Abstract
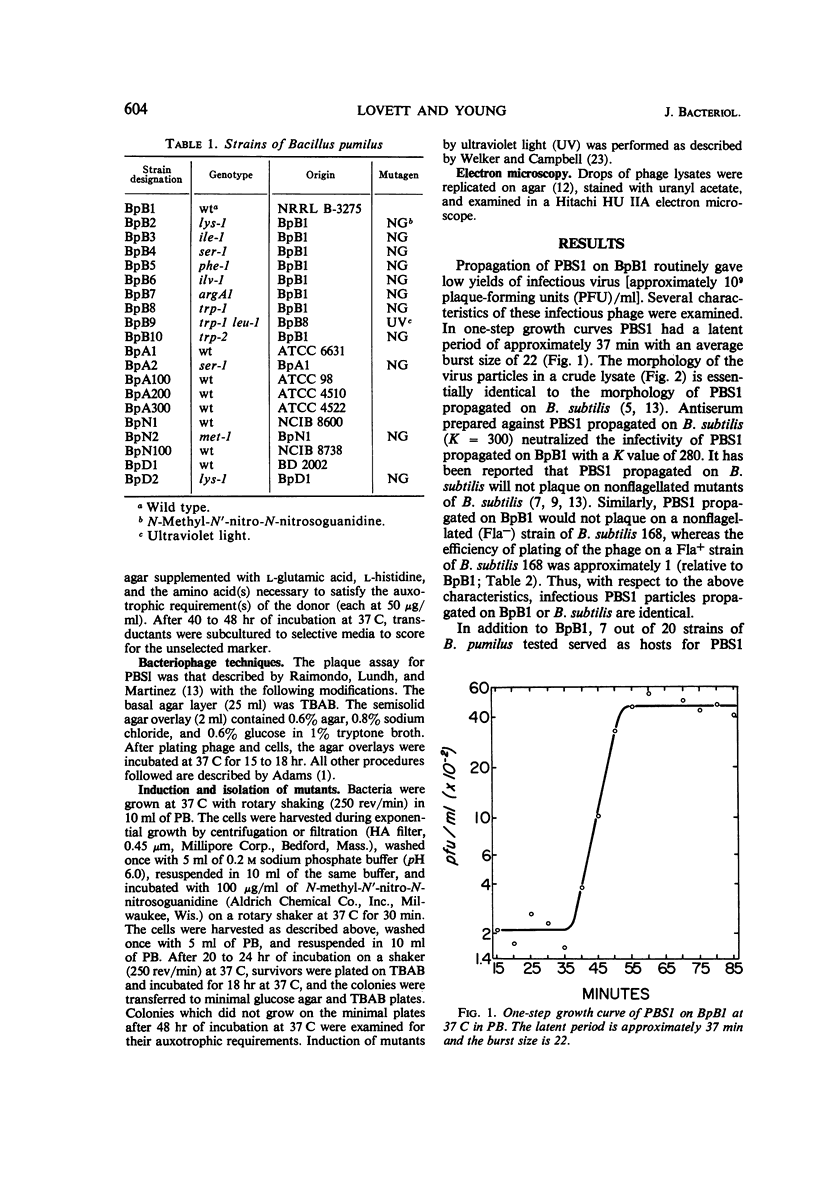
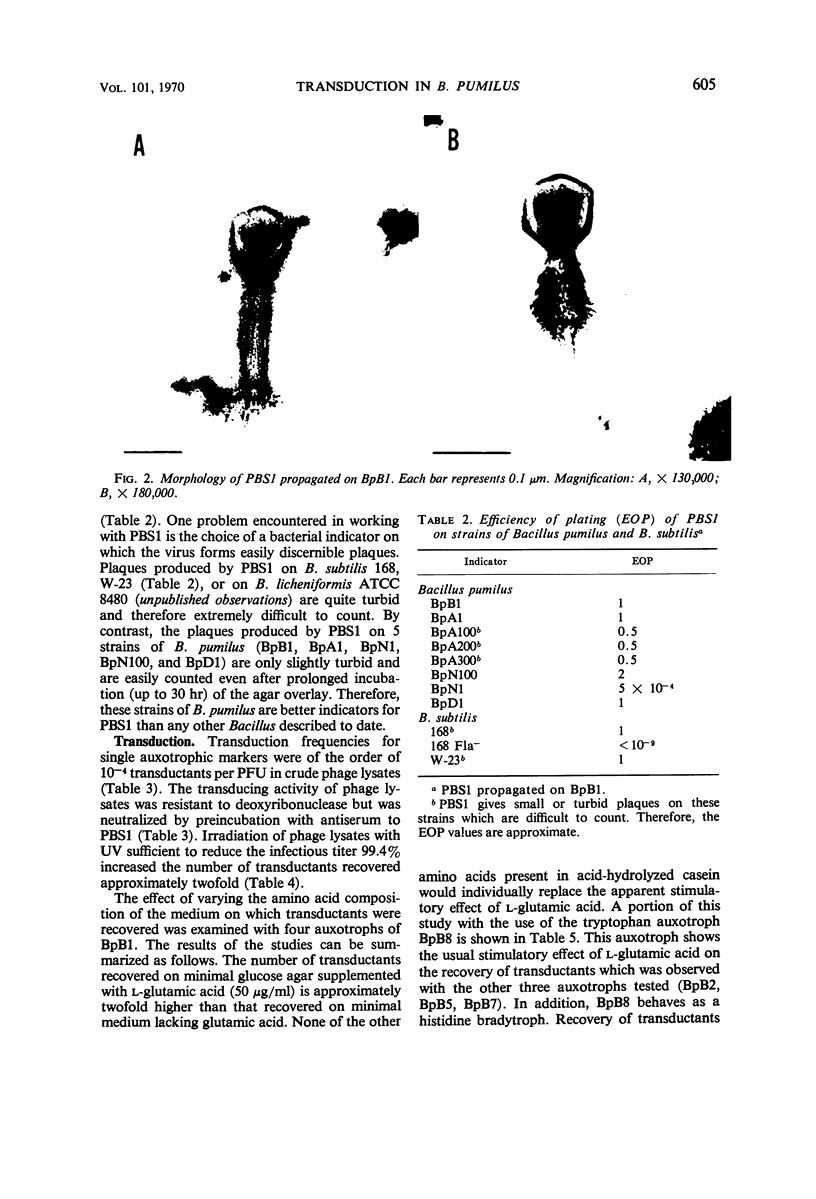
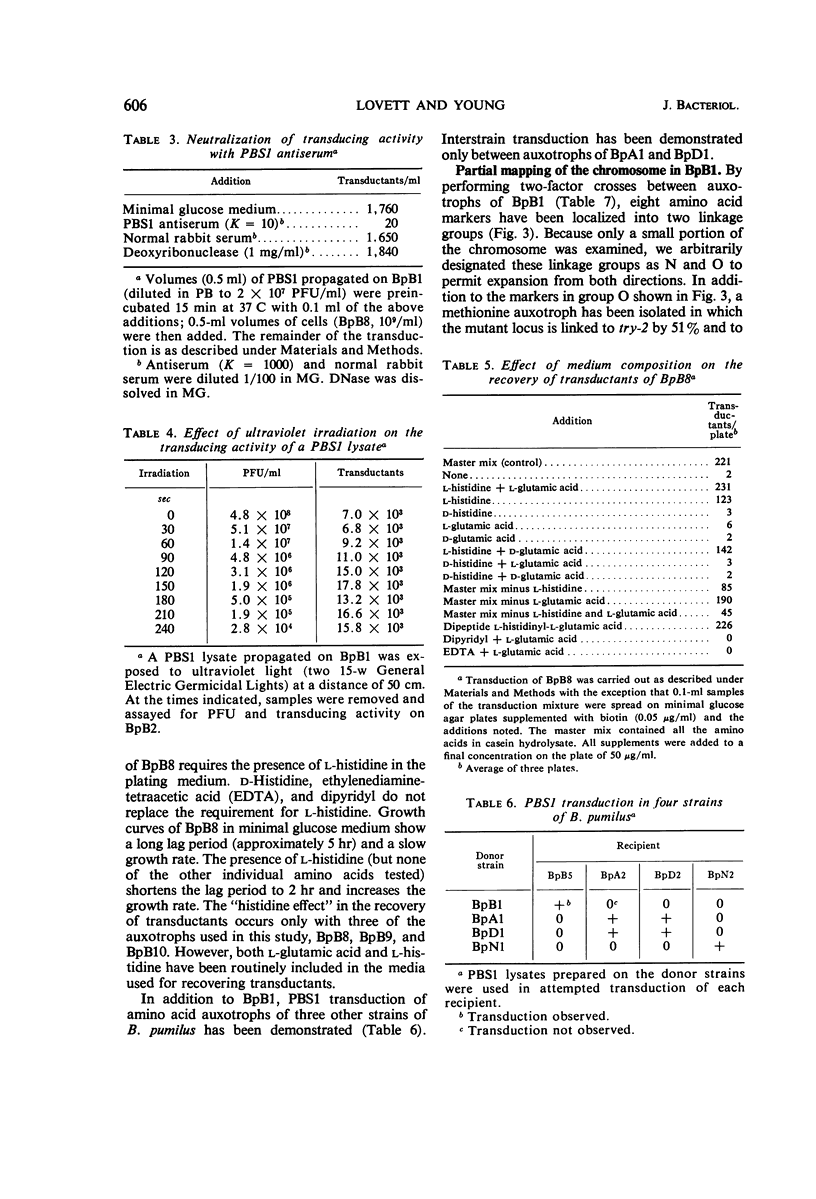
Bacteriophage PBS1 mediates generalized transduction in Bacillus pumilus NRRL B-3275 (BpB1). Transduction frequencies for single auxotrophic markers are of the order of 10−4 transductants per plaque-forming unit in crude phage lysates. The characteristics of PBS1 propagated on BpB1 and the properties of the system of transduction are similar to those reported for PBS1 propagated on Bacillus subtilis. By transduction, eight amino acid auxotrophic markers in BpB1 have been oriented into two linkage groups. One group contains the auxotrophic markers arginine A, leucine, and phenylalanine, and the other group contains the markers lysine, serine, tryptophan, isoleucine-valine, and isoleucine. The nature and relative order of the markers within each linkage group suggest that the arrangement of genes in these areas of the chromosome of BpB1 is similar to the arrangement of phenotypically comparable genes in two linkage groups (defined by PBS1 transduction) in B. subtilis. However, transduction of any of the above cited markers in BpB1 to prototrophy with PBS1 propagated on B. subtilis 168 could not be demonstrated. In addition to BpB1, seven other strains of B. pumilus can be infected with PBS1. Transduction has been demonstrated in three of these strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burmeister H. R., Hesseltine C. W. Induction and propagation of a Bacillus subtilis L form in natural and synthetic media. J Bacteriol. 