21, mice bearing these tumors survived longer after adoptive T cell therapy than mice bearing melanoma tumors that did not express CCL21 (Thanarajasingam et al., 2007). These data show that CCL21 primed antitumor immunity following adoptive T cell transfer. Additional data support for use of CCL21 to boost the immune response to the tumor by recruiting T cells and dendritic cells into the tumor microenvironment. Administering CCL21 prior to delivery of a plasmid DNA melanoma cancer vaccine induced strong immunity against the melanocyte antigen, TRP2 (Yamano et al., 2006). This observation could prove very useful in the clinic in the treatment of metastatic melanoma.
CXCR4 also is important for cytotoxic lymphocyte trafficking to melanoma tumor cells. As tumor cells secrete CXCL12 into the tumor microenvironment, CXCR4 expressing cytotoxic lymphocytes (Zhang et al., 2006b) and dendritic cells (Fushimi et al., 2006) move into the tumor microenvironment. This can be blocked with antibodies to CXCL12, CXCR4, very high concentrations of CXCL12, or a small molecule antagonist of CXCR4 (Zhang et al., 2006a; Zhang et al., 2005; Zhang et al., 2006b).
Chemokine serum levels are also reported to be biomarkers for inflammatory diseases as well as cancer. CXCL8 is a potential clinical biomarker for mutant B-RAF (V600E) mediated tumor growth since inhibition of B-RAF in melanoma tumor bearing mice reduces CXCL8 plasma levels (Crawford et al., 2008). Production of CXCL8 by human melanoma cells induces MMP-2 activity and increases both tumor growth and metastasis (Luca et al., 1997). Extensive data show that tumor cells also stimulate the production of CXCL1 and CXCL2 chemokines in fibroblasts, and these cancer associated fibroblasts play an important role in tumor progression and metastasis (Gallagher et al., 2005). Neutrophils recruited into the tumor microenvironment also are induced to produce CXCL8, which in turn promotes a microenvironment that is supportive of metastasis (Peng et al., 2007). Chemokines produced by tissues in predicted sites for metastasis are reported to be important in establishing the `premetastatic niche' through the recruitment of bone marrow derived progenitor stem cells (Croker and Allan, 2008; Kucia et al., 2005; Shiozawa et al., 2008).
Angiostatic chemokines as a therapeutic modality
Delivery of angiostatic chemokines to melanoma inhibits the growth of melanoma xenografts in mice. For example, xenograft models using human melanoma cells transfected to express CXCL10 grew more slowly than parental or vector control cells (Yang and Richmond, 2004). Moreover, expression of a mutant form of CXCL10 that cannot bind to CXCR3 in the melanoma cells resulted in tumor growth at a rate equivalent to that of parental melanoma cells. Mutation of the GAG binding domain alone did not reduce the CXCL10 growth inhibitory capacity (Yang and Richmond, 2004). These data argue that CXCL10 activation of the CXCR3 expressed on melanoma cells, endothelial cells, or immune cells is an important negative regulator of melanoma tumor growth. Interestingly, when B16 melanoma cells are given a vaccine strain encoding flk1 and one encoding CXCL10, the tumor growth was reduced, CTL responses were enhanced, angiogenesis and cell proliferation were reduced, and apoptosis was enhanced (Lu et al., 2008). Potentially, delivery of CXCL10 or factors that induce CXCL10 can be used for melanoma therapy. IL-12 biotherapy provides a potential example of this type of therapy, since IL-12 induces IFNγ, which in turn can induce CXCL9, 10 and 11 which are angiostatic (Lesinski et al., 2004; Okada et al., 2004; Palmer et al., 2001). Though a number of problems have arisen with IL-12 biotherapy, it is proposed that delivery of the IL-12 directly to the tumor cells may offer therapeutic potential through induction of an anti-angiogenic program (Airoldi et al., 2007). How much this is dependent upon induction of angiostatic chemokines remains in question, however.
Delivery of mutant CCL2 to melanoma bearing mice also inhibits tumor angiogenesis and tumor growth (Koga et al., 2008). The mutant CCL2 reduced TNFα, IL-1 and VEGF in the tumor microenvironment, likely due to the reduction in recruitment of tumor associated macrophages.
Therapy for melanoma based upon antagonizing chemokines
It has been postulated that therapies which can antagonize CXCR4, CCR7, CCR9, CCR10, CXCR2, CCL5, or CXCL8 will be useful for melanoma therapy. A number of peptide antagonists and small molecule inhibitors of chemokines and chemokine receptors are already being tested in preclinical models for therapeutic effects on melanoma tumors and other tumors as well. CXCR4 has been targeted by a number of antagonists. One example of this is single subcutaneous delivery of the 4F-bTE peptide antagonist for CXCR4 in microcapsules of biodegradable poly D,L-lactic acid blocks lung metastasis of melanoma (Takenaga et al., 2004). Another example is the T22 peptide antagonist of CXCR4, which has also been shown to block lung metastasis of B16 melanoma cells expressing CXCR4. However, the T22 antagonist did not block the tumor growth of the CXCR4 expressing B16 melanoma cells (Lee et al., 2006). This suggests that combinational therapies will be needed to be used to block both growth and metastasis of melanoma tumors. The bicyclam AMD3100 is still another antagonist that targets CXCR4. AMD3100 has been successfully tested in patients with multiple myeloma, leukemia, non-Hodgkin's lymphoma to mobilize CD34+ cells from the bone marrow (Devine et al., 2004; Fierro et al., 2008; Fruehauf et al., 2006). Though caution is needed with regard to detrimental cardiovascular effects based upon our knowledge from CXCR4−/− mice, CXCR4 may prove to be a useful target for melanoma and other cancer types.
