Abstract
Francisella tularensis, the causative agent of tularemia, interacts with host cells of innate immunity in an atypical manner. For most Gram-negative bacteria, the release of lipopolysaccharide (LPS) from their outer membranes stimulates an inflammatory response. When LPS from the attenuated live vaccine strain (LVS) or the highly virulent Schu S4 strain of F. tularensis was incubated with human umbilical vein endothelial cells, neither species of LPS induced expression of the adhesion molecule E-selectin or secretion of the chemokine CCL2. Moreover, a high concentration (10 μg/ml) of LVS or Schu S4 LPS was required to stimulate production of CCL2 by human monocyte-derived macrophages (huMDM). A screen for alternative proinflammatory factors of F. tularensis LVS identified the heat shock protein GroEL as a potential candidate. Recombinant LVS GroEL at a concentration of 10 μg/ml elicited secretion of CXCL8 and CCL2 by huMDM through a TLR4-dependent mechanism. When 1 μg of LVS GroEL/ml was added to an equivalent amount of LVS LPS, the two components synergistically activated the huMDM to produce CXCL8. Schu S4 GroEL was less stimulatory than LVS GroEL and showed a lesser degree of synergy when combined with Schu S4 LPS. These findings suggest that the intrinsically low proinflammatory activity of F. tularensis LPS may be increased in the infected human host through interactions with other components of the bacterium.
Francisella tularensis is a highly infectious, Gram-negative bacterium that causes the often-lethal illness called tularemia. This aerobic, nonmotile coccobacillus is distinguished by two main subspecies that are pathogenic for humans. F. tularensis subsp. tularensis (also known as type A) is predominantly found in North America and causes severe, acute disease. F. tularensis subsp. holarctica (type B) is found throughout Eurasia and causes a milder disease that is seldom fatal (44). A live vaccine strain (LVS) derived from type B F. tularensis, which is pathogenic in mice but attenuated in humans, is commonly used to study this organism (21, 27). The molecular basis for attenuation of the LVS is not known (21). The type A Schu S4 strain of F. tularensis is extremely virulent in humans and is classified as a category A select agent, meaning it has great potential for an adverse impact on public health if released intentionally and a moderate to high potential for large-scale dissemination (52).
F. tularensis is a facultative intracellular organism that replicates within both human and murine macrophages, thereby evading assault from the host immune system (2, 8, 26). However, only human macrophages secrete substantial amounts of cytokines and chemokines in response to this organism. In particular, F. tularensis LVS stimulates the production of interleukin-1β (IL-1β), CXCL8, and CCL2 by these cells (8). To date, few inflammatory components of F. tularensis LVS have been identified. Typically, lipopolysaccharide (LPS) released from the Gram-negative bacterial outer membrane acts as a potent proinflammatory mediator. LPS can be liberated as a result of bacterial multiplication or death, allowing the exposure of its toxic moiety, lipid A (62). However, the LPS of F. tularensis LVS has an atypical lipid A and core structure (64), as well as a diminished ability to provoke secretion of inflammatory cytokines such as IL-1β and tumor necrosis factor alpha (TNF-α) from either murine or human cells of innate immunity (1, 20, 34, 53). It is likely, then, that F. tularensis elicits inflammation via components other than LPS. Recently, it has been shown that two lipoproteins of F. tularensis, LpnA and FTT1103, stimulate expression of chemokines by human and murine cells of innate immunity (24, 60). As shown herein, the heat shock protein GroEL from F. tularensis also provokes changes in host cells.
Over the past decade, heat shock proteins have been shown to be potent activators of the innate immune system. Heat shock proteins are highly conserved and expressed in both prokaryotes and eukaryotes (50). They function as chaperones, facilitating the noncovalent assembly and disassembly of proteins. During exposure of cells to environmental stresses such as increased temperature, extreme pH, free radicals, and degradative enzymes, chaperones protect cellular proteins from aggregation and promote refolding (56). However, recent evidence shows that heat shock proteins also can trigger strong immunological responses, functioning as potent immunogens and inflammatory agents (35). Heat shock proteins of both mammalian and microbial origin stimulate monocytes, macrophages, and dendritic cells to produce nitric oxide and a variety of cytokines, including TNF-α, IL-1β, IL-6, and IL-12 (39, 42, 66). Heat shock proteins also activate endothelial cells to express adhesion molecules such as E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) (10, 28, 39, 63). These molecules bind to circulating leukocytes and aid their extravasation in response to chemoattractants, such as CXCL8 and CCL2 (30). In fact, Asea et al. (4) have coined the term “chaperokine” to describe the dual role of heat shock proteins as molecular chaperones and cytokines.
One of the best-characterized heat shock proteins is chaperonin 60 (Cpn60). It is commonly accepted that Cpn60 from both prokaryotes and eukaryotes are intracellular proteins, and yet Cpn60 also can be released from cells to initiate a robust immune response (35, 42). Like the Cpn60 of Escherichia coli, the Cpn60 of F. tularensis is commonly referred to as GroEL. Although little is known about F. tularensis GroEL, Ericsson et al. (22) observed that its levels are increased under conditions of stress, such as heat shock and exposure to hydrogen peroxide. In addition, transcription of groEL is controlled by the heat shock sigma factor RpoH (29), and GroEL is found in the cytosol of host cells that are infected with F. tularensis (40). To date, the inflammatory potential of F. tularensis GroEL has not been reported.
Few studies have compared proinflammatory components derived from type A or type B strains of F. tularensis. We therefore examined the reactions of human cells of innate immunity to LPS and GroEL from the virulent type A Schu S4 strain and the attenuated type B LVS. LPS and GroEL both stimulated human macrophages to a limited extent, but these components had little to no effect on human endothelial cells. Of particular interest is the observation that GroEL and LPS acted together to elicit a synergistic response in macrophages, but this synergy was more pronounced with factors derived from the LVS. These results suggest that F. tularensis LPS, through its interaction with GroEL, may have a greater capacity to provoke inflammation in human hosts than previously appreciated.
MATERIALS AND METHODS
Culture of bacteria.
Stocks of F. tularensis LVS, a gift from Karen Elkins (Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Rockville, MD), and the Schu S4 strain (catalog no. NR-643), from the Biodefense and Emerging Infections Research Resources Repository (Manassas, VA), were prepared as previously described (23). Bacteria were grown on Mueller-Hinton (MH) II agar supplemented with 1% bovine hemoglobin and 1% IsoVitaleX Enrichment (all from BD Biosciences, Lincoln Park, NJ). After 2 to 3 days of growth on the plate, a single colony was inoculated into MH broth supplemented with 2% IsoVitaleX Enrichment, 0.1% glucose, 625 μM CaCl2, 530 μM MgCl2, and 335 μM ferric pyrophosphate. The bacteria were grown to late log phase for 16 to 18 h at 37°C with shaking at 100 rpm. Aliquots of bacterial culture then were centrifuged, and the pellets were resuspended in culture medium appropriate to the type of mammalian cell under study. The concentrations of bacteria were initially estimated by the optical density at 600 nm (OD600) of the suspension culture, and actual numbers of viable bacteria were determined by streaking dilutions on agar plates and counting the colonies 3 days later. Material from gentamicin-killed bacteria was obtained by incubating F. tularensis overnight at 37°C in the presence of 50 μg of gentamicin (Invitrogen, Carlsbad, CA)/ml. The following day, samples were centrifuged for 20 min at 4,200 × g, and the supernatants were collected. The material released by the killed bacteria into these supernatants is hereafter referred to as “releasate.” Treatment of F. tularensis with gentamicin in this manner causes extensive breakdown of the organisms, as revealed by negative-stain electron microscopy (23).
Culture of endothelial cells.
Primary human umbilical vein endothelial cells (HUVEC) were obtained by perfusion of the veins with collagenase (36). These cells were grown to confluence in 60-mm dishes in medium 199 (M199; Invitrogen) supplemented with 20% heat-inactivated (hi; 56°C for 30 min) fetal bovine serum (FBS; HyClone Laboratories, Logan, UT), 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 μg of amphotericin B/ml. Subsequently, the cells were trypsinized, passaged onto 48-well plates or 96-well Primaria plates (BD Biosciences) in antibiotic-free medium, and used for experiments upon attaining confluence.
Isolation of human monocyte-derived macrophages.
Human monocytes were isolated from the blood of healthy human donors after informed consent using 0.12% disodium EDTA as an anticoagulant. A mononuclear cell fraction was collected by overlaying the blood onto Accu-Prep Lymphocytes (Accurate Chemical and Scientific Corp., Westbury, NY) as previously described (18). Monocytes then were isolated from this fraction by negative selection by using immunomagnetic beads (Monocyte Isolation Kit II), according to the instructions of the manufacturer (Miltenyi Biotec; Auburn, CA). Cells were added to 24- or 48-well plates at concentrations of 2 × 105 and 1 × 105 cells per well, respectively, and cultured for 5 days in RPMI medium (Invitrogen) containing 10% hiFBS and 50 ng of recombinant human macrophage colony-stimulating factor from R&D Systems (Minneapolis, MN)/ml to induce the formation of human monocyte-derived macrophages (huMDM). All experiments were performed with cells isolated from individual donors.
Preparation of murine bone marrow-derived macrophages.
Murine bone marrow-derived macrophages (muBMDM) were isolated as previously described (13, 24). Marrow was flushed from the femurs of female C57BL/6 mice (Taconic Laboratories, Germantown, NY), and cells were cultured in Dulbecco modified Eagle medium (DMEM) containing 2 mM l-glutamine, 1 mM sodium pyruvate (all from Invitrogen), 20% hiFBS, and 30% medium previously conditioned by L929 cells (47) to promote differentiation. After 5 days, the muBMDM were detached from plates with ice-cold phosphate-buffered saline (PBS; Invitrogen) and resuspended in DMEM supplemented as described above, but with the content of hiFBS and L929 cell-conditioned medium reduced to 10 and 15%, respectively. MuBMDM were seeded in 48-well plates at a concentration of 1.5 × 105 cells/well and used for experiments on the following day. All murine experiments were approved by the Stony Brook University Institutional Animal Care and Use Committee.
Purification of LPS.
