Abstract
The heat-labile enterotoxins (HLT) expressed by Vibrio cholerae (cholera toxin) and Escherichia coli (LT-I, LT-IIa, and LT-IIb) are potent systemic and mucosal adjuvants. Co-administration of the enterotoxins with a foreign antigen (Ag) produces an augmented immune response to that antigen. Although each enterotoxin has potent adjuvant properties, the means by which the enterotoxins induce various immune responses are distinctive for each adjuvant. Various mutants have been engineered to dissect the functions of the enterotoxins required for their adjuvanticity. The capacity to strongly bind to one or more specific ganglioside receptors appears to drive the distinctive immunomodulatory properties associated with each enterotoxin. Mutant enterotoxins with ablated or altered ganglioside binding affinities have been employed to investigate the role of gangliosides in enterotoxin-dependent immunomodulation.
Keywords: enterotoxin, adjuvant, immunomodulation, ganglioside, signal transduction
INTRODUCTION
Heat-labile enterotoxins (HLT) are potent mucosal and systemic adjuvants. Yet, only in the last ten years have detailed investigations begun to unravel the events by which HLTs augment cellular and humoral immune responses to foreign antigens (Ag). These experiments have revealed that HLTs stimulate the immune system using a series of complex mechanisms [1]. A plethora of diverse interactions between HLT and various immunocompetent cell types [2] and a multitude of cellular responses elicited in those cell types have been identified. Some of these immununomodulatory activities are believed to be induced, either directly or indirectly, as a response to dramatic increases in the intracellular cAMP levels conferred by the toxic (catalytic) activities of the HLT [3-10]. cAMP is a strong secondary messenger molecule employed by many types of cells to regulate gene expression. On the other hand, not all immune responses elicited by HLTs ensue from the enterotoxins' catalytic properties [7,11,12]. Agents that raise cAMP levels in the cell (e.g. forskalin) do not elevate immune responses to Ags [13]. Binding of HLTs to their specific receptors on the surfaces of immunocompetent cells is often sufficient to trigger signal transduction events [14,15] which are likely required for immune responsiveness and immunomodulation. Recent studies have shown that binding of cholera toxin (CT), an HLT produced by Vibrio cholerae, to specific cell surface receptors on immunocompetent cells promotes redistribution of components of the plasma membrane within lipid microdomains (rafts) involved in signal transduction [16]. Signals which are promulgated by binding of HLTs to their receptors foster up-regulation and down-regulation expression of a number of genes that encode essential immune functions [1]. The processes by which immunomodulatory activities are promoted by binding of HLTs to receptors have not been fully characterized or enumerated. Recent investigations using mutant HLTs with altered receptor binding activities, however, have revealed a number of mechanisms by which HLTs likely augment immune responses. This review will concentrate on those mutant HLTs, the immune responses which are (or are not) induced by those mutants, and the receptors in the plasma membrane which are involved in productive HLT-immunocompetent cell interactions.
Gangliosides
Gangliosides are members of an extensive family of sialylated glycosphigolipids which are ubiquitously expressed on the plasma membrane of most, if not all eukaryotic cells [17]. Structurally, gangliosides are oligoglycosylceramides which contain N-acetylneuraminic acid (sialic acid or NeuAc) residues or less commonly N-glycolyl-neuraminic acid (NeuGc) joined via glycosidic linkages to one or more of the monosaccharide units [18]. The ceramide core is usually embedded in the lipid bilayer of the cell while the sugar groups are normally exposed to the surface of the cell. The diversity in the family arises from the various arrangements by which the sugar groups are arranged on the ceramide core [18](Fig. 1). This diversity is reflected in the distribution of gangliosides in nature. The types of gangliosides vary immensely both between different species and between different cell types within a single species (e.g., resting T cells are enriched in ganglioside GM3 (GM3) and ganglioside GD1a (GD1a)[19,20]; B cell neoplasms were shown to predominate in ganglioside GM2 (GM2)[21]; mouse macrophages express GD1a, ganglioside GM1b, and ganglioside GM1a, in combination with a population of minor gangliosides [22]). Interestingly, gangliosides are not always found inserted into plasma membranes. Human serum contains levels of GD1a near to that ganglioside's critical micellar concentration [23,24]. While gangliosides are best known as receptors for numerous bacterial toxins [25], these oligoglycosylceramides also have strong bioactive roles in normal cellular metabolism. Many gangliosides participate, directly or indirectly, in regulating responses of cells. For example, gangliosides have been established as receptors for platelet-derived growth factor, epidermal growth factor, and insulin [26,27]. Other gangliosides have immunosuppressive effects on T cells, B cells, and macrophages [23,28-31]. Immunomodulatory responses in those immunocompetent cells are likely induced by signals which are translated across the membrane after binding of ligands to gangliosides [26,32-35].
Fig. 1.
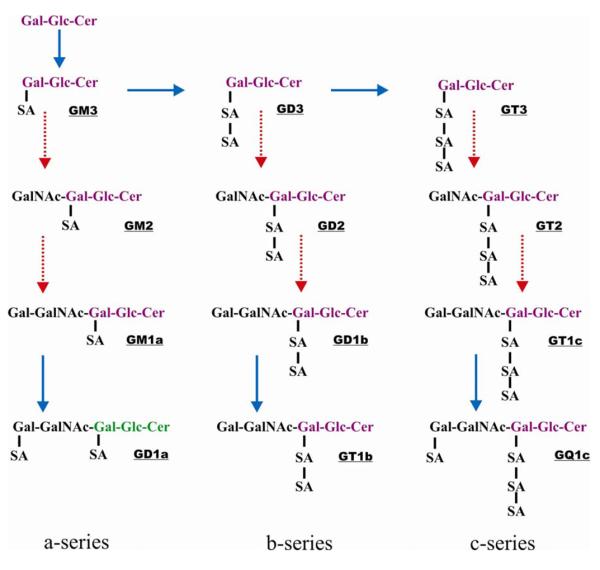
Pathways for the biosynthesis of the common a-, b-, and c-series of gangliosides. Synthesis of the various gangliosides involves the sequential activities of two types of enzymes: sialyltransferases (noted in solid lines) and glycosyltransferases (noted in dotted lines). Modified from - The Lipid Library. “Gangliosides: Structure, occurrence, biology and analysis”. http://www.lipidlibrary.co.uk/Lipids/gang/. Copyright of W.W. Christie and/ where stated The Oily Press Ltd or others. Mr. Christie can be contacted by email at William.Christie@scri.ac.uk.
Crosslinking of gangliosides
Whereas binding of a ligand to a single ganglioside in the plasma membrane may be sufficient to trigger certain cellular responses, it is likely that ligands which have the capacity to crosslink gangliosides are powerful agents for inducing responses in some immunocompetent cells [36]. Crosslinking of ganglioside GD3 (GD3) with an anti-GD3 monoclonal antibody induced proliferation of T cells, secretion of interferon-γ (IFN- γ) and interleukin-6 (IL-6), and increased expression of HLA class II molecules and interleukin-2 (IL-2) receptors [37-39]. Hammond et al. [16] hypothesized that clustering of a small number of lipids in a cell's membrane potentially could reorganize many other membrane components in a rapid manner. When artificial lipid vesicles were treated with the B pentamer of CT (CTB) which has the capacity to bind to five GM1 gangliosides (see below), the GM1 gangliosides in the vesicles were crosslinked. Crosslinking of GM1 induced phase-separation of the lipids within the vesicles which was correlated with a dramatic redistribution of a transmembrane peptide [16]. Crosslinking of ganglioside GM1 (GM1) in Jurkat T cells was correlated with phosphorylation of phospholipase C, a signaling molecule found within lipid-rich domains (rafts) of the plasma membrane [40]. CTB also was capable of substituting for co-stimulation upon activation of T cells, presumably by signaling after crosslinking GM1 in rafts [41] . It would be predicted, therefore, that any molecule which interacts with a ganglioside on the surface of an immunocompetent cell has the potential to elicit one or more immunomodulatory responses. The HLT produced by Escherichia coli and Vibrio cholerae which have five ganglioside-binding site, can crosslink gangliosides [42]. Both CT and LT-I strongly exhibit immunostimulatory properties [1,43-45].
Type I and Type II heat-labile enterotoxins
The HLT of E. coli and Vibrio cholerae belong to a family of structurally-related proteins that induce diarrheal symptoms in humans and animals [46]. The family is divided into two major groups based on genetic, biochemical, and immunological characteristics [46](Fig. 2). The Type I subfamily consists of CT from V. cholerae, the LT enterotoxin (referred herein as LT-I) of E. coli, and antigenically-related enterotoxin from several other enteric bacteria [46]. The Type II HLT subfamily is comprised of LT-IIa enterotoxin of E. coli, and LT-IIb, its partially cross-reacting antigenic variant [46]. The toxicity of Type I and Type II enterotoxins is conferred by an endogenous enzymatic activity which catalyzes an ADP ribosylation of the Gsa subunit of the adenylate cyclase complex in the plasma membrane of enterocytes and other cells [47-49]. Ribosylation of the Gsa subunit stimulates runaway synthesis of cAMP in the cell which in turn initiates a series of cellular events, one of which is a major change in transmembranal ion flux [46,48]. Type I and Type II heat-labile enterotoxins are oligomeric proteins composed of a single A polypeptide which is non-covalently bound to a pentameric array of B polypeptides [46,50]. Fragment A1, derived by proteolytic cleavage and reduction of an intrachain disulfide bond in the A polypeptide, is the toxic moiety, while the A2 fragment interacts in a non-covalent manner with the B pentamer to promote holotoxin assembly [47,50]. Binding of the enterotoxins to specific receptors on the plasma membrane of target cells is mediated solely by the B polypeptides [47,51]. Structural constraints to maintain toxic activity has highly conserved the amino acid sequences of the A polypeptides of the Type I and Type II HLT [46]. The B polypeptides are less conserved. While the B polypeptides of CT and LT-I exhibit over 80% identity at the amino acid level, the B polypeptides of LT-IIa and LT-IIb have little homology (<14%) to the B polypeptides of CT or LT-I, or to each other [46].