The Duffy antigen receptor for chemokines (DARC) acts in part as a decoy receptor, binding CXC chemokines CXCL1, CXCL5, CXCL8, CCL5 and other chemokines. There are reports that persons who are Dfy−/− exhibit increased incidence of prostate cancer, suggesting this receptor may play a role as a negative regulator of tumor progression (Lentsch, 2002; Shen et al., 2006; Stephan et al., 2005). To determine whether DARC could serve as a negative regulator of melanoma tumor growth by providing a ligand sink for angiogenic chemokines and chemokines that recruit macrophages to the tumor microenvironment, we developed transgenic mice over-expressing DARC on the endothelial cells under the transcriptional regulation of the preproendothelin promoter/enhancer (PPEP). These PPEP-mDARC transgenic mice exhibited reduced melanoma tumor growth and reduced angiogenesis in association with the tumor (Horton et al., 2007). These data support the concept that targeting ligands for CXCR2 (CXCL1–3, and CXCL5–8) or the ligand CCL5 may offer therapeutic benefit for melanoma patients.
Antibodies to chemokines offer promise as a therapeutic modality for treatment of malignant melanoma. Humanized antibodies to CXCL8 have been shown to inhibit melanoma tumor growth, angiogenesis and metastasis (Melnikova and Bar-Eli, 2006; Zigler et al., 2008). Antagonists for CXCR2 are also under consideration for melanoma therapy. In vitro studies show that chemotherapy with paclitaxel dangerously induces CXCL1 and CXCL8 expression, but fortunately this induction can be inhibited with MEK inhibitors (Taxman et al., 2003). Schering Plough, AstraZeneca and Glaxo-Smith-Kline have developed CXCR2 antagonists that show some promise for cancer treatment (Gonsiorek et al., 2007; Wang et al., 2006; Wilson et al., 2008). Thus there is hope for utilizing CXC chemokine/chemokine receptor antagonist for future melanoma therapy.
Clearly we need drugs that appropriately and specifically target chemokines and we need appropriate molecular diagnostics on each melanoma patient tumor. With these in hand, we should be able to better predict the specific pathways affected in the patient tumor so that therapy can be individualized for each patient based upon the molecular genotype of the tumor. However, these genetic profiles will prove to be complex and depending upon the combination of genetic abnormalities any one patient has, therapeutic response will need to be differentially targeted. The complexity of the tumor profile is a direct consequence of mutations and loss of tumor suppressors. For example, mutation of BRAF, loss of P53, amplification of aurora kinases, loss of PTEN, mutation of N-Ras, loss of MITF, loss of INK4a/ARF are a few of the types of genetic alterations that often occur in melanoma. As research efforts continue toward improved diagnostics and development of drugs that specifically target the affected genes or pathways, there is hope for improved treatment of advanced melanoma patients.
New horizons for examining chemokines as prognostic indicators in cancer
We are learning that inflammation plays a key role in the development of many cancers. It remains unclear exactly how chronic inflammation is involved in initiation or progression of cancer, but recent work has shown that epigenetic processes may be involved (Hahn et al., 2008). Chemokine amplification leads to persistent leukocyte infiltration which is associated with tumorigenesis in a number of cancers including cancers of the lung, colon, liver, cervix, prostate, bladder, ovary, esophagus, skin and lymphatics (Balkwill et al., 2005; Balkwill and Mantovani, 2001; Mantovani, 2009; Mantovani et al., 2008).
Chemokine expression profiles have been shown to predict recurrence and disease free survival in many non-melanoma cancers. In prostate cancer, one study shows CX3CL1 and IL-15 expression in prostate tissue were associated with recurrence-free survival following prostatectomy, while CCL4 expression was associated with recurrence (Blum et al., 2008). Another study examining cancer cell expression of the receptor for CX3CL1, CX3CR1, shows that high expression of CX3CR1 in pancreatic ductal adenocarcinoma is associated with neurotropism of these cancer cells, likely due to the expression of CX3CL1 by neurons (Marchesi et al., 2008).
CXCR4 expression in primary tumors after neo-adjuvant chemotherapy has been demonstrated to predict poor outcome for patients with locally advanced breast cancer (Holm et al., 2009). Elevation in CXCR4 predicts recurrence in HER-2 negative breast cancer (Holm et al., 2007). Over-expression of CXCR4 and VEGF predict early distant relapse in stage II–III colorectal cancer patients (Ottaiano et al., 2006).
CCR7 expression in metastatic squamous cell carcinoma (SCC) is associated with lower incidence of disease free survival and lower overall survival (Tsuzuki et al., 2006). CCR7 positive SCCs were associated with large lymph node metastases, progressive disease, local recurrence and death due to cancer. Learning how to prevent this process will be important for future therapies (Tsuzuki et al., 2006).
Polymorphisms in VEGF (C936T and CXCL8 T251A) are shown to predict tumorrecurrence in stage III colon cancer, even though these mutations are silent mutations (Lurje et al., 2008). Polymorphism of CXCL8 is also associated with risk of recurrence for patients with rectal cancer (Gordon et al., 2006).
