Abstract
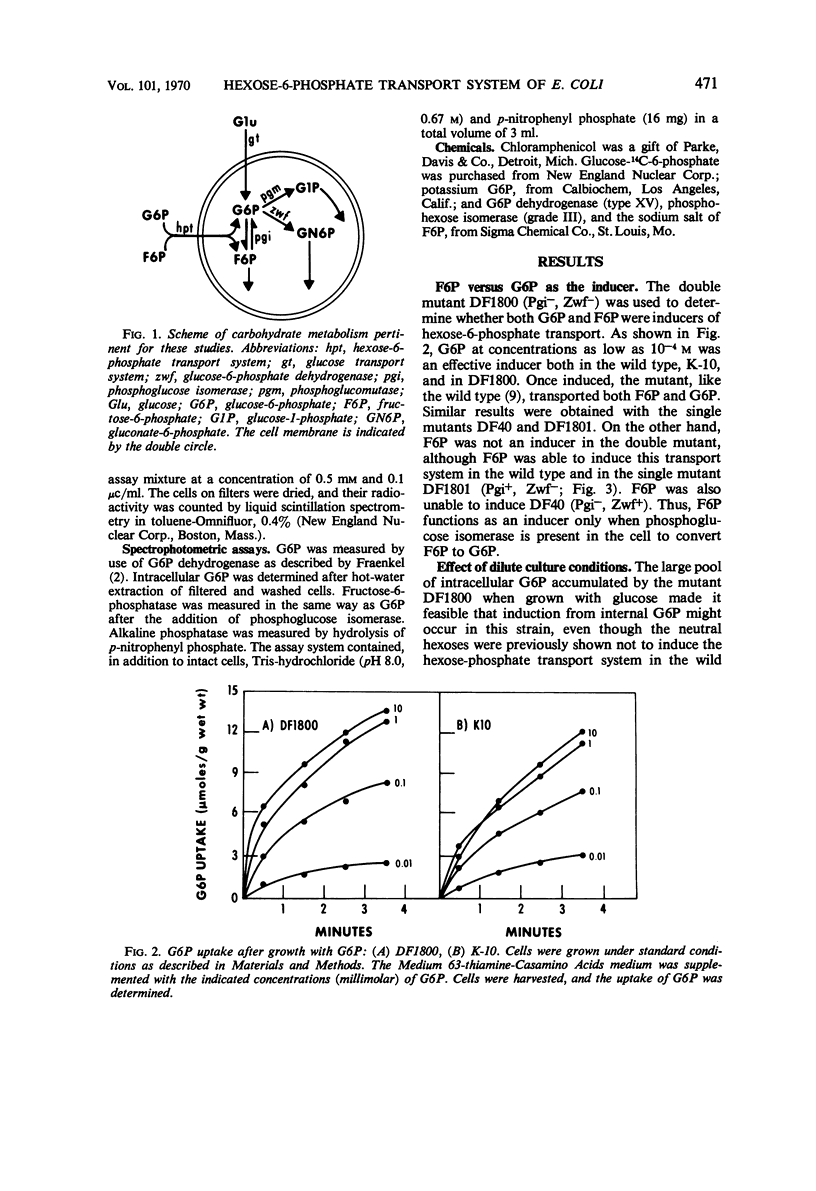
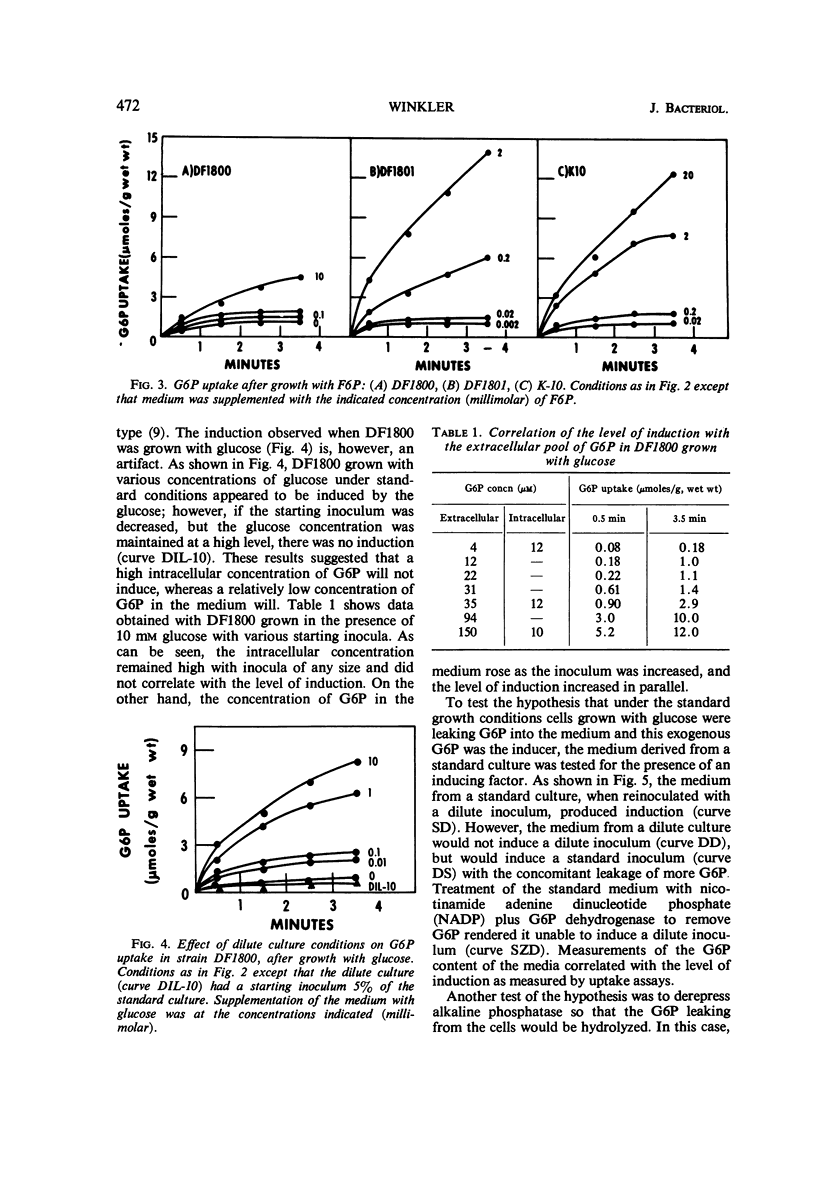
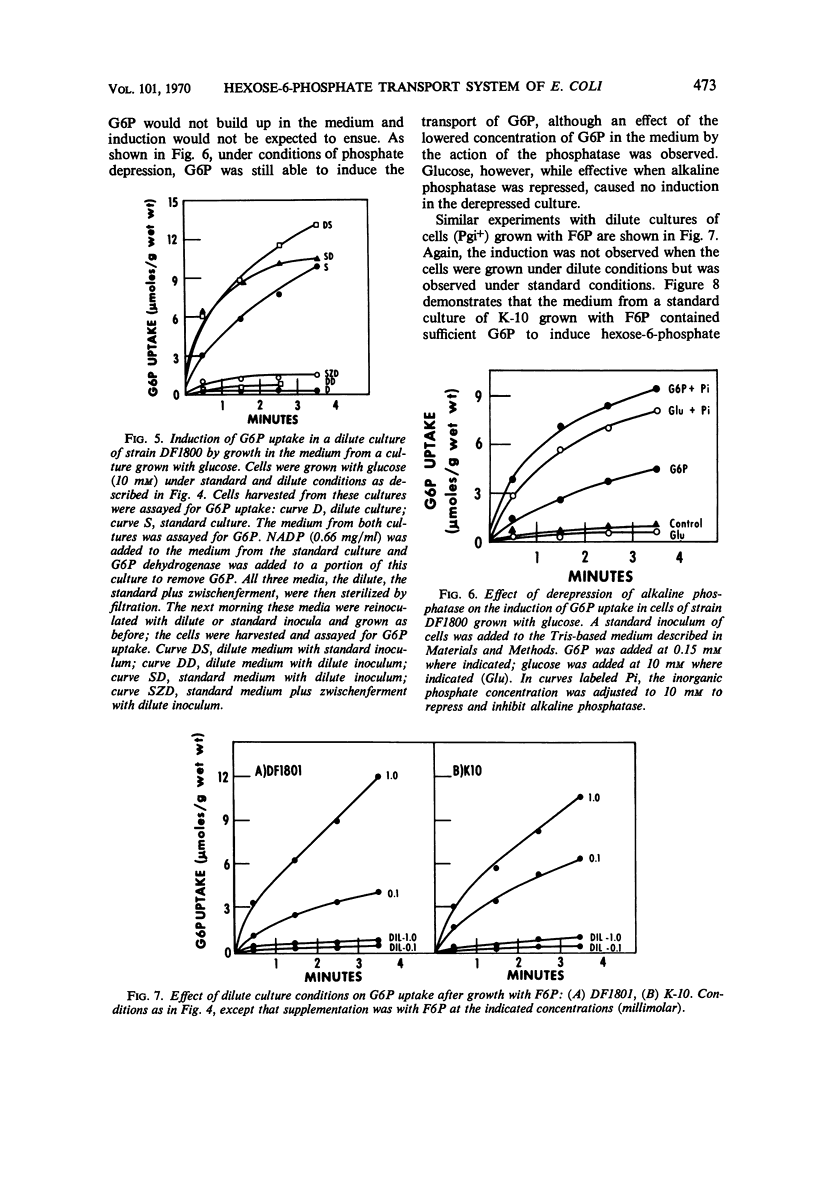
The induction of the hexose-6-phosphate transport system was investigated. Glucose-6-phosphate (G6P) at concentrations as low as 10−4m was able to induce this system in wild-type cells, as well as in mutants lacking phosphoglucose isomerase or G6P dehydrogenase. Growth in the presence of fructose-6-phosphate (F6P) induced the system only if the cells contained phosphoglucose isomerase. Furthermore, glucose and F6P were found to induce the system only if the extracellular concentration of G6P became appreciable in the medium as a consequence of the leakage of intracellular G6P formed from the glucose or F6P. Intracellular G6P was not an inducer even at high concentrations. The metabolism of glucose inhibited the induction of the hexose-6-phosphate transport system. Hypotheses for this compartmentalization of inducer and membrane-associated induction are presented.