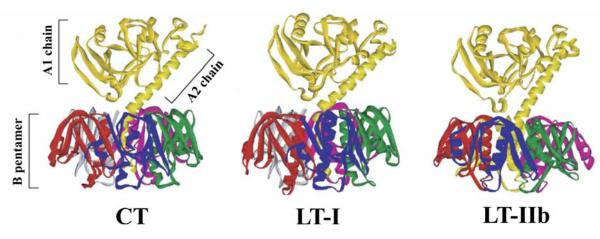
Fig. 2.
Ribbon diagrams of the crystal structures of CT [135], LT-I [135-137], and LT-IIb [138]. The A polypeptide, containing the catalytically-active A1 chain, is non-covalently bound to the B pentamer by interactions between the extended alpha helix of the A2 chain. A proteolytically-sensitive site is located in a site between the A1 and A2 chains bounded by two cysteines which form a disulfide bond. The five ganglioside binding pockets are located on the opposite side of the B pentamer in respect to the A polypeptide. Figure is modified from [139].
The receptors for CT, LT-I, LT-IIa, and LT-IIb are one or more gangliosides which are bound by the B pentamers of the HLT [42,51]. Each B pentamer has five binding sites for its respective ganglioside receptor, thus enabling the holotoxin to crosslink multiple gangliosides. The divergence in amino acid sequences of the B polypeptides of CT, LT-I, LT-IIa, and LT-IIb confers distinctive ganglioside binding patterns on the HLT [46]. CT and LT-I bind strongly to to ganglioside GM1. LT-I, however, also has affinity for polyglycosylceramides, asialo-GM1, GM2, and polylactosamine-containing glycoproteins [52-54]. LT-IIa and LT-IIb exhibit a more divergent pattern of ganglioside specificity. LT-IIa binds most avidly to ganglioside GD1b (GD1b), has lesser binding avidity for GD1a, and has a low but measurable avidity for GM1 [51]. LT-IIb binds with high affinity only to GD1a [51], but also binds weakly to several other minor and unidentified gangliosides (T.D.C. and Hesham F. Nawar, unpubl.). It is thought that the enterotoxins evolved different ganglioside-binding activities and specificities as a means to bestow host specificity in respect to their ability to intoxicate particular animal species, tissues or cell types [55-58]. It is becoming clear, however, that these differences in ganglioside-binding activities also dictate the types of immunocompetent cells to which these HLT can bind, and at least in part, determine the cellular and molecular pathways within those immunocompetent cells which the four HLT stimulate to augment immune responses to co-administered Ags.
Adjuvant properties of HLT
The capacities of CT and LT-I to augment immune responses to co-administered Ags have been reviewed elsewhere [1,8,44,45]. Thus, only the most important features relating to the immunomodulating activities of those two HLT will be summarized here. Northrup and Fauci [59] initially reported that CT, when delivered by the intravenous route with a foreign Ag, behaved as an adjuvant. Subsequently, Elson and Ealding [60] detected adjuvant activity when CT was administered by an alternate route of administration (per oris). It was noted in both studies that the adjuvant effects were observed only when CT and the Ag were administered simultaneously. The ability of CT to augment immune responses was not limited to short term effects. Reactivity to Ag, and to CT, was detectable long after the initial immune response. These data indicated that CT also augmented long-term memory responses to a co-administered Ag [61,62]. While the precise mechanism(s) by which CT exerts its adjuvant effect is still unclear, the response is usually predominantly correlated with a Th2-type polarization, a model that is supported by measurements of cytokine production and antibody isotype expression [63-66]. After immunization with CT as an adjuvant, interleukin 4 (IL-4) levels, a cytokine needed for B cell differentiation, is significantly elevated in gut-associated tissues and in spleen, while the levels of interferon gamma (INF-g), a cytokine needed to evoke cellular immune responses, either decreased or remained static [67,68]. CT also selectively inhibited production of IL-2 and IFN-γ from Th1 cells while increasing the production of IL-4 and IL-5 from Th2 cells [67,68]. per oris administration of CT predominately induced production of immunoglobulin G1 (IgG1) and secretory IgA (SIgA) in immunized animals [66,67]. CT was also reported to enhance production of IgG, IgE, and IgA in animal models [69,70]. When cultured Ag-specific lymph node cells were treated with LT-I, an increased activation of B cells and CD4+ T lymphocytes reflected in increased CD25 expression, was observed with a concomitant complete depletion of CD8+ lymphocytes [71]. Supernatants from the mixed lymphocytes cultures treated with LT-I contained no IL-4, IL-5, and IL-12, had decreased IFN-γ, but contained increased amounts of IL-2. This distribution of cytokines likely reflected the activation status of B cells and CD4+ T cells in the LT-I-treated population [71]. Recent investigations by Arce et al. [72] demonstrated that the Th2-like immunomodulating activities of CT are not due solely to its effects on T cells. In splenic populations, CT facilitates differentiation of naive B cells into plasma cells [72]. This B cell response was also T cell-dependent. Facilitated differentiation of B cells required the presence of CT-treated T cells in the culture [72]. While many of the effects of CT and LT-I are observed in B cells and T cells, the immunomodulating properties of CT and LT-I have also be attributed to direct effects on lymphoreticular cells. After CT administration, Ag-presentation was enhanced in macrophages [73]. MHC class II molecule expression and peptide presentation by macrophages was reported to be influenced by CT [73]. Several studies demonstrated that treatment with CT induced maturation and mobilization of DCs [74-76]. Dendritic cells (DC) treated with CT produced and secreted IL-1 [77]. CT-treated DC also up-regulated expression of HLA-DR, B7.1, and B7.2, and primed naive CD4+CD45RA+ T cells in vitro, thus driving those cells toward a Th2 phenotype [63]. CT also inhibited synthesis of IL-12p70 and TNF-α, RANTES, MIP-1α, and MIP-1β by lipopolysaccharide-activated or CD40 ligand-activated DC [63]. Human monocyte-derived DC are matured by both CT and LT-I which up regulate expression of CD80, CD83, CD86, and HLA-DR in a cAMP-dependent manner [78]. Animal studies showed that treatment with CT had a positive effect on uptake of models Ags from the lumen of the gut, presumably by affecting the efficiency of Ag-presenting cells [79]. Yet, while CT and LT-I generally induce Th2-type immune responses, there is gathering data that both Type I enterotoxins stimulate Th1 responses. Cytotoxic lymphocytes were evoked in several different immunization models when either CT or LT-I were employed as mucosal adjuvants to a bystander antigen ([80-83]. In fact, even the B pentamer of CT was shown to induce genital antigen-specific CTLs in a murine model after intravaginal so-immunization with the antigen [84]. Furthermore, treatment of dendritic cells with CT lead to the induction of cytotoxic CD8+ T cells in a B7.1-dependent manner [85]. Administration of CT-treated DC was correlated with rejection of an antigen-specific tumor in mice which required CD8+ T cells [86]. CT also appears to have a capacity for inducing regulatory T cells by modulating DC activation [87]. The mechanisms by which the other HLTs promote development of regulatory T cells have yet to be fully described [87].
Detoxified HLT
While it is evident that CT and LT-I are potent adjuvants, their inherent toxicities have precluded their use as adjuvants in human vaccines [1]. This omission is particularly relevant for mucosal vaccines. Mouse models demonstrated that CT and LT-I, when administered via the intranasal route, efficiently bound to the nasal neuroepithelium and subsequently trafficked by retrograde transport along the underlying olfactory nerves to the olfactory bulbs in the brain [88,89]. Retrograde transport of CT likely exerted an inflammatory effect on brain tissues. After intranasal administration of CT, transcriptional expression of the proinflammatory mediators IL-1β, TNF-α, COX-2, MCP-1, MIP-1α, and cyclooxygenase-2 were all elevated in the murine brain [90]. Retrograde transport of CT and LT-I require binding to GM1 since mutant enterotoxins (below) having no detectible binding to GM1 did not elicit the inflammatory effects [90]. As expected, these observations raised serious concerns for the use of CT and LT-I in vaccines, a consideration that appeared to be justified after a significant number of cases of Bell's palsy, a form of temporary facial paralysis resulting from damage or trauma to one of the two facial nerves, was detected in individuals who had been administered an intranasal LT-I-containing vaccine against influenza virus [91]. In an attempt to produce an HLT in which the potentially serious toxicities were ablated, recombinant engineering was employed to produce non-toxic forms of CT and LT-I. In initial attempts, mutant holotoxins were engineered which targeted single-point amino acid substitutions at amino acids in the A polypeptides which were considered to be critical for ribosylation activity. While many of these mutant holotoxins [e.g., CT(E29H)[92] LT-I(H44A)[93], LT-I(A69G)[3], LT-I(S61F)[94,95], LT-I(S63K)[10], LT-I(S63Y)[96], and LT-I(A72R)[97], LT-I(E112K)[94,95]] (mutants will be noted by the single letter code for the amino acid in wt HLT, the position of the amino acid in the polypeptide, and the single letter code for the substituted amino acid) retained adjuvant activities, further evaluation demonstrated that mutant enterotoxins CT(E29H), LT-I(H44A), LT-I(A72R), and LT-I(A69G) retained residual enzymatic activity. Additional mutant enterotoxins were engineered to alter other properties of the enterotoxins required for full toxic activity including LT-I(R192G)[9], a mutant holotoxin in which a proteolytically-sensitive site within the A2 domain of the A polypeptide required for full toxicity was disrupted with a glycine for arginine substitution at amino acid position 192 of the A polypeptide. This mutant enterotoxin, which exhibited reduced capacity to increase intracellular cAMP [3], had adjuvant properties similar to that of wt LT-I [9].
