Abstract
Purpose of the review
Regulation of lipoprotein receptor activity influences lipoprotein metabolism, related physiology and pathophysiology. Adaptor proteins that bind to the LDL or HDL receptors apparently link these receptors to cellular components essential for their normal functioning.
Here we focus on the influence of PDZK1 on the HDL receptor SR-BI, with emphasis on the roles played by its individual PDZ domains, the impact in regulating HDL metabolism and the relevance for cardiovascular disease.
Recent findings
PDZK1 plays an essential role in maintaining hepatic SR-BI levels and controlling HDL metabolism, protects against the development of atherosclerosis in a murine model and also mediates SR-BI-dependent regulation of endothelial cell biology by HDL, suggesting that PDZK1 plays multiple roles in normal physiology and may influence associated pathology. All four PDZ domains of PDZK1 appear necessary to promote normal hepatic expression, function and intracellular localization of SR-BI.
Summary
SR-BI mediates several features of HDL metabolism and function, some of which depend on SR-BI’s interaction with PDZK1. Exploration of the structure and function of PDZK1 and the mechanisms by which it controls SR-BI will provide additional insights into HDL metabolism and may provide the basis for new therapeutic modalities for cardiovascular disease.
Keywords: PDZ domains, high-density lipoprotein receptor, atherosclerosis, endothelium
Introduction
The level of plasma high-density lipoprotein (HDL) cholesterol is inversely associated with the risk of developing atherosclerosis [1, 2], apparently in part because of its role in promoting reverse cholesterol transport (RCT) [3–6]. In reverse cholesterol transport, cholesterol is removed from peripheral organs and transported to the liver where it or its metabolic products (e.g., bile acids) are excreted into the bile. Reverse cholesterol transport appears to be one of the essential mechanisms by which HDL inhibits the development of atherosclerosis and protects against one of the most common forms of cardiovascular disease. Among the cell surface receptors playing major roles in regulating plasma cholesterol levels are members of the low-density lipoprotein (LDL) receptor superfamily (LDL receptor, LRP, etc.), which are regulated by intracellular adaptors, including ARH (autosomal recessive hypercholesterolemia), disabled 1 and disabled 2 [7–11]. The HDL receptor, scavenger receptor class B type I (SR-BI) [12], is one of several cell surface proteins that are regulated by the intracellular adaptor PDZK1 [13–15], previously reviewed in [16]. Here we focus on the regulation of SR-BI’s activity by PDZK1.
SR-BI is an HDL receptor
SR-BI is a 509 amino acid, integral membrane glycoprotein [6]. The bulk of the protein comprises a heavily N-glycosylated extracellular loop, the remaining portions of the molecule defining two transmembrane domains and short intracellular amino and carboxy termini. SR-BI is predominantly expressed in the liver, gastro-intestinal tract and steroidogenic organs [12], although it can also be detected in other types of cells (e.g., macrophages, endothelial cells [6]). SR-BI binding to HDL leads to SR-BI-mediated bulk transfer of its core cholesteryl esters to the cell via a distinctive mechanism called selective lipid uptake [12]. In addition, SR-BI mediates bidirectional flux of unesterified cholesterol between cells and lipoproteins ([17], reviewed in [6]).Targeted disruption of the SR-BI gene in mice leads to an ~2.2-fold increase in plasma cholesterol in abnormally large and unesterified cholesterol enriched HDL particles [18, 19], accompanied by a decrease in biliary cholesterol secretion [20]. SR-BI’s role in reverse cholesterol transport is thought to underlie its potent atheroprotective effects in mice ([21–23], reviewed in [6]). Indeed, increasing the expression of hepatic SR-BI promotes reverse cholesterol transport and reduces atherosclerosis, despite accompanying decreased plasma HDL cholesterol concentration [24–28]. In addition, combined targeted disruptions of the apoE and SR-BI genes results in a novel murine model of coronary heart disease, because it leads not only to severe occlusive coronary arterial atherosclerosis, but also myocardial infarction, cardiac dysfunction and premature death [19, 21]. In the liver, and to a lesser extent in the gastro-intestinal tract, the expression of SR-BI is controlled by its adaptor protein PDZK1 [13, 14].
