Abstract
Objective
L-homocysteine and/or L-homocystine interact in vivo with albumin and other extracellular proteins by forming mixed-disulfide conjugates. Because of its extremely rich cysteine content, we hypothesized that metallothionein, a ubiquitous intracellular zinc-chaperone and superoxide anion radical scavenger, reacts with L-homocysteine and that homocysteinylated-metallothionein suffers loss of function.
Methods and Results
35S-homocysteinylated-metallothionein was resolved in lysates of cultured human aortic endothelial cells in the absence and presence of reduced glutathione by SDS-PAGE and identified by Western blotting and phosphorimaging. Using zinc-Sepharose chromatography, L-homocysteine was shown to impair the zinc-binding capacity of metallothionein even in the presence of reduced glutathione. L-Homocysteine induced a dose-dependent increase in intracellular free zinc in zinquin-loaded human aortic endothelial cells within 30 minutes, followed by the appearance of early growth response protein-1 within 60 minutes. In addition, intracellular reactive oxygen species dramatically increased 6 hours after L-homocysteine treatment. In vitro studies demonstrated that L-homocysteine is a potent inhibitor of the superoxide anion radical scavenging ability of metallothionein.
Conclusion
These studies provide the first evidence that L-homocysteine targets intracellular metallothionein by forming a mixed-disulfide conjugate and that loss of function occurs after homocysteinylation. The data support a novel mechanism for disruption of zinc and redox homeostasis.
Keywords: early growth response protein-1, endothelial dysfunction, hyperhomocysteinemia, metallothionein, superoxide anion radical scavenging, zinc homeostasis
Elevated plasma total homocysteine (tHcy)1 is associated with accelerated vascular pathology in humans2 and in rodent models.3–5 Elevated tHcy is a marker for impaired 1-carbon metabolism, affecting processes associated with transsulfuration and/or remethylation of homocysteine. Because vascular cells and tissues have a limited capacity to metabolize homocysteine,6 they may be particularly sensitive to the detriments associated with hyperhomocystinemia, namely oxidative stress, reduced nitric oxide (NO) bioavailability, endoplasmic reticulum stress, and hypercoagulability.7–10 Although hyperhomocystinemia is considered a risk factor for stroke, coronary artery disease, and peripheral vascular disease, the mechanisms for its pathogenicity have not been elucidated at the molecular level. Homocysteine and homocystine can interact with proteins to form mixed-disulfide conjugates and alter protein function.11 This paradigm, known as the “homocysteine molecular targeting hypothesis,” is supported by impaired binding of tissue plasminogen activator to homocysteinylated-annexin II,12 diminished binding of homocysteinylated-fibronectin to fibrin,13 decreased dimethylarginine dimethylaminohydrolase (DDAH) activity,14 and impaired calcium binding function in homocysteinylated-fibrillin-1 fragments.15
Because cysteine accounts for ≈35% of the amino acids in metallothionein (MT), we hypothesized that it may be targeted by homocysteine and suffer loss of function. MT is a 6-kDa intracellular protein essential for detoxifying heavy metals and regulating zinc/copper homeostasis.16,17 In addition, MT scavenges reactive oxygen species (ROS).18 Although MT has a high affinity for zinc, the metal can be released during NO signaling and by oxidized glutathione. 19–22 For these reasons, MT plays a critical role in the regulation of cellular redox, NO signaling, and zinc homeostasis. 20–23 Because of the importance of zinc in signal transduction and enzymatic function, impaired zinc binding could have deleterious consequences across multiple biochemical processes. Moreover, the indiscriminate release of zinc caused by homocysteine could abnormally influence zinc-dependent intracellular protein expression. Studies using neural cells and bronchial epithelial cells demonstrated that a sudden rise in intracellular free zinc from glutamatergic vesicles induced early growth response protein 1 (Egr-1) expression.24,25 Therefore, in the present study we establish that homocysteine targets MT in human aortic endothelial cells (HAECs), homocysteine impairs the ability of MT to coordinate zinc, homocysteine increases intracellular free zinc and induces the expression of Egr-1 protein, and homocysteine impairs superoxide anion radical scavenging.
Methods
Isolation of HAECs
Discarded thoracic aortic segments were obtained from donor hearts during heart transplantation with approval from the Institutional Review Board. Under a sterile field, the intima of the aortic ring was digested with Dulbecco phosphate-buffered saline (DPBS) (minus Ca2+ and Mg2+) containing collagenase (Worthington, Type 2, 2000 U/mL) and dispase (2 U/mL) for 10 minutes at 37°C. Cells were obtained by gentle scraping. After centrifugation, the cells were seeded into a fibronectin-coated 6-well plate using EBM-2 media (Cambrex BioSciences). For all experiments, HAECs were used between passages 2 to 4. Positive immunostaining for von Wille-brand factor served as an indicator of endothelial origin.
Preparation and Purification of 35S-D,L-Homocysteine Thiolactone
35S-l-methionine (50 µCi/µmol; 20 µmol total) was refluxed under argon for 24 hours in 7.5 mol/L hydriodic acid according to Baernstein.26 After evaporating, the residue was dissolved in water and 35S-D,L-homocysteine thiolactone was purified by semi-preparative high-performance liquid chromatography (Catanescu et al, to be submitted). Immediately before use, 35S-D,L-homocysteine thiolactone was converted to 35S-D,L-homocysteine by base hydrolysis and the concentration of −SH groups determined using Ellman’s reagent.27 Note: We have confirmed that the 35S-homocysteine thiolactone produced by the aforementioned method is racemic. However, when non-labeled L-homocysteine thiolactone is converted to L-homocysteine by base hydrolysis, as used throughout this work, racemization does not occur.
Identification of 35S-Homocysteinylated-MT in HAECs
HAECs were cultured to 80% confluence in T-163 cm2 flasks and incubated with 50 µmol/L 35S-D,L-homocysteine for 12 hours at 37°C in a humidified CO2 incubator. After washing 3 to 5 times with PBS, cells were trypsinized by adding 5 mL of 0.05% trypsin containing 0.53 mmol/L EDTA and incubated for 10 minutes at 37°C followed by trypsin neutralization. Cell pellets were obtained using a Beckman J-6 M/E centrifuge at 1000 rpm for 10 minutes at 4°C. The cells were then lysed in 0.5 mL distilled water and 0.5 mL of Laemmli sample preparation buffer without β-mercaptoethanol (BME) was added. The sample was equally divided and BME was added to one-half of the sample to a final concentration of 0.75 mol/L BME. The other half of the sample received an equivalent volume of water. The samples were resolved using small-pore Laemmli SDS-PAGE gels (12% T; 1.4% C). Gels were transferred to an Immobilon-PSQ 0.2 µm polyvinylidene fluoride membrane (Millipore) using the discontinuous semi-dry method of Kyhse-Andersen,28 probed with anti-MT antibody (E9 clone, DakoCytomation) and developed with diaminobenzidine. The Western blot was exposed to a phosphorimager screen (Molecular Dynamics). To evaluate the effect of reduced glutathione on MT-homocysteinylation, HAECs were treated with 50 µmol/L 35S-D,L-homocysteine as described. After harvesting the cellular pellet, the lysate was treated with 10 mmol/L reduced glutathione for 2 hours at 37°C and processed as stated.
