Abstract
Extensive morphological modification occurs during mammalian spermiogenesis when spermatids change their spherical shape into cells with a compact head and a long tail. In this study, freeze-fracture was used to elucidate the alteration of the nuclear envelope during this process. Nuclear condensation resulted in a great reduction of spermatid nuclear volume and the formation of the redundant nuclear envelope. During nuclear condensation, distribution patterns of nuclear pores were greatly affected by the developing acrosome and manchette. As the acrosome enlarged to cap the nucleus, the pores redistributed caudally in the nuclear membranes and became exclusively localized to the redundant nuclear envelope. Manchette microtubules play an important role in shaping the nucleus, and formation of the manchette was associated with exclusion of nuclear pores from the underlying nuclear envelope; therefore, it is likely that the redistribution of nuclear pores was aided by manchette development. The appearance of an electron-lucent nuclear region surrounded by the nascent redundant nuclear envelope indicated a pathway for transporting degradation products through the nuclear pores to the residual cytoplasm. The packaging of the nuclear pores into the redundant nuclear envelope suggests that they play a role in late stages of sperm maturation or in fertilization, as most other unnecessary organelles of sperm are discarded during spermiogenesis or during shedding of the cytoplasmic droplet.
Keywords: freeze-fracture technique, manchette, nuclear pore, redundant nuclear envelope
Introduction
Spermiogenesis is the process whereby spermatids undergo elaborate morphological changes to form mature sperm. It consists four phases: Golgi phase, cap phase, acrosome phase and maturation phase (Clermont, 1972). Nuclear condensation is one of the most conspicuous events that occurs in spermatids during the acrosome and maturation phases (Russell et al. 1990). A great reduction in nuclear volume results in the packaging of the excess nuclear envelope into the redundant nuclear envelope in spermatids of many mammals (Fawcett & Ito, 1965; Franklin, 1968; Toshimori et al. 1985; Kerr, 1991). Although termed ‘redundant nuclear envelope’ originally because its function was unknown, its persistence in the neck region of mature sperm inspired speculation that it plays a role in fertilization, where redundant nuclear envelope could be re-incorporated into the sperm nucleus during formation of the male pronucleus (Fawcett & Ito, 1965). We have shown that a portion of the redundant nuclear envelope in mature sperm regulates sperm hyperactivated motility by serving as an intracellular calcium store (Ho & Suarez, 2003). Recently, discoveries of the functioning of the ubiquitin-proteasome system in sperm have again drawn attention to the redundant nuclear envelope and also to the nuclear pore complexes specifically localized there (Haraguchi et al. 2007; Crimmins et al. 2009).
During spermatid nuclear condensation, histones are gradually replaced by transition proteins, and finally by protamines (Meistrich et al. 2003). Many protein turnover and degradation events are therefore executed within the spermatid nucleus and cytoplasm. Proteasomes and polyubiquitinated proteins, which are designated to be degraded, are localized in a non-condensed nuclear region surrounded by the redundant nuclear envelope. The degraded protein products are proposed to be transported into the neck cytoplasm through the nuclear pores (Haraguchi et al. 2007) and may become a part of the residual body or the cytoplasmic droplet which is eventually shed from the sperm tail during final maturation events in the epididymis.
Besides remodeling of the spermatid chromatin, the formation of the manchette participates in nuclear condensation and shaping (Russell et al. 1990). The manchette consists of an array of microtubules that forms around the postacrosomal spermatid nucleus. Rod-like linkers between manchette microtubules and the outer nuclear membrane observed in rat and mouse spermatids during the acrosome phase were proposed to play a role in nuclear shaping (Russell et al. 1991). During nuclear reduction and re-shaping, nuclear pores are moved to the redundant nuclear envelope; however, it is not known whether the manchette plays a role in movement of the pores.