PDZK1 and PDZ domains
PDZK1 (Figure 1) was first identified in human carcinomas [29]. Initial studies suggested that PDZK1 was exclusively expressed in cells of epithelial origin (proximal tubular cells of the kidney, hepatocytes, intestinal mucosa, adrenal cortical cells among others) [29], however recent studies have demonstrated that it is also expressed in endothelial cells [30, 31]. Its name is based on the presence of four PDZ-protein interaction domains in its sequence. PDZ domains (named after three proteins containing such domains: post-synaptic density protein (PSD95), Drosophila discs large (Dlg) and the tight junction protein zonula occludens-1 (ZO-1)) [32] are composed of about 80–90 amino acids. The human genome encodes over 250 PDZ domains incorporated into over 100 proteins. They fold into relatively compact structures that characteristically contain six β-strands and two α-helices [33]. Because a significant number of PDZ-domain proteins contain multiple PDZ domains, they are able to promote clustering or scaffolding of groups of proteins, often cell surface and related proteins (e.g., cell surface receptors and ion channels), and are involved in a wide variety of cellular functions. Usually, PDZ domains interact with the five most carboxy terminal amino acids of their binding partners (target, proteins). Some recognize as many as seven carboxy terminal residues [34].
Figure 1.
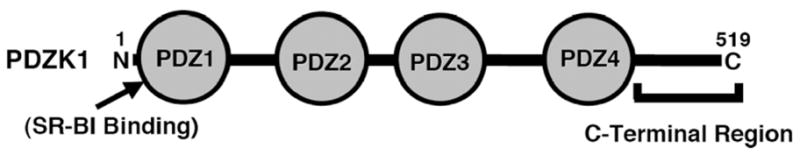
Model of the primary sequence of PDZK1 [45]
The most C-terminal amino acid (position 0) of the binding target of a PDZ domain is almost always hydrophobic. Early analysis of target sequences suggested three classes of targets: position -2 is either a serine or threonine (Class I), hydrophobic (Class II) or aspartate or glutamate (Class III) [35, 36]. However, more recent studies suggest that the PDZ domains themselves cannot be divided rigorously into simple groups based on these three classes of targets, but rather that their sequences have been optimized to very specifically allow the recognition of their binding partners [37, 38]. The C-terminus of mammalian SR-BI (…KG(T/S)VLQEA(R/K)L) (from 10 species, http://blast.ncbi.nlm.nih.gov/Blast.cgi) binds to the first PDZ domain (PDZ1, Figure 1) of PDZK1 [13].
There is a large and growing literature describing PDZK1-dependent control of many other proteins [39], including ion channels and transporters in the kidney and small intestines [15, 40, 41].
Effects of inactivation of the PDZK1 gene on SR-BI in mice
Targeted disruption of the PDZK1 gene results in an ~1.7-fold increase in plasma cholesterol carried in abnormally large HDL particles that are not abnormally enriched in unesterified cholesterol, a result similar to, but not identical to or as severe as, that observed in SR-BI knockout mice [14, 42]. The increase of plasma cholesterol can be attributed primarily to an ~95% decrease in SR-BI protein expression in the liver. There is also an ~50% decreased protein expression in the small intestines, but no change in steroidogenic organs [14]. The differences in lipoprotein metabolism between SR-BI and PDZK1 KO mice are likely due to the residual expression of SR-BI in hepatocytes and intestinal epithelial cells in PDZK1 KO mice and also to the fact that SR-BI expression in steroidogenic organs is PDZK1 independent. In addition, the minor SR-BI variant SR-BII is not regulated by PDZK1 [14]. These findings led to the realization that PDZK1 is a tissue (primarily liver) specific, functionally relevant adaptor protein for SR-BI, reminiscent of the tissue specificity of ARH’s effects on LDL receptor activity [7, 11].