Binding of MT and Homocysteinylated-MT to Zinc-Sepharose Beads
Zinc-chelating Sepharose beads, prepared according to Porath et al,29 were packed into a 14-cm Econo-Column (ID 2.5 cm; Bio-Rad) to a final height of 3 cm. HAEC lysates (2 mL containing equal amounts of total protein) from cells cultured in the absence and presence of 100 µmol/L L-homocysteine in a humidified CO2 incubator for 2 hours at 37°C were applied to the columns. The flow-through fractions (≈2 mL) were collected and the columns were then washed with 20 mL of PBS. The MT that bound to the zinc-Sepharose column was eluted with 2 mL of PBS containing 10 mmol/L EDTA. Protein in the flow-through fractions and EDTA-fractions was precipitated using 4 volumes of cold acetone, centrifuged at 14 000g for 5 minutes at 4°C using a Beckman J2-HS centrifuge, air-dried, and resuspended in 0.1 mL of Laemmli sample preparation buffer containing 0.75 mol/L BME. MT was detected by Western blotting as previously stated. To evaluate the effect of reduced glutathione, bovine liver homogenates were heat-treated for 10 minutes at 60°C followed by centrifugation (1000g) for 10 minutes at 4°C. The supernatant was removed and divided equally. One-half of the supernatant was treated with 10 mmol/L reduced glutathione and the other half treated with 10 mmol/L reduced glutathione plus 100 µmol/L L-homocysteine. Both reactions were incubated for 2 hours at 37°C. The reaction mixtures were applied to zinc-Sepharose columns and MT in the fractions was determined as previously stated.
Effect of L-Homocysteine on Intracellular Free Zinc in HAECs
Confluent HAECs were cultured in fibronectin-coated Tek chamber slides (Nalgene Nunc Int) and loaded with 20 µmol/L Zinquin-AM (TEFLABS) for 30 minutes at 37°C in a CO2 incubator. After washing with DPBS–fetal bovine serum (FBS), the cells were treated with DPBS-FBS containing L-homocysteine at final concentrations of 0, 50, 100, and 500 µmol/L for 1 hour at 37°C in a CO2 incubator. After a final washing, slides were transferred to a thermostatically controlled chamber (37°C) filled with DPBS-FBS. Internalized Zinquin-AM fluorescence, using an excitation of 365 nm and an emission of 420 nm, was visualized immediately using a Leica DMLB microscope coupled with an Optronics camera and Magnafire software (Goleta, Calif). Fluorescence was converted to [Zn2+]i according to the procedure of Chen et al,30 which uses the formula:
where Kd is the dissociation constant of the Zinquin-Zn2+ complex (80 nmol/L); F is the cellular fluorescence intensity; Fmin is the fluorescence intensity in cells incubated in Zn2+-free solution; Fmax is the fluorescence intensity in cells incubated in the Zn2+-saturated solution. EGTA (100 µmol/L) and pyrithione (20 µmol/L) were used to measure Fmin and Fmax, respectively. A total of 20 HAECs per each condition was used to quantify intracellular free zinc and the values reported as mean ±SD. For a kinetic study, HAECs were loaded with Zinquin-AM and 50 µmol/L L-homocysteine at 37°C for 0.0, 0.5, 1, 2, 3, 4, 5, 6, 12, and 24 hours. At each time point, internalized Zinquin fluorescence was visualized as described.
Homocysteine-Mediated Reactive Oxygen Species in HAECs
Confluent HAEC were incubated in DPBS-FBS containing 5 µmol/L 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA), and 50 µmol/L L-homocysteine for 0, 0.5, 1, 2, 3, 4, 5, 6, 12, and 24 hours at 37°C in a CO2 incubator. At each time point, internalized CM-H2DCFDA fluorescence was determined at 520 nm (excitation=485 nm) as described.
Determination of Egr-1 Expression
Confluent HAECs were incubated at 37°C in a CO2 incubator in the absence and presence of 50 µmol/L L-homocysteine in DPBS-FBS for 0, 0.5, 1, 2, and 4 hours. At each time point, cells were harvested, lysed and processed as previously stated. Gels were transferred and membranes probed with Egr-1 antibody (Santa Cruz Biotechnology). To ensure equal loading, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was probed using anti-GAPDH (CHEMICON).
Scavenging of Superoxide Anion Radicals by MT and Homocysteinylated MT
Superoxide scavenging was assessed using the method of Hunaiti,31 which is based on the ability of superoxide anions, generated by riboflavin photolysis, to reduce nitroblue tetrazolium to formazan. The MT reaction (0.5 mL total) consisted of 30 µg MT, 60 µmol/L riboflavin, 8 mmol/L N,N,N′,N′-tetramethylethylenediamine, 50 mmol/L K2HPO4 (pH 7.8), and 850 µmol/L nitroblue tetrazolium in the absence and presence of 50 µmol/L L-homocysteine. The superoxide dismutase (SOD) reaction (0.5 mL total) consisted of 6000 U of SOD, 60 µmol/L riboflavin, 8 mmol/L N,N,N′,N′-tetramethylethylenediamine, 50 mmol/L K2HPO4 (pH 7.8), and 850 µmol/L nitroblue tetrazolium. Reactions were initiated by exposure to light (Osram Dulux® 13 Watt fluorescent lamp; Osrum, Sylvania, Ill) for 10 minutes. All reactions were performed in triplicate and expressed as percent formazan production normalized to the control reactions minus MT or SOD.
Statistical Analysis
Data were tested for homogeneity of variance using a Levene test. An ANOVA was performed on parametric data while a Kruskal-Wallis ANOVA was performed on nonparametric data. Parametric homogeneous subsets were identified using Scheffe post-hoc tests while a Tamhane post-hoc was used for nonparametric data. All statistical tests were performed using SPSS statistical software. The 5% level of confidence was arbitrarily used for assigning statistically significant differences.