LPS was purified by a procedure similar to that used by Phillips et al. (48). Lawns of F. tularensis were scraped from 10 100-mm-diameter agar plates and placed into PBS containing 0.5% phenol for 1 h at 37°C. Subsequently, the bacteria were pelleted at 6,000 × g for 15 min and resuspended in 10 mM EDTA, 60 mM Tris base, and 2% sodium dodecyl sulfate (SDS) at pH 6.8. This solution was then boiled for 5 min and immediately cooled to 65°C. Next, 1 mg of proteinase K (Sigma-Aldrich Chemical Co., St. Louis, MO) was added to the sample, which was incubated at 65°C for 1 h and at 37°C overnight with shaking. To this sample, 3 volumes of ethanol and sodium acetate to a final concentration of 0.3 M were added, and the tube was placed at −20°C overnight. The sample was centrifuged at 3,200 × g for 15 min, and the resulting pellet was washed three times using 97% ethanol and 0.3 M sodium acetate. Finally, the precipitate was resuspended in DNase/RNase-free water (Invitrogen) and centrifuged at 48,000 × g for 20 min. The LPS, contained in the supernatant, was sedimented by ultracentrifugation at 147,000 × g for 75 min. This pellet was washed again in water and centrifuged at 147,000 × g for 75 min. The pellet of LPS was resuspended in water and lyophilized overnight. The dry weight of the LPS was measured, and the LPS was dissolved in PBS. The purity of the LPS was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) on 12% gels. Silver staining of heavily loaded gels (10 μg of LPS per lane) revealed no protein bands in LPS purified from the LVS and only one very faint band at ∼30 kDa in LPS isolated from the Schu S4 strain (data not shown).
FPLC.
Prior to performing fast protein liquid chromatography (FPLC), a releasate from gentamicin-killed LVS organisms was prepared as described above and concentrated 10-fold using a Centricon Plus-20 centrifugal filter device with a 5-kDa molecular mass cutoff (Millipore Corp., Billerica, MA). The releasate was next centrifuged at 21,000 × g for 20 min to remove insoluble components. A Superdex 70 10/300 GL gel filtration column (GE Healthcare, Piscataway, NJ) was washed with two column volumes of water, followed by one volume of PBS. Subsequently, 1 ml of concentrated releasate was injected into the column. PBS was used to elute the sample at a flow rate of 0.5 ml/min. The sample was separated into 100 fractions of 250 μl. A portion of each fraction was added to an equivalent volume of M199 containing 40% FBS. These samples were then used to stimulate HUVEC, and the levels of E-selectin were determined by whole-cell enzyme-linked immunosorbent assay (ELISA) as described below. Active fractions were subjected to SDS-PAGE on 12% gels and stained using Coomassie brilliant blue. Protein bands were excised from the gel and sent to the Stony Brook University Proteomics Center, which performed in-gel digestion with trypsin, high-performance liquid chromatographic separation (Dionex LC Packings, Bannockburn, IL), and analysis by using liquid chromatography/tandem mass spectrometry (API QSTAR Pulsar i LC/MS/MS System; Applied Biosystems, Foster City, CA). The data obtained from the mass spectrometer were searched with Mascot (Matrix Science, Boston, MA) to identify peptides that were present in the sample.
Cloning, expression, and purification of F. tularensis GroEL.
The sequences of GroEL from the LVS (GenBank accession number AM233362) and the Schu S4 strain (GenBank accession number AJ749949) of F. tularensis were used for primer design and sequence analysis. Forward 5′-GAAACATATGATGGCTGCAAAACAAG-3′ and reverse 5′-ACAACTCGAGGACTATTACATCATGCCAGG-3′ primers were used to amplify a 1,660-bp region from the LVS or Schu S4 strain genome with restriction enzyme cleavage sites (NdeI and XhoI) inserted at the ends of the PCR product. This product was initially ligated into a pGEM vector (Promega, Madison, WI) and then transferred into a pET14b vector (Novagen, Madison, WI) to add an N-terminal sequence of six histidine residues to the gene. Purification of the recombinant protein was performed by the Northeast Biodefense Center Protein Expression Core at the Wadsworth Center, Albany, NY. The plasmid was placed into BL21 Star(DE3)/pLysS E. coli cells (Invitrogen), and induction of protein expression was carried out for 3 h in the presence of IPTG (isopropyl-β-d-thiogalactopyranoside). The cells were then sonicated, and the supernatant was applied to a HisTrap HP Column (GE Healthcare). Fractions containing GroEL were collected and then passed over a Waters HPLC AP-1 column (Waters Chromatography Division, Millipore Corp.) packed with Protein-Pak Q 15 HR anion-exchange resin. Endotoxin was depleted using Endoclean (Biovintage, San Diego, CA), and levels were less than 0.0005 endotoxin units per μg of GroEL as measured by the QCL-1000 Limulus amebocyte lysate assay (Cambrex, Walkersville, MD). In a preliminary study (data not shown), moreover, the marginal stimulation of HUVEC by 10 μg of LVS GroEL/ml (see Fig. 4) was not altered significantly by the addition of 20 μg of polymyxin B sulfate (7,940 U/mg; Sigma-Aldrich)/ml, which inactivates E. coli LPS (12). Nevertheless, GroEL was incubated routinely with this concentration of polymyxin B in appropriate cell culture medium for 1 h at 37°C before use in experiments to ensure that residual E. coli LPS did not contribute to any observed effects.
FIG. 4.
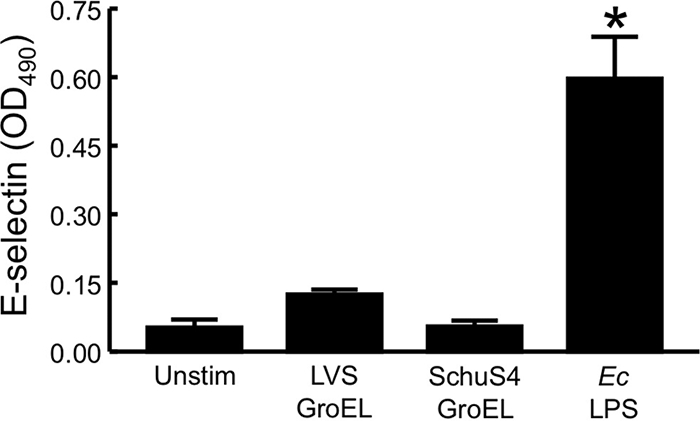
Recombinant GroEL from F. tularensis LVS or Schu S4 does not induce expression of E-selectin by HUVEC. LVS GroEL or Schu S4 GroEL (10 μg/ml) was preincubated for 1 h with 20 μg of polymyxin B/ml. E. coli (Ec) LPS at 0.5 ng/ml was used as a positive control. Prepared samples or culture medium alone (Unstim) was incubated with HUVEC for 4 h at 37°C. The levels of E-selectin were determined by whole-cell ELISA. Bars represent the means ± the SD of four replicate samples. *, Significantly greater than unstimulated samples (P < 0.001). This experiment was repeated two more times, and only once did LVS GroEL slightly but significantly stimulate HUVEC.
The concentration of GroEL was determined by using the BCA protein assay kit from Pierce (Rockford, IL). The purity was assessed by spotting 10 pmol of protein on a matrix-assisted laser desorption/ionization plate, adding 1 μl of sinapinic acid (10 mg/ml in an aqueous solution of 50% methanol and 0.1% trifluoroacetic acid), air drying, and analyzing on a Voyager DE-STR (API) Biospectrometry Workstation (Applied Biosystems). Tryptic digests of the preparations of recombinant proteins also were analyzed by liquid chromatography-tandem mass spectroscopy using an LTQ XL instrument from Thermo Fisher Scientific, Inc. (Waltham, MA).
Western blot analysis.
Samples were separated by SDS-PAGE on 12% gels and transblotted onto HyBond ECL nitrocellulose membranes (GE Healthcare). To detect F. tularensis GroEL, a mouse monoclonal antibody (MAb) raised against the recombinant LVS GroEL was used at a dilution of 1:1,000. The MAb was produced as described by Savitt et al. (54) after intraperitoneal inoculation of a BALB/c mouse with three 50-μg doses of GroEL in Ribi adjuvant at 2-week intervals. For fluorescent detection, an Alexa Fluor 680-conjugated goat anti-mouse IgG secondary antibody from Invitrogen was used at a dilution of 1:10,000. The signal was detected by using an Odyssey infrared imaging system (LI-COR, Lincoln, NE).
Quantitation of E-selectin.
HUVEC were seeded in 96-well tissue culture plates at 2.3 × 104 cells/well, cultured to confluence, and incubated with 100 μl of control medium (M199 with 20% hiFBS and 25 mM HEPES) or appropriate samples at 37°C for 4 h. An ELISA to detect expression of E-selectin on the surfaces of living, intact monolayers of HUVEC was performed according to the method of Sellati et al. (55). For the ELISA, a MAb to human E-selectin (R&D Systems) was used.
Quantitation of cytokines.
HUVEC, huMDM, and muBMDM were isolated as described above and seeded in 48-well plates. To assess the production of chemokines and cytokines by these primary cells, F. tularensis, components of the bacteria, or control medium was added to each well for 24 h in a volume of 0.5 ml. LPS from E. coli serotype 0111:B4 (Sigma-Aldrich) was used as a positive control in many of the experiments. To determine whether LVS GroEL synergizes with LPS from E. coli, a highly purified preparation of E. coli K-12 LPS (InvivoGen, San Diego, CA) was used. This last experiment was necessarily performed in the absence of polymyxin B.
For experiments in which Toll-like receptors (TLR) were blocked, huMDM were preincubated for 1 h at 37°C with 10 μg/ml of a MAb to either TLR2 (TL2.1) or TLR4 (HTA125), or a combination of both MAbs. Similarly, an isotype-matched (IgG2a) control antibody was used at 10 or 20 μg/ml. Blocking and control MAbs were purchased from eBiosciences (San Diego, CA). The cells were then treated, in the continued presence of antibodies, with 2 μg of GroEL/ml and 20 μg of polymyxin B sulfate/ml or with 1 μg of LVS GroEL/ml and 1 μg of LVS LPS/ml for 24 h. Conditioned media were collected and clarified by centrifugation. Amounts of CCL2, as well as human CXCL8 and murine macrophage inflammatory protein 2 (muMIP-2), in the supernatants were quantitated using ELISA kits specific for human (Antigenix America, Inc., Franklin Square, NY) or murine (R&D Systems) cytokines. The decision to focus on CCL2 and CXCL8 was prompted by our previous observation that huMDM produce large amounts of these chemokines in response to living F. tularensis LVS, but only small amounts of IL-1β and no TNF-α (8).
Intracellular growth of F. tularensis.
To examine their ability to replicate within huMDM, the LVS and Schu S4 strain were suspended in 1.0 ml of RPMI medium with 5% hiFBS and incubated with macrophages in 24-well plates for 2 h at 37°C. The huMDM then were washed extensively and incubated with 5 μg of gentamicin/ml for 1 h to kill extracellular bacteria (23). To measure intracellular numbers of viable bacteria, some samples were lysed with 1.0 ml of 1% (wt/vol) saponin (Sigma-Aldrich) in PBS for 10 min. Serial dilutions in MH broth were plated to determine the extent of uptake. Other infected cultures were incubated for an additional 13 h in antibiotic-free medium before lysis to assess the degree to which the bacteria had replicated. To determine whether infection of the huMDM induced their death within this period of time, cell-free conditioned media collected from the cultures were assayed for content of lactate dehydrogenase (Cytotox 96 nonradioactive cytotoxicity assay; Promega).
