Abstract
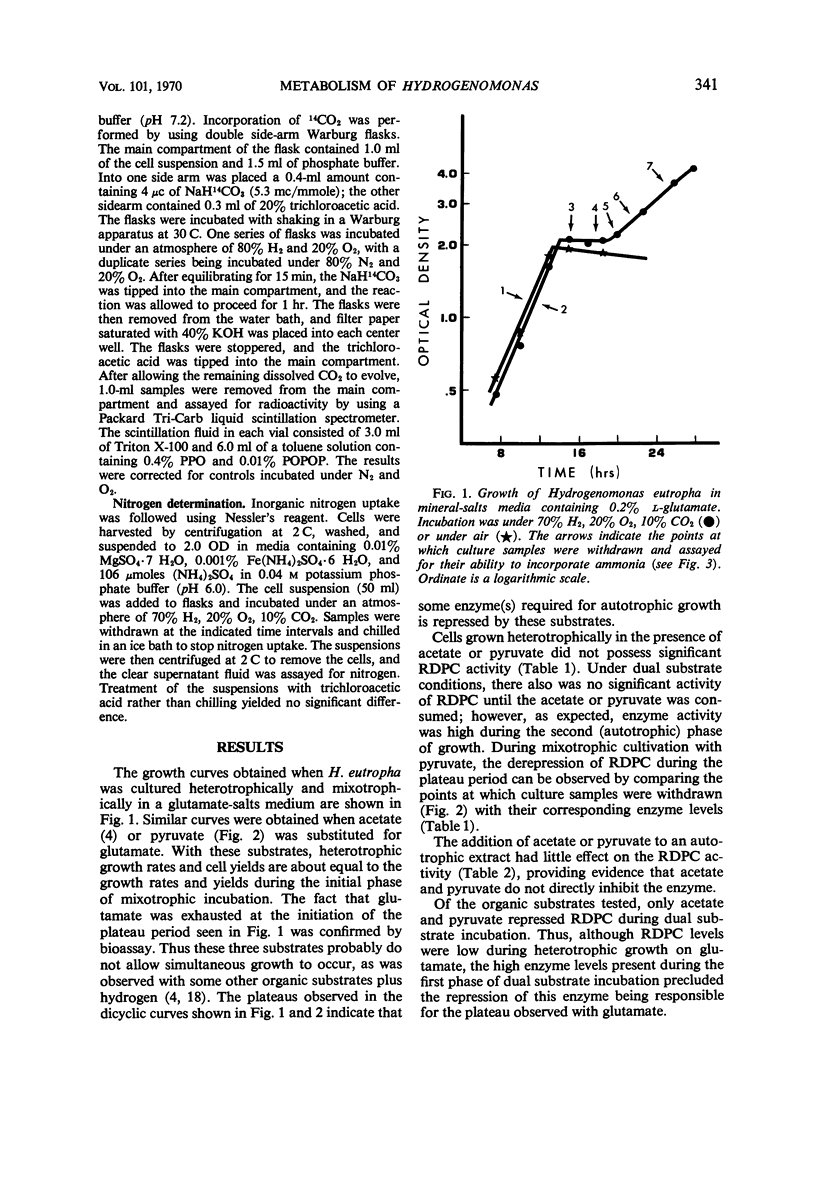
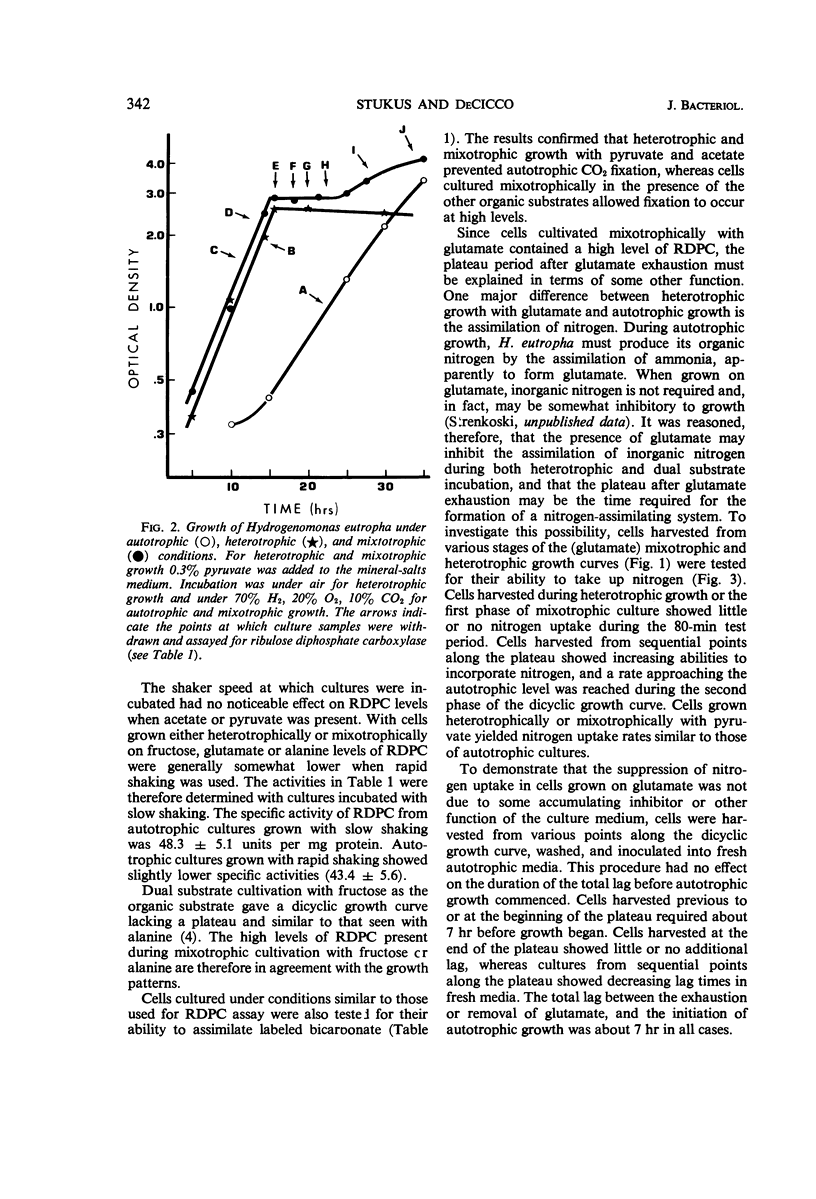
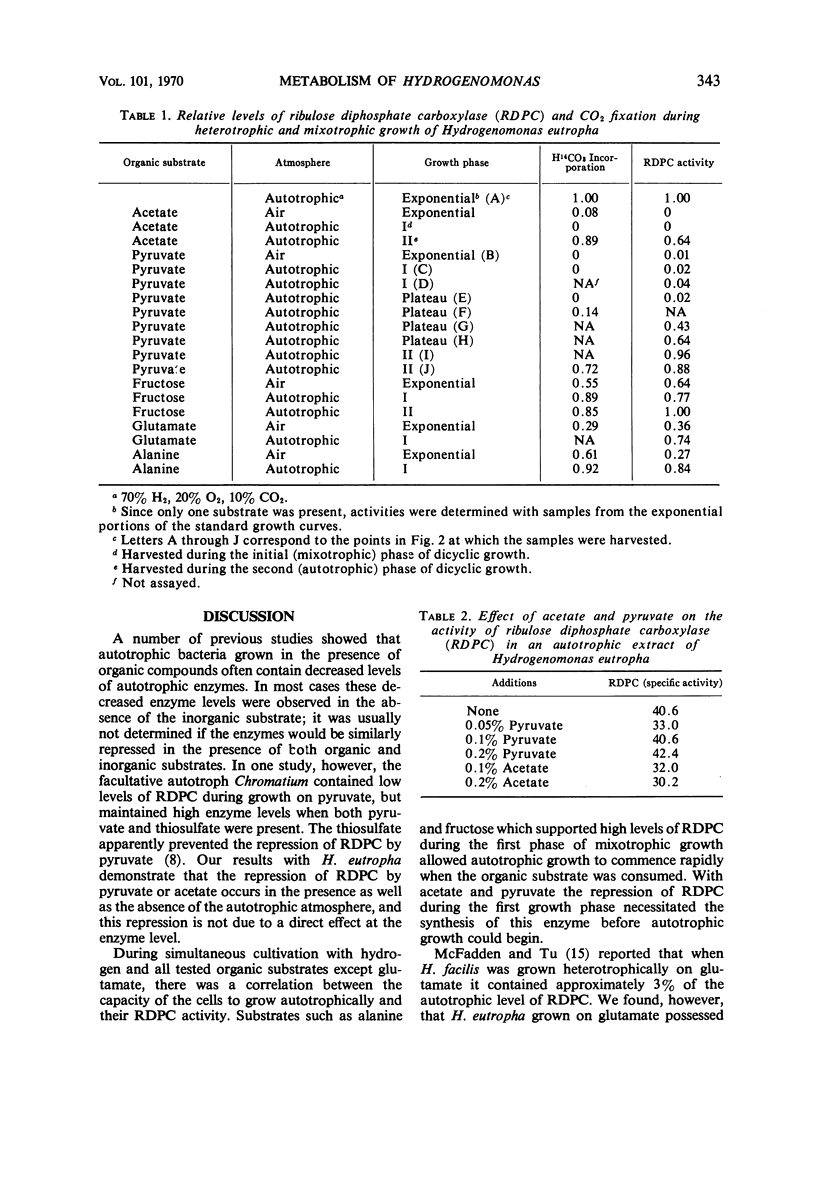
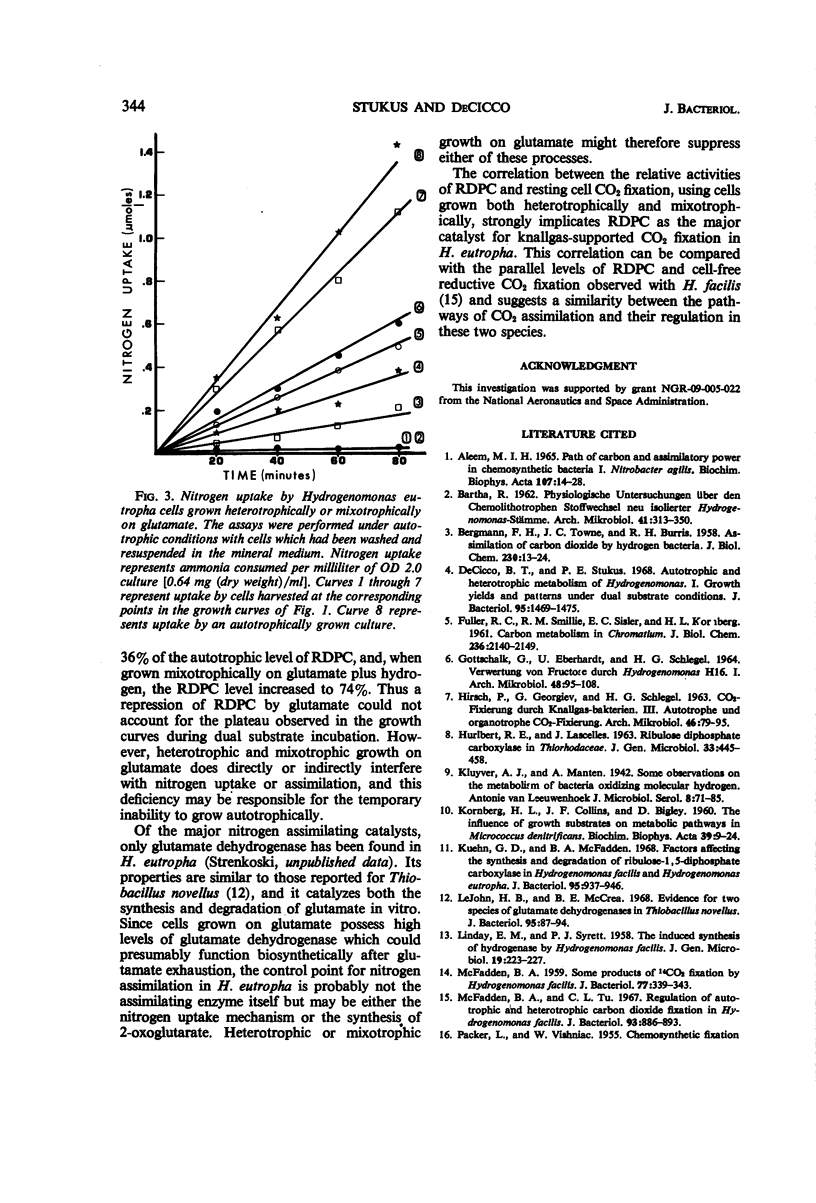
The effects of a number of organic substrates on the autotrophic metabolism of Hydrogenomonas eutropha were examined. Dual substrate (mixotrophic) cultivation in the presence of hydrogen plus either fructose or alanine allowed autotrophic growth to begin immediately after the exhaustion of the organic substrate. On the other hand, the presence of acetate, pyruvate, or glutamate caused a lengthy lag to occur before autotrophic growth commenced. With acetate or pyruvate this lag (plateau) in the dicyclic growth curve was due to the repression of ribulose diphosphate carboxylase (RDPC) synthesis during mixotrophic growth. During heterotrophic growth with glutamate, RDPC was partially repressed; however, during mixotrophic growth, RDPC activity was high. Thus the delay of autotrophic growth was not due to a repression of RDPC by glutamate. The data suggest that glutamate interferes with autotrophic metabolism by repressing the incorporation of inorganic nitrogen. The repression of these vital autotrophic functions by acetate, pyruvate, and glutamate occurred both in the presence and absence of hydrogen, i.e., during both heterotrophic and mixotrophic cultivation. The derepression