Results
Identification of 35S-Homocysteinylated-MT in Cultured HAECs
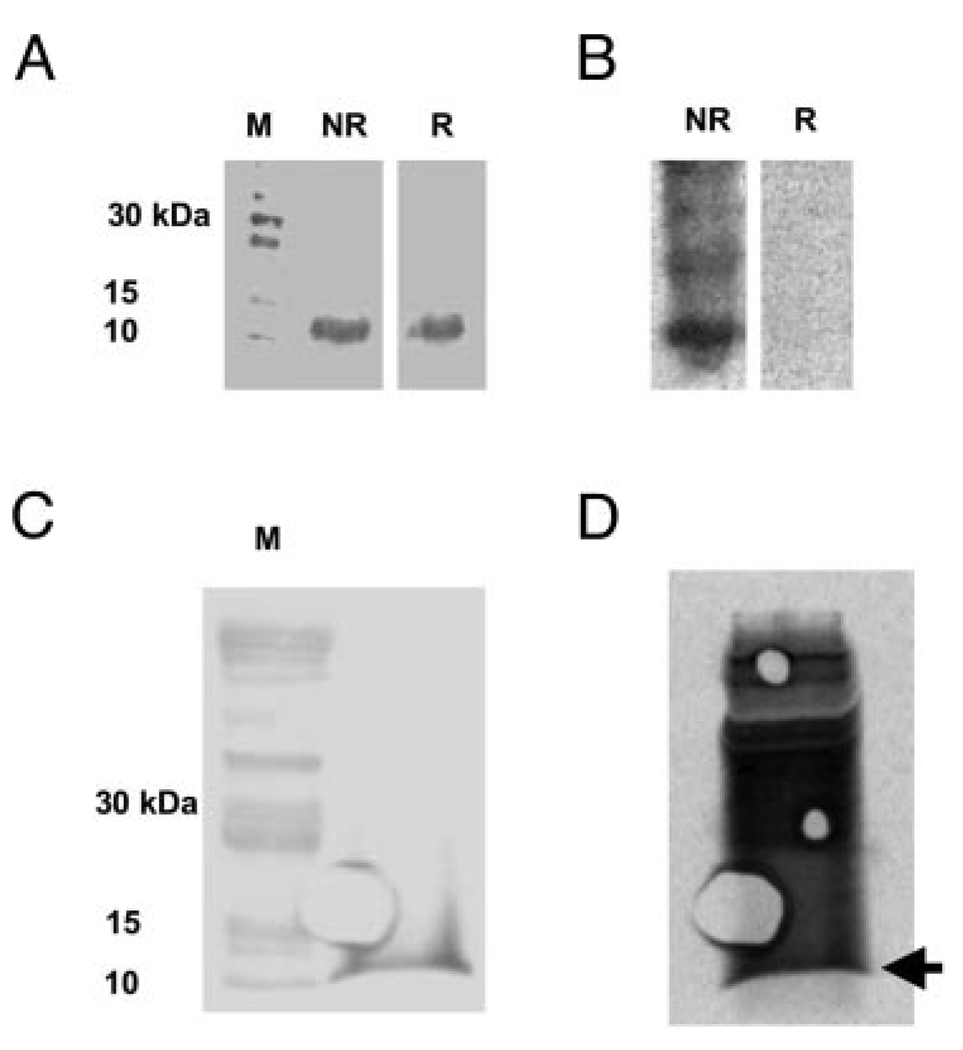
Direct evidence for the S-homocysteinylation of MT under cell culture conditions was obtained by incubating HAECs with 35S-D,L-homocysteine. Western blots of cell lysates run on nonreducing (NR) and reducing (R) SDS-PAGE gels are shown in Figure 1a. A ≈10 kDa band corresponding to MT is shown in both the NR and R lanes. Phosphorimage analysis of the Western blots depicted in Figure 1a is shown in Figure 1b. Several bands including the one corresponding to MT at 10 kDa are present in the NR lane but are not present in the R lane containing BME, which removes the 35S-homocysteine label. Conversely, Western blot (Figure 1c) and phosphorimaging (Figure 1d) demonstrate that treating the 35S-homocysteine labeled cell lysate with 10 mmol/L GSH did not remove the 35S-homocysteine label.
Figure 1.
Identification of 35S-D,L-homocysteinylated-MT in HAEC. A, Western blot of HAEC lysate probed with anti-MT antibody in the absence (NR) and presence (R) of β-mercaptoethanol (BME). B, Phosphorimage of the same Western blot depicted in (A) demonstrating 35S-homocysteinylated proteins. Several bands including the one corresponding to MT are present in the NR lane but not in the R lane. C, Western blot of HAEC lysate from 35S-D,L-homocysteine-treated cells exposed to 10 mmol/L reduced glutathione. D, Phosphorimage of the blot in (C) demonstrating the retention of the 35S-homocysteine label after glutathione treatment. Arrow denotes the position of MT.
Inability of MT from Homocysteine-Treated HAECs to Bind to Zinc-Sepharose
Although a single molecule of MT is capable of binding 7 atoms of zinc,19–22 intracellular MT is never fully saturated32 and will be retained by zinc-Sepharose columns as shown in Scheme 1.33
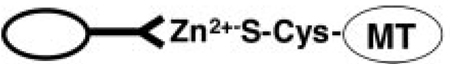 |
(1) |
We reasoned that if some or all of the sulfhydryl groups in MT became homocysteinylated, the protein would loose its ability to coordinate zinc-Sepharose as shown in Scheme 2.