Immunoprecipitation.
Immunoprecipitation was performed with Protein G Plus beads from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). A bacterial releasate was prepared and added to a 200-μl volume of beads for 1 h at 25°C on a rotating shaker. These beads were previously incubated for 1 h at 25°C with 50 μg/ml of mouse MAb against LVS GroEL and/or LPS (54), which were capable of detecting native F. tularensis LVS components (data not shown). As a control, beads were also incubated with IgG1 and/or IgG3 isotype-matched MAbs directed against an irrelevant antigen (R&D Systems). The samples were centrifuged for 5 min at 1,000 × g, and the supernatants were collected. Samples of bacterial releasate or the bacterial releasate after the removal of GroEL and LPS were subjected to Western blot analysis. For coimmunoprecipitation studies, 30 μg of recombinant LVS or Schu S4 GroEL were mixed with 30 μg of LVS LPS or Schu S4 LPS, respectively, in a volume of 1.5 ml for 1 h at 25°C on a rotating shaker. Subsequently, 400 μl of the mixed components were added to a 200-μl volume of beads that had been previously incubated with 20 μg of anti-LPS MAb or IgG3 isotype-matched MAb. The samples were then processed as described above and analyzed by Western blotting.
Statistics.
Statistical significance of all data was determined by using an unpaired analysis of variance and the Tukey-Kramer multiple-comparisons test (Instat version 3.01, GraphPad Software, San Diego, CA).
RESULTS
F. tularensis LPS is not a potent stimulator of cells of innate immunity.
Endothelial cells responded strongly to concentrations of E. coli LPS of <1 ng/ml (Fig. 1). However, when HUVEC were stimulated with up to 10 μg of LPS/ml from either F. tularensis LVS or the Schu S4 strain, they expressed little of the adhesion molecule E-selectin (Fig. 1) or the chemokine CCL2 (data not shown). In contrast, huMDM produced significant levels of CCL2 in response to 10 μg of LVS or Schu S4 LPS/ml in three independent experiments (Fig. 2). When equal concentrations (i.e., either 1 or 10 μg/ml) of LPS from the LVS or Schu S4 strain were evaluated in the same experiment, potency was the same in five of six paired comparisons. Thus, there was no difference in the proinflammatory activities of LPS from these attenuated and virulent strains of F. tularensis, at least with respect to the measured parameters. Notably, stimulation of huMDM required much greater amounts of F. tularensis LPS than E. coli LPS (Fig. 2). LPS from F. tularensis also was substantially less potent in eliciting secretion of CCL2 compared to the bacterium itself (8). Therefore, it was decided to look for other factors of F. tularensis that generate a proinflammatory response in human cells of innate immunity.
FIG. 1.
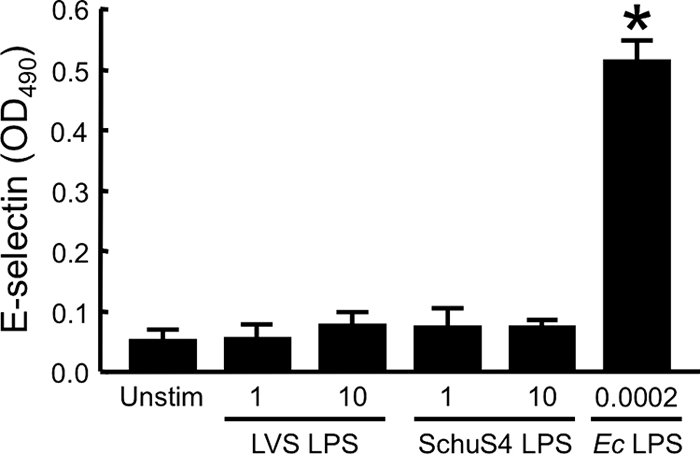
LPS from F. tularensis LVS or Schu S4 does not induce expression of E-selectin by HUVEC. HUVEC were incubated for 4 h at 37°C with medium alone (Unstim), 1 or 10 μg of F. tularensis LVS LPS/ml, 1 or 10 μg of F. tularensis Schu S4 LPS/ml, or 0.0002 μg of E. coli (Ec) LPS/ml. The levels of E-selectin were determined by whole-cell ELISA. Bars represent the means ± the standard deviations (SD) of four replicate samples. *, Significantly greater than the unstimulated samples (P < 0.001). This experiment was repeated two more times, yielding similar results.
FIG. 2.
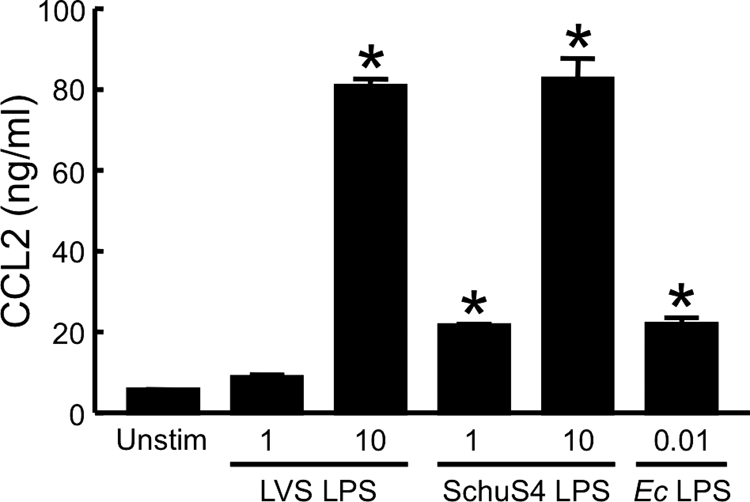
Human macrophages secrete CCL2 in response to F. tularensis LPS. HuMDM were incubated for 24 h at 37°C with medium alone (Unstim), 1 or 10 μg of F. tularensis LVS LPS/ml, 1 or 10 μg of F. tularensis Schu S4 LPS/ml, or 0.01 μg of E. coli (Ec) LPS/ml. Cell-free conditioned media were collected, and CCL2 was measured by ELISA. Bars represent the means ± the SD of three replicate samples. *, Significantly different from unstimulated control (P < 0.001). These experiments were repeated two more times, yielding similar results.
F. tularensis LVS GroEL is a proinflammatory factor for human macrophages.
To identify additional inflammatory components of F. tularensis, material released from bacteria killed with gentamicin was fractionated by using FPLC. Three fractions of ca. 60, 30, and 6 kDa stimulated the production of E-selectin by HUVEC (data not shown), but only the 60-kDa fraction contained a visible band after SDS-PAGE. Analysis of the band using mass spectroscopy identified it as the heat shock protein GroEL. A recombinant form of F. tularensis LVS GroEL was therefore generated. Because GroEL is a chaperone with the potential to bind many other proteins, the purity of the preparation was of concern. However, analysis of the intact recombinant LVS GroEL by mass spectroscopy revealed no discrete peaks other than the two ascribable to the singly and doubly charged ionic forms of the protein (Fig. 3A).
FIG. 3.
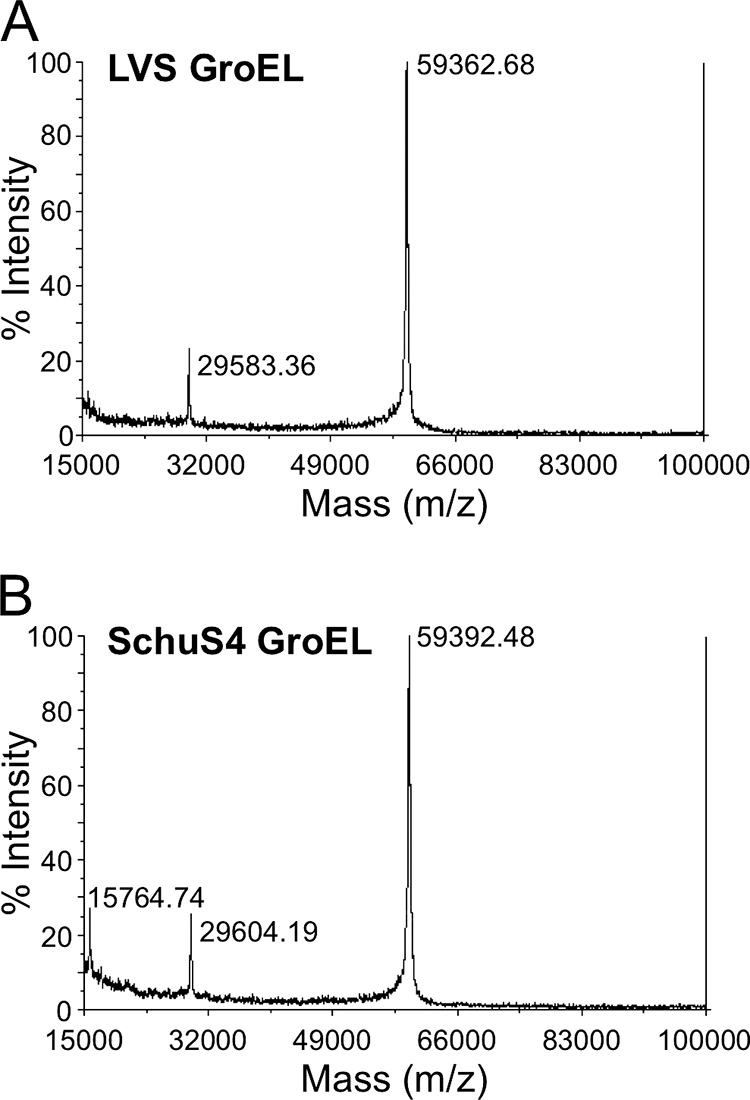
Preparations of recombinant GroEL are of high purity. Preparations of intact recombinant GroEL of LVS (A) or Schu S4 (B) origin were subjected to mass spectroscopy. Peaks at 59.4 and 29.6 kDa represent the singly or doubly charged ionic forms of the GroEL proteins, respectively. A 15.8-kDa species is present in preparations of GroEL derived from the Schu S4 strain but not the LVS.
To evaluate the proinflammatory activity of GroEL toward endothelial cells, expression of E-selectin on HUVEC stimulated with the recombinant protein was measured. Despite the fact that GroEL was present in the 60-kDa active fraction, concentrations of the recombinant molecule as high as 10 μg/ml induced expression of endothelial E-selectin only marginally (Fig. 4). Nonetheless, it was decided to test whether GroEL might be a proinflammatory factor for other cells of innate immunity. Indeed, in contrast to its lack of activity toward HUVEC, GroEL at a concentration of 10 μg/ml stimulated huMDM to secrete significant amounts of both CXCL8 and CCL2 (Fig. 5).