Morphological specializations are often associated with functions. To realize how and when the redundant nuclear envelope performs its functions, a detailed understanding of the development of the redundant nuclear envelope is necessary. The distribution pattern of nuclear pores along the spermatid nucleus during spermiogenesis may also be important for nuclear condensation and shaping. Although the migration of nuclear pore complexes towards the caudal nucleus has been shown under fluorescence microscopy (Sutovsky et al. 1999; Crimmins et al. 2009), there has not to date been direct ultrastructural evidence showing the distribution pattern of nuclear pores and their relationship with the manchette and formation of the redundant nuclear envelope.
Materials and methods
Animals and tissue collection
Mature male ICR mice were sacrificed by CO2 inhalation and cervical dislocation. Testes were removed and prepared as follows for ultrastructural observations. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Tzu Chi University.
Transmission electron microscopy
Small blocks of testis were pre-fixed with 2.5% glutaraldehyde prepared in 0.1 m cacodylate buffer containing 2% tannic acid at 0–4 °C for 1 h, and post-fixed with 1% OsO4 in 0.1 m cacodylate buffer for 1 h at room temperature. After fixation, specimens were dehydrated through a graded series of ethanol and embedded in Spurr’s resin. Serial ultrathin sections of approximately 80 nm were made with a Leica Ultracut R ultramicrotome (Leica, Heerbrugg, Switzerland) and stained with uranyl acetate and lead citrate. Sections were examined with a Hitachi H-7100 or an H-7500 transmission electron microscope at 75 kV or 80 kV.
Freeze-fracture
Testes fixed with 2.5% glutaraldehyde/0.1 m cacodylate buffer were washed in buffer and cryoprotected with 30% glycerol. Specimens were frozen rapidly in liquid propane, and transferred into Balzer BAF-400D freeze-etching apparatus. Specimens were fractured and etched at −105 °C, then coated with carbon and shadowed with Pt/C at a 45° angle. Replicas were floated on ice-cold methanol and washed by chromic acid and sodium hypochlorite before being collected on the 300-mesh grids. Replicas were observed with a Hitachi H-7100 at 75 kV.
Statistical analysis
Spermatid sectional images at early and late maturation phases were randomly selected. The electron-lucent areas surrounded by the developing redundant nuclear envelopes were measured by ImageJ (http://rsbweb.nih.gov/ij/) and the values were adjusted according to magnification. Data were expressed as mean ± SEM, and were analyzed using minitab statistical software (Minitab Inc., State College, PA, USA). One-tail Student’s t-test was used to test for statistically significant differences.
Results
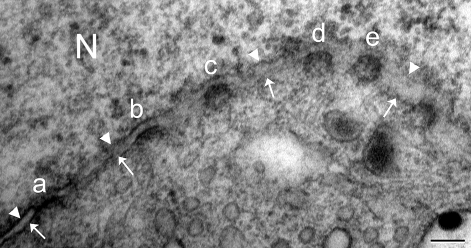
Nuclear pores are openings on the nuclear envelope that are formed from the fusion of the inner and outer nuclear membranes. Under the transmission electron microscope, nuclear pore appeared from a side view as an electron-dense, fuzzy diaphragm-like structure crossing the pore opening (Fig. 1a). However, a face-on view revealed the circular appearance of the nuclear pore, while thickened electron-dense inner or outer membrane indicated oblique sectioning through the nuclear pore (Fig. 1).
Fig. 1.
Nuclear pores (a–e) appear differently according to sectioning planes. (a) A side-view of nuclear pore shows electron-dense diaphragm-like structure connecting fused inner (arrowheads) and outer (arrows) nuclear membrane. (b–d) Oblique sectioning through nuclear pores shows thickened nuclear membrane. (e) Face-on view of nuclear pore shows circular electron-dense structure. N, nucleus. Scale bar: 100 nm.