1968 May;95(5):1857–1861. doi: 10.1128/jb.95.5.1857-1861.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Smith I., Morell P., Marmur J. Gene conservation in Bacillus species. I. Conserved genetic and nucleic acid base sequence homologies. Proc Natl Acad Sci U S A. 1965 Aug;54(2):491–498. doi: 10.1073/pnas.54.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserling F. A. The structure of Bacillus subtilis bacteriophage PBS 1. J Ultrastruct Res. 1967 Feb;17(3):342–347. doi: 10.1016/s0022-5320(67)80053-4. [DOI] [PubMed] [Google Scholar]
- Ezekiel D. H., Hutchins J. E. Mutations affecting RNA polymerase associated with rifampicin resistance in Escherichia coli. Nature. 1968 Oct 19;220(5164):276–277. doi: 10.1038/220276a0. [DOI] [PubMed] [Google Scholar]
- Frankel R. W., Joys T. M. Adsorption Specificity of Bacteriophage PBS1. J Bacteriol. 1966 Aug;92(2):388–389. doi: 10.1128/jb.92.2.388-389.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I. D., Gwinn D. D., Thorne C. B. Interspecies transformation between Bacillus subtilis and Bacillus licheniformis. Biochem Biophys Res Commun. 1966 May 25;23(4):543–548. [PubMed] [Google Scholar]