FIG. 5.
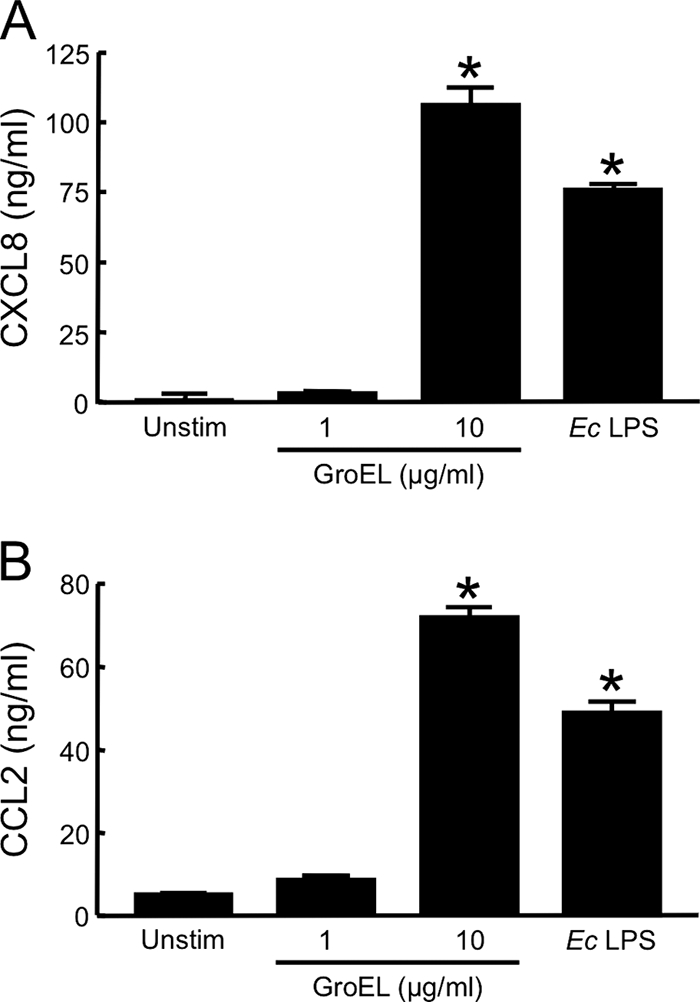
Human macrophages secrete proinflammatory cytokines in response to recombinant F. tularensis LVS GroEL. HuMDM were incubated with 1 or 10 μg of recombinant LVS GroEL/ml for 24 h at 37°C in the presence of 20 μg of polymyxin B/ml. For controls, cells were treated with either 10 ng of E. coli (Ec) LPS/ml or medium only (Unstim). Cell-free, conditioned media were collected and assayed for CXCL8 (A) or CCL2 (B) by ELISA. Bars indicate the means ± the SD of three replicate samples. *, Significantly greater than unstimulated samples (P < 0.001). Each experiment was repeated once with similar results.
F. tularensis LVS LPS and GroEL synergistically stimulate huMDM.
Both F. tularensis LVS LPS and GroEL stimulated huMDM to a limited extent. However, we previously observed that the response of huMDM to killed bacteria was greater than that seen with either of these bacterial components alone (8). It was therefore decided to test for cooperativity between the two stimuli. As seen in previous experiments (Fig. 2 and 5), secretion of only slight amounts of chemokines was triggered when huMDM were incubated with either 1 μg of LVS LPS/ml or 1 μg of recombinant GroEL/ml. When the LVS LPS and GroEL were combined, however, the cells showed a synergistic response (Fig. 6A). This observation was confirmed in four independent experiments. The degree of synergy (defined as the amount of CXCL8 elicited by the combined stimuli divided by the sum of the amounts of CXCL8 induced by each stimulus alone) averaged 2.1 ± 0.7. Although relatively high doses of GroEL were required to stimulate the macrophages, semiquantitative Western blot analysis detected ∼2 μg of GroEL/ml in a sample containing 5 × 108 F. tularensis organisms/ml (data not shown). Furthermore, these results show that lower levels of GroEL and LPS can act together to stimulate production of CXCL8. In contrast, LVS GroEL did not cooperate with highly purified LPS from E. coli to synergistically activate huMDM. In two experiments, LVS GroEL did not augment the secretion of CXCL8 by macrophages exposed to E. coli LPS at all; in a third, combining the two stimuli elicited a less-than-additive effect (data not shown).
FIG. 6.
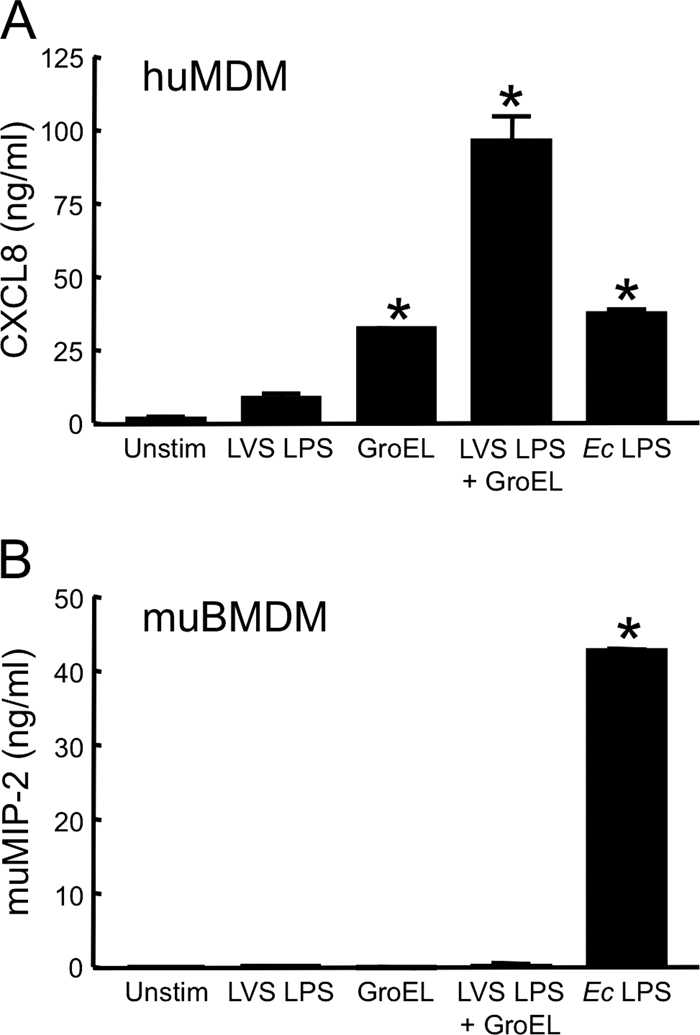
F. tularensis LVS LPS and recombinant GroEL synergistically activate human, but not murine, macrophages. HuMDM (A) or muBMDM (B) were treated with 1 μg of LVS GroEL/ml, 1 μg of LVS LPS/ml, or a combination of the two stimuli (each at 1 μg/ml) for 24 h at 37°C in the presence of 20 μg of polymyxin B/ml. For controls, cells were incubated with either 10 ng of E. coli (Ec) LPS/ml or medium only (Unstim). Cell-free, conditioned media were collected and assayed for CXCL8 (A) or muMIP-2 (B) by ELISA. Bars indicate the means ± the SD of three replicate samples. *, Significantly greater than unstimulated samples (P < 0.001). The experiment with huMDM was repeated three more times with similar results. The experiment with muBMDM was repeated once, yielding similar results.
Our initial screening of material released from killed F. tularensis and separated by FPLC showed that GroEL was present in a fraction that stimulated HUVEC. Therefore, the capacity of HUVEC to respond synergistically to LPS and GroEL was tested. However, endothelial cells did not secrete CXCL8 or express E-selectin when treated concurrently with 1 μg/ml of both agents (data not shown). Therefore, HUVEC do not respond to F. tularensis LPS and GroEL either singly or together.
Because we found that primary murine macrophages secrete very small amounts of proinflammatory cytokines when stimulated with live or killed F. tularensis LVS (8), it was determined whether lack of responsiveness to LVS GroEL and LPS could account for this observation. To this end, muBMDM were incubated with either 1 μg of LVS LPS/ml, 1 μg of GroEL/ml, or a combination of the two and assayed for production of muMIP-2, a CXC chemokine (Fig. 6B). Similar to results seen with the whole bacterium, the cells were unresponsive to either F. tularensis LVS LPS or GroEL alone and showed no activation when the agents were combined. Addition of GroEL alone at a concentration of 100 μg/ml also failed to elicit secretion of CCL2 or muMIP-2 (data not shown), although the muBMDM responded vigorously to 10 ng of E. coli LPS/ml (Fig. 6B).
F. tularensis GroEL stimulates huMDM through a TLR4-dependent mechanism.
Cpn60 proteins from other bacterial species act through TLR2- and/or TLR4-dependent pathways (3, 10, 11, 16, 17, 57, 68). Thus, the ability of huMDM to respond to LVS GroEL was evaluated in the presence of blocking antibodies against human TLR2 or TLR4 (Fig. 7A). Previously, we have shown that this same MAb to TLR2 prevents stimulation of huMDM by tripalmitoyl-Cys-Ser-(Lys)4 and LpnA, a lipoprotein of F. tularensis (24). In three of four experiments, however, pretreatment with 10 μg of anti-TLR2 MAb/ml did not alter production of CXCL8 by macrophages stimulated with GroEL. An increased concentration of anti-TLR2 (20 μg/ml) was similarly without effect (data not shown). In contrast, 10 μg of anti-TLR4 MAb/ml significantly inhibited the response to GroEL in all experiments. The blocking activity of this antibody was specific, as confirmed using an isotype-matched control antibody. In no instance did the combination of both blocking antibodies inhibit the response to GroEL more than did anti-TLR4 alone.
FIG. 7.