Hepatic overexpression of SR-BI in SR-BI/PDZK1 double knockout mice restores normal expression of SR-BI on the hepatocyte cell surface, suggesting that PDZK1 is required for maintaining adequate steady state cell-surface levels of SR-BI, but is not essential for its surface localization or function, at least in the case of SR-BI transgene overexpression [43]. In transgenic mice, hepatic protein expression and function of transgenic human SR-BI (also called CLA-1) is increased when a human PDZK1 transgene is co-expressed [44].
Role of individual PDZ domains of PDZK1 in controlling SR-BI
When Ikemoto et al. first reported that PDZK1 binds to SR-BI, they showed that the PDZ1 domain of PDZK1 was responsible for this interaction [13]. Fenske et al. [45, 46] have explored the roles of PDZK1’s four PDZ domains and the PDZ domain-free C-terminal region (Figure 1) in regulating hepatic SR-BI. They generated wild-type and PDZK1 KO mice with hepatic expression of PDZK1 transgenes encoding proteins with nested C-terminal truncations, including: PDZ1, PDZ1.2, PDZ1.2.3 or PDZ1.2.3.4, which contain only the first one, two, three or four N-terminal PDZ domains, respectively, but not the remaining C-terminal sequences. Hepatic expression of PDZ1 was not able to restore normal expression or function of SR-BI in PDZK1 KO mice. Immunoperoxidase studies showed that small amounts of SR-BI expressed in hepatocytes of these mice were mislocalized in the cytoplasm, instead of being associated with the plasma membrane. Nevertheless, the PDZ1 transgene exhibited a dominant negative effect when expressed in wild-type mice, inducing phenotypes similar to the PDZK1 KO mice expressing the PDZ1 transgene (reduced and mislocalized SR-BI, hypercholesterolemia due to large HDL). These results indicate that the PDZ1 domain can control the abundance and localization, and therefore the function, of hepatic SR-BI and that structural features of PDZK1 in addition to its SR-BI-binding PDZ1 domain are required for normal hepatic SR-BI regulation. PDZK1 KO mice expressing the PDZ1.2 and PDZ1.2.3 transgenes exhibited phenotypes similar to those with the PDZ1 transgene (although there was a low level of hepatocyte cell surface expression and function of SR-BI in PDZ1.2.3 expressing mice). In contrast, there was restoration of virtually wild-type levels and function of heptatic SR-BI in PDZK1 KO mice expressing hepatic PDZ1.2.3.4. Thus, in this system all four PDZ domains of PDZK1, but not the C-terminal region, are necessary for apparently normal hepatic regulation of SR-BI abundance, localization and function.
Additional studies will be needed to define precisely the roles that PDZ domains 2 to 4 play in normal PDZK1 function. One or more of these domains may 1) facilitate intramolecular PDZK1 folding or act as spacers to maintain the appropriate three dimensional relationship between two or more of the PDZ domains, 2) participate in intermolecular interactions possibly leading to dimerization/oligomerization of PDZK1 essential for SR-BI function, and/or 3) bind to other proteins that promote stable expression of SR-BI at the cell surface. Indeed, Lalonde and Bretscher have recently reported evidence for intramolecular association of PDZK1’s C-terminus with its PDZ1 domain and for PDZ3-mediated dimerization of PDZK1 [47]. Future studies, especially those exploring the roles of PDZ2–4 in binding other cellular components (scaffold function), may be facilitated by recently described models designed to predict the target binding sequence of a PDZ domain based on that domain’s primary sequence [34, 37, 38]
Dietary, genetic, hormonal and pharmacologic regulation of hepatic PDZK1 and SR-BI
Much of the analysis of PDZK1 has focused on its regulation of its target proteins. To date there have only been a relatively limited number of reports focused on the regulation of the expression and activity of PDZK1 itself.