- Joys T. M. Correlation between susceptibility to bacteriophage PBS1 and motility in Bacillus subtilis. J Bacteriol. 1965 Dec;90(6):1575–1577. doi: 10.1128/jb.90.6.1575-1577.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEONARD C. G., MATTHEIS M. J. DIFFERENT TRANSFORMING CHARACTERISTICS OF COLONIAL VARIANTS FROM AUXOTROPHIC MUTANTS OF BACILLUS LICHENIFORMIS. J Bacteriol. 1965 Aug;90:558–559. doi: 10.1128/jb.90.2.558-559.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Identification of Bacillus subtilis NRRL B-3275 as a strain of Bacillus pumilus. J Bacteriol. 1969 Nov;100(2):658–661. doi: 10.1128/jb.100.2.658-661.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Melnick J. L., Rongey R., Mayor H. D. Physical assay and growth cycle studies of a defective adeno-satellite virus. J Virol. 1967 Feb;1(1):171–180. doi: 10.1128/jvi.1.1.171-180.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo L. M., Lundh N. P., Martinez R. J. Primary adsorption site of phage PBS1: the flagellum of Bacillus subtilis. J Virol. 1968 Mar;2(3):256–264. doi: 10.1128/jvi.2.3.256-264.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Revised linkage map of Salmonella typhimurium. Bacteriol Rev. 1967 Dec;31(4):354–372. doi: 10.1128/br.31.4.354-372.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J., Prestidge L. Conditions for competence in the Bacillus licheniformis transformation system. J Bacteriol. 1969 Jul;99(1):70–77. doi: 10.1128/jb.99.1.70-77.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR M. J., THORNE C. B. TRANSDUCTION OF BACILLUS LICHENIFORMIS AND BACILLUS SUBTILIS BY EACH OF TWO PHAGES. J Bacteriol. 