During Golgi phase (steps 1–3), spermatids were characterized by elaborate perinuclear Golgi apparatus, whereas the acrosomal vesicles were small and usually not in contact with the nucleus. The nuclear pores appeared to be scarce and for the most part randomly distributed, except in association with annulate lamellae, where pores were more consistently present (Fig. 2). In cap phase (steps 4–7), acrosomal vesicle attached and flattened on the surface of the nucleus to become the acrosomal cap. At the sites where the acrosomal cap developed, the perinuclear space narrowed and the nuclear envelope underneath the acrosomal cap thickened; this is called the modified nuclear envelope (Fig. 3). The electron-dense modified nuclear envelope became devoid of nuclear pores and this area expanded from the early to the late cap phase to cover one third to a half of the nucleus (Figs 3 and 4). Outside of the region of the modified nuclear envelope, nuclear pores were found in clusters around the nucleus (Fig. 3B). Even at late cap phase, nuclear pores were still visible at the lateral sides of the nucleus (Fig. 4B).
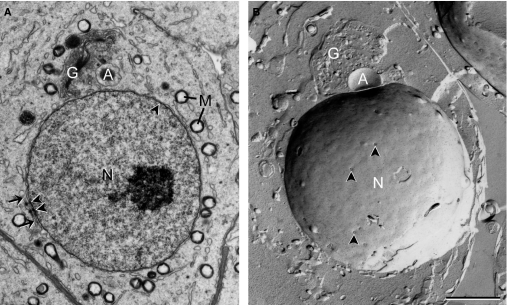
Fig. 2.
Golgi phase spermatids observed in an ultrathin section (A) and a freeze-fracture replica (B). Nuclear pores (arrowheads) are randomly distributed in most part of the nuclear envelope except at places associated with annulate lamella (arrow), where nuclear pores are more consistently present. G, Golgi complex; A, acrosomal vesicle; N, nucleus; M, mitochondria. Scale bar: 2 μm.
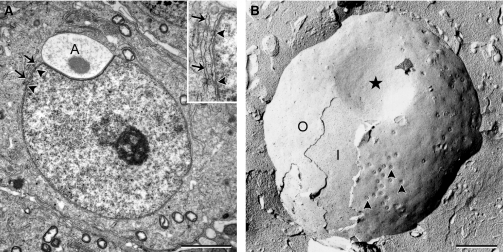
Fig. 3.
Early cap phase spermatids observed in an ultrathin section (A) and a freeze-fracture replica (B). Aggregation of nuclear pores (arrowheads) is visible at this stage in freeze-fracture replica, although not obvious in ultrathin sections. Associations of annulate lamella (arrows) and nuclear pores are still found at this stage (see inset for higher magnification). Note that no nuclear pores are found in the nuclear envelope where the acrosomal cap (A) is attached (*). I, inner nuclear membrane; O, outer nuclear membrane. Scale bars: 2 μm (A), 1 μm (B).
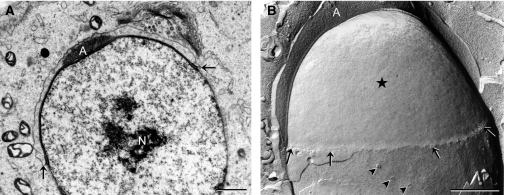
Fig. 4.
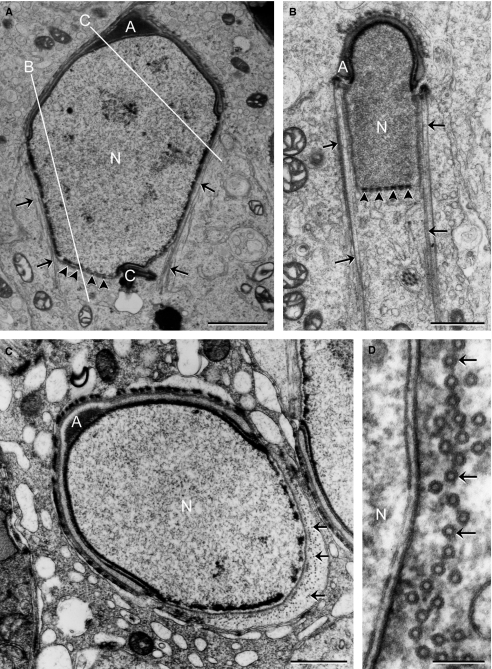
Late cap phase spermatids. As the developing acrosomal cap (A) expands to cap the nucleus, the modified nuclear envelope (*) also expands and there are no nuclear pores in this area. A few nuclear pores (arrowheads) are found on lateral surfaces of the nucleus, caudal to the acrosomal cap (B). N, nucleus; arrows, caudal margin of the developing acrosomal cap. Scale bars: 1 μm.