Niemeier et al. [48] have reported that feeding mice an atherogenic diet (high cholesterol, high cholic acid) can reduce hepatic levels of PDZK1 protein as well as SR-BI protein. Several other studies also report coordinate regulation of PDZK1 and SR-BI. Robichaud et al. have reported that the reduced plasma HDL cholesterol level in mice deficient in a key enzyme (phosphatidylethanolamine N-methyltransferase) in one of the two pathways for phosphatidylcholine synthesis is associated with a 1.5-fold increase in the hepatic levels of both PDZK1 and SR-BI protein [49]. Nakamura et al. [50] discovered that the C-terminal region of PDZK1, which does not contain PDZ domains (Figure 1), can be phosphorylated on serine(s) in cultured cells and in vivo in rat liver. In cultured cells, such phosphorylation can - at least in some conditions - facilitate the ability of PDZK1 to optimally increase SR-BI protein levels. They also showed that the extent of this phosphorylation, as well as the levels of hepatic PDZK1 and SR-BI and levels of plasma HDL can be increased in rats by glucagon treatment. Thus, PDZK1 levels can be subject to hormonal control.
Maradones et al. [51] showed that treatment of mice with fibrates (ciprofibrate or fenofibrate), peroxisome proliferator-activated receptor alpha (PPARα agonists, dramatically suppresses hepatic levels of PDZK1 and SR-BI proteins (but not mRNAs) and consequently increases plasma levels of abnormally large HDL particles, a phenotype similar to that observed in SR-BI and PDZK1 knockout mice. Fibrate treatment of cultured rat hepatocytes also suppresses SR-BI protein expression. Lan and Silver have shown that at least some of the decrease in murine hepatic SR-BI protein levels by fibrate (fenofibrate) treatment may be due to enhanced, PDZK1-independent, degradation of SR-BI [52].
As a consequence, results from one of the first reports examining the promoter of PDZK1 were somewhat unexpected. Tachibana et al. reported that the human PDZK1 gene is a positively regulated target of PPARα [53]). They identified consensus sequences for the peroxisome proliferator responsive element (PPRE) and the estrogen receptor responsive element in the promoter region of the human PDZK1 gene. Earlier, Walker et al. reported that estradiol induces PDZK1 expression in an estrogen receptor positive ovarian cancer cell line [54]. Tachibana et al. showed that this PPRE in cultured cells mediates fibrate (PPARα agonist) induction of reporter gene expression [53]. They also showed that a different PPARα agonist (GW7647) could induce an approximately 2.3-fold increase in PDZK1 protein expression in a human hepatoma cell line. The apparently inconsistent results for PPARα agonists in the human and murine systems remain to be explained.
Role of PDZK1 and SR-BI in endothelial cells
Lipoproteins can significantly influence endothelial cell biology [55]. For example, HDL leads to inhibition of adhesion of inflammatory cells to the endothelium, decreased endothelial cell apoptosis and anti-inflammatory effects on endothelial cells [56]. HDL has been shown to induce multiple signaling systems and downstream consequences (e.g., activation of eNOS and cell migration) in endothelial cells via SR-BI, which is expressed on their surfaces [30, 57–61]. PDZK1 is required for several HDL-mediated effects on endothelial cells, including activation of src, endothelial NO synthase and cell migration, and for carotid artery reendothelialization following perivascular electric injury [30, 62–64]. Thus, HDL/SR-BI/PDZK1 interaction seems to be essential in maintaining integrity of the endothelial monolayer [30].