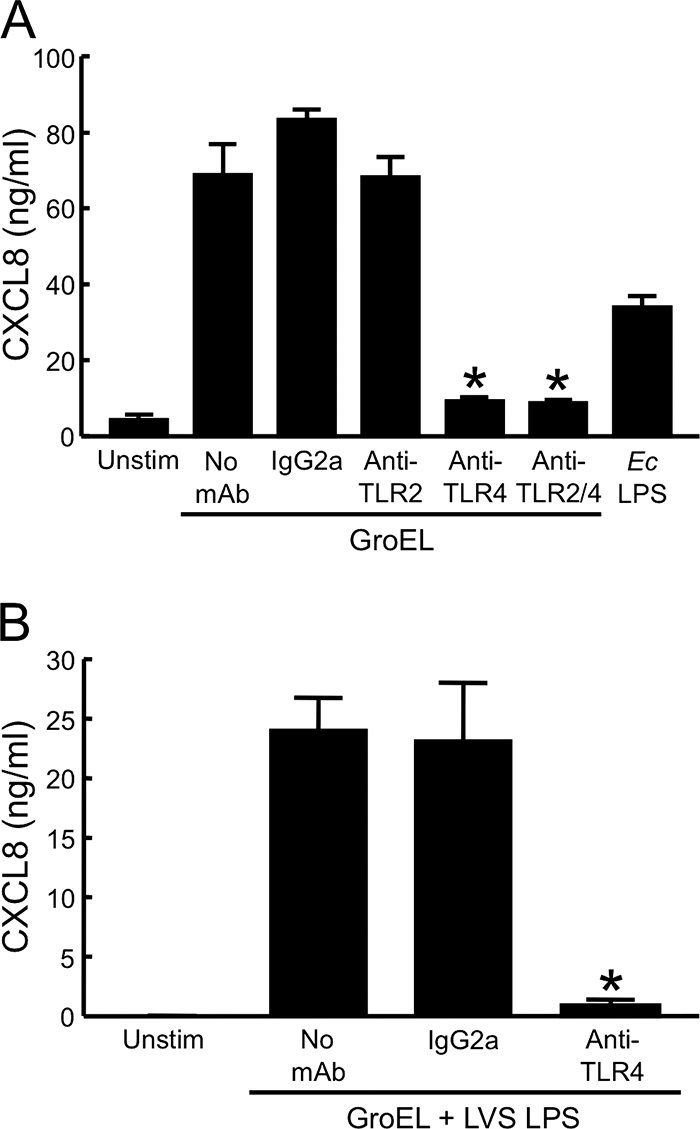
F. tularensis LVS GroEL stimulates human macrophages through a TLR4-dependent mechanism. (A) HuMDM were preincubated for 1 h at 37°C with either 10 μg of a MAb to TLR2/ml, 10 μg of a MAb to TLR4/ml, or a combination of both. Similarly, an isotype-matched control IgG2a MAb was used at 20 μg/ml. The cells were then stimulated with 2 μg of LVS GroEL/ml in the presence of antibody and 20 μg of polymyxin B/ml. For controls, cells were incubated with either 10 ng of E. coli (Ec) LPS/ml or medium only (Unstim). (B) HuMDM were incubated in medium only (Unstim) or with a combination of 1 μg of LVS GroEL/ml and 1 μg of LVS LPS/ml in the absence of antibody or in the presence of 10 μg of a MAb to TLR4/ml or an isotype-matched control IgG2a MAb. Cell-free, conditioned media were collected and assayed for CXCL8 by ELISA. Bars indicate the means ± the SD of three replicate samples. *, Significantly less than GroEL in the absence of a MAb (P < 0.001). The experiment in panel A was repeated three more times with similar results.
Although the LPS of F. tularensis is a weak agonist, it has been reported to exert its limited effects through TLR4 (20). Accordingly, we observed that activation of huMDM by the combination of GroEL and LPS from the LVS was almost completely blocked by an antibody to this receptor (Fig. 7B).
GroEL proteins from an attenuated and a virulent strain of F. tularensis differ in proinflammatory activity.
To compare the activities of recombinant GroEL from virulent and attenuated strains of F. tularensis, GroEL from the Schu S4 strain was cloned, expressed, and purified in a manner identical to that used for LVS GroEL. Like its LVS counterpart, the recombinant Schu S4 GroEL was of high purity. However, examination of an undigested Schu S4 GroEL preparation by mass spectroscopy revealed a small peak at 15.8 kDa that was not observed in the preparation of LVS GroEL (Fig. 3). Tryptic digests of the LVS and Schu S4 GroEL samples were subjected to mass spectroscopy to attempt to identify this protein, but no contaminant unique to the Schu S4 GroEL preparation was observed. Although traces of peptides derived from proteins in this molecular weight range were detected, they were present in both the LVS and Schu S4 GroEL digests.
The recombinant GroEL from the Schu S4 strain was first tested for its ability to stimulate HUVEC. Even at a concentration of 10 μg/ml, Schu S4 GroEL had no activity toward HUVEC (Fig. 4). GroEL from the LVS and Schu S4 strains were then compared with respect to their abilities to stimulate huMDM. At equivalent doses, LVS GroEL elicited a much greater response in human macrophages than did GroEL from F. tularensis Schu S4 (Fig. 8). This result was confirmed in three independent experiments, using leukocytes from different donors.
FIG. 8.
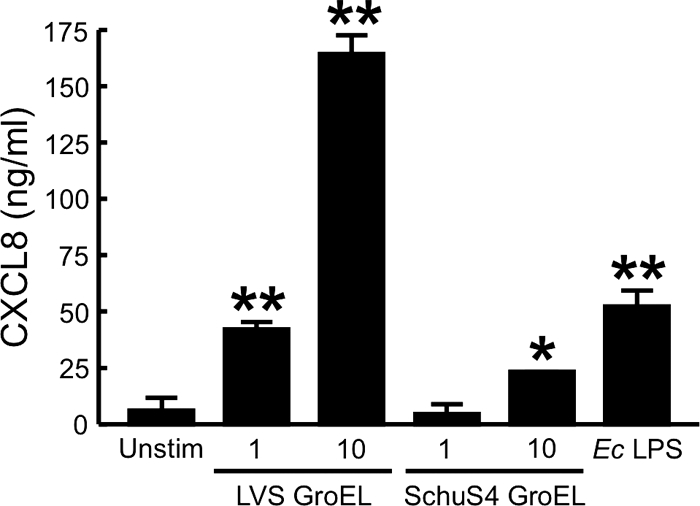
Recombinant GroEL from F. tularensis Schu S4 elicits marginal production of CXCL8 by human macrophages. HuMDM were stimulated with 1 or 10 μg of GroEL/ml from the LVS or the Schu S4 strain for 24 h at 37°C in the presence of 20 μg of polymyxin B/ml. For controls, cells were incubated with either 10 ng of E. coli (Ec) LPS/ml or medium only (Unstim). Cell-free, conditioned media were collected and assayed for CXCL8 by ELISA. Bars indicate the means ± the SD of three replicate samples. *, Significantly greater than unstimulated samples (P < 0.01); **, significantly greater than unstimulated samples (P < 0.001). This experiment was repeated two more times with similar results.
As previously described (Fig. 6A), GroEL and LPS from the LVS synergistically stimulated huMDM. To determine whether this same phenomenon occurs with GroEL and LPS from the Schu S4 strain, huMDM were incubated with either 1 μg of Schu S4 LPS/ml, 1 μg/ml of recombinant GroEL from the Schu S4 strain, or a combination of the two, and secretion of CXCL8 was measured (Fig. 9). In three experiments, a slight synergistic response was seen when the macrophages were exposed to both stimuli. In contrast to LVS GroEL and LPS, where the synergistic index averaged 2.1 ± 0.7, the degree of synergy for Schu S4 components averaged only 1.4 ± 0.1. However, the difference between these synergistic indices is not statistically significant. As seen with LPS and GroEL from the LVS, Schu S4 LPS and GroEL did not synergistically stimulate HUVEC (data not shown).
FIG. 9.
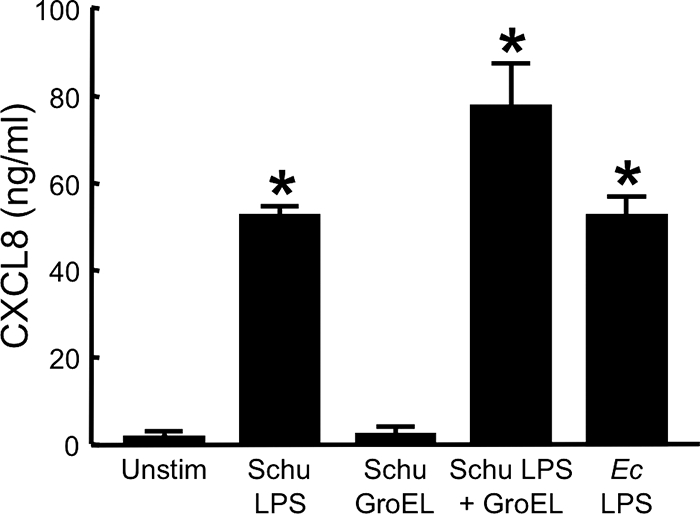
F. tularensis Schu S4 LPS and recombinant GroEL synergistically activate human macrophages. HuMDM were treated with 1 μg of Schu S4 GroEL/ml, 1 μg of Schu S4 LPS/ml, or a combination of the two stimuli (each at 1 μg/ml) for 24 h at 37°C in the presence of 20 μg of polymyxin B/ml. For controls, cells were incubated with either 10 ng of E. coli (Ec) LPS/ml or medium only (Unstim). Cell-free, conditioned media were collected and assayed for CXCL8 by ELISA. Bars indicate the means ± the SD of three replicate samples. *, Significantly greater than unstimulated samples (P < 0.001). The experiment was repeated two more times with similar results.
To examine whether the higher proinflammatory activity of components from the LVS is reflected in the behavior of the intact organisms, the abilities of the LVS and Schu S4 strain to stimulate huMDM were compared side-by-side. When added at the same initial multiplicity of infection (MOI), the two strains induced secretion of similar levels of CXCL8 (Fig. 10A). However, the Schu S4 strain infected the macrophages more efficiently and replicated intracellularly to much higher numbers than did the LVS (Fig. 10B). This higher bacterial burden did not lead to increased death of the huMDM during the 16-h assay; fewer than 2% of macrophages died whether infected with the LVS or the Schu S4 strain (data not shown). These results are consistent with the hypothesis that the highly virulent Schu S4 strain incites less inflammation in its human host than the attenuated F. tularensis LVS.
FIG. 10.