of the affected systems during the plateau phase of the dicyclic growth curves was demonstrated. Carbon dioxide assimilation by whole cells agreed well with the RDPC activity of extracts from cells grown under similar conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleem M. I. Path of carbon and assimilatory power in chemosynthetic bacteria. I. Nitrobacter agilis. Biochim Biophys Acta. 1965 Aug 24;107(1):14–28. doi: 10.1016/0304-4165(65)90384-3. [DOI] [PubMed] [Google Scholar]
- BARTHA R. [Physiological studies on the chemolithotropic metabolism of recently isolated Hydrogenomonas strains]. Arch Mikrobiol. 1962;41:313–350. [PubMed] [Google Scholar]
- BERGMANN F. H., TOWNE J. C., BURRIS R. H. Assimilation of carbon dioxide by hydrogen bacteria. J Biol Chem. 1958 Jan;230(1):13–24. [PubMed] [Google Scholar]
- DeCicco B. T., Stukus P. E. Autotrophic and heterotrophic metabolism of Hydrogenomonas. I. Growth yields and patterns under dual substrate conditions. J Bacteriol. 1968 Apr;95(4):1469–1475. doi: 10.1128/jb.95.4.1469-1475.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK G., EBERHARDT U., SCHLEGEL H. G. VERWERTUNG VON FRUCTOSE DURCH HYDROGENOMONAS H 16. (I.) Arch Mikrobiol. 1964 Apr 2;48:95–108. [PubMed] [Google Scholar]
- HIRSCH P., GEORGIEV G., SCHLEGEL H. G. CO2-FIXIERUNG DURCH KNALLGASBAKTERIEN. III. AUTOTROPHE UND ORGANOTROPHE CO2-FIXIERUNG. Arch Mikrobiol. 1963 Jul 18;46:79–95. [PubMed] [Google Scholar]