Full text
PDF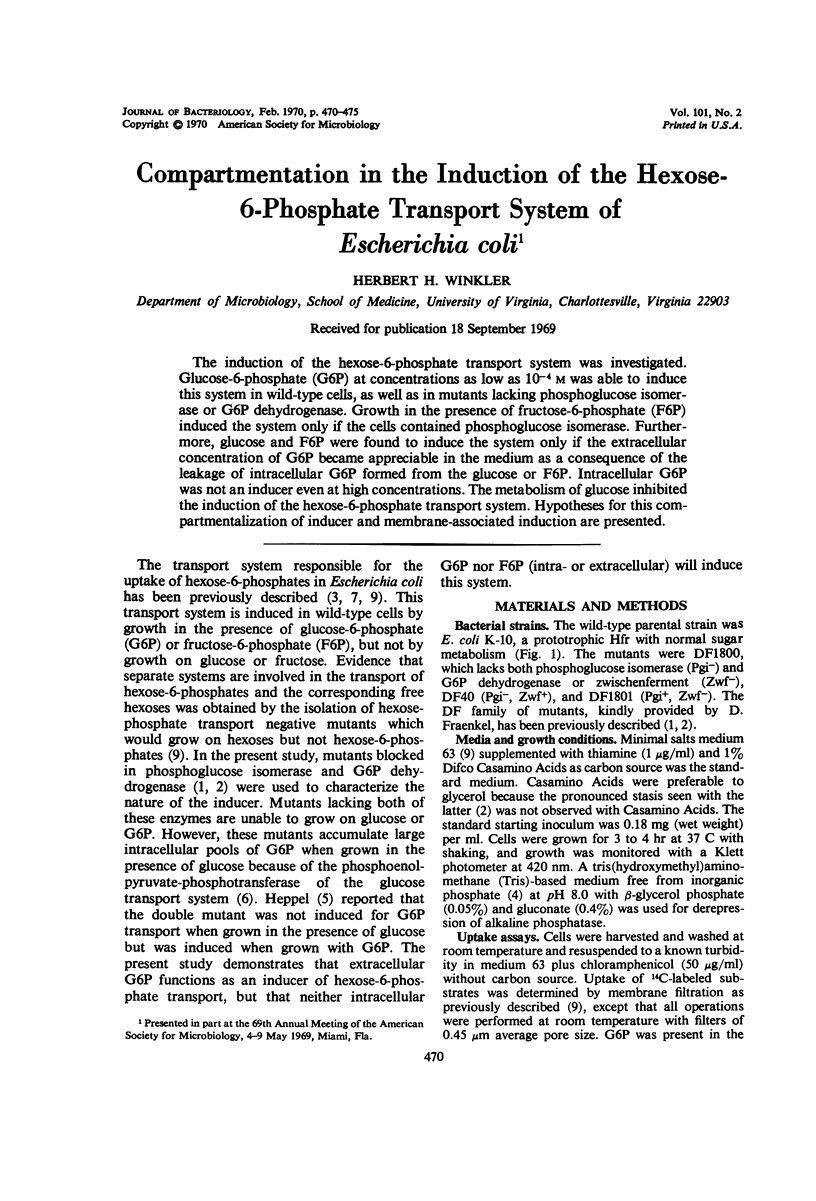

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FRAENKEL D. G., FALCOZ-KELLY F., HORECKER B. L. THE UTILIZATION OF GLUCOSE 6-PHOSPHATE BY GLUCOKINASELESS AND WILD-TYPE STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Nov;52:1207–1213. doi: 10.1073/pnas.52.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. Selection of Escherichia coli mutants lacking glucose-6-phosphate dehydrogenase or gluconate-6-phosphate dehydrogenase. J Bacteriol. 1968 Apr;95(4):1267–1271. doi: 10.1128/jb.95.4.1267-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. The accumulation of glucose 6-phosphate from glucose and its effect in an Escherichia coli mutant lacking phosphoglucose isomerase and glucose 6-phosphate dehydrogenase. J Biol Chem. 1968 Dec 25;243(24):6451–6457. [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogell B. M., Maity B. R., Frumkin S., Shapiro S. Induction of an active transport system for glucose 6-phosphate in Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):406–415. doi: 10.1016/0003-9861(66)90047-6. [DOI] [PubMed] [Google Scholar]
- SERCARZ E. E., GORINI L. DIFFERENT CONTRIBUTION OF EXOGENOUS AND ENDOGENOUS ARGININE TO REPRESSOR FORMATION. J Mol Biol. 1964 Feb;8:254–262. doi: 10.1016/s0022-2836(64)80135-2. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. A hexose-phosphate transport system in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):231–240. doi: 10.1016/0304-4165(66)90170-x. [DOI] [PubMed] [Google Scholar]