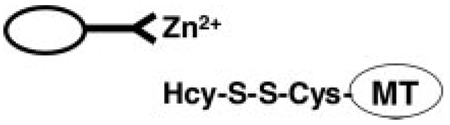 |
(2) |
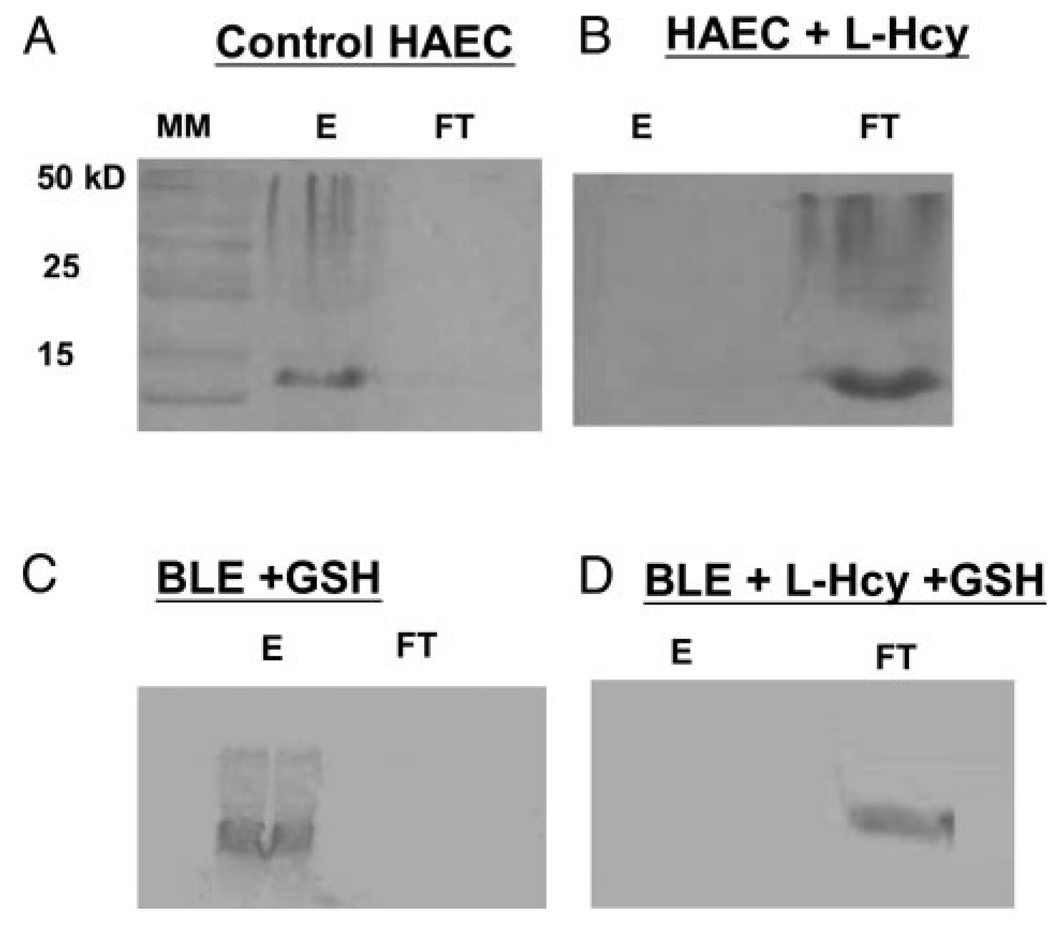
Lysates from HAECs incubated with or without L-homocysteine were applied to zinc-Sepharose columns. In separate experiments, bovine liver extracts (BLE) were incubated with 10 mmol/L GSH or 10 mmol/L GSH plus 100 µmol/L L-homocysteine. Both the flow-through (FT) and EDTA elutions (E) from the zinc-Sepharose columns were analyzed for MT by Western blotting. As shown in Figure 2a, the MT in lysates from HAECs incubated without L-homocysteine was retained by the zinc-Sepharose column and was recovered after elution with EDTA (Figure 2a, E). Little or no MT was detected in the FT (Figure 2a, FT). However, as shown in Figure 2b, MT in lysates from HAECs incubated with 100 µmol/L L-homocysteine appeared in the FT (Figure 2b, FT). Little or no MT was retained by the zinc-Sepharose column (Figure 2b, E). As shown in Figure 2c, MT in bovine liver extracts treated with 10 mmol/L GSH was retained in the column and recovered after EDTA elution (Figure 2c, E). Conversely, MT in the bovine liver extracts treated with 10 mmol/L GSH plus 100 µmol/L L-homocysteine were not retained in the column and were detected in the FT (Figure 2d, FT). This experiment demonstrates that MT obtained from homocysteine-treated HAEC and BLE loses its ability to bind zinc. In addition, GSH was unable to reverse impaired zinc binding caused by L-homocysteine. MT in lysates from HAEC treated with 100 µmol/L L-cysteine behaved like MT in control cells, ie, L-cysteine did not prevent binding of MT to Zn-Sepharose (data not shown). These results demonstrate that the targeting of MT by homocysteine is thiol specific and not affected by physiological levels of GSH or L-cysteine.
Figure 2.
Inability of L-homocysteinylated MT to bind to zinc-Sepharose. A, Western blot of MT in either the EDTA eluant (E) and flow-through (FT) fractions obtained by applying untreated HAEC lysate and (B) homocysteine-treated HAEC lysate to zinc-Sepharose. C, Western blot of E and FT fractions obtained by applying bovine liver extract (BLE) treated with 10 mmol/L glutathione and (D) BLE treated with 10 mmol/L glutathione plus 100 µmol/L L-homocysteine to zinc-Sepharose. The presence of MT in the EDTA eluant (E) signifies MT retention on the column while the presence of MT in the flow-through (FT) signifies MT was not retained on the column.