1963 Sep;86:452–461. doi: 10.1128/jb.86.3.452-461.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne C. B., Stull H. B. Factors affecting transformation of Bacillus licheniformis. J Bacteriol. 1966 Mar;91(3):1012–1020. doi: 10.1128/jb.91.3.1012-1020.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne C. B. Transduction in Bacillus cereus and Bacillus anthracis. Bacteriol Rev. 1968 Dec;32(4 Pt 1):358–361. [PMC free article] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Marino P., Colvill A. J. Mutant of E. coli containing an altered DNA-dependent RNA polymerase. Nature. 1968 Oct 19;220(5164):275–276. doi: 10.1038/220275a0. [DOI] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Lawton W. D., MacQuillan A. M. Sequential replication of the chromosome of Bacillus licheniformis. J Bacteriol. 1968 Jun;95(6):2062–2069. doi: 10.1128/jb.95.6.2062-2069.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker N. E., Campbell L. L. Unrelatedness of Bacillus amyloliquefaciens and Bacillus subtilis. J Bacteriol. 1967 Oct;94(4):1124–1130. doi: 10.1128/jb.94.4.1124-1130.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E., Smith C., Reilly B. E. Chromosomal location of genes regulating resistance to bacteriophage in Bacillus subtilis. J Bacteriol. 1969 Jun;98(3):1087–1097. doi: 10.1128/jb.98.3.1087-1097.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]