Abstract
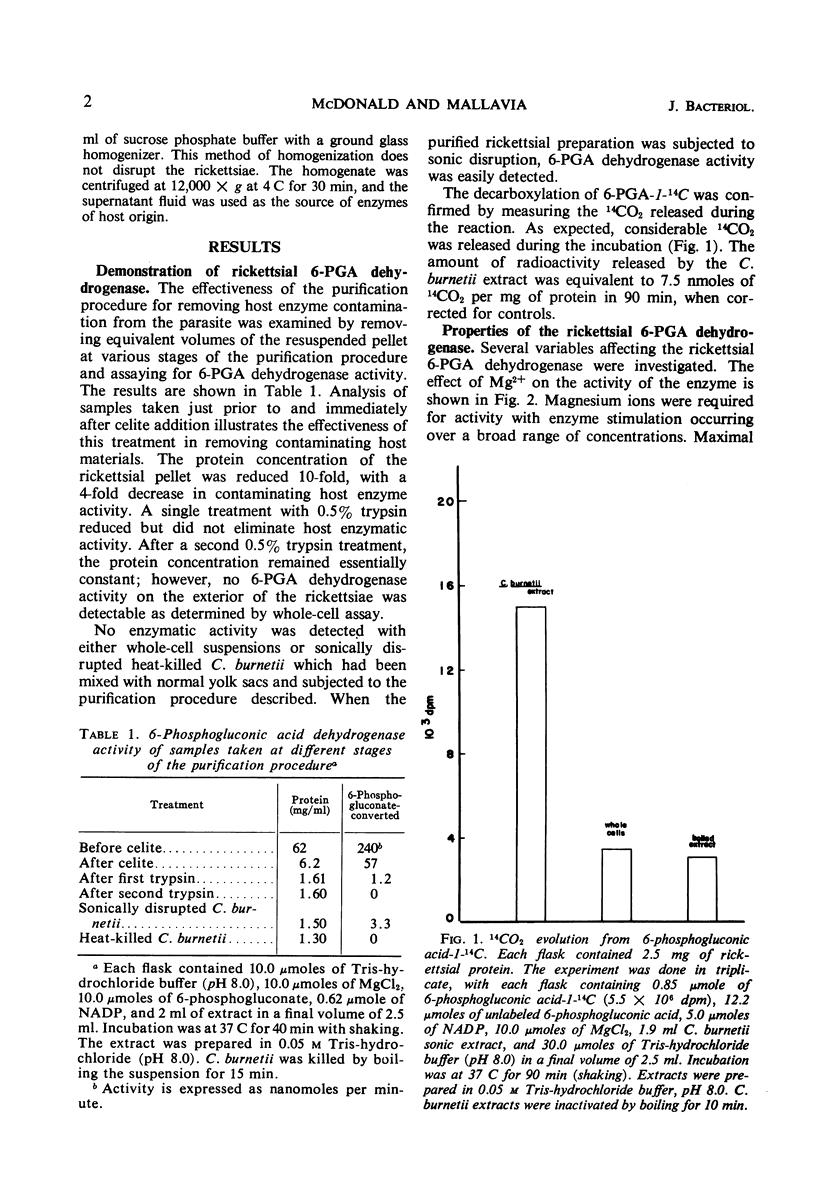
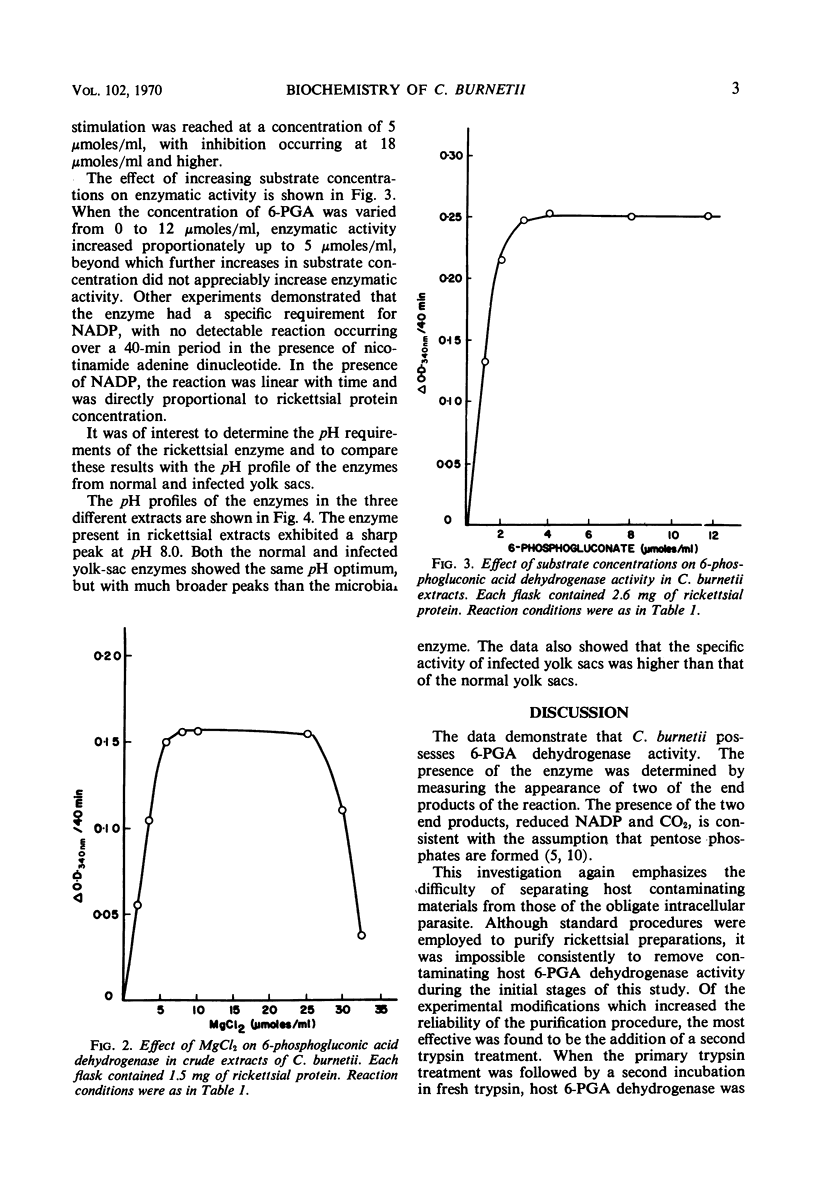
Purified preparations of the rickettsial agent, Coxiella burnetii, have been examined for their ability to decarboxylate 6-phosphogluconate. The enzyme 6-phosphogluconic acid dehydrogenase [6-phospho-d-gluconate: NADP (nicotinamide adenine dinucleotide phosphate) oxidoreductase (decarboxylating), EC 1.1.1.44] was detected in extracts, but not in whole-cell preparations of C. burnetii. Both extracts and whole cells were shown to be free from contaminating host enzyme activity. Partial characterization of the enzyme has shown that it is substrate-dependent, specific for NADP, and requires magnesium for activity. The pH optimum of the rickettsial enzyme is 8.0.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS D. H., ROWSON K. E., SALAMAN M. H. The effect of tumours, of leukaemia, and of some viruses associated with them, on the plasma lactic dehydrogenase activity of mice. Br J Cancer. 1961 Dec;15:860–867. doi: 10.1038/bjc.1961.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R. Phosphorylation accompanying the oxidation of glutamate by the Madrid E strain of typhus rickettsiae. J Biol Chem. 1956 May;220(1):353–361. [PubMed] [Google Scholar]
- Bovarnick M. R. INCORPORATION OF ACETATE-1-C INTO LIPID BY TYPHUS RICKETTSIAE. J Bacteriol. 1960 Oct;80(4):508–512. doi: 10.1128/jb.80.4.508-512.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. S., SCOTT D. B. M. Formation of pentose phosphate from 6-phosphogluconate. Science. 1950 May 19;111(2890):543–544. doi: 10.1126/science.111.2890.543. [DOI] [PubMed] [Google Scholar]
- CONSIGLI R. A., PARETSKY D. Oxidation of glucose 6-phosphate and isocitrate by Coxiella burnetii. J Bacteriol. 1962 Jan;83:206–207. doi: 10.1128/jb.83.1.206-207.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens F. Oxidation of phosphohexonate and pentose phosphoric acids by yeast enzymes: Oxidation of phosphohexonate. II. Oxidation of pentose phosphoric acids. Biochem J. 1938 Sep;32(9):1626–1644. doi: 10.1042/bj0321626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Levels of enzymes of the direct oxidative pathway of carbohydrate metabolism in mammalian tissues and tumours. Biochem J. 1954 Jan;56(1):171–175. doi: 10.1042/bj0560171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin C. S., Tyrrell D. A., Head B., Rees R. J. Inhibition of haemaggregation by lepromin and other mycobacterial substances. Nature. 1967 Dec 9;216(5119):1019–1020. doi: 10.1038/2161019a0. [DOI] [PubMed] [Google Scholar]