Non-toxic B pentamers
Although mutant holotoxins LT-I(S61F), LT-I(E112K), LT-I(S63Y) and others exhibited diminished capacity to increase levels of cAMP within cells, there was continuing concern that these mutant HLT retained residual capacity to affect cellular cAMP production that was below the level of detection of the current assays or that the non-immunocompetent cell types which were usually employed in the bioassays to measure cAMP accumulation were irrelevant, i.e. cAMP accumulation and the respective cAMP-dependent responses induced by the mutant holotoxins might differ in immunocompetent cells. These concerns led to experiments to determine whether the non-toxic B pentamers of HLT exhibited adjuvant properties. In the absence of the A polypeptides, B pentamers of the HLT (CTB and LT-IB) exhibit no detectable toxicity in standard Y-1 adrenal cell bioassays [3]. While usually not as potent as the respective holotoxins, multiple studies established that CTB and LT-IB has potent adjuvant immunomodulatory activity in vivo. For example, a strong serum IgG antibody response against influenza HA was obtained in rabbits after immunization with the Ag in the presence of CTB [98,99]; immunization of mice with the SBR Ag of Streptococcus mutans coupled to CTB stimulated a potent anti-SBR SIgA response [100]; immunization of mice with herpes simplex virus glycoproteins in the presence of LT-IB potentiated antibody and T cell responses that correlated with protection against ocular HSV-1 infection [101]; and, CTB used as an intranasal adjuvant induced protective responses against challenge with influenza virus [102-104]. Treatment with CTB also enhanced the Ag-presenting function of macrophages [73]. Although proinflammatory cytokines in the olfactory bulbs were not measured, neither CTB nor LT-IB used at 0.1μg/dose/mouse caused histological changes in the brain of intranasally immunized mice [105]. And, CTB and LT-IB have been shown to promoter tolerance to antigens when administered orally (for a review, see [106]). These studies, and many others, provided strong evidence that CTB and LT-IB alone, at least in combination with particular Ags and administered by particular routes, often exhibited adjuvant properties [1,9,44,45,107]. These studies also provided strong evidence that binding to GM1 was critically important in the immunostimulatory properties of CT and LT-I. Yet, the importance of GM1 binding for the adjuvant properties of CT and LT-I have only been intensively evaluated in the last few years.
Mutant Type I HLTs with altered ganglioside binding activities
GM1 is ubiquitously distributed in the cells of most mammals (i.e. no animals or cell lines were available that lacked expression of GM1) [108-110]. Thus, it has been difficult to design experiments to directly evaluate the correlation between CT or LT-I and GM1 binding, adjuvanticity, and immunomodulation. In 1991, however, mutant CT holotoxins having single-point substitutions at amino acid position 33 in the B polypeptide were engineered [111,112]. Of these mutant holotoxins, CT(G33D) has been the most extensively studied. Structural studies demonstrated that the G33D amino acid substitution had no affect either on pentameric assembly of the mutant B polypeptides or on non-covalent association of the mutant pentamers with A polypeptide to form holotoxin [111,112]. The mutant holotoxin also exhibited a decreased capacity to intoxicate cells, an effect presumably due to the inability of the mutant holotoxin to bind strongly to GM1 on the cell surface [111,112]. Similar G33D mutants of LT-I exhibited binding and toxic characteristics analogous to those of CT(G33D)[71,113]. Recognizing their potential as agents for dissecting the association between immunomodulation and GM1 binding, CTB(G33D) and LT-IB(G33D) holotoxins and B pentamers were employed by a bevy of investigators to determine if the immunomodulatory activities of CT and LT-I were dependent upon binding to GM1. In the majority of cases, abrogation of GM1-binding activity completely abolished, in vivo and in vitro, the immunomodulatory properties of CT and LT-I. For example, lymphocyte cultures treated with the wt B pentamer of LT-I (LT-IB) displayed an increase in the proportion of B cells [71], many of which expressed CD25, CD40, B7, ICAM-1, and MHC II, all of which indicated an activated state required for increased immune responsiveness [114]. Treatment with LT-B induced a complete depletion of CD8+ T cells by apoptosis, an increase in activation of CD4+ T cells, elevated production of IL-2, IL10, and IL-6, and decreased production of IL-12 and IFN-γ, all of which would tend to polarize the cells to a Th2-type of immune response [115,116]. LT-I triggered activation of caspase 8 and caspase 3 in CD8+ T cells by a Fas and Tumor Necrosis Factor (TNF) receptor-independent process [117]. None of these effects, however, was observed when lymphocyte cultures were treated with the B pentamer of LT-I(G33D) [71,113,114,116-121]. The potent adjuvant properties of LT-IB were also altered by the G33D mutation. In comparison to the antibody titers of mice immunized with wt LT-IB, mice subcutaneously immunized with LT-IB(G33D) exhibited a 160-fold decrease in anti-LT-IB antibody titer [71]. Treatment of human CD14+ monocytes with LT-IB, but not with LT-IB(G33D) triggered release of IL-10 and IL-6, two B cell modifiers [115,122,123], and inhibited expression of IL-12 [CTB, however, exerted no effects on IL-12 production, a cytokine which polarizes T cells to a Th1-type of immune response [115], suggesting that these two closely related enterotoxins have unique immunomodulatory properties]. To determine if more potent adjuvant properties would be demonstrated by LT-I holotoxin containing the G33D mutation in the B polypeptides, mice were immunized with LT-I(G33D) holotoxin. Surprisingly, the LT-I(G33D) holotoxin exhibited residual toxic activity in a mouse Y-1 adrenal cell bioassay and stimulated amounts of cAMP in Caco-2 cells equivalent to those stimulated by treatment with wt LT-I [113]. These data suggested that the amino acid substitution at G33 in the B polypeptide of LT-I did not fully abrogate binding of the mutant enterotoxin to GM1. LT-I(G33D) holotoxin, when employed as an oral adjuvant, was also incapable of augmenting the levels of IgG or IgA directed against a co-administered Ag or against the holotoxin [113]. Collectively, these data indicated that strong binding of LT-I or CT to GM1 was essential for augmenting the enterotoxins' immune responses.
Adjuvant properties of Type II HLT
Experiments using the G33D mutants confirmed that strong binding to GM1 was a key event in the immunomodulatory capacities of LT-I and CT by inducing or suppressing relevant immune responses in various immunocompetent cell types. These observations, however, stimulated a related question. Are the immunostimulatory effects solely dependent upon binding to GM1? This question has been addressed by evaluating the adjuvant properties of LT-IIa and LT-IIb and the immunomodulatory properties of their respective ganglioside binding mutants [2,124-130]. Although the immunomodulatory properties of LT-IIa and LT-IIb have not been as extensively investigated as those of CT and LT-I, recent studies have demonstrated that the mucosal and systemic adjuvant properties of LT-IIa and LT-IIb are equivalent to those of CT and LT-I [2,124-130]. Yet, LT-IIa and LT-IIb exhibited unique immunological properties [2,124-130]. While the capacities of LT-IIa and LT-IIb to augment immune responses to foreign Ags were similar, the immune responses and immunomodulatory events elicited by LT-IIa and LT-IIb in mouse immunization models and in cell cultures were very distinguishable from those evoked by CT or LT-I, indicating that the Type II HLT were unique immunostimulants [2,124-130]. Cytokine and antibody isotype distributions indicated that LT-IIa and LT-IIb displayed a trend toward eliciting a more balanced Th1/Th2 response, in contrast to the predominant Th2-like responses evoked by CT and, in most cases, by LT-I [2,126-130]. Evaluations of anti-CD3-stimulated human peripheral blood mononuclear cells (PBMC) demonstrated that CT suppressed production of IL-2, TNF-α, and IL-12 to a much greater degree than did either LT-IIa or LT-IIb [2,128]. In contrast to CT, LT-IIa and LT-IIb exerted no effects on expression of CD25 and CD69 by CD4+ T cells [72]. And CT, but not LT-IIa or LT-IIb, reduced expression of CD40 ligand on CD4+ T cells [128]. Treatment of CD4+ T cells with LT-IIa or LT-IIb elicited greater amounts of TNF-α and IL12p70 by monocyte-derived DCs than did treatment with CT [128]. Apoptotic effects were also disparate between the Type I and Type II enterotoxins. LT-IIa and CT, but not LT-IIb, induced apoptosis of CD8+ T cells [2]. Unlike CT, however, neither LT-IIa nor LT-IIb inhibited mitogen-driven CD4+ T cell proliferation [2]. LT-IIa or LT-IIb, in contrast to CT, did not facilitate rapid differentiation of B cells into plasma cells [72]. Finally, a chimeric LT-IIa holotoxin composed of the SBR Ag of S. mutans genetically fused to the A2 assembly domain of the A polypeptide of LT-IIa and assembled with the wt LT-IIa B pentamers, while eliciting strong anti-SBR antibody titers when used as an immunogen, did not up-regulate expression of B7.1 or B7.2 in B220+, CD11b+, or CD11c+ splenic cells [126]. In contrast, an SBR-A2/CTB chimera significantly elevated expression of those co-stimulatory receptors on those cell types [126]. Clearly, LT-IIa and LT-IIb must elicit their adjuvant effects by mechanisms distinctive from those utilized by either CT or LT-I, and the mechanisms by which LT-IIa and LT-IIb stimulate the immune system must be relatively novel since neither LT-IIa nor LT-IIb evoke the cytokine, apoptotic, proliferative, or cell differentiation pathways which are evoked by CT and LT-I [2,72,126-130].