PDZK1’s role in preventing the development of atherosclerosis
Because PDZK1 controls hepatic SR-BI abundance and function and SR-BI is atheroprotective in murine models of atherosclerosis (LDL receptor or apoE KO mice [21, 22, 65], reviewed in [6]), it seemed likely that PDZK1 would also be atheroprotective. This has been confirmed using apoE/PDZK1 double KO and control apoE single KO mice fed with a high cholesterol, Western diet for three months [31]. Aortic root atherosclerotic plaque was 157% greater in double than control single KO mice. The PDZK1 deficiency did not lower SR-BI protein expression on the surfaces of endothelial or monocyte/macrophage cells. Disease in the apoE/PDZK1 double KO mice was not as severe as that in SR-BI/apoE double KO mice [21]. It seems likely that the effects of PDZK1 deficiency on hepatic SR-BI and HDL metabolism play an important role in the atheroprotective effect of PDZK1. It is also possible that the influence of PDZK1 on SR-BI-mediated signaling in endothelial cells (see above) may also contribute to its atheroprotective activity.
Summary
Considerable progress has been made in analyzing the control of SR-BI abundance, localization and function mediated by PDZK1. However many questions remain to be answered. We don’t yet understand the precise molecular and cellular mechanisms by which PDZK1 mediates the tissue specific regulation of SR-BI. For example, it is possible that PDZK1 serves as a scaffold protein in the liver to link SR-BI to other, critically important, as yet unidentified, cellular components. The relative importance of hepatic vs. endothelial PDZK1 in atheroprotection has not been established, nor do we know if naturally occurring variation in PDZK1 structure and function influence human SR-BI and have clinical consequences (e.g., influence risk for atherosclerotic or other cardiovascular diseases). With the continued development of powerful experimental tools to study PDZK1, we can expect substantial progress in addressing these questions in the near future.
Acknowledgments
Supported by grants from the National Institutes of Health to OK (HL077780) and MK (HL64737, HL52212 and HL66105)
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Gordon T, Castelli W, Hjortland M, et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 2.Miller G, Miller N. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975:1. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- 3.Glomset J. The plasma lecithin:cholesterol acyltrans ferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 4.Krieger M. Charting the fate of the “Good Cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- 5.Krieger M, Kozarsky K. Influence of the HDL receptor SR-BI on atherosclerosis. Curr Opin Lipidol. 1999;10:491–7. doi: 10.1097/00041433-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 6 *.Rigotti A, Miettinen H, Krieger M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocrine Reviews. 2003;24:357–387. doi: 10.1210/er.2001-0037. A comprehensive review of SR-BI. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J, Kimmel M, Polanski A, Hobbs H. Molecular mechanisms of autosomal recessive hypercholesterolemia. Curr Opin Lipidol. 2003;14:121–127. doi: 10.1097/00041433-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Stolt P, Bock H. Modulation of lipoprotein receptor functions by intracellular adaptor proteins. Cell Signal. 2006;18:1560–1571. doi: 10.1016/j.cellsig.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 9.He G, Gupta S, Yi M, et al. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin and AP-2. J Biol Chem. 2002;277:44044–44049. doi: 10.1074/jbc.M208539200. [DOI] [PubMed] [Google Scholar]
- 10.Michaely P, Li W, Anderson R, et al. The modular adaptor protein ARH is required for low density lipoprotein (LDL) binding and internalization but not for LDL receptor clustering in coated pits. J Biol Chem. 2004;279:34023–34031. doi: 10.1074/jbc.M405242200. [DOI] [PubMed] [Google Scholar]