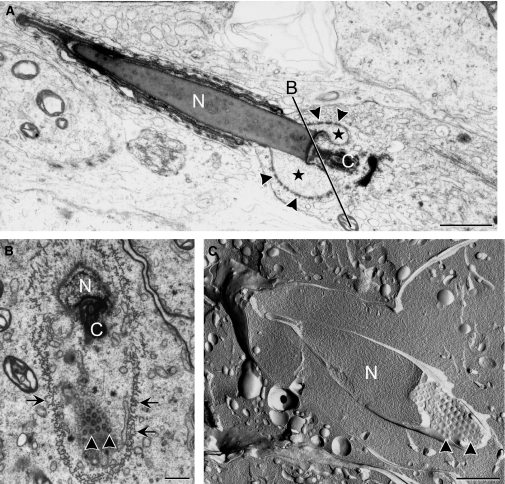
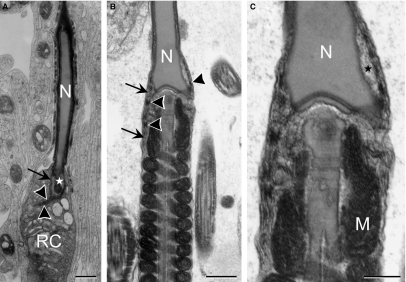
During the acrosome phase (steps 8–12), nuclear elongation/condensation began and manchette formed around the postacrosomal nucleus. An obvious nuclear pore aggregation was found at the caudal end of the nucleus; no pores were found at regions associated with either the acrosomal cap or the manchette microtubules (Fig. 5). Subsequently, in the maturation phase (steps 13–16), where nucleus became more condensed and mitochondrial sheath formed around the middle piece of tail, nuclear pores became highly concentrated and packed at the future site of formation of the redundant nuclear envelope, surrounding an electron-lucent area (Fig. 6). By measuring the electron-lucent areas on sections, a significant decrease (P<0.005) was found: the electron-lucent area in early maturation phase spermatids averaged 0.71 ± 0.19 μm2 (n = 10), whereas this area decreased to 0.20 ± 0.15 μm2 (n = 10) in late maturation phase spermatids. This indicated that the volume of the electron-lucent area, or the nuclear pocket, gradually decreased as spermatids matured, leaving the apposed redundant nuclear envelope projecting into the neck region (Fig. 7). The residual cytoplasm, which was prominent around the neck region, contained structures designated to be discarded (Fig. 7).
Fig. 5.
Acrosome phase spermatids. (A) Section through the anterior–posterior axis shows that nuclear pores (arrowheads) are only found at the caudal pole of the nucleus, where no manchette microtubules (arrows) are associated with the nuclear envelope. (B) A lateral sagittal section through the elongating spermatid as indicated by the white line B in (A). Nuclear pores are exclusively localized at the caudal end of the nucleus. (C) Section plane is indicated by the white line C in (A), where manchette microtubules (arrows) are cross-sectioned, the perinuclear space narrowed, and no pores are observed here. (D) Higher magnification shows the narrowed perinuclear space facing the cross-sectioned manchette microtubules. A, acrosomal cap; C, connecting piece; N, nucleus. Scale bars: 2 μm (A), 1 μm (B,C), 100 nm (D).
Fig. 6.