Mutant Type II HLT with altered ganglioside-binding properties
The current hypothesis is that the divergent patterns of immune responses evoked by LT-IIa and LT-IIb are due to their capacity to bind to non-GM1 ganglioside receptors which presumably induce different signal transduction pathways, elicit divergent changes in the plasma membrane, and/or differentially induce or suppress various intracellular responses of one or more types of immunocompetent cells. To test that hypothesis, mutant LT-IIa and LT-IIb holotoxins were engineered with single-point substitutions in their B polypeptides which altered their ganglioside-binding patterns [55-57]. Co-administration of mice by the intranasal route with AgI/II from S. mutans and LT-IIa(T34I), a mutant HLT which had no detectible binding activity by ganglioside-dependent ELISA for GD1b, GD1b, or GM1, the gangliosides most strongly bound by LT-IIa, failed to induce production of anti-Ag/II antibodies in either serum or saliva [130]. The failure of LT-IIa(T34I) to induce an immune response was likely due to the failure of the mutant enterotoxin to interact with one or more types of immunocompetent cells; LT-IIa(T34I) had no affinity for any class of cervical lymph node lymphocytes [130]. This failure to bind to the cells is reflected in the decreased affinity of LT-IIa(T34I) to gangliosides in vitro [130] which likely affected the mutant enterotoxin's toxic capacity. LT-IIa(T34I) exhibited little toxicity in a Y1 adrenal cell assay, nor did it increase cAMP levels in murine macrophages above the levels produced by treatment of the cells with the non-toxic LT-IIa B pentamer [129,130]. LT-IIa(T34I), however, did have some adjuvant properties. Boosting mice which had initially received AgI/II and LT-IIa(T34I) as a mucosal adjuvant with AgI/II alone had primed the mice for a strong Ag-specific humoral memory response [129]. A second set of LT-IIa mutants having single-point amino acid substitutions at amino acid position 14 in the B polypeptides revealed that toxicity was not only separable from adjuvanticity, but that adjuvanticity was directly correlated with strength of binding to gangliosides [130]. By ganglioside-dependent ELISA using purified bovine brain gangliosides, LT-IIa(T14S) and wt LT-II had equivalent affinities for GD1b, GD1a, and GM1 (and to the minor receptors GM2, GM3, anGQ1b, and GT1b) [130]. LT-IIa(T14I) and LT-IIa(T14D), however, bound to those same gangliosides with less avidity [LT-IIa > LT-II(T14S) > LT-II(T14I) >> LT-IIa(T14D)] [130]. When administered via the intranasal route, mutants LT-IIa(T14S), LT-IIa(T14I), and LT-IIa(T14D) all induced strong mucosal and systemic immune responses to the co-administered Ag which were equivalent to the responses induced by use of wt LT-IIa [130]. While LT-IIa(T14S) was capable of eliciting high levels of intracellular cAMP in mouse macrophages, LT-IIa(T14I) produced less than half the amount of cAMP in those cells [130]. In contrast, LT-IIa(T14D) produced no more cAMP in macrophages upon treatment with LT-IIa(T14D) than did macrophages treated with the fully non-toxic LT-IIa B pentamer [130]. These results suggested that immunomodulatory activities of LT-IIa required weaker binding to gangliosides than was required for toxicity.
Similar experiments were performed using LT-IIb(T13I). By ganglioside-dependent ELISA, LT-IIb(T13I0 had no affinity for GD1a, the ganglioside receptor which is strongly bound by LT-IIb, nor did it elevate cAMP levels in murine macrophages [129]. LT-IIb(T13I) however, retained the capacity to bind to splenic T cells, B cells, and macrophages, and exhibited full adjuvant capacity [129]. Thin-layer chromatographic (TLC) immunoblotting experiments revealed that binding affinity for GD1a was not completely ablated in LT-IIb(T13I). Rather, the mutant holotoxin bound weakly to GD1a and to several other minor gangliosides obtained from murine macrophages (T.D.C. and Hesham F. Nawar, unpubl.). Mice synthesize gangliosides which are decorated with either N-glycolylneurominic acids (NeuGc) or with Nacetylneurominic acids [22]. LT-IIb(T13I) bound preferentially to NeuGc-containing gangliosides in the TLC blots (T.D.C. and Hesham F. Nawar, unpubl.). Preferential binding to NeuGc gangliosides may confer the immunomodulatory activities to LT-IIb(T13I). A knock-out strain of mice in which the enzyme required to decorate gangliosides with NeuGc gangliosides, however, has recently been engineered. This KO mouse will facilitate experiments to evaluate the possibility that LT-IIb(T13I) utilizes NeuGc gangliosides as functional receptors for immunomodulation [131].
Functional interactions of Type II HLT with Toll-like receptor 2 (TLR2)
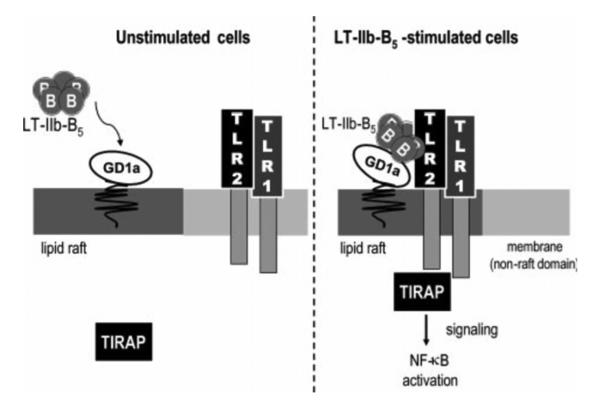
Recent reports indicate that the Type II HLT, unlike CT and LT-I, are not solely dependent upon binding to gangliosides for immunostimulatory activity. B pentamers of LT-IIa and LT-IIb (LT-IIaB and LT-IIbB, respectively), but not their respective holotoxins [132,133], functionally interact with TLR2 [125,132-134]. This effect is not observed with either CT, and likely will not be evident with LT-I. Both LT-IIaB and LT-IIbB, but not CT, induced TLR2-dependent nuclear factor κβ (NF-κβ) activation in a reporter cell [125,132-134]. Induction of IL-1β, IL-6, IL-8, and TNF-α by human THP-1 cells treated with LT-IIaB or LT-IIbB was inhibited by anti-TLR2 but not by anti-TLR4 antibodies [133]. Further evidence of the role of TLR2 in LT-IIbB-dependent immunomodulation was provided by mice having a defect in expression of TLR2. Peritoneal macrophages from TLR2−/− mice failed to respond to LT-IIaB or LT-IIbB [133]. Fluorescence resonance energy transfer (FRET) experiments demonstrated that LT-IIbB induces lipid raft recruitment of TLR2 and TIRAP, an adaptor protein, and that LT-IIbB, TLR2, and GD1a co-localize in the cell [133,134]. Interaction of LT-IIaB or LT-IIbB with TLR2 appeared to be partially mediated by binding of the B pentamer to gangliosides since LT-IIbB(T13I), a mutant which has altered GD1b binding activity, lacked the capacity to induce production of cytokines in THP-1 cells [134]. It is hypothesized that TLR2 agonist activity by LT-IIbB is elicited by a pentamer-induced formation of a multimolecular complex formed of the B pentamer, GD1a (or one of the other receptors for LT-IIb), and TLR2 [134](Fig. 3). This interaction may be a functionally novel one. CD14, a usual coreceptor for TLR2 did not have a role as an accessory protein in LT-IIbB/TLR2-induced cell activation; CD14-deficient macrophages behaved in a manner similar to that observed in CD14-proficient macrophages when both were treated with LT-IIbB [134]. But what role does interaction of LT-IIbB with TLR2 have in immunomodulation? Ag-specific immune responses to a model Ag were augmented in mice intranasally immunized with LT-IIbB (T.D.C. and Hesham F. Nawar, unpubl). It will be interesting to determine if the mucosal and systemic adjuvant activities of LT-IIbB is a TLR2-dependent function. To address that issue, immunization experiments employing TLR2-deficient mice are ongoing.
Fig. 3.
Model of the multimolecular complex formed by cooperative binding of LT-IIbB to GD1a and TLR2. After formation, the multimolecular complex is recruited into lipid rafts. Induction of TLR2 occurs on the cell surface and requires TIRAP, an adaptor protein, which co-localizes to the LT-IIbB/GD1a//TLR2 complex. Figure was originally published in [134].
EXPERT COMMENTARY
Many of the serious pathogens which cause disease in both industrial and developing countries invade and colonize individuals by infecting mucosal membranes. Thus, vaccines which stimulate protective immunity at the relevant mucosal site are desired. Unfortunately, vaccines which are administered parentally often fail to induce mucosal immunity. And, while perenteral vaccines have a reasonably good track record for safety, there is always a risk of infection and allergic responses when vaccines are administered by perenteral routes. Thus, there is a concerted effort to design safe mucosal vaccines. This goal, however, has been stymied by the failure of most vaccines when administered mucosally to induce strong and protective immune response, likely as a result of the toleragenic nature of mucosal tissues to foreign Ags. HLTs provide the means to augment the efficacy of mucosal vaccines. Yet, the inherent toxicities of the HLTs preclude their use in human mucosal vaccines. It is hoped that one or more mutants of CT, LT-I, LT-IIa, or LT-IIb will be engineered that will be non-toxic but will exert strong mucosal immune responses to foreign Ags. A non-toxic mutant enterotoxin which does not traffic along neuronal tracts would, of course, be most desirable.
It is also possible that research into the roles of gangliosides in HLT-dependent immunomodulation will provide data useful in developing other strong agonists for ganglioside-dependent immunomodulation. In that mode, the observation that the B pentamers of LT-IIa and LT-IIb interact functionally with TLR2 are especially intriguing. Toll-like receptors are potent regulators of both innate and adaptive immunity. Elucidating the mechanism(s) by which the B pentamers interact with TLR2 may reveal novel pathways and new agents for inducing innate and adaptive immune responses to useful immunogens.
FIVE-YEAR VIEW
Gangliosides are potent bioactive molecules which likely exert their effects by directly transducing signals or by aggregating transducing molecules upon crosslinking by ligands such as CT, LT-I, LT-IIa, and LT-IIb. Experiments employing mutant HLT with altered ganglioside binding activities have separated toxicity and adjuvanticity, thus making it more feasible to consider employing the mutant HLTs in human vaccines as agents to augment immune responses to the immunogen(s). The data are consistent with a model in which crosslinking of different gangliosides by CT, LT-I, LT-IIa, and LT-IIb or their respective mutant holotoxins and B pentamers stimulate distinctive immune responses. If so, mutant HLTs may be used in the future to direct immune responses into desired directions. New and exploitable mechanisms of immunostimulation may also be revealed by further investigating the properties of the B pentamers and holotoxins of wt and mutant LT-IIa and LT-IIb.