Increase in Intracellular Free Zinc in Homocysteine-Treated HAECs
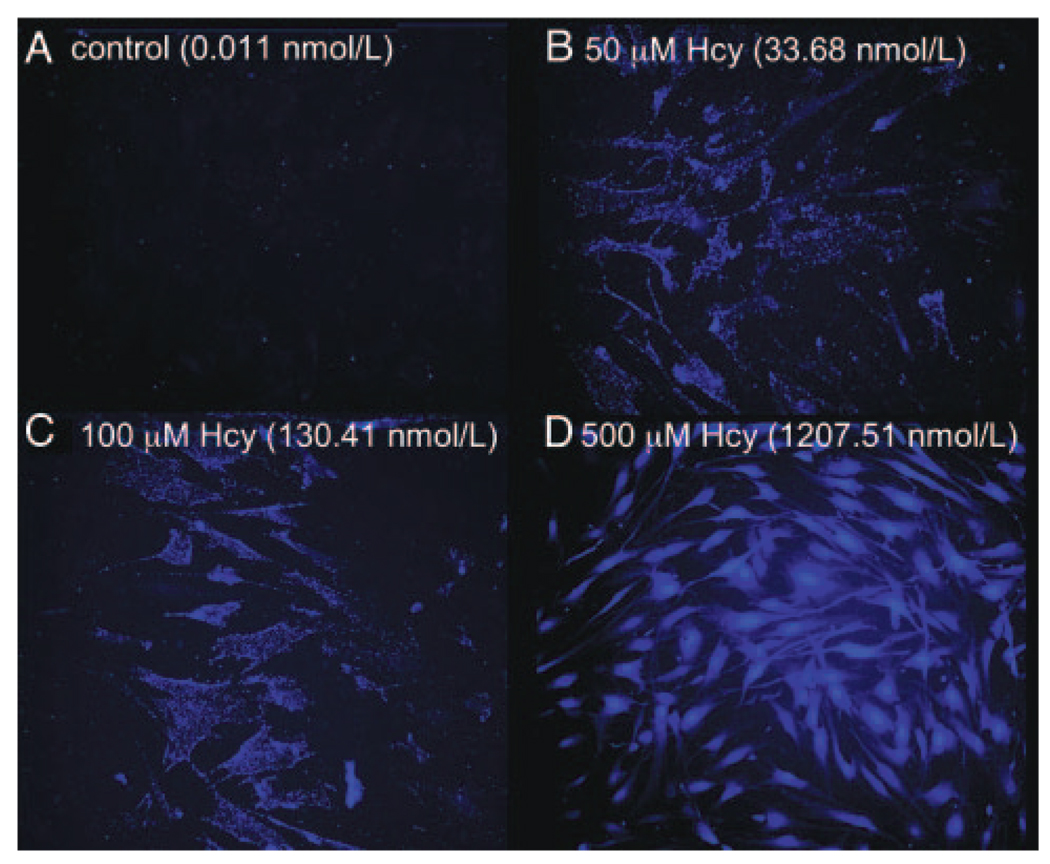
HAECs were loaded with a zinc-specific fluorophore (Zinquin-AM) to visualize intracellular free zinc in the absence and presence of different concentrations of L-homocysteine. As shown in Figure 3a, under control conditions, intracellular zinc was measured at sub nmol/L concentrations. After incubating with increasing amounts of L-homocysteine, a dose-dependent increase in free zinc was observed (Figure 3b to 3d). Specifically, after incubation with 50, 100, and 500 µmol/L L-homocysteine, intracellular free zinc increased to 34±5.1, 130±15.3, and 1208±118.4 nmol/L, respectively. L-cysteine, at similar concentrations, was unable to elicit a dose-dependent increase in intracellular free zinc (data not shown).
Figure 3.
Visualization of free intracellular Zn2+ in cultured HAECs after treatment with L-homocysteine. Panel A represents free zinc under control conditions. B to D, Free zinc after treatment with increasing doses of L-homocysteine (50, 100, and 500 µmol/L), respectively. Values represent the mean [Zn2+]i obtained from 20 cells per each condition as determined by the method of Chen et al.30
Induction of Egr-1 Protein Expression in Homocysteine-Treated HAECs
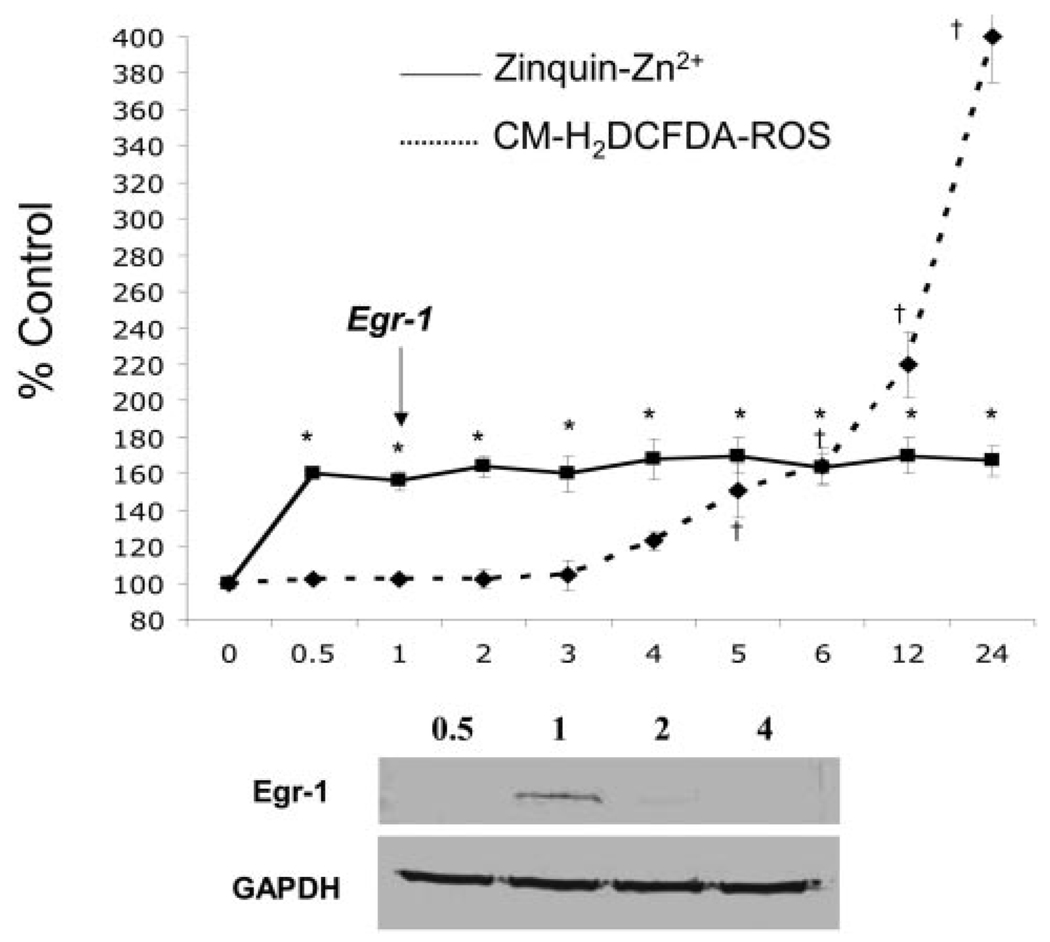
Sudden increases in zinc are known to induce the expression of immediate early genes such as Egr-1.24,25 Based on previous studies showing the induction of Egr-1 expression in response to increased free zinc,24,25 we suspected that the release of free zinc mediated by homocysteine in HAECs might also induce the expression of Egr-1. As shown in Figure 4, maximal intracellular Zinquin-AM fluorescence occurred within 30 minutes of homocysteine treatment and remained constant for 24 hours. Lysates, prepared from similarly treated HAECs at 0.5, 1, 2, and 4 hours depict a transient expression of Egr-1 protein 1 hour after L-homocysteine treatment (Figure 4). ROS increased significantly 5 to 6 hours after L-homocysteine treatment. This study shows that L-homocysteine mediates zinc release in HAECs, which is associated with the expression of Egr-1 protein. L-homocysteine-mediated zinc release occurred several hours before L-homocysteine–mediated increases in ROS (Figure 4).
