Abstract
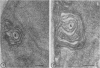
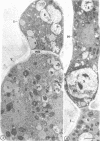
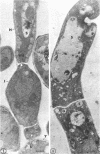
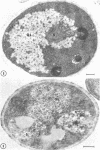
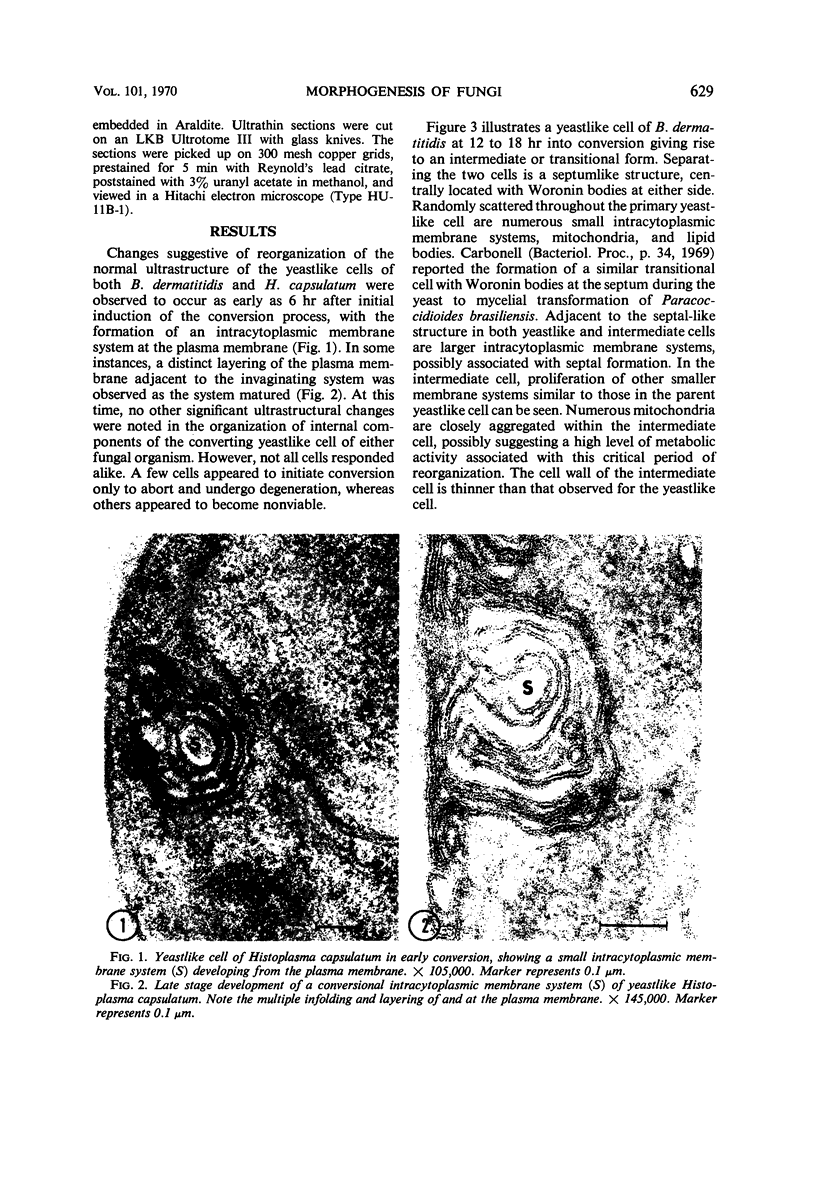
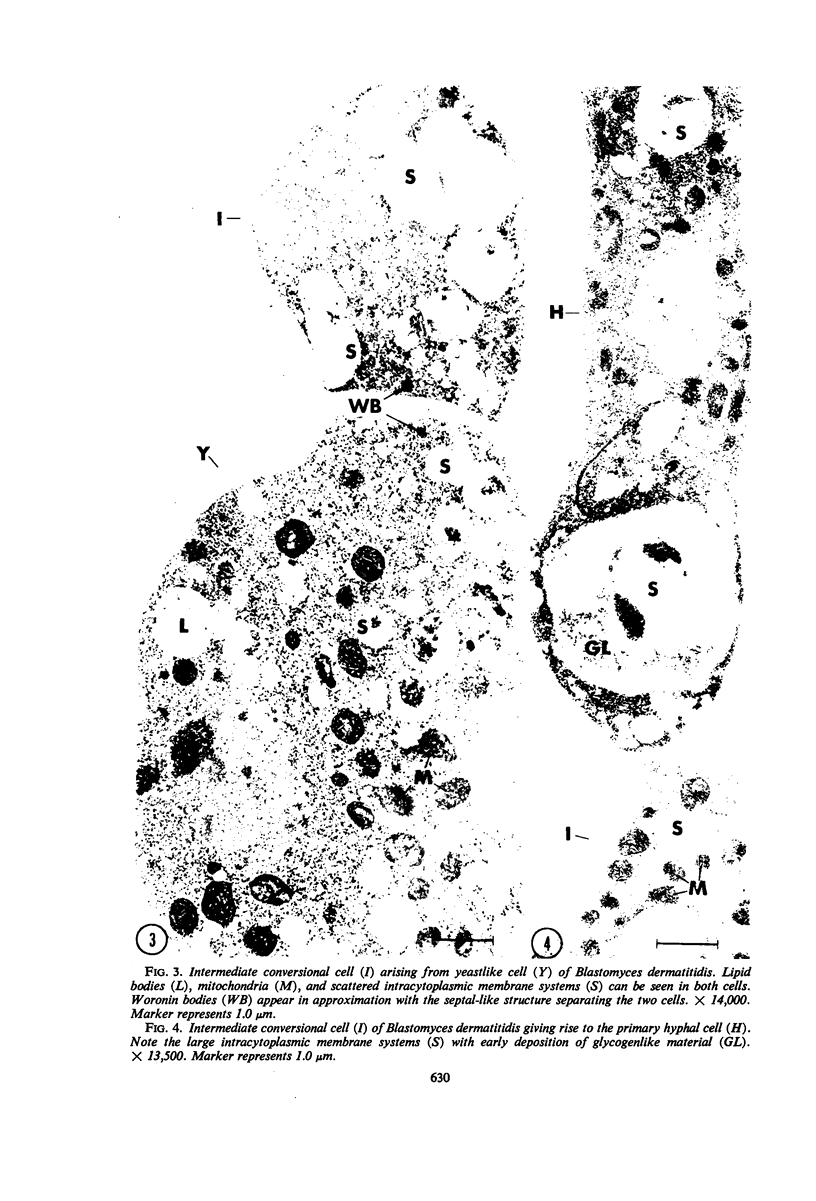
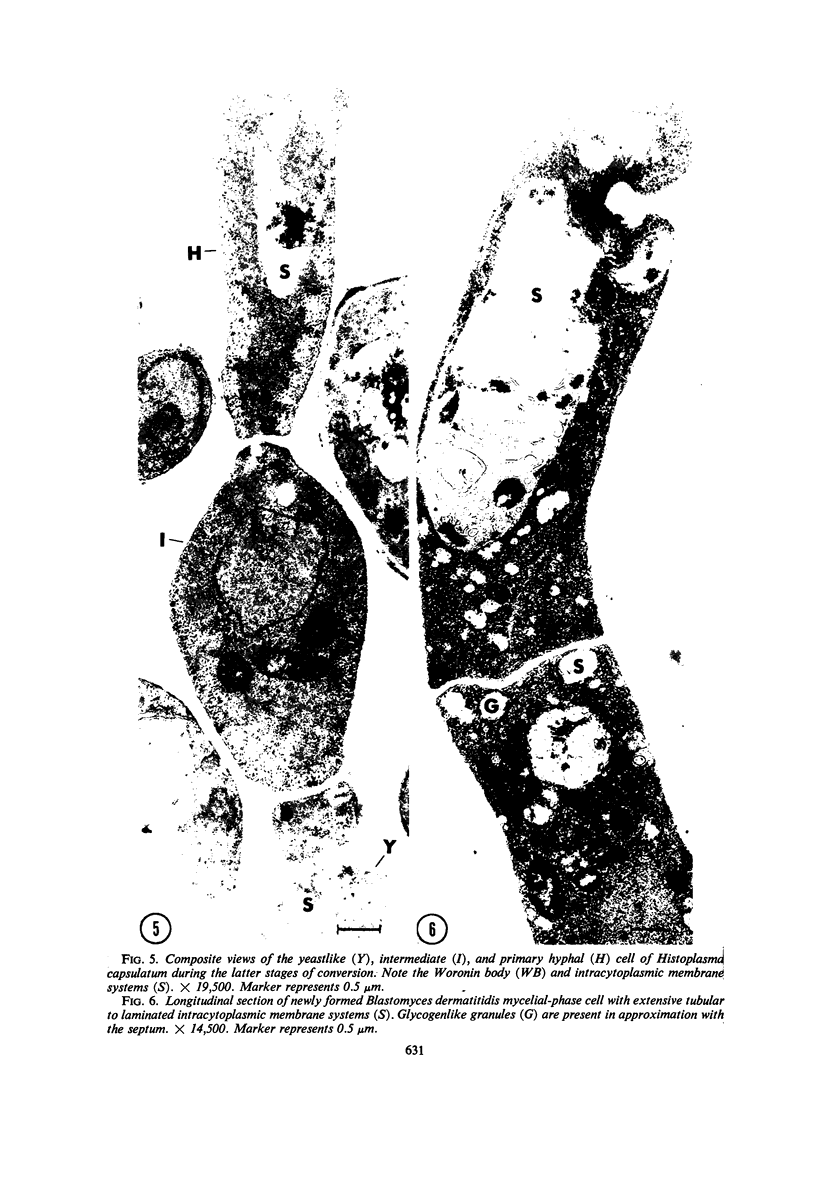
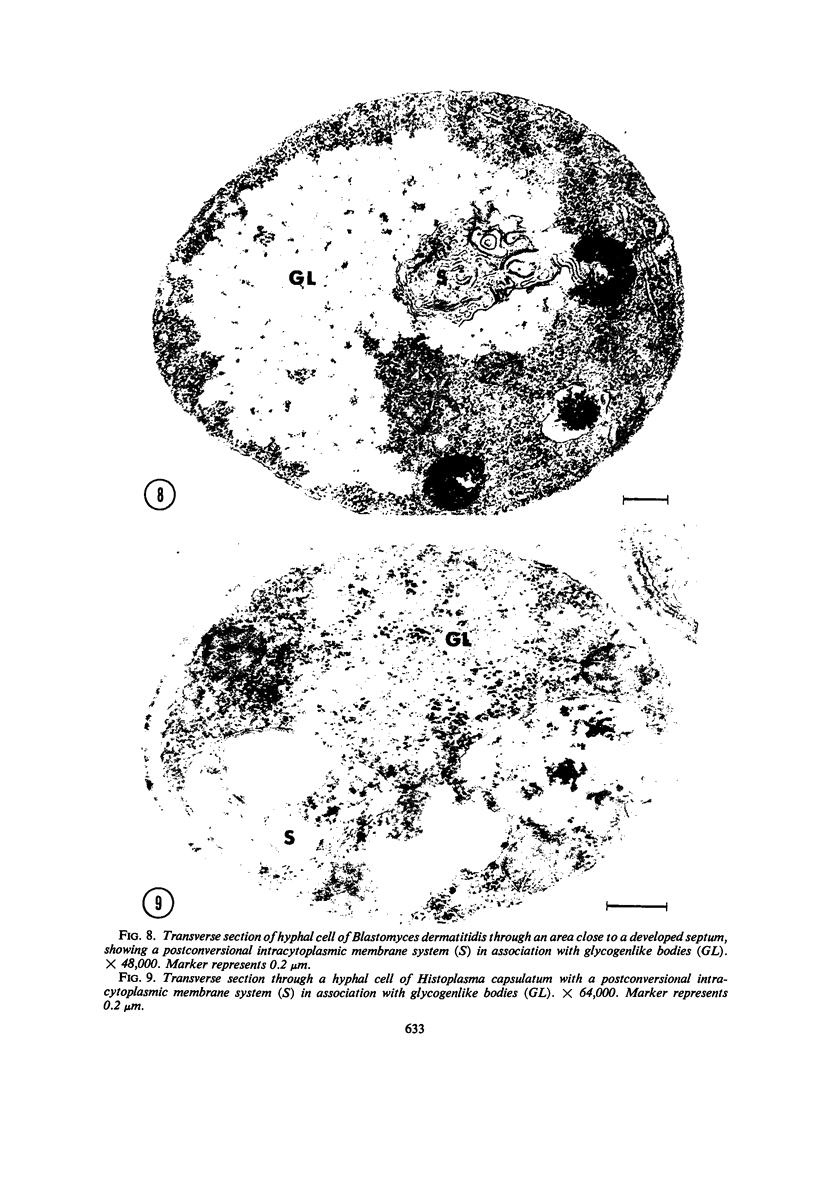
Fine details of the sequential anatomical events occurring during yeast to mold morphogenesis of the dimorphic fungal pathogens Blastomyces dermatitidis and Histoplasma capsulatum as seen in ultrathin sections are described and illustrated by electron micrographs. Discrete intracytoplasmic membrane systems intimately associated with the plasma membrane were observed to be formed within 6 to 8 hr after induction of the conversion process. Within 12 to 18 hr, an intermediate or transitional