Abstract
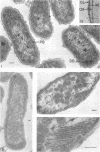
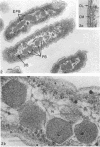
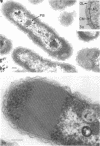
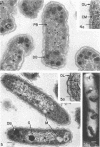
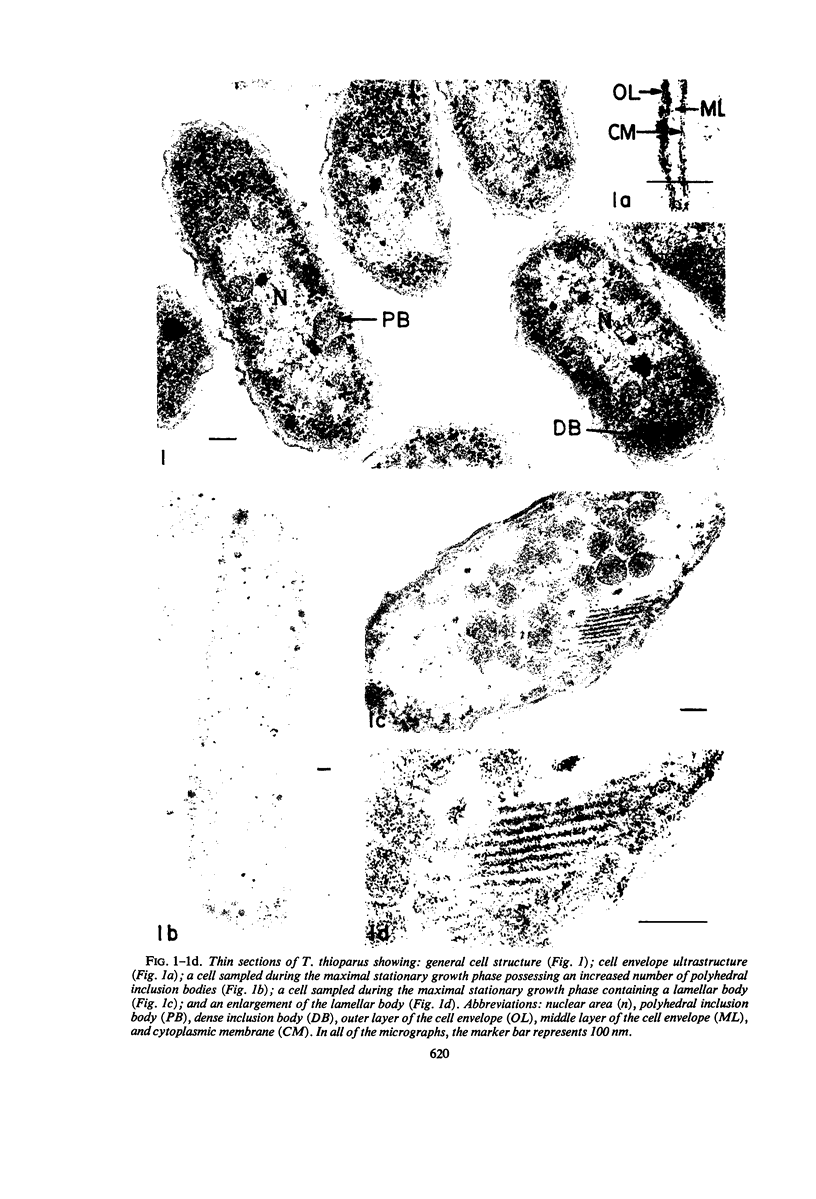
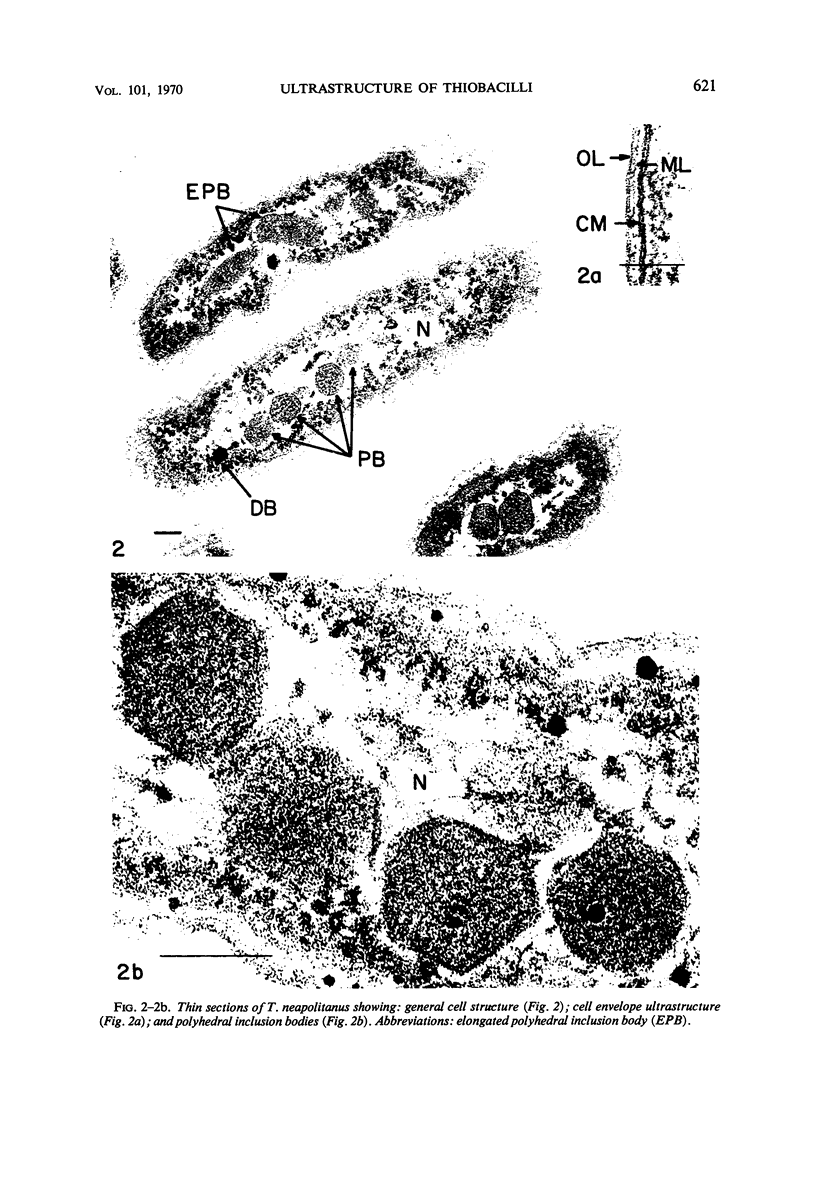
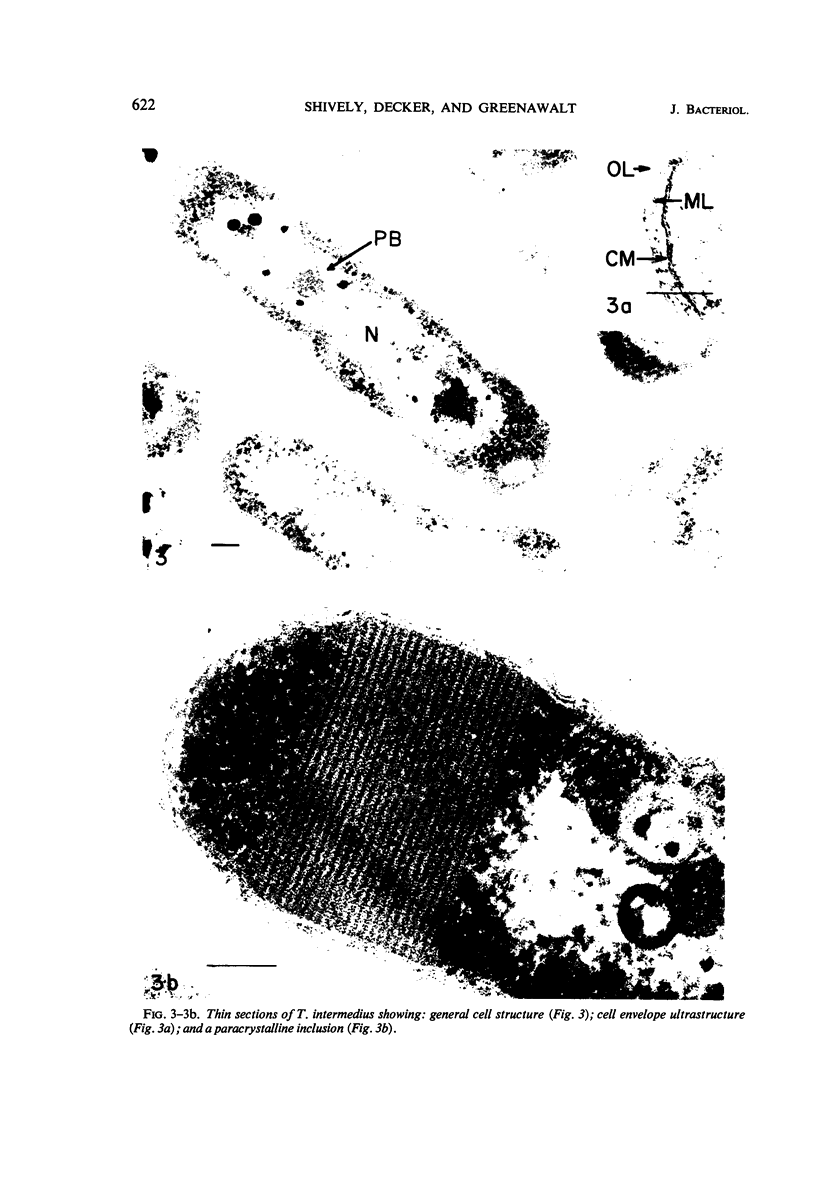
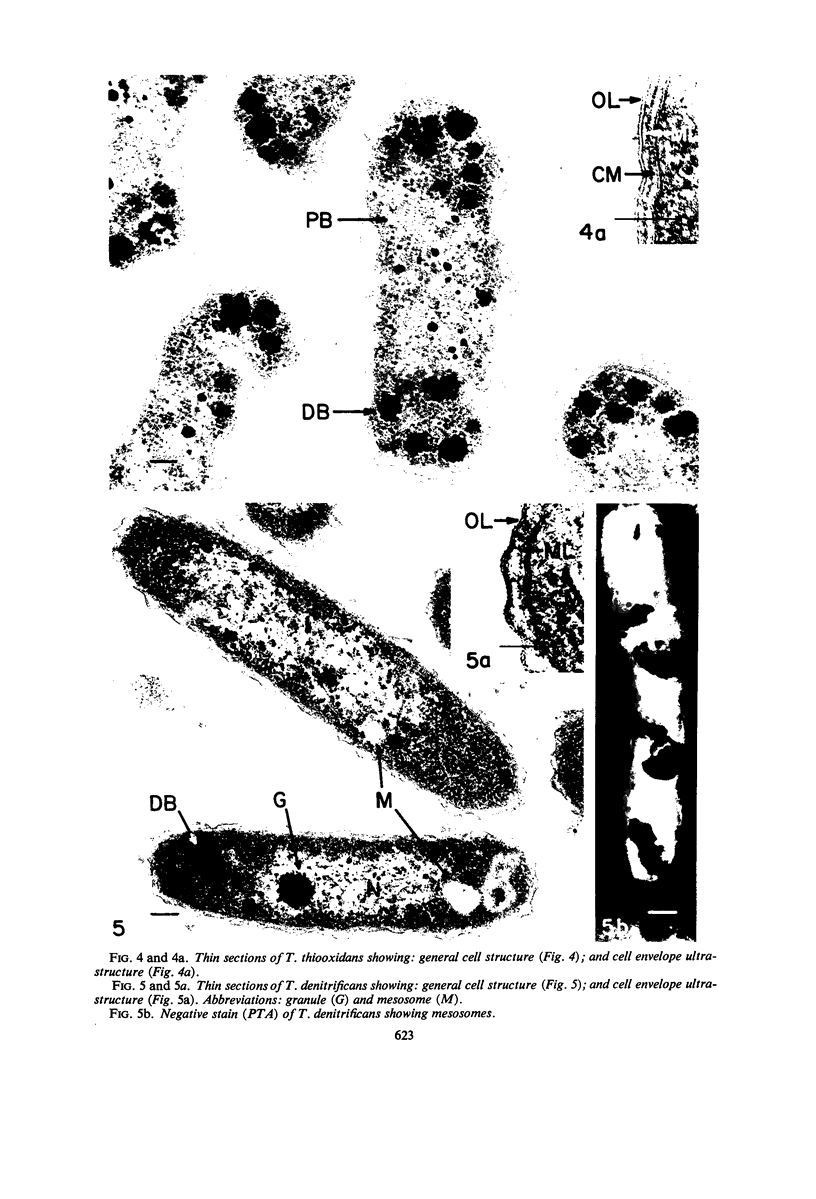
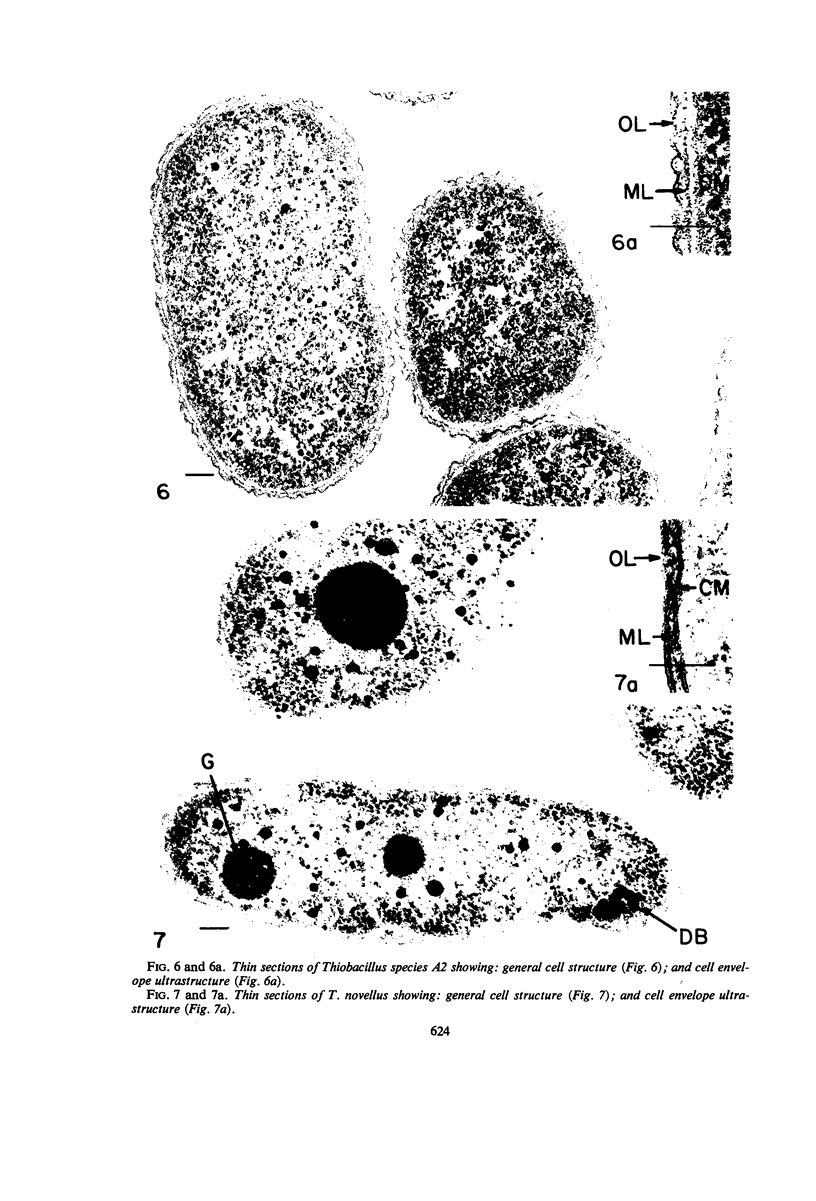
The ultrastructure of seven Thiobacillus species was studied. The structure of their cell envelopes is similar, if not identical, to that found in other gram-negative bacteria. Obvious differences were noted in the middle layer of the cell envelope of the seven cultures. Polyhedral