Figure 4.
Intracellular free Zn2+, ROS, and Egr-1 protein expression in HAEC as a function of time exposed to L-homocysteine. The solid line represents the percent change in intracellular free Zn2+ in HAEC incubated with 50 µmol/L L-homocysteine. The dashed line represents the percent change in ROS as measured with CM-H2DCFDA-loaded HAECs incubated with 50 µmol/L L-homocysteine. The inset depicts a Western blot probed with anti-Egr-1 and GAPDH antibodies at 0.5-, 1-, 2-, and 4-hour points. The arrow marks Egr-1 protein expression. Error bars represent ±SD. *Statistical significance compared with Zinquin-loaded cells at the zero time point (P<0.01). †Statistical significance compared with CM-H2DCFDA-loaded cells at the zero time point (P<0.01).
Superoxide Anion Radical Scavenging Ability of MT and Homocysteinylated MT
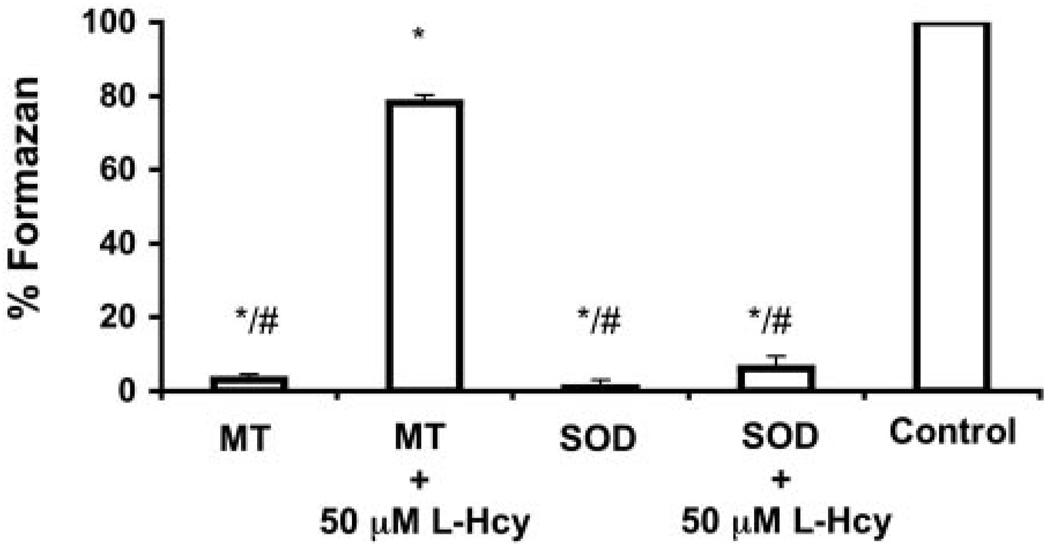
Using in vitro riboflavin photolysis to generate superoxide anion radicals, the ability of MT to scavenge these radicals was determined. As shown in Figure 5, MT in the absence of homocysteine conferred a superoxide-scavenging ability comparable to 6000 U of SOD. In the presence of 50 µmol/L of L-homocysteine, the ability of MT to scavenge superoxide anion radicals was significantly impaired while homocysteine was without effect on SOD (Figure 5). These studies demonstrate for the first time that L-homocysteine is capable of disrupting the superoxide anion radical scavenging function of MT in vitro.
Figure 5.
Ability of MT and homocysteinylated-MT to scavenge superoxide anion radicals. Bars represent the ability to scavenge superoxide anion radicals in the absence and presence of 50 µmol/L L-homocysteine (L-Hcy). Values expressed as normalized percentage of formazan relative to control reactions. Error bars represent ± SD. *Statistical significant difference relative to control (P<0.01). # Statistical significance relative to MT +50 µmol/L L-Hcy.
Discussion
The concept of molecular targeting of protein cysteine residues by L-homocysteine provides a plausible mechanism that could explain the impaired vascular function associated with hyperhomocystinemia. This hypothesis is supported by the observations that the sulfhydryl group of homocysteine and the disulfide group of homocystine interact with protein cysteine residues to form stable mixed-disulfide conjugates resulting in altered function of the targeted protein.11 Previously, our laboratory demonstrated key aspects of this paradigm with several extracellular proteins.13,34,35 However, the identification and characterization of intracellular targets has been limited. Therefore, the major finding of this work is that homocysteine targets the intracellular cysteine-rich protein MT in cultured HAECs, impairs its zinc-binding function and inhibits its ability to scavenge superoxide anion radicals. Moreover, L-homocysteine, in a dose-dependent manner increases intracellular zinc in HAECs and promotes the transient expression of Egr-1, a protein involved in atherosclerosis and restenosis.36
The pathological consequence of molecular targeting of an intracellular zinc-chaperone and anti-oxidant defense molecule is a novel mechanism for homocysteine-mediated vascular damage. Although decreased glutathione peroxidase (GPx-1) activity has been associated with elevated homocysteine, it is not clear if the decreased activity is the result of a post-translational modification of GPx-1, or a genomic effect resulting in altered GPx-1 gene expression.37,38 Therefore, identifying MT as a target of homocysteine in HAECs represents the first naturally occurring intracellular protein to be homocysteinylated in an intact cellular system.
The finding that homocysteine impairs the zinc-binding function of MT has major implications. Zinc is the second most prevalent intracellular trace element in the body and plays a vital role in both the structural and functional integrity of numerous signaling and metabolic pathways. Approximately 2000 transcription factors39 and 300 enzymes require zinc.40 However, because of the low intracellular concentration of labile zinc (fmol/L-nmol/L range),41 MT is believed to function as an intracellular zinc chaperone and maintain intracellular zinc homeostasis for zinc-requiring proteins.42 It is thus postulated that MT plays an important function in the trafficking of cytosolic zinc.42 Specifically, inhibition of Cu/apo-superoxide dismutase has been demonstrated with apo-MT, whereas its activation has been demonstrated with zinc-saturated MT.43 Therefore, the interference of the zinc-binding function could have deleterious consequences on intracellular zinc homeostasis affecting multiple biochemical processes.