cell with Woronin bodies at the septum was formed from the converting yeastlike cell. Both cells were noted to contain increased numbers of mitochondria. At approximately 48 hr from the initial induction of the conversion stimuli, the newly forming hyphal cells were observed to produce postconversional intracytoplasmic membrane systems seen normally in the ultrastructural organization of the fully established mycelial-phase cell. These membrane systems appear to be associated with normal septal formation. Although minor variations of time were observed in the occurrence of the sequential events, it is suggested that yeastlike to mycelial-phase conversion of these two fungal pathogens proceeds via a similar mechanism of ultrastructural reorganization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLONDEL B., TURIAN G. Relation between basophilia and fine structure of cytoplasm in the fungus Allomyces macrogynus Em. J Biophys Biochem Cytol. 1960 Feb;7:127–134. doi: 10.1083/jcb.7.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner M. D., Reca M. E. Vital staining of Histoplasma capsulatum with Janus Green B. Sabouraudia. 1966 Jun;5(1):26–29. [PubMed] [Google Scholar]
- Carbonell L. M. Cell wall changes during the budding process of Paracoccidioides brasiliensis and Blastomyces dermatitidis. J Bacteriol. 1967 Jul;94(1):213–223. doi: 10.1128/jb.94.1.213-223.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell L. M., Rodriguez J. Mycelial phase of Paracoccidioides brasiliensis and Blastomyces dermatitidis: an electron microscope study. J Bacteriol. 1968 Aug;96(2):533–543. doi: 10.1128/jb.96.2.533-543.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWKER L. E. FINE STRUCTURE OF FUNGI AS REVEALED BY ELECTRON MICROSCOPY. Biol Rev Camb Philos Soc. 1965 Feb;40:52–92. doi: 10.1111/j.1469-185x.1965.tb00795.x. [DOI] [PubMed] [Google Scholar]
- IMAEDA T., OGURA M. Formation of intracytoplasmic membrane system of mycobacteria related to cell division. J Bacteriol. 1963 Jan;85:150–163. doi: 10.1128/jb.85.1.150-163.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHATKIN A. J., TATUM E. L. Electron microscopy of Neurospora crassa mycelia. J Biophys Biochem Cytol. 1959 Dec;6:423–426. doi: 10.1083/jcb.6.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsenach R., Kessel M. The role of lomasomes in wall formation in Penicillium vermiculatum. J Gen Microbiol. 1965 Sep;40(3):401–404. doi: 10.1099/00221287-40-3-401. [DOI] [PubMed] [Google Scholar]