inclusion bodies were apparent in four of the organisms: T. thioparus, T. neapolitanus, T. intermedius, and T. thiooxidans. Lamellar bodies, similar to those present in certain photosynthetic bacteria were found in a few cells of T. thioparus. Structures resembling mesosomes were discovered in T. dinitrificans. A few cells of T. intermedius possessed paracrystalline bodies. Other inclusions, probably volutin and polysaccharide, were present in some of the cultures.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton L. L., Shively J. M. Thiosulfate utilization by Thiobacillus thiooxidans ATCC 8085. J Bacteriol. 1968 Feb;95(2):720–720. doi: 10.1128/jb.95.2.720-.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R., Poindexter J. S. The internal membranes of Caulobacter crescentus. J Gen Microbiol. 1966 Feb;42(2):301–308. doi: 10.1099/00221287-42-2-301. [DOI] [PubMed] [Google Scholar]
- DUGAN P. R., LUNDGREN D. G. ENERGY SUPPLY FOR THE CHEMOAUTOTROPH FERROBACILLUS FERROOXIDANS. J Bacteriol. 1965 Mar;89:825–834. doi: 10.1128/jb.89.3.825-834.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- Gibbs S. P., Sistrom W. R., Worden P. B. The photosynthetic apparatus of Rhodospirillum molischianum. J Cell Biol. 1965 Aug;26(2):395–412. doi: 10.1083/jcb.26.