- 11.Zuliani G, Arca M, Signore A, et al. Characterization of a new form of inherited hypercholesterolemia: familial recessive hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1999;19:802–809. doi: 10.1161/01.atv.19.3.802. [DOI] [PubMed] [Google Scholar]
- 12.Acton S, Rigotti A, Landschulz KT, et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–20. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 13.Ikemoto M, Arai H, Feng D, et al. Identification of a PDZ-domain-containing protein that interacts with the scavenger receptor class B type I. Proc Natl Acad Sci U S A. 2000;97:6538–43. doi: 10.1073/pnas.100114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kocher O, Yesilaltay A, Cirovic C, et al. Targeted disruption of the PDZK1 gene in mice causes tissue-specific depletion of the high density lipoprotein receptor scavenger receptor class B type I and altered lipoprotein metabolism. J Biol Chem. 2003;278:52820–52825. doi: 10.1074/jbc.M310482200. [DOI] [PubMed] [Google Scholar]
- 15.Seidler U, Singh A, Chen M, et al. Knockout mouse models for intestinal electrolyte transporters and regulatory PDZ adaptors: new insights into cystic fibrosis, secretory diarrhoea and fructose-induced hypertension. Exp Physiol. 2009;94:175–179. doi: 10.1113/expphysiol.2008.043018. [DOI] [PubMed] [Google Scholar]
- 16.Yesilaltay A, Kocher O, Rigotti A, Krieger M. Regulation of SR-BI-mediated high-density lipoprotein metabolism by the tissue-specific adaptor protein PDZK1. Curr Opin Lipidol. 2005;16:147–152. doi: 10.1097/01.mol.0000162319.54795.e5. [DOI] [PubMed] [Google Scholar]
- 17.Ji Y, Jian B, Wang N, et al. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 18.Rigotti A, Trigatti BL, Penman M, et al. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A. 1997;94:12610–5. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun A, Zhang S, Miettinen H, et al. Probucol prevents early coronary heart disease and death in the high-density lipoprotein receptor SR-BI/apolipoprotein E double knockout mouse. Proc Natl Acad Sci U S A. 2003;100:7283–7288. doi: 10.1073/pnas.1237725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mardones P, Quinones V, Amigo L, et al. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J Lipid Res. 2001;42:170–180. [PubMed] [Google Scholar]
- 21.Braun A, Trigatti BL, Post MJ, et al. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res. 2002;90:270–6. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- 22.Trigatti B, Rayburn H, Vinals M, et al. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci U S A. 1999;96:9322–7. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Eck M, Twisk J, Hoekstra M, et al. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J Biol Chem. 2003;278:23699–23705. doi: 10.1074/jbc.M211233200. [DOI] [PubMed] [Google Scholar]
- 24.Arai T, Wang N, Bezouevski M, et al. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scaveger receptor BI transgene. J Biol Chem. 1999;274:2266–2271. doi: 10.1074/jbc.274.4.2366. [DOI] [PubMed] [Google Scholar]
- 25.Kozarsky K, Donahee M, Glick J, et al. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler Thromb Vasc Biol. 2000;20:721–727. doi: 10.1161/01.atv.20.3.721. [DOI] [PubMed] [Google Scholar]
- 26.Ueda Y, Gong E, Royer L, et al. Relationship between expression levels and atherogenesis in scavenger receptor class B, type I transgenics. J Biol Chem. 2000;275:20368–20373. doi: 10.1074/jbc.M000730200. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Da Silva J, Reilly M, et al. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rader D, Alexander E, Weibel G, et al. Role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2008 doi: 10.1194/jlr.R800088-JLR200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kocher O, Comella N, Tognazzi K, Brown LF. Identification and partial characterization of PDZK1: A novel protein containing PDZ interaction domains. Lab Invest. 1998;78:117–125. [PubMed] [Google Scholar]
- 30 **.Zhu W, Saddar S, Seetharam D, et al. The scavenger receptor class B type I adaptor protein PDZK1 maintains endothelial monolayer integrity. Circ Res. 2008;102:480–487. doi: 10.1161/CIRCRESAHA.107.159079. This study shows that PDZK1 is necessary for endothelial regeneration following injury. [DOI] [PubMed] [Google Scholar]