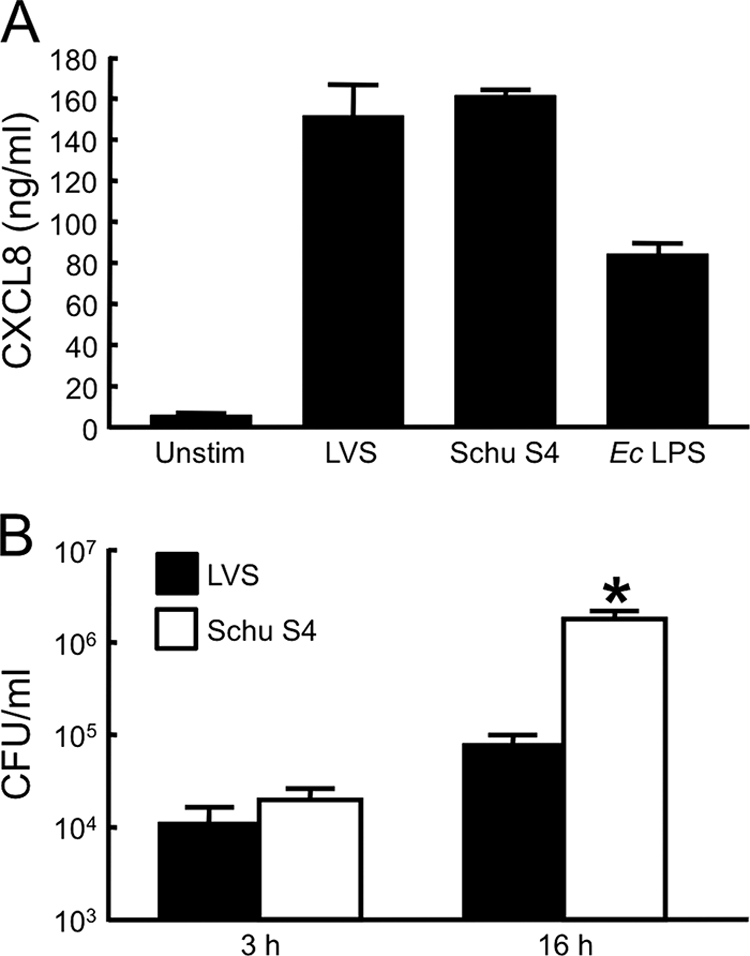
The LVS and Schu S4 strain stimulate huMDM to similar degrees, but the Schu S4 strain replicates intracellularly to much greater numbers. (A) HuMDM were incubated with either the LVS or the Schu S4 strain at an initial MOI of 17 for 24 h. For controls, huMDM were incubated with 25 ng of E. coli (Ec) LPS/ml or medium only (Unstim). Conditioned media were collected, clarified by centrifugation, and assayed for content of CXCL8 by ELISA. Bars indicate the means ± the SD of three replicate samples. (B) The number of CFU of F. tularensis within huMDM was measured 3 or 16 h after infection with the LVS or Schu S4 strain was initiated; extracellular organisms were removed by treatment of the cultures with gentamicin between 2 and 3 h postinfection. The initial MOIs were 56 for the LVS and 16 for the Schu S4 strain, a difference that resulted in similar numbers of live bacteria within the huMDM after the 2-h period of infection. Bars represent the means ± the SD of six samples from two experiments performed with huMDM isolated from different donors. *, Significantly greater than LVS samples (P < 0.001).
DISCUSSION
LPS from most enteric Gram-negative organisms potently induces proinflammatory changes in endothelial cells (19). However, LPS purified from either the LVS or the Schu S4 strain failed to stimulate HUVEC. The lack of activity of F. tularensis LPS toward endothelium undoubtedly stems from its atypical properties. Its lipid A moiety is hypoacylated and contains longer acyl side chains than LPS from enteric bacteria, and it possesses several other unusual features (31). Furthermore, F. tularensis LPS does not bind to host LPS-sensing molecules such as lipopolysaccharide-binding protein (LBP) (6). Nonetheless, when similar amounts of F. tularensis LPS from either strain were used to treat human macrophages, the cells secreted significant amounts of CCL2. LPS from the LVS and the Schu S4 strain produced equivalent responses in the macrophages. This observation correlates with structural studies, which found that LPS molecules from type A and type B subspecies are very similar (31, 34, 49). However, in keeping with previous reports that LPS from F. tularensis is a weak agonist for human and murine leukocytes (1, 20, 34, 38, 53), 100-fold greater concentrations of F. tularensis LPS were required to produce a response comparable to that caused by E. coli LPS. From these results, it seems that F. tularensis LPS is at best a minor contributor to the generation of a proinflammatory response in cells of innate immunity.
To identify alternative proinflammatory components of F. tularensis, material released from killed LVS organisms was subjected to FPLC, and individual fractions were assessed for their ability to stimulate expression of E-selectin by HUVEC. A single band was detected by SDS-PAGE in an active fraction of ∼60 kDa, and mass spectroscopy revealed this entity to be the heat shock protein GroEL. GroEL is one of the most abundant proteins in F. tularensis cultured under normal conditions (65), and its expression is increased by stressful stimuli such as reactive oxygen species and elevated temperature (22). It has been reported that GroEL is secreted by and expressed on the surface of F. tularensis (40, 45). However, given that GroEL is largely cytoplasmic, the predominant source in an infected host is likely to be release from bacteria that fail to find a replicative niche and thus die. Observations that substantial numbers of extracellular F. tularensis organisms exist in the blood (25) and lungs (9) of infected mice further support the possibility that host cells encounter significant amounts of GroEL during the course of tularemia.
Others have shown that exposure of HUVEC to Cpn60 homologues from E. coli, Chlamydia trachomatis, Mycobacterium bovis, or humans at concentrations ranging from 1 to 100 μg/ml stimulates production of IL-6, E-selectin, ICAM-1, and VCAM-1 (10, 28, 39, 63). However, when recombinant GroEL from F. tularensis LVS was used to treat HUVEC, it did not significantly induce expression of E-selectin. This was an unexpected result, due to the fact that GroEL was previously identified in a fraction of releasate that stimulated HUVEC. It is possible that GroEL, as a chaperone, associates with active factors but does not stimulate HUVEC itself. Alternatively, GroEL may act synergistically with other factors to produce a response in HUVEC. Thus, recombinant GroEL would show no activity without its cofactor. Although some other bacterial Cpn60 proteins induce expression of adhesion molecules by endothelium (10, 28, 39, 63), it is evident that differential recognition of Cpn60 species exists (32, 35, 56).
In contrast to its failure to activate HUVEC, F. tularensis GroEL stimulated human macrophages to secrete CXCL8 and CCL2. Of concern was the possibility that residual traces of LPS from the E. coli expression strain were accounting for this activity. However, GroEL samples were preincubated with polymyxin B to inhibit any E. coli LPS before addition to either the HUVEC or macrophages. Moreover, HUVEC were not stimulated by the recombinant GroEL but were highly responsive to concentrations of E. coli LPS as low as 0.2 ng/ml. Therefore, it was clear that the macrophages were responding specifically to GroEL. Indeed, Cpn60 proteins from a variety of bacterial species stimulate both human and murine macrophages to produce proinflammatory cytokines (39, 42, 66).
Using neutralizing antibodies, we found that human macrophages use TLR4 to respond to LVS GroEL. No decrease was observed in the stimulatory activity of GroEL when TLR2 was blocked, in agreement with the finding of Thakran et al. (60) that F. tularensis GroEL displays no TLR2 agonism. Recently, Ashtekar et al. (5) reported that TLR4 also mediates activation and maturation of murine dendritic cells elicited by another heat shock protein of F. tularensis, namely, DnaK. Interestingly, HUVEC, which express TLR4 (58), were unresponsive to high doses of GroEL. A possible explanation is that F. tularensis GroEL is dependent on a coreceptor(s) present on macrophages in addition to TLR4. In fact, both human and chlamydial Cpn60 must be endocytosed before initiation of cell signaling in macrophages can occur (61). HUVEC are not professional antigen-presenting cells and may not possess the appropriate receptor to internalize GroEL.
A number of investigators have sought to identify TLR pathways triggered by F. tularensis. Li et al. (41) used intact LVS organisms to stimulate HeLa cells transfected with various human TLR and found that the bacterium activates NF-κB only in cells expressing TLR2. In the murine host, TLR2 plays a major role in regulating the immune response to the LVS (37, 41, 43). These observations are seemingly at odds with our finding that GroEL activates human macrophages via TLR4. However, studies using transfected cell lines may be inadequate to identify complex interactions between F. tularensis and host cell receptors. Furthermore, results of experiments in mice cannot necessarily be extrapolated to explain what occurs in the human host. Indeed, the sequence homology of TLR2 in human and murine cells is quite low, and levels of induction of TLR expression can differ greatly between the murine and human host (51).
The finding that F. tularensis GroEL and LPS synergistically stimulate human macrophages is not unprecedented. Osterloh et al. (46) noted that human Cpn60 and E. coli LPS synergize to increase production of IL-12p40 by antigen-presenting cells. This observation led the authors to hypothesize that Cpn60 may act similarly to LBP to facilitate LPS-mediated signaling through TLR4. Indeed, human Cpn60 binds to E. coli LPS, and a region of 12 amino acids within the chaperonin molecule is responsible for most of the binding activity (33). To our knowledge, our results provide the first evidence of synergistic activation of macrophages by a bacterial Cpn60 and LPS. F. tularensis LPS alone does not signal efficiently through TLR4 (20). This deficiency is likely due to its tetra-acylated lipid A structure, which adopts a cylindrical shape commonly associated with nontoxic LPS species (7). Thus, GroEL may help F. tularensis LPS to adopt a more-stimulatory conformation that enables it to interact better with TLR4. Clearly, the exact mechanism through which this synergistic response occurs is important to elucidate in future work. We did not observe synergistic activation of huMDM by LVS GroEL and LPS derived from E. coli, suggesting that the bacterial GroEL, unlike its human counterpart (46), does not increase the already-efficient binding of LPS from enteric organisms to TLR4.
It was also of interest to assess the response of human cells of innate immunity to GroEL from the highly virulent Schu S4 strain. When huMDM were stimulated with equivalent concentrations of GroEL from either the LVS or the Schu S4 strain, much lower levels of CXCL8 were produced in response to the Schu S4 protein. Like GroEL from the LVS, Schu S4 GroEL did not stimulate expression of E-selectin by HUVEC, indicating that it too was not significantly contaminated with E. coli LPS. Schu S4 GroEL combined with Schu S4 LPS synergistically activated human macrophages. However, synergy resulting from Schu S4 factors was modest compared to the synergy index observed with LVS LPS and GroEL. According to published genome sequences, the LVS and Schu S4 GroEL molecules differ by only three amino acids. Importantly, the recombinant GroEL molecules used herein were cloned, expressed, and purified using identical protocols, and sequencing verified that the only differences between the Schu S4 and LVS entities were the expected ones. We fully anticipated that the GroEL molecules from the two strains would behave similarly. However, the literature suggests that small changes in sequence can have a great impact on activity. Enterobacter aerogenes Cpn60 acts as an insect toxin. This protein has high homology to E. coli GroEL, but the E. coli chaperonin does not possess the same toxic functions. Notably, though, substitution of a single amino acid at certain sites in E. coli GroEL is sufficient to turn the protein into an active insect neurotoxin (67).