In melanoma patients, tumor burden, VEGF and CXCL8 serum levels are independent predictive factors of progression-free survival, where elevations in the serum levels of these angiogenic factors strongly correlate with a poor overall prognosis and reduced progression-free survival (Ugurel et al., 2001). Moreover, serum levels of CXCL8 are reported to fall as stage IV melanoma patients respond to chemotherapy or immune-chemotherapy, while persistence of elevated VEGF and bFGF serum levels is indicative of treatment resistance (Brennecke et al., 2005). These studies did not examine the expression of CXCR1 or CXCR2 on the melanoma cells, so it is unclear whether the effects of CXCL8 on the tumor growth are due to an autocrine or paracrine loop.
Though the serum level of CXCL8 is associated with prognosis of melanoma patients, there are not clear studies showing that tumor CXCL8, CCR7 or CXCR4 mRNA levels correlate with metastasis. This could be due to failure to appropriately micro-dissect samples prior to mRNA isolation and amplification (Otto et al., 2007).
Conclusions
Clearly much more work and better techniques are needed to adequately characterize the prognostic value of determining chemokine and chemokine receptor expression in melanoma tumor cells and patient serum. Studies thus far show that the major chemokine/chemokine receptor interactions that appear to be important in melanoma development and progression include CXCR4/CXCL12, CXCR2/CXCL1, 5 and 8, CCL2/CCR2, CCR7/CCL19 and 21, CCR9/CCL25. The status of a number of chemokine/chemokine receptor antagonists and a number of inhibitors in Phase I, II or III clinical trials in a variety of diseases has recently been reviewed (Wells et al., 2006). Though the pharmaceutical companies are reluctant to launch clinical trials with these reagents in cancer, as they are approved for other disorders, perhaps the door will open for targeting chemokine/chemokine receptor interactions in cancer. Hopefully new methods for appropriately intervening in these interactions will bring improved therapy for melanoma patients.
Acknowledgements
This work is supported in part by a VA Senior Research Career Scientist Award and a VA Merit Award to Ann Richmond and grants from the NCI CA34590, CA 116021, CA098807. Figure 2 was designed and contributed by Nicole Neel (Vanderbilt University School of Medicine and University of North Carolina, Chapel Hill) with the help of artist, Mike Neel (no university affiliation).
References
- Airoldi I, Di Carlo E, Cocco C, Taverniti G, D'Antuono T, Ognio E, Watanabe M, Ribatti D, Pistoia V. Endogenous IL-12 triggers an antiangiogenic program in melanoma cells. Proc. Natl Acad. Sci. USA. 2007;104:3996–4001. doi: 10.1073/pnas.0609028104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amersi FF, Terando AM, Goto Y, Scolyer RA, Thompson JF, Tran AN, Faries MB, Morton DL, Hoon DS. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin. Cancer Res. 2008;14:638–645. doi: 10.1158/1078-0432.CCR-07-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balentien E, Mufson BE, Shattuck RL, Derynck R, Richmond A. Effects of MGSA/GRO alpha on melanocyte transformation. Oncogene. 1991;6:1115–1124. [PubMed] [Google Scholar]
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology. 1999;67:12–18. doi: 10.1159/000028045. [DOI] [PubMed] [Google Scholar]
- Bartolome RA, Ferreiro S, Miquilena-Colina ME, et al. The chemokine receptor CXCR4 and the metalloproteinase MT1-MMP are mutually required during melanoma metastasis to lungs. Am. J. Pathol. 2009;174:602–612. doi: 10.2353/ajpath.2009.080636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin. Exp. Metastasis. 2008;25:345–356. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int. J. Cancer. 1987;39:414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- Blum DL, Koyama T, M'Koma AE, Iturregui JM, Martinez-Ferrer M, Uwamariya C, Smith JA, Jr, Clark PE, Bhowmick NA. Chemokine markers predict biochemical recurrence of prostate cancer following prostatectomy. Clin. Cancer Res. 2008;14:7790–7797. doi: 10.1158/1078-0432.CCR-08-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- Bottazzi B, Walter S, Govoni D, Colotta F, Mantovani A. Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth, and susceptibility to IL-2 therapy of a murine melanoma. J. Immunol. 1992;148:1280–1285. [PubMed] [Google Scholar]
- Brennecke S, Deichmann M, Naeher H, Kurzen H. Decline in angiogenic factors, such as interleukin-8, indicates response to chemotherapy of metastatic melanoma. Melanoma Res. 2005;15:515–522. doi: 10.1097/00008390-200512000-00006. [DOI] [PubMed] [Google Scholar]
- Brown CE, Vishwanath RP, Aguilar B, Starr R, Najbauer J, Aboody KS, Jensen MC. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J. Immunol. 2007;179:3332–3341. doi: 10.4049/jimmunol.179.5.3332. [DOI] [PubMed] [Google Scholar]
- Cardones AR, Murakami T, Hwang ST. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via beta(1) integrin. Cancer Res. 2003;63:6751–6757. [PubMed] [Google Scholar]