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney R. P., Edwards M. R. Fine Structure of Thiobacillus thiooxidans. J Bacteriol. 1966 Aug;92(2):487–495. doi: 10.1128/jb.92.2.487-495.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J. R., Proctor H. M. Crystalline inclusions in Bacillus thuringiensis. J Bacteriol. 1969 May;98(2):824–826. doi: 10.1128/jb.98.2.824-826.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsen C., Lundgren D. G. Electron microscopy of the cell envelope of Ferrobacillus ferrooxidans prepared by freeze-etching and chemical fixation techniques. J Bacteriol. 1966 Dec;92(6):1765–1771. doi: 10.1128/jb.92.6.1765-1771.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F., Hoare D. S. New facultative Thiobacillus and a reevaluation of the heterotrophic potential of Thiobacillus novellus. J Bacteriol. 1969 Oct;100(1):487–497. doi: 10.1128/jb.100.1.487-497.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Caeseele L., Lees H. The ultrastructures of autotrophically and heterotrophically grown Thiobacillus novellus. Can J Microbiol. 1969 Jul;15(7):651–654. doi: 10.1139/m69-116. [DOI] [PubMed] [Google Scholar]
- Wang W. S., Lundgren D. G. Poly-beta-hydroxybutyrate in the chemolithotrophic bacterium Ferrobacillus ferrooxidans. J Bacteriol. 1969 Feb;97(2):947–950. doi: 10.1128/jb.97.2.947-950.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]