Maturation phase spermatids. (A) In late spermatid, condensed nucleus (N) is electron-dense, but the area surrounded by the redundant nuclear envelope is more electron lucent (*). Nuclear pores (arrowheads) exclusively localize to the redundant nuclear envelope, where they are closely packed. (B) Tangential section through the redundant nuclear envelope, as indicated by line B in (A), shows closely packed nuclear pores. (C) Freeze-fracture replica that fractured along the surface of the redundant nuclear envelope and through the nucleus, shows high density packing of nuclear pores. C, connecting piece; arrows, manchette microtubules. Scale bars: 1 μm (A), 500 nm (B), 1 μm (C).
Fig. 7.
Late maturation phase spermatids with mitochondrial helical sheath (M) formed in the middle piece. The electron-lucent area (*) surrounded by the redundant nuclear envelope (arrows) shows decreased volume compared to early maturation phase spermatids. The distal end of the redundant nuclear envelope shows tightly packed membranes with a lot of nuclear pores (arrowheads). (C) Enlarged neck region of (B), showing electron-lucent area and electron-dense nuclear pores (not labeled) in greater detail. N, spermatid nucleus; RC, residual cytoplasm. Scale bars: 500 nm (A,B), 250 nm (C).
Discussion
This study provides visual evidence of the redistribution process of nuclear pores during spermiogenesis in the mouse testis. By examining ultrathin sections along with freeze-fracture replicas, the changes of nuclear pore distribution pattern were clearly appreciated. Freeze-fracture replicas provided nice topographic images of the nuclear envelope, showing the regular organization of nuclear pores in the redundant nuclear envelope in late spermatids, which is usually hard to envision from ultrathin sections alone. On the other hand, the relationship among nuclear pores, acrosomal cap, and manchette microtubules are clearly seen in ultrathin sections.
The nuclear pores and the redundant nuclear envelope have been brought to attention lately for their role in ubiquitin-proteasome system. The ubiquitin-proteasome system regulates protein turnover, including spermatid nuclear protein replacement and nuclear condensation, a process that would diminish sperm fertilizing ability when malfunctioning (Escalier et al. 2003; Kwon et al. 2005). Proteasomes and polyubiquitinated proteins were found to localize in the electron-lucent nuclear region surrounded by the redundant nuclear envelope (Haraguchi et al. 2007). If nucleoprotein degradation occurred according to Haraguchi et al. (2007), it is possible that the degradation products are transported through the nuclear pores along the redundant nuclear envelope to the neck cytoplasm, accumulating in cytoplasmic droplets around the neck region of testicular and epididymal sperm. A stable pool of free ubiquitin is maintained by de-ubiquitinating enzymes that carefully regulate ubiquitin-proteasome proteolysis (Amerik & Hochstrasser, 2004). Immunohistochemistry experiments showed that Usp14, a de-ubiquitinating enzyme, is localized at the redundant nuclear envelope and cytoplasmic droplet of elongated spermatids and epididymal sperm (Crimmins et al. 2009), also indicating the involvement of ubiquitin-mediated proteolysis at the site of redundant nuclear envelope. In the present study, the appearance of a region of low electron density in the nucleus near the accumulating nuclear pores indicates that nucleoprotein degradation could be occurring here for transference to the residual cytoplasm. The decrease of the electron-lucent area surrounded by the redundant nuclear envelope in late spermatids (Fig. 7), as long as the prominent residual cytoplasm around the redundant nuclear envelope, suggest export of degraded nucleoproteins out of the nucleus.
By applying transmission electron microscopy and indirect immunofluorescence labeling, Tovich et al. (2004) concluded that all cap phase spermatids show focal nuclear pore complex localization at the caudal pole of the nucleus. In the present study, the freeze-fracture images clearly showed focal aggregations of nuclear pores around cap phase spermatid nucleus. However, they were not exclusively confined to the caudal pole of the nucleus; they were also visible at the lateral sides of the nucleus caudal to the margin of the acrosomal cap. Nevertheless, our results are consistent with Tovich et al.’s findings that no nuclear pore complexes were detected along the nuclear envelope abutting the manchette (Tovich et al. 2004).