- Caunt M, Hu L, Tang T, Brooks PC, Ibrahim S, Karpatkin S. Growth-regulated oncogene is pivotal in thrombin-induced angiogenesis. Cancer Res. 2006;66:4125–4132. doi: 10.1158/0008-5472.CAN-05-2570. [DOI] [PubMed] [Google Scholar]
- Chen GS, Yu HS, Lan CC, Chow KC, Lin TY, Kok LF, Lu MP, Liu CH, Wu MT. CXC chemokine receptor CXCR4 expression enhances tumorigenesis and angiogenesis of basal cell carcinoma. Br. J. Dermatol. 2006;154:910–918. doi: 10.1111/j.1365-2133.2006.07150.x. [DOI] [PubMed] [Google Scholar]
- Crawford S, Belajic D, Wei J, et al. A novel B-RAF inhibitor blocks interleukin-8 (IL-8) synthesis in human melanoma xenografts, revealing IL-8 as a potential pharmacodynamic biomarker. Mol. Cancer Ther. 2008;7:492–499. doi: 10.1158/1535-7163.MCT-07-0307. [DOI] [PubMed] [Google Scholar]
- Croker AK, Allan AL. Cancer stem cells: implications for the progression and treatment of metastatic disease. J. Cell Mol. Med. 2008;12:374–390. doi: 10.1111/j.1582-4934.2007.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, Calandra G, DiPersio JF. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J. Clin. Oncol. 2004;22:1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J. Leukoc. Biol. 2002;72:9–18. [PMC free article] [PubMed] [Google Scholar]
- Dhawan P, Su Y, Thu YM, Yu Y, Baugher P, Ellis DL, Sobolik-Delmaire T, Kelley M, Cheung TC, Ware CF, Richmond A. The lymphotoxin-beta receptor is an upstream activator of NF-kappaB-mediated transcription in melanoma cells. J. Biol. Chem. 2008;283:15399–15408. doi: 10.1074/jbc.M708272200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro FA, Brenner S, Oelschlaegel U, Jacobi A, Knoth H, Ehninger G, Illmer T, Bornhauser M. Combining SDF-1/CXCR4 antagonism and chemotherapy in relapsed acute myeloid leukemia. Leukemia. 2008;23:393–396. doi: 10.1038/leu.2008.182. [DOI] [PubMed] [Google Scholar]
- Forster R, Ohl L, Henning G. Lessons learned from lymphocytes: CC chemokine receptor-7 involved in lymphogenic metastasis of melanoma. J. Natl. Cancer Inst. 2001;93:1588–1589. doi: 10.1093/jnci/93.21.1588. [DOI] [PubMed] [Google Scholar]
- Fruehauf S, Seeger T, Maier P, et al. The CXCR4 antagonist AMD3100 releases a subset of G-CSF-primed peripheral blood progenitor cells with specific gene expression characteristics. Exp. Hematol. 2006;34:1052–1059. doi: 10.1016/j.exphem.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Fushimi T, O'Connor TP, Crystal RG. Adenoviral gene transfer of stromal cell-derived factor-1 to murine tumors induces the accumulation of dendritic cells and suppresses tumor growth. Cancer Res. 2006;66:3513–3522. doi: 10.1158/0008-5472.CAN-05-1493. [DOI] [PubMed] [Google Scholar]
- Gallagher PG, Bao Y, Prorock A, Zigrino P, Nischt R, Politi V, Mauch C, Dragulev B, Fox JW. Gene expression profiling reveals cross-talk between melanoma and fibroblasts: implications for host-tumor interactions in metastasis. Cancer Res. 2005;65:4134–4146. doi: 10.1158/0008-5472.CAN-04-0415. [DOI] [PubMed] [Google Scholar]
- Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, Mantovani A, Mordoh J, Wainstok R. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J. Invest. Dermatol. 2007;127:2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- Gebhardt C, Averbeck M, Viertel A, Kauer F, Saalbach A, Anderegg U, Simon JC. Ultraviolet-B irradiation enhances melanoma cell motility via induction of autocrine inter-leukin 8 secretion. Exp. Dermatol. 2007;16:636–643. doi: 10.1111/j.1600-0625.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Spagnoli GC, Martin I, Ploegert S, Demougin P, Heberer M, Reschner A. Three-dimensional culture of melanoma cells profoundly affects gene expression profile: a high density oligonucleotide array study. J. Cell. Physiol. 2005;204:522–531. doi: 10.1002/jcp.20320. [DOI] [PubMed] [Google Scholar]
- Gonsiorek W, Fan X, Hesk D, et al. Pharmacological characterization of Sch527123, a potent allosteric CXCR1/CXCR2 antagonist. J. Pharmacol. Exp. Ther. 2007;322:477–485. doi: 10.1124/jpet.106.118927. [DOI] [PubMed] [Google Scholar]
- Gordon MA, Gil J, Lu B, et al. Genomic profiling associated with recurrence in patients with rectal cancer treated with chemoradiation. Pharmacogenomics. 2006;7:67–88. doi: 10.2217/14622416.7.1.67. [DOI] [PubMed] [Google Scholar]
- Graves DT, Barnhill R, Galanopoulos T, Antoniades HN. Expression of monocyte chemotactic protein-1 in human melanoma in vivo. Am. J. Pathol. 1992;140:9–14. [PMC free article] [PubMed] [Google Scholar]
- Guleng B, Tateishi K, Ohta M, et al. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res. 2005;65:5864–5871. doi: 10.1158/0008-5472.CAN-04-3833. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Reeducating” tumor-associated macrophages by targeting NF-kappaB. J. Exp. Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB, Cardwell N, Luan J, Shattuck-Brandt R, Richmond A. The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J. Leukoc. Biol. 2000;67:53–62. doi: 10.1002/jlb.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MA, Hahn T, Lee DH, Esworthy RS, Kim BW, Riggs AD, Chu FF, Pfeifer GP. Methylation of poly-comb target genes in intestinal cancer is mediated by inflammation. Cancer Res. 2008;68:10280–10289. doi: 10.1158/0008-5472.CAN-08-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqq C, Nosrati M, Sudilovsky D, Crothers J, Khodabakhsh D, Pulliam BL, Federman S, Miller JR, III, Allen RE, Singer MI, et al. The gene expression signatures of melanoma progression. Proc. Natl Acad. Sci. USA. 2005;102:6092–6097. doi: 10.1073/pnas.0501564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm NT, Abreo F, Johnson LW, Li BD, Chu QD. Elevated chemokine receptor CXCR4 expression in primary tumors following neoadjuvant chemotherapy predicts poor outcomes for patients with locally advanced breast cancer (LABC) Breast Cancer Res. Treat. 2009;113:293–299. doi: 10.1007/s10549-008-9921-8. [DOI] [PubMed] [Google Scholar]