- 31 *.Kocher O, Yesilaltay A, Shen C, et al. Influence of PDZK1 on Lipoprotein Metabolism and Atherosclerosis. Biochim Biophys Acta. 2008;1782:310–316. doi: 10.1016/j.bbadis.2008.02.004. This study establishes that PDZK1 is atheroprotective in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 33.Doyle DA, Lee A, Lewis J, et al. Crystal structures of a complexed and peptide-free membrane protein-binding domain: Molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- 34.Tonikian R, Zhang Y, Sazinsky S, et al. A specificity map for the PDZ domain family. PLoS Biol. 2008;6:e239. doi: 10.1371/journal.pbio.0060239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hung A, Sheng M. PDZ domains: structural modules for protein complex assembly. J Biol Chem. 2002;277:5699–5702. doi: 10.1074/jbc.R100065200. [DOI] [PubMed] [Google Scholar]
- 36.Nourry C, Grant S, Borg J. PDZ domain proteins: plug and play! Sci STKE 2003. 2003:RE7. doi: 10.1126/stke.2003.179.re7. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Chang B, Allen J, et al. Predicting PDZ domain-peptide interactions from primary sequences. Nat Biotechnol. 2008;26:1041–1045. doi: 10.1038/nbt.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stiffler M, Chen J, Grantcharova V, et al. PDZ domain binding selectivity is optimized across the mouse proteome. Science. 2007;317:364–369. doi: 10.1126/science.1144592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang P, Wang J, Xiao Y, et al. Interaction with PDZK1 is required for expression of organic anion transporting protein 1A1 on the hepatocyte surface. J Biol Chem. 2005;280:30143–30149. doi: 10.1074/jbc.M503969200. [DOI] [PubMed] [Google Scholar]
- 40.Hillesheim J, Riederer B, Tuo B, et al. Down regulation of small intestinal ion transport in PDZK1 (CAP70/NHERF3) deficient mice. Pflugers Archiv European Journal of Physiology. 2007;454:575–586. doi: 10.1007/s00424-007-0239-x. [DOI] [PubMed] [Google Scholar]
- 41.Thomson R, Wang T, Thomson B, et al. Role of PDZK1 in membrane expression of renal brush border ion exchangers. Proc Natl Acad Sci U S A. 2005:13331–13336. doi: 10.1073/pnas.0506578102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kocher O, Pal R, Roberts M, et al. Targeted disruption of the PDZK1 gene by homologous recombination. Mol Cell Biol. 2003;23:1175–80. doi: 10.1128/MCB.23.4.1175-1180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yesilaltay A, Kocher O, Pal R, et al. PDZK1 is required for maintaining hepatic scavenger receptor, class B, type I (SR-BI) steady state levels but not its surface localization or function. J Biol Chem. 2006;281:28975–28980. doi: 10.1074/jbc.M603802200. [DOI] [PubMed] [Google Scholar]
- 44.Komori H, Arai H, Kashima T, et al. Co-expression of CLA-1 and human PDZK1 in murine liver modulates HDL cholesterol metabolism. Arterioscler Thromb Vasc Biol. 2008;28:1298–1303. doi: 10.1161/ATVBAHA.108.165845. [DOI] [PubMed] [Google Scholar]
- 45 **.Fenske S, Yesilaltay A, Pal R, et al. Normal hepatic cell-surface localization of the high-density lipoprotein receptor, SR-BI, depends on all four PDZ domains of PDZK1. J Biol Chem. 2009;284:5797–5806. doi: 10.1074/jbc.M808211200. This study establishes that all four domains of PDZK1 are necessary for normal expression, localization and function of SR-BI in hepatocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46 **.Fenske S, Yesilaltay A, Pal R, et al. Overexpression of the PDZ1 domain of PDZK1 blocks the activity of hepatic scavenger receptor, class B, type I by altering its abundance and cellular localization. J Biol Chem. 2008;283:22097–22104. doi: 10.1074/jbc.M800029200. This study shows that the first PDZ domain of PDZK1 expressed as a truncated mutant functions as a dominant negative. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lalonde D, Bretscher A. The scaffold protein PDZK1 undergoes a head-to-tail intramolecular association that negatively regulates its interaction with EBP50. Biochemistry. 