- HURLBERT R. E., LASCELLES J. RIBULOSE DIPHOSPHATE CARBOXYLASE IN THIORHODACEAE. J Gen Microbiol. 1963 Dec;33:445–458. doi: 10.1099/00221287-33-3-445. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., COLLINS J. F., BIGLEY D. The influence of growth substrates on metabolic pathways in Micrococcus denitrificans. Biochim Biophys Acta. 1960 Mar 25;39:9–24. doi: 10.1016/0006-3002(60)90117-7. [DOI] [PubMed] [Google Scholar]
- Kuehn G. D., McFadden B. A. Factors affecting the synthesis and degradation of ribulose-1,5-diphosphate carboxylase in Hydrogenomonas facilis and Hydrogenomonas eutropha. J Bacteriol. 1968 Mar;95(3):937–946. doi: 10.1128/jb.95.3.937-946.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDAY E. M., SYRETT P. J. The induced synthesis of hydrogenase by Hydrogenomonas facilis. J Gen Microbiol. 1958 Oct;19(2):223–227. doi: 10.1099/00221287-19-2-223. [DOI] [PubMed] [Google Scholar]
- LéJohn H. B., McCrea B. E. Evidence for two species of glutamate dehydrogenases in Thiobacillus novellus. J Bacteriol. 1968 Jan;95(1):87–94. doi: 10.1128/jb.95.1.87-94.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFADDEN B. A. Some products of C1402 fixation by Hydrogenomonas facilis. J Bacteriol. 1959 Mar;77(3):339–343. doi: 10.1128/jb.77.3.339-343.