- Holm NT, Byrnes K, Li BD, Turnage RH, Abreo F, Mathis JM, Chu QD. Elevated levels of chemokine receptor CXCR4 in HER-2 negative breast cancer specimens predict recurrence. J. Surg. Res. 2007;141:53–59. doi: 10.1016/j.jss.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Horton LW, Yu Y, Zaja-Milatovic S, Strieter RM, Richmond A. Opposing roles of murine duffy antigen receptor for chemokine and murine CXC chemokine receptor-2 receptors in murine melanoma tumor growth. Cancer Res. 2007;67:9791–9799. doi: 10.1158/0008-5472.CAN-07-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Xiong J, Ji K, Sun H, Wang J, Liu H. Recombined CC chemokine ligand 2 into B16 cells induces production of Th2-dominant [correction of dominanted] cytokines and inhibits melanoma metastasis. Immunol. Lett. 2007;113:19–28. doi: 10.1016/j.imlet.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J. Leukoc Biol. 2006;79:639–651. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, Inaba K, Minato N, Oshima M, Taketo MM. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64:4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler. Thromb. Vasc. Biol. 2008;28:1928–1936. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Mori T, Chen SL, Amersi FF, Martinez SR, Kuo C, Turner RR, Ye X, Bilchik AJ, Morton DL, Hoon DS. Chemokine receptor CXCR4 expression in patients with melanoma and colorectal cancer liver metastases and the association with disease outcome. Ann. Surg. 2006;244:113–120. doi: 10.1097/01.sla.0000217690.65909.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Lee CH, Midura BV, et al. Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases. Clin. Exp. Metastasis. 2008;25:201–211. doi: 10.1007/s10585-007-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga M, Kai H, Egami K, et al. Mutant MCP-1 therapy inhibits tumor angiogenesis and growth of malignant melanoma in mice. Biochem. Biophys. Res. Commun. 2008;365:279–284. doi: 10.1016/j.bbrc.2007.10.182. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- Lee CH, Kakinuma T, Wang J, Zhang H, Palmer DC, Restifo NP, Hwang ST. Sensitization of B16 tumor cells with a CXCR4 antagonist increases the efficacy of immunotherapy for established lung metastases. Mol .Cancer Ther. 2006;5:2592–2599. doi: 10.1158/1535-7163.MCT-06-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentsch AB. The Duffy antigen/receptor for chemokines (DARC) and prostate cancer. A role as clear as black and white? FASEB J. 2002;16:1093–1095. doi: 10.1096/fj.02-0066hyp. [DOI] [PubMed] [Google Scholar]
- Lesinski GB, Badgwell B, Zimmerer J, Crespin T, Hu Y, Abood G, Carson WE., III IL-12 pretreatments enhance IFN-alpha-induced Janus kinase-STAT signaling and potentiate the antitumor effects of IFN-alpha in a murine model of malignant melanoma. J. Immunol. 2004;172:7368–7376. doi: 10.4049/jimmunol.172.12.7368. [DOI] [PubMed] [Google Scholar]
- Li H, Alizadeh H, Niederkorn JY. Differential expression of chemokine receptors on uveal melanoma cells and their metastases. Invest. Ophthalmol. Vis. Sci. 2008;49:636–643. doi: 10.1167/iovs.07-1035. [DOI] [PubMed] [Google Scholar]
- Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, Shim H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem. Biophys. Res. Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XL, Jiang XB, Liu RE, Zhang SM. The enhanced anti-angiogenic and antitumor effects of combining flk1-based DNA vaccine and IP-10. Vaccine. 2008;26:5352–5357. doi: 10.1016/j.vaccine.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J. Leukoc. Biol. 1997;62:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am. J. Pathol. 1997;151:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- Lurje G, Zhang W, Schultheis AM, Yang D, Groshen S, Hendifar AE, Husain H, Gordon MA, Nagashima F, Chang HM, Lenz HJ. Polymorphisms in VEGF and IL-8 predict tumor recurrence in stage III colon cancer. Ann. Oncol. 2008;19:1734–1741. doi: 10.1093/annonc/mdn368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J. Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Marchesi F, Piemonti L, Fedele G, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68:9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- Mehrad B, Keane MP, Strieter RM. Chemokines as mediators of angiogenesis. Thromb. Haemost. 2007;97:755–762. [PMC free article] [PubMed] [Google Scholar]