KEY ISSUES.
Type I and Type II HLT are potent mucosal and systemic adjuvants which have the capacity to dramatically augment systemic and mucosal immune responses to co-administered Ags. Yet, the cellular and molecular processes which promote the immunomodulatory properties of HLTs have not been fully characterized or enumerated. These immunomodulatory properties are likely engendered by physical interactions of the HLTs with ganglioside receptors located on the surface of one or more types of immunocompetent cells.
Gangliosides belong to a large family of sugar-decorated lipids found usually on the plasma membrane of most eukaryotic cells including lymphocytes and lymphorenticular cells. Crosslinking of gangliosides has been shown to trigger signal transduction pathways that promote regulation of genes involved in immunomodulation. The Type I and Type II HLT have distinct binding properties for various gangliosides. Binding of an HLT to a particular ganglioside on the surface of an immunocompetent cell likely stimulates distinctive immune responses.
CT selectively inhibits IL-2 and IFN-γ from Th1 cells and increases IL-4 and IL-5 from Th2 cells. CT predominately induces production of IgG1, IgE, and secretory IgA in immunized animals. LT-I activates B cells and CD4+ T lymphocytes and induces apoptosis of CD8+ T cells. LT-I stimulated IL-2 synthesis, failed to stimulate production of IL-4, IL-5, and IL-12, and decreased production of IFN-γ by splenic cells. In combination with observations of the patterns of expression of co-stimulatory ligands, CT is thought to induce a Th2-type of immune response. In some cases, LT-I also induces a Th2-type of immune response. In other cases, however, a Th1-type of response is observed when LT-I is employed as an adjuvant.
The adjuvant properties of CT and LT-I are correlated with the ability of the holotoxins to induce cAMP in cells. These residual toxic activities preclude the use of the holotoxins as mucosal vaccines, particularly when administered via the intranasal route. HLTs likely traffic to the brain by retrograde transport along the olfactory nerves. Induction of proinflammatory cytokines and inflammatory histologies have been observed in the brain after immunization with CT and LT-I.
Non-toxic B pentamers of CT and LT-I have immunomodulatory properties. These properties, however, are less potent than those of their respective holotoxins. Use of the pentamers as mucosal adjuvants, however, may avoid the neurological problems associated with use of the holotoxins as adjuvants.
LT-IIa and LT-IIb, the Type II HLT, exhibit adjuvant properties equivalent to, but distinctive from those of CT and LT-I. The distinctive immunomodulatory properties of LT-IIa and LT-IIb are likely due to differences in their ganglioside binding patterns. Both LT-IIa and LT-IIb evoke a more balanced Th1/Th2-type of immune response. Immunization and in vitro experiments with LT-IIb demonstrate that strong binding to GM1 is not required for immunomodulatory activity.
The B pentamers of LT-IIa and LT-IIb, but not the CTB or LT-IB, interact functionally with TLR2 to activate NF-κβ, induce production of IL-1β, IL-6, IL-8 and TNF-α in human monocytic THP-1 cells. TLR2-dependent activity was not induced when cells were treated with either LT-IIa or LT-IIb holotoxins. FRET and fluorescent microscopic examination suggested that LT-IIbB stimulates formation of a multimolecular complex of pentamer, ganglioside, and TLR2 on the cell surface.
ACKNOWLEDGEMENTS
I would like to thank Dr. Natalie King-Lyons for reading the manuscript and providing useful critical comments. The author was supported by The National Institutes of Health research grants DE013833 and DE014357.
REFERENCES
- 1.Hajishengallis G, Arce S, Gockel CM, Connell TD, Russell MW. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. Journal of Dental Research. 2005;84(12):1104–1116. doi: 10.1177/154405910508401205. [DOI] [PubMed] [Google Scholar]
- 2.Arce S, Nawar HF, Russell MW, Connell TD. Differential binding of Escherichia coli enterotoxins LT-IIa and LT-IIb and of cholera toxin elicits differences in apoptosis, proliferation, and activation of lymphoid cells. Infection and Immunity. 2005;73(5):2718–2727. doi: 10.1128/IAI.73.5.2718-2727.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng E, Cardenas-Freytag L, Clements JD. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT) Vaccine. 1999;18(12):38–49. doi: 10.1016/s0264-410x(99)00168-1. [DOI] [PubMed] [Google Scholar]
- 4.McCluskie MJ, Weeratna RD, Clements JD, Davis HL. Mucosal immunization of mice using CpG DNA and/or mutants of the heat-labile enterotoxin of Escherichia coli as adjuvants. Vaccine. 2001;19(27):3759–3768. doi: 10.1016/s0264-410x(01)00088-3. [DOI] [PubMed] [Google Scholar]
- 5.Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- 6.Clements JD, Hartzog NM, Lyon FL. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6(3):269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 7.de Haan L, Verweij WR, Feil IK, et al. Mutants of the Escherichia coli heat-labile enterotoxin with reduced ADP-ribosylation activity or no activity retain the immunogenic properties of the native holotoxin. Infection and Immunity. 1996;64(12):5413–5416. doi: 10.1128/iai.64.12.5413-5416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Haan L, Holtrop M, Verweij WR, Agsteribbe E, Wilschut J. Mucosal immunogenicity of the Escherichia coli heat-labile enterotoxin: role of the A subunit. Vaccine. 1996;14(4):260–266. doi: 10.1016/0264-410x(95)00235-s. [DOI] [PubMed] [Google Scholar]
- 9.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. European Journal ofIimmunology. 1992;22(9):2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 10.Douce G, Turcotte C, Cropley I, et al. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(5):1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu HY, Russell MW. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine. 1998;16(23):286–292. doi: 10.1016/s0264-410x(97)00168-0. [DOI] [PubMed] [Google Scholar]
- 12.Plant A, Williams R, Jackson ME, Williams NA. The B subunit of Escherichia coli heat labile enterotoxin abrogates oral tolerance, promoting predominantly Th2-type immune responses. European Journal of Immunology. 2003;33(11):3186–3195. doi: 10.1002/eji.200324154. [DOI] [PubMed] [Google Scholar]
- 13.Wilson AD, Robinson A, Irons L, Stokes CR. Adjuvant action of cholera toxin and pertussis toxin in the induction of IgA antibody response to orally administered antigen. Vaccine. 1993;11(2):113–118. doi: 10.1016/0264-410x(93)90004-h. [DOI] [PubMed] [Google Scholar]
- 14.Wimer-Mackin S, Holmes RK, Wolf AA, Lencer WI, Jobling MG. Characterization of receptor-mediated signal transduction by Escherichia coli type IIa heat-labile enterotoxin in the polarized human intestinal cell line T84. Infection and Immunity. 2001;69(12):7205–7212. doi: 10.1128/IAI.69.12.7205-7212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf AA, Jobling MG, Wimer-Mackin S, et al. Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia. The Journal of Cell Biology. 1998;141(4):917–927. doi: 10.1083/jcb.141.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond AT, Heberle FA, Baumgart T, Holowka D, Baird B, Feigenson GW. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(18):6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnino S, Acquotti D, Riboni L, Giuliani A, Kirschner G, Tettamanti G. New chemical trends in ganglioside research. Chemistry and Physics of Lipids. 1986;42(13):3–26. doi: 10.1016/0009-3084(86)90040-x. [DOI] [PubMed] [Google Scholar]
- 18.Levery SB. Glycosphingolipid structural analysis and glycosphingolipidomics. Methods in enzymology. 2005;405:300–369. doi: 10.1016/S0076-6879(05)05012-3. [DOI] [PubMed] [Google Scholar]
- 19.Kniep B, Cinek T, Angelisova P, Horejsi V. Association of the GPI-anchored leucocyte surface glycoproteins with ganglioside GM3. Biochemical and Biophysical Research communications. 1994;203(2):1069–1075. doi: 10.1006/bbrc.1994.2291. [DOI] [PubMed] [Google Scholar]
- 20.Nohara K, Suzuki M, Inagaki F, Sano T, Kaya K. A novel disialoganglioside in rat spleen lymphocytes. The Journal of Biological Chemistry. 1992;267(21):14982–14986. [PubMed] [Google Scholar]
- 21.O'Boyle KP, Freeman K, Kalisiak A, Agregado A, Scheinberg DA. Patterns of ganglioside expression in B cell neoplasms. Leukemia & Lymphoma. 1996;21(34):255–266. doi: 10.3109/10428199209067607. [DOI] [PubMed] [Google Scholar]
- 22.Yohe HC, Ye S, Reinhold BB, Reinhold VN. Structural characterization of the disialogangliosides of murine peritoneal macrophages. Glycobiology. 1997;7(8):1215–1227. doi: 10.1093/glycob/7.8.1215. [DOI] [PubMed] [Google Scholar]
- 23.Kanda N, Watanabe S. Ganglioside GD1a enhances immunoglobulin production by human peripheral blood mononuclear cells. Experimental Hematology. 2000;28(6):672–679. doi: 10.1016/s0301-472x(00)00167-3. [DOI] [PubMed] [Google Scholar]
- 24.Ulrich-Bott B, Wiegandt H. Micellar properties of glycosphingolipids in aqueous media. Journal of Lipid Research. 1984;25(11):1233–1245. [PubMed] [Google Scholar]
- 25.Nagai Y, Iwamori M. Biology of Sialic Acids. Plenum Publishing Co.; New York: 1994. Cellular biology of gangliosides; pp. 197–230. [Google Scholar]
- 26.Hakomori S. Structure and function of sphingoglycolipids in transmembrane signalling and cell-cell interactions. Biochemical Society Transactions. 1993;21(Pt 33):583–595. doi: 10.1042/bst0210583. [DOI] [PubMed] [Google Scholar]