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A., Tu C. C. Regulation of autotrophic and heterotrophic carbon dioxide fixation in Hydrogenomonas facilis. J Bacteriol. 1967 Mar;93(3):886–893. doi: 10.1128/jb.93.3.886-893.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C., Goodman N. S. Mixotrophic growth of Hydrogenomonas eutropha. J Bacteriol. 1969 May;98(2):617–622. doi: 10.1128/jb.98.2.617-622.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTER M., VISHNIAC W. CO2 incorporation by extracts of Thiobacillus thioparus. Biochim Biophys Acta. 1955 Sep;18(1):157–158. doi: 10.1016/0006-3002(55)90033-0. [DOI] [PubMed] [Google Scholar]
- TRUDINGER P. A. Fixation of carbon dioxide by extracts of the strict autotroph Thiobacillus denitrificans. Biochem J. 1956 Oct;64(2):274–286. doi: 10.1042/bj0640274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., TRUDINGER P. A. Symposium on autotrophy. V. Carbon dioxide fixation and substrate oxidation in the chemosynthetic sulfur and hydrogen bacteria. Bacteriol Rev. 1962 Jun;26:168–175. [PMC free article] [PubMed] [Google Scholar]
- WILSON E., STOUT H. A., POWELSON D., KOFFLER H. Comparative biochemistry of the hydrogen bacteria. I. The simultaneous oxidation of hydrogen and lactate. J Bacteriol. 1953 Mar;65(3):283–287. doi: 10.1128/jb.65.3.283-287.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]