Manchette was proposed to facilitate a Ran (Ras-related GTPase)-mediated nucleocytoplasmic transport in rat spermatids because Ran-GTPase was localized to the nucleus of round spermatids and the manchette of elongating spermatids (Kierszenbaum et al. 2002). The Ran-GTPase is well known for its crucial role in nucleocytoplasmic transport, and the import and export of macromolecules occurs across the nuclear pore complexes (reviewed by Kuersten et al. 2001; Cook et al. 2007). However, as no nuclear pores were found along the nuclear envelope abutting the manchette microtubules, the idea that manchette helped transporting molecules in and out of the nucleus remains to be clarified. As Kierszenbaum et al. (2002) localized Ran-GTPase by immunofluorescence labeling, the resolution was insufficient to discriminate whether the Ran-GTPase localized inside elongating spermatid nucleus or on the manchette surrounding the nucleus. It is therefore worth doing the experiment at the electron microscopic level to verify those authors’ hypothesis. In addition, whether intermediate molecules exist to shuffle Ran-GTPase between nucleus and manchette requires further investigation.
Fawcett & Chemes (1979) were the first to use the freeze-fracture technique to study nuclear pore distribution systematically in testicular germ cells. They described changes in nuclear pore distribution in mammalian germ cells, and found a more concentrated pore aggregation in the postacrosomal region of spermatid nucleus. Although they did not follow the distribution pattern of nuclear pores through the end of spermiogenesis, they posited an interesting question concerning whether the changing distribution patterns of pores resulted from movement of pores within the nuclear envelope or dissolution and re-assembly of pores. In this study, an abrupt change of pore distribution was observed in spermatids transitioning from the cap phase to the acrosome phase. At that time, when the manchette formed, the nuclear pores suddenly redistributed from the lateral sides of the nucleus to the caudal end. It is clear that no pores were present at the nuclear envelope abutting the manchette; therefore, it is tempting to speculate that the nuclear pores are passively excluded, with the help of manchette microtubules linked to the outer nuclear membrane as they shape the nucleus (Russell et al. 1991), from the lateral side of the nucleus to the caudal end at the beginning of acrosome phase.
Changes in the nuclear pore composition were found in developing male rat germ cells, where nucleoporin NUP62 shifted from the nuclear envelope to the nuclear interior (Yang et al. 2006). In an in vitro Xenopus egg extract system, nuclear pores were estimated to form de novo at a rate of 142 nuclear pore complexes per minute to accommodate growing nuclear volume (D’Angelo et al. 2006). In spite of the available mechanisms for reorganizing and forming nuclear pore complexes, scaffold nucleoporins were only expressed and assembled into nuclear pore complexes in actively dividing cells (D’Angelo et al. 2009), which is definitely not the case in late spermatids. Besides, there were no detectable ultrastructural changes, such as loss of electron density from the nuclear pores, which is normally evident during nuclear pore complex disassembly (Kiseleva et al. 2001). Thus, it is unlikely that nuclear pore complexes are broken down and reformed during acrosome phase.
The redistribution of the nuclear pores within the nuclear envelope is therefore more plausible than de novo synthesis of nuclear pores during spermiogenesis. However, the mechanism regulating pore redistribution is still unknown. Besides manchette microtubules mentioned above, nuclear lamin might be another candidate in organizing pore distribution. Nuclear pore complexes are tightly associated with nuclear lamina, a peripheral meshwork underneath the inner nuclear membrane, composed of intermediate filament protein lamins and associated proteins (Dultz & Ellenberg, 2007; Furukawa et al. 2009). In Drosophila, different lamin subtypes possessed different properties, where A-type lamins were proved to play a role in organizing nuclear pore complex distribution (Furukawa et al. 2009). In mammals, germ cell-specific lamins were identified in spermatocytes and spermatids (Sudhakar & Rao, 1990; Furukawa & Hotta, 1993). Interestingly, the distribution patterns of germ cell-specific lamin B3 changed during spermiogenesis. In round spermatids, lamin B3 was localized in the nuclear periphery; however, during nuclear elongation, lamin B3 became concentrated to the caudal end of spermatid nuclei (Schütz et al. 2005). Therefore, it would be interesting to determine whether nuclear pore complex redistribution found in this study was regulated by redistribution of nuclear lamin.