- Meijer J, Ogink J, Roos E. Effect of the chemokine receptor CXCR7 on proliferation of carcinoma cells in vitro and in vivo. Br. J. Cancer. 2008;99:1493–1501. doi: 10.1038/sj.bjc.6604727. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Melnikova VO, Bar-Eli M. Bioimmunotherapy for melanoma using fully human antibodies targeting MCAM/MUC18 and IL-8. Pigment Cell Res. 2006;19:395–405. doi: 10.1111/j.1600-0749.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- Miao Z, Luker KE, Summers BC, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc. Natl Acad. Sci. USA. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteagudo C, Martin JM, Jorda E, Llombart-Bosch A. CXCR3 chemokine receptor immunoreactivity in primary cutaneous malignant melanoma: correlation with clinicopathological prognostic factors. J. Clin. Pathol. 2007;60:596–599. doi: 10.1136/jcp.2005.032144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad-Zeidan AA, Melnikova VO, Wang H, Raz A, Bar-Eli M. Expression profiling of Galectin-3-depleted melanoma cells reveals its major role in melanoma cell plasticity and vasculogenic mimicry. Am. J. Pathol. 2008;173:1839–1852. doi: 10.2353/ajpath.2008.080380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrowietz U, Schwenk U, Maune S, Bartels J, Kupper M, Fichtner I, Schroder JM, Schadendorf D. The chemokine RANTES is secreted by human melanoma cells and is associated with enhanced tumour formation in nude mice. Br. J. Cancer. 1999;79:1025–1031. doi: 10.1038/sj.bjc.6690164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins IM, Slingluff CL, Lee JK, Garbee CF, Shu J, Anderson SG, Mayer ME, Knaus WA, Mullins DW. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004;64:7697–7701. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- Murakami T, Cardones AR, Hwang ST. Chemokine receptors and melanoma metastasis. J. Dermatol. Sci. 2004;36:71–78. doi: 10.1016/j.jdermsci.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, Hwang ST. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- Navarini-Meury AA, Conrad C. Melanoma and innate immunity – active inflammation or just erroneous attraction? Melanoma as the source of leukocyte-attracting chemokines. Semin. Cancer Biol. 2008 doi: 10.1016/j.semcancer.2008.10.012. doi: 10.1016/jsemcancer.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Norgauer J, Metzner B, Schraufstatter I. Expression and growth-promoting function of the IL-8 receptor beta in human melanoma cells. J. Immunol. 1996;156:1132–1137. [PubMed] [Google Scholar]
- Okada Y, Okada N, Mizuguchi H, Takahashi K, Hayakawa T, Mayumi T, Mizuno N. Optimization of antitumor efficacy and safety of in vivo cytokine gene therapy using RGD fiber-mutant adenovirus vector for preexisting murine melanoma. Biochim. Biophys. Acta. 2004;1670:172–180. doi: 10.1016/j.bbagen.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Ottaiano A, Franco R, Aiello Talamanca A, et al. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II–III colorectal cancer patients. Clin. Cancer Res. 2006;12:2795–2803. doi: 10.1158/1078-0432.CCR-05-2142. [DOI] [PubMed] [Google Scholar]
- Otto K, Starz H, Becker JC, Schrama D. Overexpression of matrix metalloproteinases, chemokines, and chemokine receptors relevant for metastasis in experimental models not an indication of lymph node metastases in human melanoma. Arch. Dermatol. 2007;143:947–948. doi: 10.1001/archderm.143.7.947. [DOI] [PubMed] [Google Scholar]
- Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuck-Brandt R, Richmond A. Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine beta and gamma proteins. Int. J. Cancer. 1997;73:94–103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aid-ijc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Palmer K, Hitt M, Emtage PC, Gyorffy S, Gauldie J. Combined CXC chemokine and interleukin-12 gene transfer enhances antitumor immunity. Gene Ther. 2001;8:282–290. doi: 10.1038/sj.gt.3301386. [DOI] [PubMed] [Google Scholar]
- Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J. Invest. Dermatol. 2002;118:915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- Peng HH, Liang S, Henderson AJ, Dong C. Regulation of interleukin-8 expression in melanoma-stimulated neutrophil inflammatory response. Exp. Cell Res. 2007;313:551–559. doi: 10.1016/j.yexcr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjeesingh R, Leung R, Siu CH. Interleukin-8 secreted by endothelial cells induces chemotaxis of melanoma cells through the chemokine receptor CXCR1. FASEB J. 2003;17:1292–1294. doi: 10.1096/fj.02-0560fje. [DOI] [PubMed] [Google Scholar]
- Ren T, Chen Q, Tian Z, Wei H. Down-regulation of surface fractalkine by RNA interference in B16 melanoma reduced tumor growth in mice. Biochem. Biophys. Res. Commun. 2007;364:978–984. doi: 10.1016/j.bbrc.2007.10.124. [DOI] [PubMed] [Google Scholar]
- Richmond A. NF-kappa B, chemokine gene transcription and tumour growth. Nat. Rev. Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond A. CCR9 homes metastatic melanoma cells to the small bowel. Clin. Cancer Res. 2008;14:621–623. doi: 10.1158/1078-0432.CCR-07-2235. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Oppenheim JJ. Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation. 2003;10:359–370. doi: 10.1038/sj.mn.7800200. [DOI] [PubMed] [Google Scholar]