Our results show that L-homocysteine elicits a significant increase in intracellular free zinc. Although the stoichiometric ratio of zinc to MT is the highest of all zinc containing proteins, this study does not unequivocally prove that the increase in zinc depicted in Figure 3 is caused by the release of zinc from MT. Future studies, using a fluorescent resonance energy transfer–MT molecule will discern this uncertainty. Despite a high affinity for zinc, the release of zinc from MT occurs on NO binding and by oxidized glutathione. 20–22 Therefore, MT plays a crucial link between the cellular redox potential, NO signaling and zinc homeostasis. 20–23 In addition, zinc released from MT was shown to increase ROS formation via NADPH oxidase induction.44
In endothelial cells, intracellular adhesion molecules expression and the attachment of monocytes are driven by zinc-dependent transcription factors, and it is thought that their activation is associated with zinc release from MT.20–23,44 Furthermore, increased intracellular free zinc is known to induce Egr-1 in neural tissue and epithelial cells.24,25 Consistent with this notion is our finding that homocysteine causes a transient expression of Egr-1 within 1 hour of homocysteine incubation. This finding is significant because there are consensus sequences for Egr-1 in the promoters of various mediators of atherosclerosis including monocyte chemotactic protein-1, tissue necrosis factor-α, and intracellular adhesion molecule-1. Moreover, the upregulation of Egr-1 by homocysteine could potentially explain the downstream activation of monocyte chemotactic protein-1 within 2 to 3 hours of homocysteine incubation as previously reported by our laboratory. 45 Coupled with the observation that homocysteine impairs the superoxide scavenging ability of MT, homocysteine could possibly be amplifying oxidative stress by inactivating MT’s ability to react rapidly with superoxide anion radicals.
The targeting of MT by L-homocysteine represents a novel mechanism to explain the molecular basis for homocysteine-mediated pathology. In future studies, we will determine the stoichiometry of the homocysteine/MT reaction under in vitro and in vivo conditions as well as the actual mechanism(s) of the interactions between MT and L-homocysteine (or L-homocystine).
Acknowledgments
Sources of Funding
This work was supported by National Heart Lung and Blood Institute of the National Institutes of Health grant no. HL52234 (to D.W.J.).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.McCully KS, Wilson RB. Homocysteine theory of arteriosclerosis. Atherosclerosis. 1975;22:215–227. doi: 10.1016/0021-9150(75)90004-0. [DOI] [PubMed] [Google Scholar]
- 2.Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, Andria G, Boers GHJ, Bromberg IL, Cerone R, Fowler B, Grobe H, Schmidt H, Schweitzer L. The natural history of homocystinuria due to cystathionine-β-synthase deficiency. Am J Hum Genet. 1985;37:1–31. [PMC free article] [PubMed] [Google Scholar]
- 3.Hofmann MA, Lolla E, Lu Y, Ryu Gleason M, Wolf BM, Tanji N, Ferran LJ, Jr, Kohl B, Rao V, Kisiel W, Stern DM, Schmidt AM. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107:675–683. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Moller J, Danielsen CC, Bentzon J, Ravn HB, Austin RC, Falk E. Dietary supplement with methionine and homocysteine promotes early atherogenesis but not plaque rupture in apoE-deficient mice. Arterioscler Thromb Vas Biol. 2001;21:1470–1476. doi: 10.1161/hq0901.096582. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Jiang X, Yang F, Gaubatz JW, Ma L, Magera MJ, Yang X, Berger PB, Durante W, Pownall HJ, Schafer AI. Hyperhomocysteinemia accelerates atherosclerosis in cystathionine β-synthase and apolipoprotein E double knock-out mice with and without dietary perturbation. Blood. 2003;101:3901–3907. doi: 10.1182/blood-2002-08-2606. [DOI] [PubMed] [Google Scholar]
- 6.Chen P, Poddar R, Tipa EV, DiBello PM, Moravec CD, Robinson K, Green R, Kruger WD, Garrow TA, Jacobsen DW. Homocysteine metabolism in cardiovascular cells and tissues: implications for hyperhomocysteinemia and cardiovascular disease. Adv Enzyme Regul. 1999;39:93–109. doi: 10.1016/s0065-2571(98)00029-6. [DOI] [PubMed] [Google Scholar]
- 7.Weiss N, Heydrick SJ, Postea O, Keller C, Keaney JF, Jr, Loscalzo J. Influence of hyperhomocysteinemia on the cellular redox state–impact on homocysteine-induced endothelial dysfunction. Clin Chem Lab Med. 2003;41:1455–1461. doi: 10.1515/CCLM.2003.223. [DOI] [PubMed] [Google Scholar]
- 8.Moat SJ, McDowell IF. Homocysteine and endothelial function in human studies. Semin Vasc Med. 2005;5:172–182. doi: 10.1055/s-2005-872402. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Werstuck GH, Lhotak S, de Koning AB, Sood SK, Hossain GS, Moller J, Ritskes-Hoitinga M, Falk E, Dayal S, Lentz SR, Austin RC. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation. 2004;110:207–213. doi: 10.1161/01.CIR.0000134487.51510.97. [DOI] [PubMed] [Google Scholar]
- 10.Cattaneo M. Hyperhomocysteinemia, atherosclerosis and thrombosis. Thromb Haemost. 1999;81:165–176. [PubMed] [Google Scholar]
- 11.Jacobsen DW, DiBello PM, Catanescu O, Barbato JC. Molecular targeting by homocysteine: A mechanism for vascular pathogenesis. Clin Chem Lab Med. 2005;43:1076–1083. doi: 10.1515/CCLM.2005.188. [DOI] [PubMed] [Google Scholar]
- 12.Hajjar KA, Mauri L, Jacovina AT, Zhong FM, Mirza UA, Padovan JC, Chait BT. Tissue plasminogen activator binding to the annexin II tail domain - Direct modulation by homocysteine. J Biol Chem. 1998;273:9987–9993. doi: 10.1074/jbc.273.16.9987. [DOI] [PubMed] [Google Scholar]
- 13.Majors AK, Sengupta S, Willard B, Kinter MT, Pyeritz RE, Jacobsen DW. Homocysteine binds to human plasma fibronectin and inhibits its interaction with fibrin. Arterioscler Thromb Vas Biol. 2002;22:1354–1359. doi: 10.1161/01.atv.0000023899.93940.7c. [DOI] [PubMed] [Google Scholar]
- 14.Stuhlinger MK, Tsao PS, Her J-H, Kimoto M, Balint RF, Cooke JP. Homocysteine impairs the nitric oxide synthase pathway role of asymmetric dimethylarginine. Circulation. 2001;104:2569–2575. doi: 10.1161/hc4601.098514. [DOI] [PubMed] [Google Scholar]