To elucidate the activity of native GroEL from the LVS, we attempted to immunoprecipitate GroEL from bacterial releasates. However, Western blot analysis showed a substantial decrease in GroEL even when LVS releasates were subjected to immunoprecipitation using control antibodies (data not shown). When recombinant LVS GroEL and LPS were combined and immunoprecipitation was performed to look for interactions between the two molecules, we again saw considerable nonspecific binding of GroEL to beads coated with control MAb (data not shown). Because of this phenomenon, we were unable to assess the contribution of native LVS GroEL to the proinflammatory activity of releasates from killed LVS. In contrast, when Schu S4 releasates were incubated with beads coated with control MAbs, little GroEL was removed (data not shown). Thus, the three amino acid differences between LVS and Schu S4 GroEL appear to influence the physical properties of the protein.
As mentioned above, an intriguing finding was that LVS GroEL had greater proinflammatory activity than Schu S4 GroEL and produced a substantially stronger synergism when combined with LPS. We cannot rule out the possibility that the 15.8-kDa contaminant seen in preparations of Schu S4 GroEL accounts for this difference. Attempts to remove the contaminant by centrifugal filtration were unsuccessful, implying that it is bound to the Schu S4 GroEL or self-aggregates. The contaminant was consistently found in preparations of Schu S4 GroEL but not in LVS GroEL prepared identically, suggesting that its presence is linked to the different sequences of the two GroEL proteins. A search of genomic sequences available in GenBank revealed that the Schu S4 GroEL protein is identical to that of two other highly virulent type A strains, FSC033 (GenBank accession number AAYE00000000) and FSC198 (AM286280); however, it has been speculated that the FSC198 strain may be a recent derivative of the Schu S4 strain (15). GroEL proteins from the LVS and four type B strains [OSU18 (CP000437), FTNF002-00 (CP000803), FSC257 (AAUD00000000), and FSC200 (AASP00000000)] share the same sequence, which differs from that of Schu S4 GroEL at positions 46, 107, and 438. GroEL from the type B strain FSC022 (AAYD00000000) varies from GroEL of the other five subsp. holarctica strains only at position 107, where, like Schu S4 GroEL, it contains alanine. To determine whether differences in GroEL translate into differences in the abilities of type A and type B organisms to induce inflammation, we exposed huMDM to living F. tularensis LVS or Schu S4. Despite the fact that the Schu S4 strain grew intracellularly to ∼10-fold higher numbers than the LVS, the amounts of CXCL8 induced by the two strains were similar. On a per-bacterium basis, then, the Schu S4 strain appears to be substantially less stimulatory. Interpretation of these results is complicated by the observation that F. tularensis can actively suppress proinflammatory changes in human dendritic cells (14) and peripheral blood mononuclear cells (59). Nevertheless, our findings raise the possibility that the higher proinflammatory activity of GroEL in type B F. tularensis, combined with the ability of this chaperonin to synergize with LPS of the organism, might be linked to the relatively low virulence of this subspecies in humans.
Acknowledgments
This study was supported by Public Health Service grants AI055621 (J.L.B. and M.B.F.) and AI056320 (T.J.S.) from the National Institute of Allergy and Infectious Diseases. We are grateful for assistance with purification of the recombinant proteins from the Northeast Biodefense Center Protein Expression Core at the Wadsworth Center (Northeast Biodefense Center U54-AI057158-Lipkin).
Analysis of recombinant proteins was carried out with expert help from Antonius Koller and Robert Rieger of the Stony Brook University Proteomics Center and support from a shared equipment grant (NIH/NCRR 1 S10 RR023680-1). We thank Anne Savitt and Gloria Monsalve for providing the monoclonal antibodies used in this study and Indralatha Jayatilaka for expert technical assistance.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 1 February 2010.
REFERENCES
- 1.Ancuta, P., T. Pedron, R. Girard, G. Sandström, and R. Chaby. 1996. Inability of the Francisella tularensis lipopolysaccharide to mimic or to antagonize the induction of cell activation by endotoxins. Infect. Immun. 64:2041-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony, L. S. D., R. D. Burke, and F. E. Nano. 1991. Growth of Francisella spp. in rodent macrophages. Infect. Immun. 59:3291-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argueta, J. G., S. Shiota, N. Yamaguchi, Y. Masuhiro, and S. Hanazawa. 2006. Induction of Porphyromonas gingivalis GroEL signaling via binding to Toll-like receptors 2 and 4. Oral Microbiol. Immunol. 21:245-251. [DOI] [PubMed] [Google Scholar]
- 4.Asea, A., S. K. Kraeft, E. A. Kurt-Jones, M. A. Stevenson, L. B. Chen, R. W. Finberg, G. C. Koo, and S. K. Calderwood. 2000. HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 6:435-442. [DOI] [PubMed] [Google Scholar]
- 5.Ashtekar, A. R., P. Zhang, J. Katz, C. C. Deivanayagam, P. Rallabhandi, S. N. Vogel, and S. M. Michalek. 2008. TLR4-mediated activation of dendritic cells by the heat shock protein DnaK from Francisella tularensis. J. Leukoc. Biol. 84:1434-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker, J. H., J. Weiss, M. A. Apicella, and W. M. Nauseef. 2006. Basis for the failure of Francisella tularensis lipopolysaccharide to prime human polymorphonuclear leukocytes. Infect. Immun. 74:3277-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beutler, B., and E. T. Rietschel. 2003. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 3:169-176. [DOI] [PubMed] [Google Scholar]
- 8.Bolger, C. E., C. A. Forestal, J. K. Italo, J. L. Benach, and M. B. Furie. 2005. The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines. J. Leukoc. Biol. 77:893-897. [DOI] [PubMed] [Google Scholar]
- 9.Bosio, C. M., H. Bielefeldt-Ohmann, and J. T. Belisle. 2007. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J. Immunol. 178:4538-4547. [DOI] [PubMed] [Google Scholar]
- 10.Bulut, Y., E. Faure, L. Thomas, H. Karahashi, K. S. Michelsen, O. Equils, S. G. Morrison, R. P. Morrison, and M. Arditi. 2002. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J. Immunol. 168:1435-1440. [DOI] [PubMed] [Google Scholar]
- 11.Bulut, Y., K. Shimada, M. H. Wong, S. Chen, P. Gray, R. Alsabeh, T. M. Doherty, T. R. Crother, and M. Arditi. 2009. Chlamydial heat shock protein 60 induces acute pulmonary inflammation in mice via the Toll-like receptor 4- and MyD88-dependent pathway. Infect. Immun. 77:2683-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavaillon, J.-M., and N. Haeffner-Cavaillon. 1986. Polymyxin-B inhibition of LPS-induced interleukin-1 secretion by human monocytes is dependent upon the LPS origin. Mol. Immunol. 23:965-969. [DOI] [PubMed] [Google Scholar]
- 13.Celada, A., P. W. Gray, E. Rinderknecht, and R. D. Schreiber. 1984. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J. Exp. Med. 160:55-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chase, J. C., J. Celli, and C. M. Bosio. 2009. Direct and indirect impairment of human dendritic cell function by virulent Francisella tularensis Schu S4. Infect. Immun. 77:180-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhuri, R. R., C. P. Ren, L. Desmond, G. A. Vincent, N. J. Silman, J. K. Brehm, M. J. Elmore, M. J. Hudson, M. Forsman, K. E. Isherwood, D. Gurycova, N. P. Minton, R. W. Titball, M. J. Pallen, and R. Vipond. 2007. Genome sequencing shows that European isolates of Francisella tularensis subspecies tularensis are almost identical to US laboratory strain Schu S4. PLoS One 2:e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa, C. P., C. J. Kirschning, D. Busch, S. Dürr, L. Jennen, U. Heinzmann, S. Prebeck, H. Wagner, and T. Miethke. 2002. Role of chlamydial heat shock protein 60 in the stimulation of innate immune cells by Chlamydia pneumoniae. Eur. J. Immunol. 32:2460-2470. [DOI] [PubMed] [Google Scholar]
- 17.Da Costa, C. U., N. Wantia, C. J. Kirschning, D. H. Busch, N. Rodriguez, H. Wagner, and T. Miethke. 2004. Heat shock protein 60 from Chlamydia pneumoniae elicits an unusual set of inflammatory responses via Toll-like receptor 2 and 4 in vivo. Eur. J. Immunol. 34:2874-2884. [DOI] [PubMed] [Google Scholar]
- 18.Dame, T. M., B. L. Orenzoff, L. E. Palmer, and M. B. Furie. 2007. IFN-gamma alters the response of Borrelia burgdorferi-activated endothelium to favor chronic inflammation. J. Immunol. 178:1172-1179. [DOI] [PubMed] [Google Scholar]
- 19.Dauphinee, S. M., and A. Karsan. 2006. Lipopolysaccharide signaling in endothelial cells. Lab. Invest. 86:9-22. [DOI] [PubMed] [Google Scholar]
- 20.Dueñas, A. I., M. Aceves, A. Orduña, R. Díaz, C. M. Sanchez, and C. García-Rodríguez. 2006. Francisella tularensis LPS induces the production of cytokines in human monocytes and signals via Toll-like receptor 4 with much lower potency than Escherichia coli LPS. Int. Immunol. 18:785-795. [DOI] [PubMed] [Google Scholar]
- 21.Ellis, J., P. C. F. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ericsson, M., A. Tärnvik, K. Kuoppa, G. Sandström, and A. Sjöstedt. 1994. Increased synthesis of DnaK, GroEL, and GroES homologs by Francisella tularensis LVS in response to heat and hydrogen peroxide. Infect. Immun. 62:178-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forestal, C. A., J. L. Benach, C. Carbonara, J. K. Italo, T. J. Lisinski, and M. B. Furie. 2003. Francisella tularensis selectively induces proinflammatory changes in endothelial cells. J. Immunol. 171:2563-2570. [DOI] [PubMed] [Google Scholar]
- 24.Forestal, C. A., H. Gil, M. Monfett, C. E. Noah, G. J. Platz, D. G. Thanassi, J. L. Benach, and M. B. Furie. 2008. A conserved and immunodominant lipoprotein of Francisella tularensis is proinflammatory but not essential for virulence. Microb. Pathog. 44:512-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forestal, C. A., M. Malik, S. V. Catlett, A. G. Savitt, J. L. Benach, T. J. Sellati, and M. B. Furie. 2007. Francisella tularensis has a significant extracellular phase in infected mice. J. Infect. Dis. 196:134-137. [DOI] [PubMed] [Google Scholar]