- Scala S, Ierano C, Ottaiano A, et al. CXC chemokine receptor 4 is expressed in uveal malignant melanoma and correlates with the epithelioid-mixed cell type. Cancer Immunol. Immunother. 2007;56:1589–1595. doi: 10.1007/s00262-007-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J. Immunol. 1993;151:2667–2675. [PubMed] [Google Scholar]
- Schutyser E, Su Y, Yu Y, Gouwy M, Zaja-Milatovic S, Van Damme J, Richmond A. Hypoxia enhances CXCR4 expression in human microvascular endothelial cells and human melanoma cells. Eur. Cytokine Netw. 2007;18:59–70. doi: 10.1684/ecn.2007.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl H, Richtig E, Tilz H, Stefan M, Schmidbauer U, Asslaber M, Zatloukal K, Herlyn M, Schaider H. Profiles of chemokine receptors in melanocytic lesions: de novo expression of CXCR6 in melanoma. Hum. Pathol. 2007;38:768–780. doi: 10.1016/j.humpath.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Shen H, Schuster R, Stringer KF, Waltz SE, Lentsch AB. The Duffy antigen/receptor for chemokines (DARC) regulates prostate tumor growth. FASEB J. 2006;20:59–64. doi: 10.1096/fj.05-4764com. [DOI] [PubMed] [Google Scholar]
- Shields JD, Emmett MS, Dunn DB, Joory KD, Sage LM, Rigby H, Mortimer PS, Orlando A, Levick JR, Bates DO. Chemokine-mediated migration of melanoma cells towards lymphatics–a mechanism contributing to metastasis. Oncogene. 2007;26:2997–3005. doi: 10.1038/sj.onc.1210114. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22:941–950. doi: 10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti O, Goteri G, Lucarini G, Filosa A, Pieramici T, Rubini C, Biagini G, Offidani A. Potential role of CCL27 and CCR10 expression in melanoma progression and immune escape. Eur. J. Cancer. 2006;42:1181–1187. doi: 10.1016/j.ejca.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Singh S, Varney M, Singh RK. Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Res. 2009;69:411–415. doi: 10.1158/0008-5472.CAN-08-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos GT, Psallidas I, Moustaki A, et al. A central role for tumor-derived monocyte chemoattractant protein-1 in malignant pleural effusion. J. Natl. Cancer Inst. 2008;100:1464–1476. doi: 10.1093/jnci/djn325. [DOI] [PubMed] [Google Scholar]
- Stephan C, Schnorr D, Loening SA, Jung K. Eur. Urol. 2005;48:1059–1060. doi: 10.1016/j.eururo.2005.08.011. Re: Roddam AW, Duffy MJ, Hamdy FC, et al. Use of prostate-specific antigen (PSA) isoforms for the detection of prostate cancer in men with a PSA Level of 2–10 ng/ml: systematic review and meta-analysis. Eur. Urol. 48, 386–399. author reply 1060–1051. [DOI] [PubMed] [Google Scholar]
- Struyf S, Burdick MD, Peeters E, Van den Broeck K, Dillen C, Proost P, Van Damme J, Strieter RM. Platelet factor-4 variant chemokine CXCL4L1 inhibits melanoma and lung carcinoma growth and metastasis by preventing angiogenesis. Cancer Res. 2007;67:5940–5948. doi: 10.1158/0008-5472.CAN-06-4682. [DOI] [PubMed] [Google Scholar]
- Takenaga M, Tamamura H, Hiramatsu K, Nakamura N, Yamaguchi Y, Kitagawa A, Kawai S, Nakashima H, Fujii N, Igarashi R. A single treatment with microcapsules containing a CXCR4 antagonist suppresses pulmonary metastasis of murine melanoma. Biochem. Biophys. Res. Commun. 2004;320:226–232. doi: 10.1016/j.bbrc.2004.05.155. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Fujimoto A, Tanaka M, Yamano T, Hsueh E, Hoon DS. CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin. Cancer Res. 2004;10:2351–2358. doi: 10.1158/1078-0432.ccr-03-0195. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Kitago M, Hoon DS. Effects of chemokines on tumor metastasis. Cancer Treat. Res. 2007;135:177–184. doi: 10.1007/978-0-387-69219-7_13. [DOI] [PubMed] [Google Scholar]
- Taxman DJ, MacKeigan JP, Clements C, Bergstralh DT, Ting JP. Transcriptional profiling of targets for combination therapy of lung carcinoma with paclitaxel and mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor. Cancer Res. 2003;63:5095–5104. [PubMed] [Google Scholar]
- Thanarajasingam U, Sanz L, Diaz R, Qiao J, Sanchez-Perez L, Kottke T, Thompson J, Chester J, Vile RG. Delivery of CCL21 to metastatic disease improves the efficacy of adoptive T-cell therapy. Cancer Res. 2007;67:300–308. doi: 10.1158/0008-5472.CAN-06-1017. [DOI] [PubMed] [Google Scholar]
- Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat. Immunol. 2008;9:953–959. doi: 10.1038/ni.f.207. [DOI] [PubMed] [Google Scholar]
- Tsuzuki H, Takahashi N, Kojima A, Narita N, Sunaga H, Takabayashi T, Fujieda S. Oral and oropharyngeal squamous cell carcinomas expressing CCR7 have poor prognoses. Auris. Nasus. Larynx. 2006;33:37–42. doi: 10.1016/j.anl.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J. Clin. Oncol. 2001;19:577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- Ugurel S, Schrama D, Keller G, Schadendorf D, Brocker EB, Houben R, Zapatka M, Fink W, Kaufman HL, Becker JC. Impact of the CCR5 gene polymorphism on the survival of metastatic melanoma patients receiving immunotherapy. Cancer Immunol. Immunother. 2008;57:685–691. doi: 10.1007/s00262-007-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deventer HW, O'Connor W, Jr, Brickey WJ, Aris RM, Ting JP, Serody JS. C-C chemokine receptor 5 on stromal cells promotes pulmonary metastasis. Cancer Res. 2005;65:3374–3379. doi: 10.1158/0008-5472.CAN-04-2616. [DOI] [PubMed] [Google Scholar]
