Abstract
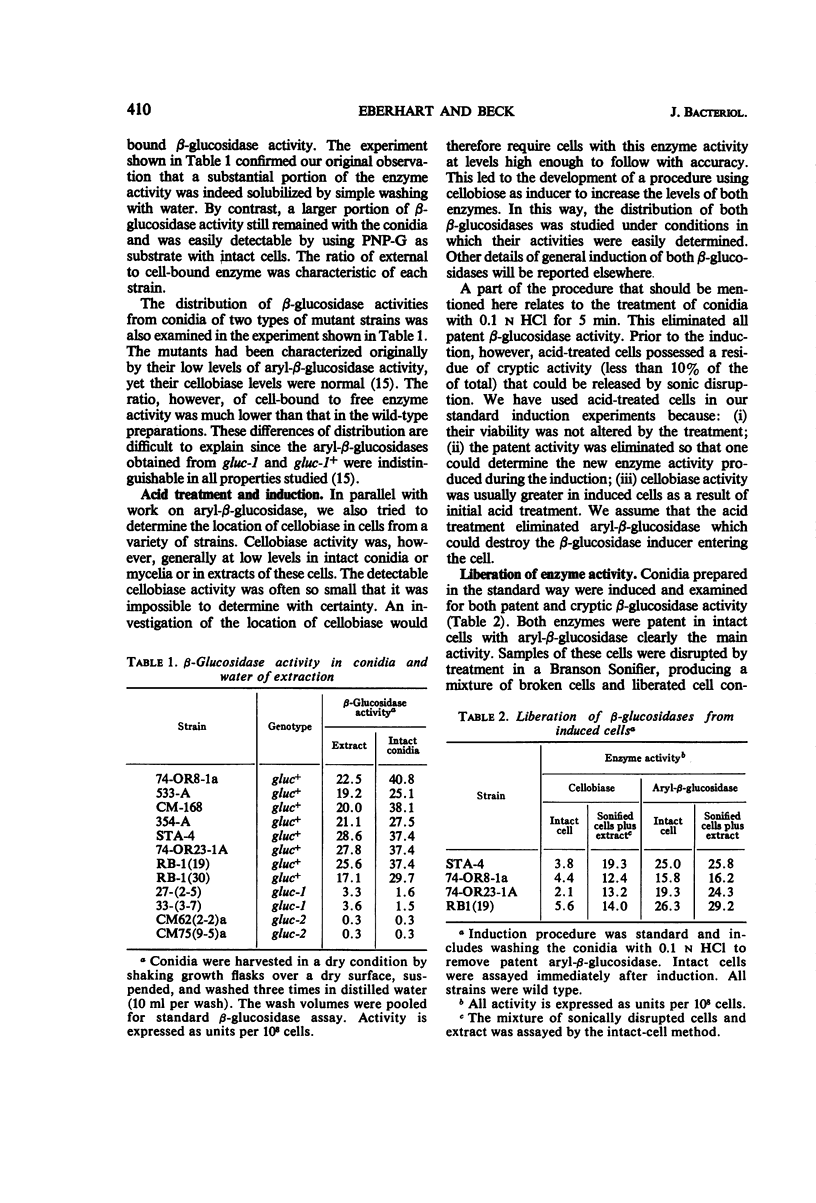
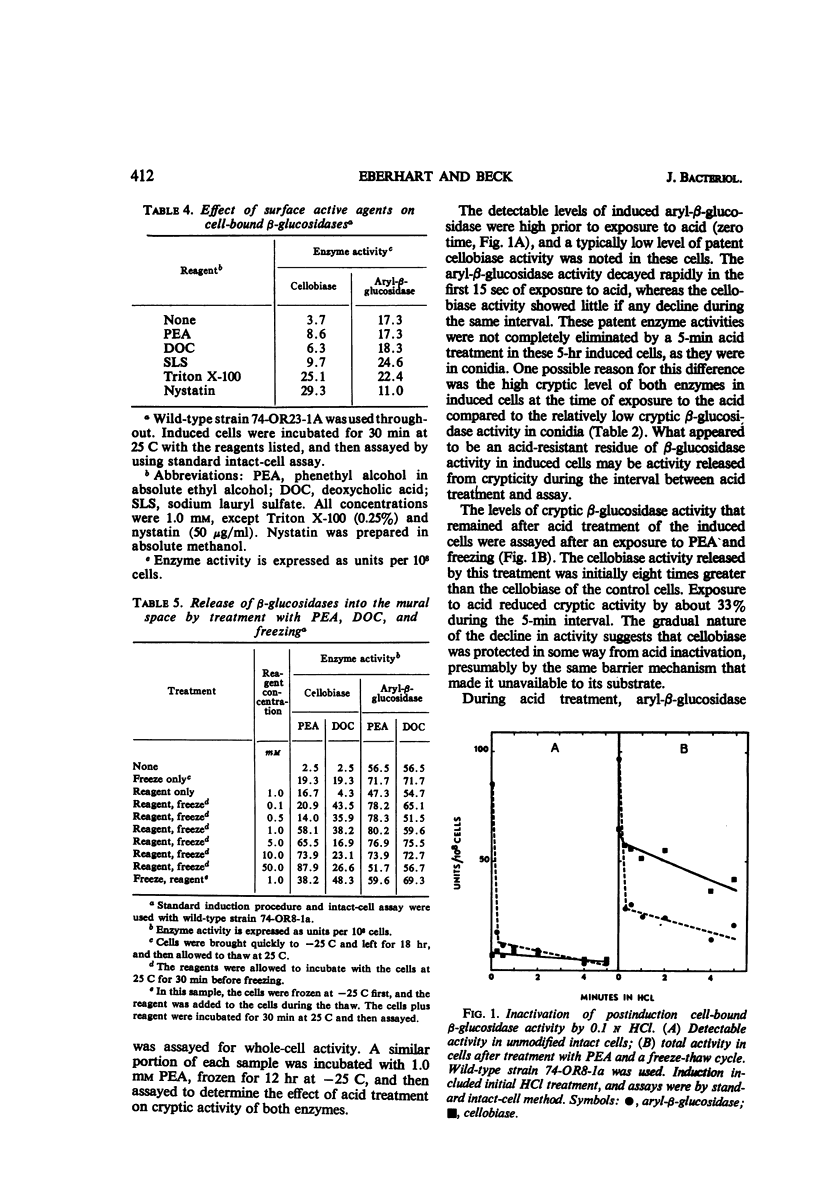
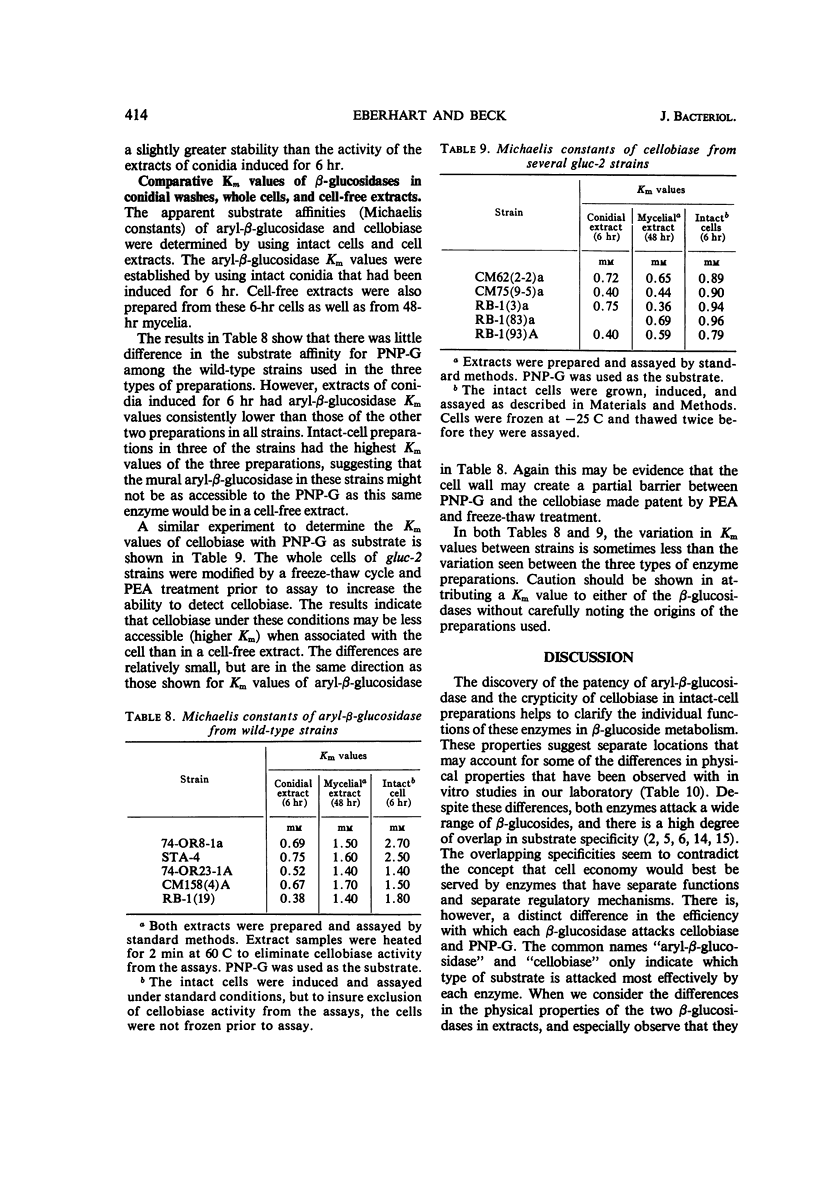
The β-glucosidases (EC 3.2.1.21) of Neurospora crassa were studied with respect to their location in conidia and young mycelia. Aryl-β-glucosidase of conidia was nearly equally divided between extracellular and bound activity. Bound aryl-β-glucosidase was almost all available to substrate. An induction procedure was used to maximize both β-glucosidases in 4 to 6-hr cells. Aryl-β-glucosidase was entirely bound but still mostly (90%) detectable, whereas cellobiase was mostly internal and cryptic. A freeze-thaw cycle or treatment with phenethyl alcohol or deoxycholic acid made the cellobiase detectable without releasing it from the cell. A 10 to 20% increase in cell-bound aryl-β-glucosidase could be obtained by this treatment. Dilute HCl (0.1 n) destroyed the patent aryl-β-glucosidase but not the cryptic aryl-β-glucosidase or the cryptic cellobiase activity in intact cells. This suggested that most aryl-β-glucosidase activity was exterior to the cell membrane but still within the mural space. The thermal stability of patent aryl-β-glucosidase and released cellobiase was found to be higher than in corresponding cell-free extracts. Measurements of Km suggested a slightly lower affinity for substrate p-nitrophenyl-β-d-glucopyranoside by the enzymes in intact cells compared to enzymes in extracts.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGER L. S., EBERHART B. M. Extracellular beta-transglucosidase activity from conidia of Neurospora crassa. Biochem Biophys Res Commun. 1961 Oct 23;6:62–66. doi: 10.1016/0006-291x(61)90186-3. [DOI] [PubMed] [Google Scholar]