The results presented in this study elucidate changing patterns of nuclear pore distribution during mouse spermiogenesis. The concentrated arrangement of nuclear pores in the redundant nuclear envelope in sperm indicates that they serve a function; if not, they would have been broken down or otherwise discarded in the residual cytoplasm. Possibilities include that the pores play an important role during nuclear protein replacement and nuclear condensation, or even in formation of the pronucleus during fertilization. The roles of the redundant nuclear envelope merit further investigation.
Acknowledgments
The author thanks Prof. Susan Suarez for valuable suggestions, and Ms Mei-Jun Yang of Electron Microscopy Laboratory, Tzu Chi University, for technical support. This work was supported by grant 95-2311-B-320-004 from the National Science Council, Taiwan.
References
- Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004;1695:189–207. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Cook A, Bono F, Jinek M, et al. Structural biology of nucleocytoplasmic transport. Annu Rev Biochem. 2007;76:647–671. doi: 10.1146/annurev.biochem.76.052705.161529. [DOI] [PubMed] [Google Scholar]
- Crimmins S, Sutovsky M, Chung PC, et al. Transgenic rescue of ataxia mice reveals a male-specific sterility defect. Dev Biol. 2009;325:33–42. doi: 10.1016/j.ydbio.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo MA, Anderson DJ, Richard E, et al. Nuclear pores form de novo from both sides of the nuclear envelope. Science. 2006;312:440–443. doi: 10.1126/science.1124196. [DOI] [PubMed] [Google Scholar]
- D’Angelo MA, Raices M, Panowski SH, et al. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dultz E, Ellenberg J. Nuclear envelope. Curr Biol. 2007;17:R154–R156. doi: 10.1016/j.cub.2006.12.035. [DOI] [PubMed] [Google Scholar]
- Escalier D, Bai XY, Silvius D, et al. Spermatid nuclear and sperm periaxonemal anomalies in the mouse Ube2b null mutant. Mol Reprod Dev. 2003;65:298–308. doi: 10.1002/mrd.10290. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Chemes HE. Changes in distribution of nuclear pores during differentiation of the male germ cells. Tissue Cell. 1979;11:147–162. doi: 10.1016/0040-8166(79)90015-6. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Ito S. The fine structure of bat spermatozoa. Am J Anat. 1965;116:567–610. doi: 10.1002/aja.1001160306. [DOI] [PubMed] [Google Scholar]
- Franklin LE. Formation of the redundant nuclear envelope in monkey spermatids. Anat Rec. 1968;161:149–161. doi: 10.1002/ar.1091610202. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Hotta Y. cDNA cloning of a germ cell specific lamin B3 from mouse spermatocytes and analysis of its function by ectopic expression in somatic cells. EMBO J. 1993;12:97–106. doi: 10.1002/j.1460-2075.1993.tb05635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Ishida K, Tsunoyama TA, et al. A-type and B-type lamins initiate layer assembly at distinct areas of the nuclear envelope in living cells. Exp Cell Res. 2009;315:1181–1189. doi: 10.1016/j.yexcr.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Haraguchi CM, Mabuchi T, Hirata S, et al. Possible function of caudal nuclear pocket: degradation of nucleoproteins by ubiquitin-proteasome system in rat spermatids and human sperm. J Histochem Cytochem. 2007;55:585–595. doi: 10.1369/jhc.6A7136.2007. [DOI] [PubMed] [Google Scholar]
- Ho HC, Suarez SS. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol Reprod. 2003;68:1590–1596. doi: 10.1095/biolreprod.102.011320. [DOI] [PubMed] [Google Scholar]