- HORECKER B. L., SMYRNIOTIS P. Z. Phosphogluconic acid dehydrogenase from yeast. J Biol Chem. 1951 Nov;193(1):371–381. [PubMed] [Google Scholar]
- Kalter S. S., Eugster A. K., Albert P. J., Cohen D. Enzyme studies in the embryonate egg and in tissue culture following inoculation with different viruses. Arch Gesamte Virusforsch. 1967;20(2):180–189. doi: 10.1007/BF01241271. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MALLAVIA L., PARETSKY D. STUDIES ON THE PHYSIOLOGY OF RICKETTSIAE. V. METABOLISM OF CARBAMYL PHOSPHATE BY COXIELLA BURNETII. J Bacteriol. 1963 Aug;86:232–238. doi: 10.1128/jb.86.2.232-238.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallavia L. P., Paretsky D. Physiology of rickettsiae. VII. Amino acid incorporation by Coxiella burnetii and by infected hosts. J Bacteriol. 1967 May;93(5):1479–1483. doi: 10.1128/jb.93.5.1479-1483.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARETSKY D., CONSIGLI R. A., DOWNS C. M. Studies on the physiology of rickettsiae. III. Glucose phosphorylation and hexokinase activity in Coxiella burnetii. J Bacteriol. 1962 Mar;83:538–543. doi: 10.1128/jb.83.3.538-543.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTREMOLI S., DE FLORA A., GRAZI E., MANGIAROTTIG, BONSIGNORE A., HORECKER B. L. Crystalline D-gluconate 6-phosphate dehydrogenase. J Biol Chem. 1961 Nov;236:2975–2980. [PubMed] [Google Scholar]
- Rapoport M. I., Lust G., Beisel W. R. Host enzyme induction of bacterial infection. Arch Intern Med. 1968 Jan;121(1):11–16. [PubMed] [Google Scholar]
- Rees H. B., Jr, Weiss E. Glutamate catabolism of Rickettsia rickettsi and factors affecting retention of metabolic activity. J Bacteriol. 1968 Feb;95(2):389–396. doi: 10.1128/jb.95.2.389-396.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly L. I., Doelle H. W. Glucose-6-phosphate dehydrogenase in cell free extracts of Zymomonas mobilis. Arch Mikrobiol. 1968;63(3):197–213. doi: 10.1007/BF00412836. [DOI] [PubMed] [Google Scholar]