- CIRILLO V. P., HARSCH M., LAMPEN J. O. ACTION OF THE POLYENE ANTIBIOTICS FILIPIN, NYSTATIN AND N-ACETYLCANDIDIN ON THE YEAST CELL MEMBRANE. J Gen Microbiol. 1964 May;35:249–259. doi: 10.1099/00221287-35-2-249. [DOI] [PubMed] [Google Scholar]
- EBERHART B. M. Exogenous enzymes of Neurospora conidia and mycelia. J Cell Comp Physiol. 1961 Aug;58:11–16. doi: 10.1002/jcp.1030580103. [DOI] [PubMed] [Google Scholar]
- EBERHART B., CROSS D. F., CHASE L. R. BETA-GLUCOSIDASE SYSTEM OF NEUROSPORA CRASSA. I. BETA-GLUCOSIDASE AND CELLULASE ACTIVITIES OF MUTANT AND WILD-TYPE STRAINS. J Bacteriol. 1964 Apr;87:761–770. doi: 10.1128/jb.87.4.761-770.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylar E. H. On the biological role of glycoproteins. J Theor Biol. 1966 Jan;10(1):89–113. doi: 10.1016/0022-5193(66)90179-2. [DOI] [PubMed] [Google Scholar]
- HILL E. P., SUSSMAN A. S. DEVELOPMENT OF TREHALASE AND INVERTASE ACTIVITY IN NEUROSPORA. J Bacteriol. 1964 Dec;88:1556–1566. doi: 10.1128/jb.88.6.1556-1566.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN J. G. AN INDUCIBLE SYSTEM FOR THE HYDROLYSIS AND TRANSPORT OF BETA-GLUCOSIDES IN YEAST. I. CHARACTERISTICS OF THE BETA-GLUCOSIDASE ACTIVITY OF INTACT AND OF LYSED CELLS. J Gen Physiol. 1965 May;48:873–886. doi: 10.1085/jgp.48.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSKY S. C. Nystatin binding by protoplasts and a particulate fraction of Neurospora crassa, and a basis for the selective toxicity of polyene antifungal antibiotics. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1049–1056. doi: 10.1073/pnas.48.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester G. Inhibition of Growth, Synthesis, and Permeability in Neurospora crassa by Phenethyl Alcohol. J Bacteriol. 1965 Jul;90(1):29–37. doi: 10.1128/jb.90.1.29-37.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHADEVAN P. R., EBERHART B. ARYL BETA-GLUCOSIDASE OF SOME NEUROSPORA STRAINS. Biochim Biophys Acta. 1964 Jul 15;90:214–215. doi: 10.1016/0304-4165(64)90147-3. [DOI] [PubMed] [Google Scholar]
- MAHADEVAN P. R., EBERHART B. THE BETA-GLUCOSIDASE SYSTEM OF NEUROSPORA CRASSA. 3. FURTHER STUDIES ON AN ARYL BETA-GLUCOSIDASE MUTANT. Arch Biochem Biophys. 1964 Oct;108:30–35. doi: 10.1016/0003-9861(64)90351-0. [DOI] [PubMed] [Google Scholar]
- MAHADEVAN P. R., EBERHART B. THE BETA-GLUCOSIDASE SYSTEM OF NEUROSPORA CRASSA. II. PURIFICATION AND CHARACTERIZATION OF ARYL BETA-GLUCOSIDASE. Arch Biochem Biophys. 1964 Oct;108:22–29. doi: 10.1016/0003-9861(64)90350-9. [DOI] [PubMed] [Google Scholar]
- MANDELS G. R. Localization of carbohydrases at the surface of fungus spores by acid treatment. Exp Cell Res. 1953 Sep;5(1):48–55. doi: 10.1016/0014-4827(53)90093-7. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. ENZYMICALLY ACTIVE SUBUNITS OF NEUROSPORA INVERTASE. Biochim Biophys Acta. 1964 Aug 26;89:291–302. doi: 10.1016/0926-6569(64)90217-2. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. THE LOCALIZATION OF BETA-FRUCTOFURANOSIDASE IN NEUROSPORA. Biochim Biophys Acta. 1963 Nov 8;77:455–465. doi: 10.1016/0006-3002(63)90521-3. [DOI] [PubMed] [Google Scholar]