- 26.Fortier, A. H., D. A. Leiby, R. B. Narayanan, E. Asafoadjei, R. M. Crawford, C. A. Nacy, and M. S. Meltzer. 1995. Growth of Francisella tularensis LVS in macrophages: the acidic intracellular compartment provides essential iron required for growth. Infect. Immun. 63:1478-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortier, A. H., M. V. Slayter, R. Ziemba, M. S. Meltzer, and C. A. Nacy. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect. Immun. 59:2922-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galdiero, M., G. C. de l'Ero, and A. Marcatili. 1997. Cytokine and adhesion molecule expression in human monocytes and endothelial cells stimulated with bacterial heat shock proteins. Infect. Immun. 65:699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grall, N., J. Livny, M. Waldor, M. Barel, A. Charbit, and K. L. Meibom. 2009. Pivotal role of the Francisella tularensis heat-shock sigma factor RpoH. Microbiology 155:2560-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grandel, U., and F. Grimminger. 2003. Endothelial responses to bacterial toxins in sepsis. Crit. Rev. Immunol. 23:267-299. [DOI] [PubMed] [Google Scholar]
- 31.Gunn, J. S., and R. K. Ernst. 2007. The structure and function of Francisella lipopolysaccharide. Ann. N. Y. Acad. Sci. 1105:202-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Habich, C., K. Kempe, R. van der Zee, V. Burkart, and H. Kolb. 2003. Different heat shock protein 60 species share pro-inflammatory activity but not binding sites on macrophages. FEBS Lett. 533:105-109. [DOI] [PubMed] [Google Scholar]
- 33.Habich, C., K. Kempe, R. van der Zee, R. Rumenapf, H. Akiyama, H. Kolb, and V. Burkart. 2005. Heat shock protein 60: specific binding of lipopolysaccharide. J. Immunol. 174:1298-1305. [DOI] [PubMed] [Google Scholar]
- 34.Hajjar, A. M., M. D. Harvey, S. A. Shaffer, D. R. Goodlett, A. Sjöstedt, H. Edebro, M. Forsman, M. Byström, M. Pelletier, C. B. Wilson, S. I. Miller, S. J. Skerrett, and R. K. Ernst. 2006. Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect. Immun. 74:6730-6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson, B., E. Allan, and A. R. Coates. 2006. Stress wars: the direct role of host and bacterial molecular chaperones in bacterial infection. Infect. Immun. 74:3693-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang, A. J., M. B. Furie, S. C. Nicholson, J. Fischbarg, L. S. Liebovitch, and S. C. Silverstein. 1988. Effects of human neutrophil chemotaxis across endothelial cell monolayers on the permeability of these monolayers to ions and macromolecules. J. Cell Physiol. 135:355-366. [DOI] [PubMed] [Google Scholar]
- 37.Katz, J., P. Zhang, M. Martin, S. N. Vogel, and S. M. Michalek. 2006. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect. Immun. 74:2809-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kieffer, T. L., S. Cowley, F. E. Nano, and K. L. Elkins. 2003. Francisella novicida LPS has greater immunobiological activity in mice than F. tularensis LPS, and contributes to F. novicida murine pathogenesis. Microbes Infect. 5:397-403. [DOI] [PubMed] [Google Scholar]
- 39.Kol, A., T. Bourcier, A. H. Lichtman, and P. Libby. 1999. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J. Clin. Invest. 103:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee, B. Y., M. A. Horwitz, and D. L. Clemens. 2006. Identification, recombinant expression, immunolocalization in macrophages, and T-cell responsiveness of the major extracellular proteins of Francisella tularensis. Infect. Immun. 74:4002-4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, H., S. Nookala, X. R. Bina, J. E. Bina, and F. Re. 2006. Innate immune response to Francisella tularensis is mediated by TLR2 and caspase-1 activation. J. Leukoc. Biol. 80:766-773. [DOI] [PubMed] [Google Scholar]
- 42.Maguire, M., A. R. Coates, and B. Henderson. 2002. Chaperonin 60 unfolds its secrets of cellular communication. Cell Stress Chaperones 7:317-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malik, M., C. S. Bakshi, B. Sahay, A. Shah, S. A. Lotz, and T. J. Sellati. 2006. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect. Immun. 74:3657-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLendon, M. K., M. A. Apicella, and L. A. Allen. 2006. Francisella tularensis: taxonomy, genetics, and immunopathogenesis of a potential agent of biowarfare. Annu. Rev. Microbiol. 60:167-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melillo, A., D. D. Sledjeski, S. Lipski, R. M. Wooten, V. Basrur, and E. R. Lafontaine. 2006. Identification of a Francisella tularensis LVS outer membrane protein that confers adherence to A549 human lung cells. FEMS Microbiol. Lett. 263:102-108. [DOI] [PubMed] [Google Scholar]
- 46.Osterloh, A., U. Kalinke, S. Weiss, B. Fleischer, and M. Breloer. 2007. Synergistic and differential modulation of immune responses by Hsp60 and lipopolysaccharide. J. Biol. Chem. 282:4669-4680. [DOI] [PubMed] [Google Scholar]
- 47.Pang, Z. J., Y. Chen, and F. Q. Xing. 2001. Effect of L929 cell-conditioned medium on antioxidant capacity in RAW264.7 cells. Br. J. Biomed. Sci. 58:212-216. [PubMed] [Google Scholar]
- 48.Phillips, N. J., B. Schilling, M. K. McLendon, M. A. Apicella, and B. W. Gibson. 2004. Novel modification of lipid A of Francisella tularensis. Infect. Immun. 72:5340-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prior, J. L., R. G. Prior, P. G. Hitchen, H. Diaper, K. F. Griffin, H. R. Morris, A. Dell, and R. W. Titball. 2003. Characterization of the O antigen gene cluster and structural analysis of the O antigen of Francisella tularensis subsp. tularensis. J. Med. Microbiol. 52:845-851. [DOI] [PubMed] [Google Scholar]
- 50.Ranford, J. C., and B. Henderson. 2002. Chaperonins in disease: mechanisms, models, and treatments. Mol. Pathol. 55:209-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rehli, M. 2002. Of mice and men: species variations of Toll-like receptor expression. Trends Immunol. 23:375-378. [DOI] [PubMed] [Google Scholar]
- 52.Rotz, L. D., A. S. Khan, S. R. Lillibridge, S. M. Ostroff, and J. M. Hughes. 2002. Public health assessment of potential biological terrorism agents. Emerg. Infect. Dis. 8:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandström, G., A. Sjöstedt, T. Johansson, K. Kuoppa, and J. C. Williams. 1992. Immunogenicity and toxicity of lipopolysaccharide from Francisella tularensis LVS. FEMS Microbiol. Immunol. 105:201-210. [DOI] [PubMed] [Google Scholar]
- 54.Savitt, A. G., P. Mena-Taboada, G. Monsalve, and J. L. Benach. 2009. Francisella tularensis infection-derived monoclonal antibodies provide detection, protection, and therapy. Clin. Vaccine Immunol. 16:414-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sellati, T. J., M. J. Burns, M. A. Ficazzola, and M. B. Furie. 1995. Borrelia burgdorferi upregulates expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Infect. Immun. 63:4439-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart, G. R., and D. B. Young. 2004. Heat-shock proteins and the host-pathogen interaction during bacterial infection. Curr. Opin. Immunol. 16:506-510. [DOI] [PubMed] [Google Scholar]
- 57.Takenaka, R., K. Yokota, K. Ayada, M. Mizuno, Y. Zhao, Y. Fujinami, S. N. Lin, T. Toyokawa, H. Okada, Y. Shiratori, and K. Oguma. 2004. Helicobacter pylori heat-shock protein 60 induces inflammatory responses through the Toll-like receptor-triggered pathway in cultured human gastric epithelial cells. Microbiology 150:3913-3922. [DOI] [PubMed] [Google Scholar]
- 58.Talreja, J., M. H. Kabir, B. Filla, D. J. Stechschulte, and K. N. Dileepan. 2004. Histamine induces Toll-like receptor 2 and 4 expression in endothelial cells and enhances sensitivity to Gram-positive and Gram-negative bacterial cell wall components. Immunology 113:224-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Telepnev, M., I. Golovliov, and A. Sjöstedt. 2005. Francisella tularensis LVS initially activates but subsequently downregulates intracellular signaling and cytokine secretion in mouse monocytic and human peripheral blood mononuclear cells. Microb. Pathog. 38:239-247. [DOI] [PubMed] [Google Scholar]
- 60.Thakran, S., H. Li, C. L. Lavine, M. A. Miller, J. E. Bina, X. R. Bina, and F. Re. 2008. Identification of Francisella tularensis lipoproteins that stimulate the Toll-like receptor (TLR) 2/TLR1 heterodimer. J. Biol. Chem. 283:3751-3760. [DOI] [PubMed] [Google Scholar]
- 61.Vabulas, R. M., P. Ahmad-Nejad, C. da Costa, T. Miethke, C. J. Kirschning, H. Hacker, and H. Wagner. 2001. Endocytosed HSP60s use Toll-like receptor 2 (TLR2) and TLR4 to activate the Toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem. 276:31332-31339. [DOI] [PubMed] [Google Scholar]
- 62.Van Amersfoort, E. S., T. J. Van Berkel, and J. Kuiper. 2003. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 16:379-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verdegaal, E. M. E., S. T. Zegveld, and R. van Furth. 1996. Heat shock protein 65 induces CD62e, CD106, and CD54 on cultured human endothelial cells and increases their adhesiveness for monocytes and granulocytes. J. Immunol. 157:369-376. [PubMed] [Google Scholar]
- 64.Vinogradov, E., M. B. Perry, and J. W. Conlan. 2002. Structural analysis of Francisella tularensis lipopolysaccharide. Eur. J. Biochem. 269:6112-6118. [DOI] [PubMed] [Google Scholar]
- 65.Waldo, R. H., E. D. Cummings, S. T. Sarva, J. M. Brown, C. M. Lauriano, L. A. Rose, R. J. Belland, K. E. Klose, and G. M. Hilliard. 2007. Proteome cataloging and relative quantification of Francisella tularensis tularensis strain Schu4 in 2D PAGE using preparative isoelectric focusing. J. Proteome Res. 6:3484-3490. [DOI] [PubMed] [Google Scholar]
- 66.Wallin, R. P., A. Lundqvist, S. H. Moré, A. von Bonin, R. Kiessling, and H. G. Ljunggren. 2002. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 23:130-135. [DOI] [PubMed] [Google Scholar]
- 67.Yoshida, N., K. Oeda, E. Watanabe, T. Mikami, Y. Fukita, K. Nishimura, K. Komai, and K. Matsuda. 2001. Chaperonin turned insect toxin. Nature 411:44. [DOI] [PubMed] [Google Scholar]
- 68.Zhao, Y., K. Yokota, K. Ayada, Y. Yamamoto, T. Okada, L. Shen, and K. Oguma. 2007. Helicobacter pylori heat-shock protein 60 induces interleukin-8 via a Toll-like receptor (TLR)2 and mitogen-activated protein (MAP) kinase pathway in human monocytes. J. Med. Microbiol. 56:154-164. [DOI] [PubMed] [Google Scholar]