- Kerr JB. Ultrastructure of the seminiferous epithelium and intertubular tissue of the human testis. J Electron Microsc Tech. 1991;19:215–240. doi: 10.1002/jemt.1060190208. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum AL, Gil M, Rivkin E, et al. Ran, a GTP-binding protein involved in nucleocytoplasmic transport and microtubule nucleation, relocates from the manchette to the centrosome region during rat spermiogenesis. Mol Reprod Dev. 2002;63:131–140. doi: 10.1002/mrd.10164. [DOI] [PubMed] [Google Scholar]
- Kiseleva E, Rutherford S, Cotter LM, et al. Steps of nuclear pore complex disassembly and reassembly during mitosis in early Drosophila embryos. J Cell Sci. 2001;114:3607–3618. doi: 10.1242/jcs.114.20.3607. [DOI] [PubMed] [Google Scholar]
- Kuersten S, Ohno M, Mattaj IW. Nucleocytoplasmic transport: Ran, beta and beyond. Trends Cell Biol. 2001;11:497–503. doi: 10.1016/s0962-8924(01)02144-4. [DOI] [PubMed] [Google Scholar]
- Kwon J, Mochida K, Wang YL, et al. Ubiquitin C-terminal hydrolase L-1 is essential for the early apoptotic wave of germinal cells and for sperm quality control during spermatogenesis. Biol Reprod. 2005;73:29–35. doi: 10.1095/biolreprod.104.037077. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, Mohapatra B, Shirley CR, et al. Roles of transition nuclear proteins in spermiogenesis. Chromosoma. 2003;111:483–488. doi: 10.1007/s00412-002-0227-z. [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Sinha Hikim AP, et al. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- Russell LD, Russell JA, MacGregor GR, et al. Linkage of manchette microtubules to the nuclear envelope and observations of the role of the manchette in nuclear shaping during spermiogenesis in rodents. Am J Anat. 1991;192:97–120. doi: 10.1002/aja.1001920202. [DOI] [PubMed] [Google Scholar]
- Schütz W, Alsheimer M, Ollinger R, et al. Nuclear envelope remodeling during mouse spermiogenesis: postmeiotic expression and redistribution of germline lamin B3. Exp Cell Res. 2005;307:285–291. doi: 10.1016/j.yexcr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Sudhakar L, Rao MR. Stage-dependent changes in localization of a germ cell-specific lamin during mammalian spermatogenesis. J Biol Chem. 1990;265:22526–22532. [PubMed] [Google Scholar]
- Sutovsky P, Ramalho-Santos J, Moreno RD, et al. Onstage selection of single round spermatids using a vital, mitochondrion-specific fluorescent probe MitoTracker™ and high resolution differential interference contrast microscopy. Hum Reprod. 1999;14:2301–2312. doi: 10.1093/humrep/14.9.2301. [DOI] [PubMed] [Google Scholar]
- Toshimori K, Higashi R, Oura C. Distribution of intramembranous particles and filipin-sterol complexes in mouse sperm membranes: polyene antibiotic filipin treatment. Am J Anat. 1985;174:455–470. doi: 10.1002/aja.1001740408. [DOI] [PubMed] [Google Scholar]
- Tovich PR, Sutovsky P, Oko RJ. Novel aspect of perinuclear theca assembly revealed by immunolocalization of non-nuclear somatic histones during bovine spermiogenesis. Biol Reprod. 2004;71:1182–1194. doi: 10.1095/biolreprod.104.030445. [DOI] [PubMed] [Google Scholar]
- Yang WX, Jefferson H, Sperry AO. The molecular motor KIFC1 associates with a complex containing nucleoporin NUP62 that is regulated during development and by the small GTPase RAN. Biol Reprod. 2006;74:684–690. doi: 10.1095/biolreprod.105.049312. [DOI] [PubMed] [Google Scholar]