- 27.Zeller CB, Marchase RB. Gangliosides as modulators of cell function. The American Journal of Physiology. 1992;262(6 Pt 1):C1341–1355. doi: 10.1152/ajpcell.1992.262.6.C1341. [DOI] [PubMed] [Google Scholar]
- 28.Krifuks O, Bergelson LD, Schlesinger M. The down-modulation of CD4 induced by the GM1 ganglioside is regulated by phosphatases and kinases: evidence from enzyme inhibitors and anti-CD45 antibodies. Cell Immunol. 1998;187(1):45–51. doi: 10.1006/cimm.1998.1311. [DOI] [PubMed] [Google Scholar]
- 29.Morrison WJ, Young K, Offner H, Vandenbark AA. Ganglioside (GM1) distinguishes the effects of CD4 on signal transduction through the TCR/CD3 complex in human lymphocytes. Cell Mol Biol Res. 1993;39(2):159–165. [PubMed] [Google Scholar]
- 30.Saggioro D, Sorio C, Calderazzo F, et al. Mechanism of action of the monosialoganglioside GM1 as a modulator of CD4 expression. Evidence that GM1-CD4 interaction triggers dissociation of p56lck from CD4, and CD4 internalization and degradation. J. Biol. Chem. 1993;268(2):1368–1375. [PubMed] [Google Scholar]
- 31.Shen W, Falahati R, Stark R, Leitenberg D, Ladisch S. Modulation of CD4 Th cell differentiation by ganglioside GD1a in vitro. J Immunol. 2005;175(8):4927–4934. doi: 10.4049/jimmunol.175.8.4927. [DOI] [PubMed] [Google Scholar]
- 32.Fishman PH. Recent advances in identifying the functions of gangliosides. Chemistry and Physics of Lipids. 1986;42(13):137–151. doi: 10.1016/0009-3084(86)90049-6. [DOI] [PubMed] [Google Scholar]
- 33.Hannun YA, Linardic CM. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochimica et Biophysica Acta. 1993;1154(34):223–236. doi: 10.1016/0304-4157(93)90001-5. [DOI] [PubMed] [Google Scholar]
- 34.Nagai Y, Iwamori M. Ganglioside distribution at different levels of organization and its biological implications. Advances in Experimental Medicine and Biology. 1984;174:135–146. doi: 10.1007/978-1-4684-1200-0_12. [DOI] [PubMed] [Google Scholar]
- 35.Hakomori S, Igarashi Y. Functional role of glycosphingolipids in cell recognition and signaling. J. Bbiochem. 1995;118(6):1091–1103. doi: 10.1093/oxfordjournals.jbchem.a124992. [DOI] [PubMed] [Google Scholar]
- 36.Gouy H, Deterre P, Debre P, Bismuth G. Cell calcium signaling via GM1 cell surface gangliosides in the human Jurkat T cell line. J Immunol. 1994;152(7):3271–3281. [PubMed] [Google Scholar]
- 37.Ortaldo JR, Mason AT, Longo DL, Beckwith M, Creekmore SP, McVicar DW. T cell activation via the disialoganglioside GD3: analysis of signal transduction. J Leukoc Biol. 1996;60(4):533–539. doi: 10.1002/jlb.60.4.533. [DOI] [PubMed] [Google Scholar]
- 38.Norihisa Y, McVicar DW, Ghosh P, et al. Increased proliferation, cytotoxicity, and gene expression after stimulation of human peripheral blood T lymphocytes through a surface ganglioside (GD3) J Immunol. 1994;152(2):485–495. [PubMed] [Google Scholar]
- 39.Bukowski JF, Roncarolo MG, Spits H, et al. T cell receptor-dependent activation of human lymphocytes through cell surface ganglioside GT1b: implications for innate immunity. European J. Immunol. 2000;30(11):3199–3206. doi: 10.1002/1521-4141(200011)30:11<3199::AID-IMMU3199>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 40.Veri MC, DeBell KE, Seminario MC, et al. Membrane raft-dependent regulation of phospholipase Cgamma-1 activation in T lymphocytes. Mol. Cell. Biol. 2001;21(20):6939–6950. doi: 10.1128/MCB.21.20.6939-6950.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. Science. 5402. Vol. 283. New York, N.Y: 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains; pp. 680–682. [DOI] [PubMed] [Google Scholar]
- 42.Fishman PH, Moss J, Osborne JC., Jr Interaction of choleragen with the oligosaccharide of ganglioside GM1: evidence for multiple oligosaccharide binding sites. Biochemistry. 1978;17(4):711–716. doi: 10.1021/bi00597a024. [DOI] [PubMed] [Google Scholar]
- 43.de Haan L, Verweij W, Agsteribbe E, Wilschut J. The role of ADP-ribosylation and G(M1)-binding activity in the mucosal immunogenicity and adjuvanticity of the Escherichia coli heat-labile enterotoxin and Vibrio cholerae cholera toxin. Immunol Cell Biol. 1998;76(3):270–279. doi: 10.1046/j.1440-1711.1998.00745.x. [DOI] [PubMed] [Google Scholar]
- 44.Lycke N. From toxin to adjuvant: basic mechanisms for the control of mucosal IgA immunity and tolerance. Immunol Lett. 2005;97(2):193–198. doi: 10.1016/j.imlet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Williams NA, Hirst TR, Nashar TO. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20(2):95–101. doi: 10.1016/s0167-5699(98)01397-8. [DOI] [PubMed] [Google Scholar]
- 46.Holmes RK, Jobling MG, Connell TD. Cholera toxin and related enterotoxins of gram-negative bacteria. In: Moss BIJ, Vaughn M, Tu AT, editors. Bacterial toxins and virulence factors in disease. Marcel Dekker, Inc.; New York: 1995. pp. 225–255. [Google Scholar]
- 47.Mekalanos JJ, Collier RJ, Romig WR. Enzymic activity of cholera toxin. I. New method of assay and the mechanism of ADP-ribosyl transfer. J. Biol. Chem. 1979;254(13):5849–5854. [PubMed] [Google Scholar]
- 48.Moss J, Vaughan M. Toxin ADP-ribosyltransferases that act on adenylate cyclase systems. Methods in Enzymology. 1984;106:411–418. doi: 10.1016/0076-6879(84)06044-4. [DOI] [PubMed] [Google Scholar]
- 49.Moss J, Vaughan M. Activation of cholera toxin and Escherichia coli heat-labile enterotoxins by ADP-ribosylation factors, a family of 20 kDa guanine nucleotide-binding proteins. Molecular Microbiology. 1991;5(11):2621–2627. doi: 10.1111/j.1365-2958.1991.tb01971.x. [DOI] [PubMed] [Google Scholar]
- 50.Gill DM, Clements JD, Robertson DC, Finkelstein RA. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infection and Immunity. 1981;33(3):677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukuta S, Magnani JL, Twiddy EM, Holmes RK, Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infection and Immunity. 1988;56(7):1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Critchley DR, Magnani JL, Fishman PH. Interaction of cholera toxin with rat intestinal brush border membranes. Relative roles of gangliosides and galactoproteins as toxin receptors. J. Biol. Chem. 1981;256(16):8724–8731. [PubMed] [Google Scholar]
- 53.Orlandi PA, Critchley DR, Fishman PH. The heat-labile enterotoxin of Escherichia coli binds to polylactosaminoglycan-containing receptors in CaCo-2 human intestinal epithelial cells. Biochemistry. 1994;33(43):12886–12895. doi: 10.1021/bi00209a021. [DOI] [PubMed] [Google Scholar]
- 54.Yamada KM, Critchley DR, Fishman PH, Moss J. Exogenous gangliosides enhance the interaction of fibronectin with ganglioside-deficient cells. Experimental Cell Research. 1983;143(2):295–302. doi: 10.1016/0014-4827(83)90054-x. [DOI] [PubMed] [Google Scholar]
- 55.Connell TD, Holmes RK. Characterization of hybrid toxins produced in Escherichia coli by assembly of A and B polypeptides from type I and type II heat-labile enterotoxins. Infection and Immunity. 1992;60(4):1653–1661. doi: 10.1128/iai.60.4.1653-1661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Connell TD, Holmes RK. Molecular genetic analysis of ganglioside GD1b-binding activity of Escherichia coli type IIa heat-labile enterotoxin by use of random and site-directed mutagenesis. Infection and Immunity. 1992;60(1):63–70. doi: 10.1128/iai.60.1.63-70.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Connell TD, Holmes RK. Mutational analysis of the ganglioside-binding activity of the type II Escherichia coli heat-labile enterotoxin LT-IIb. Molecular Microbiology. 1995;16(1):21–31. doi: 10.1111/j.1365-2958.1995.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 58.Connell TD, Metzger DJ, Wang M, Jobling MG, Holmes RK. Initial studies of the structural signal for extracellular transport of cholera toxin and other proteins recognized by Vibrio cholerae. Infection and Immunity. 1995;63(10):4091–4098. doi: 10.1128/iai.63.10.4091-4098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Northrup RS, Fauci AS. Adjuvant effect of cholera enterotoxin on the immune response of the mouse to sheep red blood cells. The Journal of Infectious Diseases. 1972;125(6):672–673. doi: 10.1093/infdis/125.6.672. [DOI] [PubMed] [Google Scholar]
- 60.Elson CO, Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984;132(6):2736–2741. [PubMed] [Google Scholar]
- 61.Vajdy M, Lycke N. Stimulation of antigen-specific T- and B-cell memory in local as well as systemic lymphoid tissues following oral immunization with cholera toxin adjuvant. Immunology. 1993;80(2):197–203. [PMC free article] [PubMed] [Google Scholar]
- 62.Vajdy M, Lycke NY. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology. 1992;75(3):488–492. [PMC free article] [PubMed] [Google Scholar]
- 63.Gagliardi MC, Sallusto F, Marinaro M, Langenkamp A, Lanzavecchia A, De Magistris MT. Cholera toxin induces maturation of human dendritic cells and licenses them for Th2 priming. European J. Immunol. 2000;30(8):2394–2403. doi: 10.1002/1521-4141(2000)30:8<2394::AID-IMMU2394>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 64.Okahashi N, Yamamoto M, Vancott JL, et al. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infection and Immunity. 1996;64(5):1516–1525. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vajdy M, Kosco-Vilbois MH, Kopf M, Kohler G, Lycke N. Impaired mucosal immune responses in interleukin 4-targeted mice. The Journal of Experimental Medicine. 1995;181(1):41–53. doi: 10.1084/jem.181.1.41. [DOI] [PubMed] [Google Scholar]