- Mahadevan P. R., Menon C. P. Laminarinase of Neurospora crassa. I. Enzyme activity associated with conidia & conidial wall. Indian J Biochem. 1968 Mar;5(1):6–8. [PubMed] [Google Scholar]
- Markert C. L., Møller F. MULTIPLE FORMS OF ENZYMES: TISSUE, ONTOGENETIC, AND SPECIES SPECIFIC PATTERNS. Proc Natl Acad Sci U S A. 1959 May;45(5):753–763. doi: 10.1073/pnas.45.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkres K. D., Woodward D. O. On the genetics of enzyme locational specificity. Proc Natl Acad Sci U S A. 1966 May;55(5):1217–1224. doi: 10.1073/pnas.55.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. G., Eberhart B. Regulation of cellulase and cellobiase in Neurospora crassa. Biochem Biophys Res Commun. 1966 Sep 8;24(5):782–785. doi: 10.1016/0006-291x(66)90394-9. [DOI] [PubMed] [Google Scholar]
- PREISS J. W. The localization of invertase in the yeast cell with low voltage electrons. Arch Biochem Biophys. 1958 May;75(1):186–195. doi: 10.1016/0003-9861(58)90409-0. [DOI] [PubMed] [Google Scholar]
- Trevithick J. R., Metzenberg R. L. Molecular sieving by Neurospora cell walls during secretion of invertase isozymes. J Bacteriol. 1966 Oct;92(4):1010–1015. doi: 10.1128/jb.92.4.1010-1015.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevithick J. R., Metznberg R. L. The invertase isozyme formed by Neurospora protoplasts. Biochem Biophys Res Commun. 1964 Jul 1;16(4):319–325. doi: 10.1016/0006-291x(64)90033-6. [DOI] [PubMed] [Google Scholar]