2009 doi: 10.1021/bi802089k. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niemeier A, Kovacs W, Strobl W, Stangl H. Atherogenic diet leads to posttranslational down-regulation of murine hepatocyte SR-BI expression. Atherosclerosis. 2009;202:169–175. doi: 10.1016/j.atherosclerosis.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Robichaud J, Francis G, Vance D. A role for hepatic scavenger receptor class B, type I in decreasing high density lipoprotein levels in mice that lack phosphatidylethanolamine N-methyltransferase. J Biol Chem. 2008;283:35496–35506. doi: 10.1074/jbc.M807433200. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura T, Shibata N, Nishimoto-Shibata T, et al. Regulation of SR-BI protein levels by phosphorylation of its associated protein, PDZK1. Proc Natl Acad Sci U S A. 2005;102:13404–13409. doi: 10.1073/pnas.0506679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mardones P, Pilon A, Bouly M, et al. Fibrate down-regulate hepatic scavenger receptor class B type I protein expression in mice. J Biol Chem. 2003;278:7884–7890. doi: 10.1074/jbc.M211627200. [DOI] [PubMed] [Google Scholar]
- 52.Lan D, Silver DL. Fenofibrate induces a novel degradation pathway for scavenger receptor B-I independent of PDZK1. J Biol Chem. 2005;280:23390–23396. doi: 10.1074/jbc.M502777200. [DOI] [PubMed] [Google Scholar]
- 53.Tachibana K, Anzai N, Ueda C, et al. Regulation of the human PDZK1 expression by peroxisome proliferator-activated receptor alpha. FEBS letters. 2008 doi: 10.1016/j.febslet.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 54.Walker G, MacLeod K, Williams A, et al. Estrogen-regulated gene expression predicts response to endocrine therapy in patients with ovarian cancer. Gynecol Oncol. 2007;106:461–468. doi: 10.1016/j.ygyno.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Rader D, Dugi K. The endothelium and lipoproteins: insights from recent cell biology and animal studies. Semin Thromb Hemost. 2000;26:521–528. doi: 10.1055/s-2000-13208. [DOI] [PubMed] [Google Scholar]
- 56.Nofer J, Kehrel B, Fobker M, et al. HDL and atherosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161:1–16. doi: 10.1016/s0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 57.Hatzopoulos A, Rigotti A, Rosenberg R, Krieger M. Temporal and spatial pattern of expression of the HDL receptor SR-BI during murine embryogenesis. J Lipid Res. 1998;39:495–508. [PubMed] [Google Scholar]
- 58.Yuhanna I, Zhu Y, Cox B, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 59.Li X, Titlow W, Jackson B, et al. High density lipoprotein binding to scavenger receptor, Class B, type I activates endothelial nitric-oxide synthase in a ceramide-dependent manner. J Biol Chem. 2002;277:11058–11063. doi: 10.1074/jbc.M110985200. [DOI] [PubMed] [Google Scholar]
- 60.Mineo C, Deguchi H, Griffin J, Shaul PW. Endothelial and anti-thrombotic actions of HDL. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 61.Mineo C, Shaul P. Role of high-density lipoprotein and scavenger receptor B type I in the promotion of endothelial repair. Trends Cardiovasc Med. 2007;17:156–161. doi: 10.1016/j.tcm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Kimura T, Tomura H, Mogi C, et al. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem. 2006;281:37457–37467. doi: 10.1074/jbc.M605823200. [DOI] [PubMed] [Google Scholar]
- 63.Assanasen C, Mineo C, Seetharam D, et al. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J Clin Invest. 2005;115:969–977. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seetharam D, Yuhanna I, Marcel Y, et al. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. Circ Res. 2006;98:63–72. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Covey S, Krieger M, Wang W, et al. Scavenger receptor class B type I-mediated protection against atherosclerosis in LDL receptor-negative mice involves its expression in bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2003;23:1589–1594. doi: 10.1161/01.ATV.0000083343.19940.A0. [DOI] [PubMed] [Google Scholar]