- 66.Xu-Amano J, Kiyono H, Jackson RJ, et al. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. The Journal of Experimental Medicine. 1993;178(4):1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marinaro M, Staats HF, Hiroi T, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155(10):4621–4629. [PubMed] [Google Scholar]
- 68.Munoz E, Zubiaga AM, Merrow M, Sauter NP, Huber BT. Cholera toxin discriminates between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. The Journal of Experimental Medicine. 1990;172(1):95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lycke N, Strober W. Cholera toxin promotes B cell isotype differentiation. J Immunol. 1989;142(11):3781–3787. [PubMed] [Google Scholar]
- 70.Lycke NY. Cholera toxin promotes B cell isotype switching by two different mechanisms. cAMP induction augments germ-line Ig H-chain RNA transcripts whereas membrane ganglioside GM1-receptor binding enhances later events in differentiation. J Immunol. 1993;150(11):4810–4821. [PubMed] [Google Scholar]
- 71.Nashar TO, Webb HM, Eaglestone S, Williams NA, Hirst TR. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(1):226–230. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arce S, Nawar HF, Muehlinghaus G, Russell MW, Connell TD. In vitro induction of immunoglobulin A (IgA)- and IgM-secreting plasma blasts by cholera toxin depends on T-cell help and is mediated by CD154 up-regulation and inhibition of gamma interferon synthesis. Infection and Immunity. 2007;75(3):1413–1423. doi: 10.1128/IAI.01367-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matousek MP, Nedrud JG, Harding CV. Distinct effects of recombinant cholera toxin B subunit and holotoxin on different stages of class II MHC antigen processing and presentation by macrophages. J Immunol. 1996;156(11):4137–4145. [PubMed] [Google Scholar]
- 74.Grdic D, Ekman L, Schon K, et al. Splenic marginal zone dendritic cells mediate the cholera toxin adjuvant effect: dependence on the ADP-ribosyltransferase activity of the holotoxin. J Immunol. 2005;175(8):5192–5202. doi: 10.4049/jimmunol.175.8.5192. [DOI] [PubMed] [Google Scholar]
- 75.Anjuere F, Luci C, Lebens M, et al. In vivo adjuvant-induced mobilization and maturation of gut dendritic cells after oral administration of cholera toxin. J Immunol. 2004;173(8):5103–5111. doi: 10.4049/jimmunol.173.8.5103. [DOI] [PubMed] [Google Scholar]
- 76.Shreedhar VK, Kelsall BL, Neutra MR. Cholera toxin induces migration of dendritic cells from the subepithelial dome region to T- and B-cell areas of Peyer's patches. Infection and Immunity. 2003;71(1):504–509. doi: 10.1128/IAI.71.1.504-509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90(9):3245–3287. [PubMed] [Google Scholar]
- 78.Bagley KC, Abdelwahab SF, Tuskan RG, Fouts TR, Lewis GK. Cholera toxin and heat-labile enterotoxin activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cyclic AMP-dependent pathway. Infection and Immunity. 2002;70(10):5533–5539. doi: 10.1128/IAI.70.10.5533-5539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lycke N, Karlsson U, Sjolander A, Magnusson KE. The adjuvant action of cholera toxin is associated with an increased intestinal permeability for luminal antigens. Scandinavian Journal of Immunology. 1991;33(6):691–698. doi: 10.1111/j.1365-3083.1991.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 80.Belyakov IM, Derby MA, Ahlers JD, et al. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(4):1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manuri PR, Nehete B, Nehete PN, et al. Intranasal immunization with synthetic peptides corresponding to the E6 and E7 oncoproteins of human papillomavirus type 16 induces systemic and mucosal cellular immune responses and tumor protection. Vaccine. 2007;25(17):3302–3310. doi: 10.1016/j.vaccine.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simmons CP, Hussell T, Sparer T, Walzl G, Openshaw P, Dougan G. Mucosal delivery of a respiratory syncytial virus CTL peptide with enterotoxin-based adjuvants elicits protective, immunopathogenic, and immunoregulatory antiviral CD8+ T cell responses. J Immunol. 2001;166(2):1106–1113. doi: 10.4049/jimmunol.166.2.1106. [DOI] [PubMed] [Google Scholar]
- 83.Simmons CP, Mastroeni P, Fowler R, et al. MHC class I-restricted cytotoxic lymphocyte responses induced by enterotoxin-based mucosal adjuvants. J Immunol. 1999;163(12):6502–6510. [PubMed] [Google Scholar]
- 84.Luci C, Hervouet C, Rousseau D, Holmgren J, Czerkinsky C, Anjuere F. Dendritic cell-mediated induction of mucosal cytotoxic responses following intravaginal immunization with the nontoxic B subunit of cholera toxin. J Immunol. 2006;176(5):2749–2757. doi: 10.4049/jimmunol.176.5.2749. [DOI] [PubMed] [Google Scholar]
- 85.Jang MH, Kweon MN, Hiroi T, Yamamoto M, Takahashi I, Kiyono H. Induction of cytotoxic T lymphocyte responses by cholera toxin-treated bone marrow-derived dendritic cells. Vaccine. 2003;21(15):1613–1619. doi: 10.1016/s0264-410x(02)00734-x. [DOI] [PubMed] [Google Scholar]
- 86.Eriksson K, Sun JB, Nordstrom I, et al. Coupling of antigen to cholera toxin for dendritic cell vaccination promotes the induction of MHC class I-restricted cytotoxic T cells and the rejection of a cognate antigen-expressing model tumor. European Journal of Immunology. 2004;34(5):1272–1281. doi: 10.1002/eji.200324368. [DOI] [PubMed] [Google Scholar]
- 87.Lavelle EC, McNeela E, Armstrong ME, Leavy O, Higgins SC, Mills KH. Cholera toxin promotes the induction of regulatory T cells specific for bystander antigens by modulating dendritic cell activation. J Immunol. 2003;171(5):2384–2392. doi: 10.4049/jimmunol.171.5.2384. [DOI] [PubMed] [Google Scholar]
- 88.van Ginkel FW, Jackson RJ, Yoshino N, et al. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infection and Immunity. 2005;73(10):6892–6902. doi: 10.1128/IAI.73.10.6892-6902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000;165(9):4778–4782. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 90.Armstrong ME, Lavelle EC, Loscher CE, Lynch MA, Mills KH. Proinflammatory responses in the murine brain after intranasal delivery of cholera toxin: implications for the use of AB toxins as adjuvants in intranasal vaccines. The Journal of Infectious Diseases. 2005;192(9):1628–1633. doi: 10.1086/491739. [DOI] [PubMed] [Google Scholar]
- 91.Mutsch M, Zhou W, Rhodes P, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. The New England Journal of Medicine. 2004;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 92.Tebbey PW, Scheuer CA, Peek JA, et al. Effective mucosal immunization against respiratory syncytial virus using purified F protein and a genetically detoxified cholera holotoxin, CT-E29H. Vaccine. 2000;18(24):2723–2734. doi: 10.1016/s0264-410x(00)00058-x. [DOI] [PubMed] [Google Scholar]
- 93.Kato M, Imamura S, Kawase H, Miyama A, Tsuji T. Histidine-44 of the A subunit of Escherichia coli enterotoxin is involved in its enzymatic and biological activities. FEMS microbiology letters. 1997;152(2):219–225. doi: 10.1111/j.1574-6968.1997.tb10431.x. [DOI] [PubMed] [Google Scholar]
- 94.Yamamoto M, McGhee JR, Hagiwara Y, Otake S, Kiyono H. Genetically manipulated bacterial toxin as a new generation mucosal adjuvant. Scandinavian Journal of Immunology. 2001;53(3):211–217. doi: 10.1046/j.1365-3083.2001.00883.x. [DOI] [PubMed] [Google Scholar]
- 95.Yamamoto S, Takeda Y, Yamamoto M, et al. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. The Journal of Experimental Medicine. 1997;185(7):1203–1210. doi: 10.1084/jem.185.7.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park EJ, Chang JH, Kim JS, Yum JS, Chung SI. The mucosal adjuvanticity of two nontoxic mutants of Escherichia coli heat-labile enterotoxin varies with immunization routes. Exp Mol Med. 2000;32(2):72–78. doi: 10.1038/emm.2000.13. [DOI] [PubMed] [Google Scholar]
- 97.Pizza M, Fontana MR, Giuliani MM, et al. A genetically detoxified derivative of heat-labile Escherichia coli enterotoxin induces neutralizing antibodies against the A subunit. The Journal of Experimental Medicine. 1994;180(6):2147–2153. doi: 10.1084/jem.180.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hirabayashi Y, Kurata H, Funato H, et al. Comparison of intranasal inoculation of influenza HA vaccine combined with cholera toxin B subunit with oral or parenteral vaccination. Vaccine. 1990;8(3):243–248. doi: 10.1016/0264-410x(90)90053-o. [DOI] [PubMed] [Google Scholar]
- 99.Tamura S, Funato H, Nagamine T, Aizawa C, Kurata T. Effectiveness of cholera toxin B subunit as an adjuvant for nasal influenza vaccination despite pre-existing immunity to CTB. Vaccine. 1989;7(6):503–505. doi: 10.1016/0264-410x(89)90273-9. [DOI] [PubMed] [Google Scholar]
- 100.Hajishengallis G, Hollingshead SK, Koga T, Russell MW. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154(9):4322–4332. [PubMed] [Google Scholar]
- 101.Richards CM, Aman AT, Hirst TR, Hill TJ, Williams NA. Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heatlabile enterotoxin B subunit as an adjuvant. Journal of Virology. 2001;75(4):1664–1671. doi: 10.1128/JVI.75.4.1664-1671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takase H, Murakami Y, Endo A, Ikeuchi T. Antibody responses and protection in mice immunized orally against influenza virus. Vaccine. 1996;14(1718):1651–1656. doi: 10.1016/s0264-410x(96)00128-4. [DOI] [PubMed] [Google Scholar]