- Varney ML, Johansson SL, Singh RK. Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am. J. Clin. Pathol. 2006;125:209–216. doi: 10.1309/VPL5-R3JR-7F1D-6V03. [DOI] [PubMed] [Google Scholar]
- Varney ML, Li A, Dave BJ, Bucana CD, Johansson SL, Singh RK. Expression of CXCR1 and CXCR2 receptors in malignant melanoma with different metastatic potential and their role in interleukin-8 (CXCL-8)-mediated modulation of metastatic phenotype. Clin. Exp. Metastasis. 2003;20:723–731. doi: 10.1023/b:clin.0000006814.48627.bd. [DOI] [PubMed] [Google Scholar]
- Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006;66:3071–3077. doi: 10.1158/0008-5472.CAN-05-2871. [DOI] [PubMed] [Google Scholar]
- Wang J, Shiozawa Y, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J. Biol. Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- Wang JM, Taraboletti G, Matsushima K, Van Damme J, Mantovani A. Induction of haptotactic migration of melanoma cells by neutrophil activating protein/interleukin-8. Biochem. Biophys. Res. Commun. 1990;169:165–170. doi: 10.1016/0006-291x(90)91449-3. [DOI] [PubMed] [Google Scholar]
- Wells TN, Power CA, Shaw JP, Proudfoot AE. Chemokine blockers–therapeutics in the making? Trends Pharmacol. Sci. 2006;27:41–47. doi: 10.1016/j.tips.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Wetzel K, Struyf S, Van Damme J, Kayser T, Vecchi A, Sozzani S, Rommelaere J, Cornelis JJ, Dinsart C. MCP-3 (CCL7) delivered by parvovirus MVMp reduces tumorigenicity of mouse melanoma cells through activation of T lymphocytes and NK cells. Int. J. Cancer. 2007;120:1364–1371. doi: 10.1002/ijc.22421. [DOI] [PubMed] [Google Scholar]
- Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J. Natl. Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- Wilson C, Wilson T, Johnston PG, Longley DB, Waugh DJ. Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells. Mol. Cancer Ther. 2008;7:2649–2661. doi: 10.1158/1535-7163.MCT-08-0148. [DOI] [PubMed] [Google Scholar]
- Yamano T, Kaneda Y, Huang S, Hiramatsu SH, Hoon DS. Enhancement of immunity by a DNA melanoma vaccine against TRP2 with CCL21 as an adjuvant. Mol. Ther. 2006;13:194–202. doi: 10.1016/j.ymthe.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Yang J, Amiri KI, Burke JR, Schmid JA, Richmond A. BMS-345541 targets inhibitor of kappaB kinase and induces apoptosis in melanoma: involvement of nuclear factor kappaB and mitochondria pathways. Clin. Cancer Res. 2006;12:950–960. doi: 10.1158/1078-0432.CCR-05-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Luan J, Yu Y, Li C, DePinho RA, Chin L, Richmond A. Induction of melanoma in murine macrophage inflammatory protein 2 transgenic mice heterozygous for inhibitor of kinase/alternate reading frame. Cancer Res. 2001;61:8150–8157. [PubMed] [Google Scholar]
- Yang J, Pan WH, Clawson GA, Richmond A. Systemic targeting inhibitor of kappaB kinase inhibits melanoma tumor growth. Cancer Res. 2007;67:3127–3134. doi: 10.1158/0008-5472.CAN-06-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Richmond A. Constitutive IkappaB kinase activity correlates with nuclear factor-kappaB activation in human melanoma cells. Cancer Res. 2001;61:4901–4909. [PubMed] [Google Scholar]
- Yang J, Richmond A. The angiostatic activity of interferon-inducible protein-10/CXCL10 in human melanoma depends on binding to CXCR3 but not to glycosaminoglycan. Mol. Ther. 2004;9:846–855. doi: 10.1016/j.ymthe.2004.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, Yee H, Voura EB, Newcomb EW. Hypoxiainducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab. Invest. 2006;86:1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- Zhang T, Somasundaram R, Berencsi K, Caputo L, Gimotty P, Rani P, Guerry D, Swoboda R, Herlyn D. Migration of cytotoxic T lymphocytes toward melanoma cells in three-dimensional organotypic culture is dependent on CCL2 and CCR4. Eur. J. Immunol. 2006a;36:457–467. doi: 10.1002/eji.200526208. [DOI] [PubMed] [Google Scholar]
- Zhang T, Somasundaram R, Berencsi K, et al. CXC chemokine ligand 12 (stromal cell-derived factor 1 alpha) and CXCR4-dependent migration of CTLs toward melanoma cells in organotypic culture. J. Immunol. 2005;174:5856–5863. doi: 10.4049/jimmunol.174.9.5856. [DOI] [PubMed] [Google Scholar]
- Zhang T, Somasundaram R, Berking C, et al. Preferential involvement of CX chemokine receptor 4 and CX chemokine ligand 12 in T-cell migration toward melanoma cells. Cancer Biol. Ther. 2006b;5:1304–1312. doi: 10.4161/cbt.5.10.3153. [DOI] [PubMed] [Google Scholar]
- Zigler M, Villares GJ, Lev DC, Melnikova VO, Bar-Eli M. Tumor immunotherapy in melanoma: strategies for overcoming mechanisms of resistance and escape. Am. J. Clin. Dermatol. 2008;9:307–311. doi: 10.2165/00128071-200809050-00004. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]