- 15.Hubmacher D, Tiedemann K, Bartels R, Brinckmann J, Vollbrandt T, Batge B, Notbohm H, Reinhardt DP. Modification of the structure and function of fibrillin-1 by homocysteine suggests a potential pathogenetic mechanism in homocystinuria. J Biol Chem. 2005;280:34946–34955. doi: 10.1074/jbc.M504748200. [DOI] [PubMed] [Google Scholar]
- 16.Hamer DH. Metallothionein Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- 17.Palmiter RD. The elusive function of metallothioneins. Proc Natl Acad Sci U S A. 1998;95:8428–8430. doi: 10.1073/pnas.95.15.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Chen H, Epstein PN. Metallothionein protects islets from hypoxia and extends islet graft survival by scavenging most kinds of reactive oxygen species. J Biol Chem. 2004;279:765–771. doi: 10.1074/jbc.M307907200. [DOI] [PubMed] [Google Scholar]
- 19.Nettesheim DG, Engeseth HR, Otvos JD. Products of metal exchange reactions of metallothionein. Biochemistry. 1985;24:6744–6751. doi: 10.1021/bi00345a003. [DOI] [PubMed] [Google Scholar]
- 20.Maret W. Oxidative metal release from metallothionein via zinc-thiol/disulfide interchange. Proc Natl Acad Sci U S A. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacob C, Maret W, Vallee BL. Control of zinc transfer between thionein, metallothionein, and zinc proteins. Proc Natl Acad Sci U S A. 1998;95:3489–3494. doi: 10.1073/pnas.95.7.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang LJ, Maret W, Vallee BL. The glutathione redox couple modulates zinc transfer from metallothionein to zinc-depleted sorbitol dehydrogenase. Proc Natl Acad Sci U S A. 1998;95:3483–3488. doi: 10.1073/pnas.95.7.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maret W, Vallee BL. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc Natl Acad Sci U S A. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckmann AM, Wilce PA. Egr transcription factors in the nervous system. Neurochem Int. 1997;31:477–510. doi: 10.1016/s0197-0186(96)00136-2. discussion 517–476. [DOI] [PubMed] [Google Scholar]
- 25.Samet JM, Graves LM, Quay J, Dailey LA, Devlin RB, Ghio AJ, Wu W, Bromberg PA, Reed W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am J Physiol. 1998;275:L551–L558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- 26.Baernstein HD. A modification of the method for determining methionine in proteins. J Biol Chem. 1934;106:451–456. [Google Scholar]
- 27.Duerre JA, Miller CH. Preparation of L-homocysteine from L-homocysteine thiolactone. Anal Biochem. 1966;17:310–315. doi: 10.1016/0003-2697(66)90209-0. [DOI] [PubMed] [Google Scholar]
- 28.Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Meth. 1984;10:203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- 29.Porath J, Carlsson J, Olsson I, Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975;258:598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Feller GM, Barbato JC, Periyasamy S, Xie ZJ, Koch LG, Shapiro JI, Britton SL. Cardiac performance in inbred rat genetic models of low and high running capacity. J Physiol. 2001;535:611–617. doi: 10.1111/j.1469-7793.2001.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunaiti A. Radial diffusion as a simple and rapid method for screening superoxide dismutase activity. Ann Clin Biochem. 1987;24(Pt 5):511–512. doi: 10.1177/000456328702400515. [DOI] [PubMed] [Google Scholar]
- 32.Lehman LD, Klaassen CD. Separation and quantitation of metallothioneins by high-performance liquid chromatography coupled with atomic absorption spectrophotometry. Anal Biochem. 1986;153:305–314. doi: 10.1016/0003-2697(86)90097-7. [DOI] [PubMed] [Google Scholar]
- 33.Honda RT, Araujo RM, Horta BB, Val AL, Demasi M. One-step purification of metallothionein extracted from two different sources. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;820:205–210. doi: 10.1016/j.jchromb.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Sengupta S, Chen H, Togawa T, DiBello PM, Majors AK, Büdy B, Ketterer ME, Jacobsen DW. Albumin thiolate anion is an intermediate in the formation of albumin-S-S-homocysteine. J Biol Chem. 2001;276:30111–30117. doi: 10.1074/jbc.M104324200. [DOI] [PubMed] [Google Scholar]
- 35.Lim A, Sengupta S, McComb ME, Theberge R, Wilson WG, Costello CE, Jacobsen DW. In vitro and In vivo interactions of homocysteine with human plasma transthyretin. J Biol Chem. 2003;278:49707–49713. doi: 10.1074/jbc.M306748200. [DOI] [PubMed] [Google Scholar]
- 36.Blaschke F, Bruemmer D, Law RE. Egr-1 is a major vascular pathogenic transcription factor in atherosclerosis and restenosis. Rev Endocr Metab Disord. 2004;5:249–254. doi: 10.1023/B:REMD.0000032413.88756.ee. [DOI] [PubMed] [Google Scholar]
- 37.Upchurch GR, Jr, Welch GN, Fabian AJ, Freedman JE, Johnson JL, Keaney JF, Jr, Loscalzo J. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 1997;272:17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 38.Handy DE, Zhang Y, Loscalzo J. Homocysteine down-regulates cellular glutathione peroxidase (GPx1) by decreasing translation. J Biol Chem. 2005;280:15518–15525. doi: 10.1074/jbc.M501452200. [DOI] [PubMed] [Google Scholar]
- 39.Vallee BL, Coleman JE, Auld DS. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc Natl Acad Sci U S A. 1991;88:999–1003. doi: 10.1073/pnas.88.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 41.Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 42.Costello LC, Guan Z, Franklin RB, Feng P. Metallothionein can function as a chaperone for zinc uptake transport into prostate and liver mitochondria. J Inorg Biochem. 2004;98:664–666. doi: 10.1016/j.jinorgbio.2004.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu SX, Fabisiak JP, Tyurin VA, Borisenko GG, Pitt BR, Lazo JS, Kagan VE. Reconstitution of apo-superoxide dismutase by nitric oxide-induced copper transfer from metallothioneins. Chem Res Toxicol. 2000;13:922–931. doi: 10.1021/tx0000623. [DOI] [PubMed] [Google Scholar]
- 44.Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci. 2000;20:RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW. Homocysteine induces expression and secretion of MCP-1 and IL-8 in human aortic endothelial cells: implications for vascular disease. Circulation. 2001;103:2717–2723. doi: 10.1161/01.cir.103.22.2717. [DOI] [PubMed] [Google Scholar]