- 103.Tamura S, Kurata H, Funato H, Nagamine T, Aizawa C, Kurata T. Protection against influenza virus infection by a two-dose regimen of nasal vaccination using vaccines combined with cholera toxin B subunit. Vaccine. 1989;7(4):314–320. doi: 10.1016/0264-410x(89)90192-8. [DOI] [PubMed] [Google Scholar]
- 104.Tamura S, Samegai Y, Kurata H, Nagamine T, Aizawa C, Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988;6(5):409–413. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- 105.Hagiwara Y, Iwasaki T, Asanuma H, et al. Effects of intranasal administration of cholera toxin (or Escherichia coli heat-labile enterotoxin) B subunits supplemented with a trace amount of the holotoxin on the brain. Vaccine. 2001;19(1314):1652–1660. doi: 10.1016/s0264-410x(00)00412-6. [DOI] [PubMed] [Google Scholar]
- 106.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nature Medicine. 2005;11(4 Suppl):S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 107.Smith DJ, King WF, Barnes LA, Trantolo D, Wise DL, Taubman MA. Facilitated intranasal induction of mucosal and systemic immunity to mutans streptococcal glucosyltransferase peptide vaccines. Infection and Immunity. 2001;69(8):4767–4773. doi: 10.1128/IAI.69.8.4767-4773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ebel F, Schmitt E, Peter-Katalinic J, Kniep B, Muhlradt PF. Gangliosides: differentiation markers for murine T helper lymphocyte subpopulations TH1 and TH2. Biochemistry. 1992;31(48):12190–12197. doi: 10.1021/bi00163a031. [DOI] [PubMed] [Google Scholar]
- 109.Rodden FA, Wiegandt H, Bauer BL. Gangliosides: the relevance of current research to neurosurgery. Journal of Neurosurgery. 1991;74(4):606–619. doi: 10.3171/jns.1991.74.4.0606. [DOI] [PubMed] [Google Scholar]
- 110.Rosenfelder G, Herbst H, Braun DG. Glycolipids as markers of murine T and B lymphoblastoid tumour cell lines. FEBS letters. 1980;114(2):213–218. doi: 10.1016/0014-5793(80)81117-3. [DOI] [PubMed] [Google Scholar]
- 111.Jobling MG, Holmes RK. Analysis of structure and function of the B subunit of cholera toxin by the use of site-directed mutagenesis. Molecular Microbiology. 1991;5(7):1755–1767. doi: 10.1111/j.1365-2958.1991.tb01925.x. [DOI] [PubMed] [Google Scholar]
- 112.Jobling MG, Holmes RK. Mutational analysis of ganglioside GM(1)-binding ability, pentamer formation, and epitopes of cholera toxin B (CTB) subunits and CTB/heat-labile enterotoxin B subunit chimeras. Infection and Immunity. 2002;70(3):1260–1271. doi: 10.1128/IAI.70.3.1260-1271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guidry JJ, Cardenas L, Cheng E, Clements JD. Role of receptor binding in toxicity, immunogenicity, and adjuvanticity of Escherichia coli heat-labile enterotoxin. Infection and Immunity. 1997;65(12):4943–4950. doi: 10.1128/iai.65.12.4943-4950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nashar TO, Hirst TR, Williams NA. Modulation of B-cell activation by the B subunit of Escherichia coli enterotoxin: receptor interaction up-regulates MHC class II, B7, CD40, CD25 and ICAM-1. Immunology. 1997;91(4):572–578. doi: 10.1046/j.1365-2567.1997.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383(6603):787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 116.Turcanu V, Hirst TR, Williams NA. Modulation of human monocytes by Escherichia coli heat-labile enterotoxin B-subunit; altered cytokine production and its functional consequences. Immunology. 2002;106(3):316–325. doi: 10.1046/j.1365-2567.2002.01429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salmond RJ, Pitman RS, Jimi E, et al. CD8+ T cell apoptosis induced by Escherichia coli heat-labile enterotoxin B subunit occurs via a novel pathway involving NF-kappaB-dependent caspase activation. European Journal of Immunology. 2002;32(6):1737–1747. doi: 10.1002/1521-4141(200206)32:6<1737::AID-IMMU1737>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 118.de Haan L, Verweij WR, Feil IK, et al. Role of GM1 binding in the mucosal immunogenicity and adjuvant activity of the Escherichia coli heat-labile enterotoxin and its B subunit. Immunology. 1998;94(3):424–430. doi: 10.1046/j.1365-2567.1998.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nashar TO, Betteridge ZE, Mitchell RN. Evidence for a role of ganglioside GM1 in antigen presentation: binding enhances presentation of Escherichia coli enterotoxin B subunit (EtxB) to CD4(+) T cells. Int Immunol. 2001;13(4):541–551. doi: 10.1093/intimm/13.4.541. [DOI] [PubMed] [Google Scholar]
- 120.Salmond RJ, Williams R, Hirst TR, Williams NA. The B subunit of Escherichia coli heat-labile enterotoxin induces both caspase-dependent and -independent cell death pathways in CD8+ T cells. Infection and Immunity. 2004;72(10):5850–5857. doi: 10.1128/IAI.72.10.5850-5857.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Truitt RL, Hanke C, Radke J, Mueller R, Barbieri JT. Glycosphingolipids as novel targets for T-cell suppression by the B subunit of recombinant heat-labile enterotoxin. Infection and Immunity. 1998;66(4):1299–1308. doi: 10.1128/iai.66.4.1299-1308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muraguchi A, Hirano T, Tang B, et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. The Journal of Experimental medicine. 1988;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Connell TD, Metzger D, Sfintescu C, Evans RT. Immunostimulatory activity of LT-IIa, a type II heat-labile enterotoxin of Escherichia coli. Immunol Lett. 1998;62(2):117–120. doi: 10.1016/s0165-2478(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 125.Hajishengallis G, Nawar H, Tapping RI, Russell MW, Connell TD. The Type II heat-labile enterotoxins LT-IIa and LT-IIb and their respective B pentamers differentially induce and regulate cytokine production in human monocytic cells. Infection and Immunity. 2004;72(11):6351–6358. doi: 10.1128/IAI.72.11.6351-6358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martin M, Hajishengallis G, Metzger DJ, Michalek SM, Connell TD, Russell MW. Recombinant antigen-enterotoxin A2/B chimeric mucosal immunogens differentially enhance antibody responses and B7-dependent costimulation of CD4(+) T cells. Infection and Immunity. 2001;69(1):252–261. doi: 10.1128/IAI.69.1.252-261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martin M, Metzger DJ, Michalek SM, Connell TD, Russell MW. Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins LT-IIa and LT-IIb. Infection and Immunity. 2000;68(1):281–287. doi: 10.1128/iai.68.1.281-287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martin M, Metzger DJ, Michalek SM, Connell TD, Russell MW. Distinct cytokine regulation by cholera toxin and type II heat-labile toxins involves differential regulation of CD40 ligand on CD4(+) T cells. Infection and Immunity. 2001;69(7):4486–4492. doi: 10.1128/IAI.69.7.4486-4492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nawar HF, Arce S, Russell MW, Connell TD. Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities. Infection and Immunity. 2005;73(3):1330–1342. doi: 10.1128/IAI.73.3.1330-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nawar HF, Arce S, Russell MW, Connell TD. Mutants of type II heat-labile enterotoxin LT-IIa with altered ganglioside-binding activities and diminished toxicity are potent mucosal adjuvants. Infection and Immunity. 2007;75(2):621–633. doi: 10.1128/IAI.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Naito Y, Takematsu H, Koyama S, et al. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Molecular and Cellular Biology. 2007;27(8):3008–3022. doi: 10.1128/MCB.02047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liang S, Wang M, Triantafilou K, et al. The A subunit of type IIb enterotoxin (LT-IIb) suppresses the proinflammatory potential of the B subunit and its ability to recruit and interact with TLR2. J Immunol. 2007;178(8):4811–4819. doi: 10.4049/jimmunol.178.8.4811. [DOI] [PubMed] [Google Scholar]
- 133.Hajishengallis G, Tapping RI, Martin MH, et al. Toll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxins. Infection and Immunity. 2005;73(3):1343–1349. doi: 10.1128/IAI.73.3.1343-1349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liang S, Wang M, Tapping RI, et al. Ganglioside GD1a is an essential coreceptor for Toll-like receptor 2 signaling in response to the B subunit of type IIb enterotoxin. The Journal of Biological Chemistry. 2007;282(10):7532–7542. doi: 10.1074/jbc.M611722200. [DOI] [PubMed] [Google Scholar]
- 135.Zhang RG, Scott DL, Westbrook ML, et al. The three-dimensional crystal structure of cholera toxin. Journal of Molecular Biology. 1995;251(4):563–573. doi: 10.1006/jmbi.1995.0456. [DOI] [PubMed] [Google Scholar]
- 136.Sixma TK, Kalk KH, van Zanten BA, et al. Refined structure of Escherichia coli heatlabile enterotoxin, a close relative of cholera toxin. Journal of Molecular Biology. 1993;230(3):890–918. doi: 10.1006/jmbi.1993.1209. [DOI] [PubMed] [Google Scholar]
- 137.Sixma TK, Pronk SE, Kalk KH, et al. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991;351(6325):371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- 138.van den Akker F, Sarfaty S, Twiddy EM, Connell TD, Holmes RK, Hol WG. Crystal structure of a new heat-labile enterotoxin, LT-IIb. Structure. 1996;4(6):665–678. doi: 10.1016/s0969-2126(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 139.Lencer WI, Saslowsky D. Raft trafficking of AB5 subunit bacterial toxins. Biochimica et Biophysica Acta. 2005;1746(3):314–321. doi: 10.1016/j.bbamcr.2005.07.007. [DOI] [PubMed] [Google Scholar]