Abstract
Previous investigations demonstrated that a standardized extract of ginger rhizome inhibited the growth of Helicobacter pylori in vitro with a minimum inhibitory concentration in the range 0.78 to 12.5 μg/mL. In the present work, the extract was tested in a rodent model of H. pylori-induced disease, the Mongolian gerbil, to examine the effects of the extract on both prevention and eradication of infection. The extract was administered to Mongolian gerbils at a daily dose of 100 mg/kg body weight in rations either 3 weeks prior to infection or 6 weeks post-infection. Treatment with the standardized ginger extract reduced H. pylori load as compared with controls and significantly (P<0.05) reduced both acute and chronic muscosal and submucosal inflammation, cryptitis, as well as epithelial cell degeneration and erosion induced by H. pylori. Importantly, the extract did not increase morbidity or mortality. Further investigations of the mechanism demonstrated that the ginger extract inhibited the activity of cyclooxygenase-2, with 50% inhibitory concentration (IC50) of 8.5 μg/mL in vitro, inhibited the nuclear factor-κB transcriptional response in kBZ Jurkat cells (human T lymphocytes) with an IC50 of 24.6 μg/mL, and significantly inhibited the release of interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α from lipopolysaccharide-stimulated human peripheral blood mononuclear cells with IC50 values of 3.89, 7.7, 8.5, and 8.37 μg/mL, respectively. These results suggest ginger extracts may be useful for development as agents to reduce H. pylori-induced inflammation and as for gastric cancer chemoprevention.
Keywords: Antibacterial, chemoprevention, gastric cancer, ginger, gingerol, Helicobacter pylori, Mongolian gerbil, peptic ulcer disease, Zingiber officinale
Introduction
In 1994, Helicobacter pylori was the first bacterium to be classified as a Group 1 carcinogen and a definite cause of gastric cancer in man by the International Agency for Research on Cancer (IARC, 1994). Since that time, H. pylori has been epidemiologically linked to adenocarcinoma of the distal stomach, as well as colorectal adenomas (Breuer-Katschinski et al., 1999; Scheiman & Culter, 1999; Figueiredo et al., 2002; Forman & Graham, 2004). cagA is the strain-specific H. pylori gene that has been linked to the development of pre-malignant and malignant histological lesions (Shmuely et al., 2001). Thus, susceptibility of cagA+ H. pylori strains is of note because, as compared with cagA− strains, infections caused by cagA+ strains significantly increase the risk for developing severe gastric inflammation, atrophic gastritis, and noncardia gastric adenocarcinoma (Shmuely et al., 2001). Although gastric cancer has been investigated for centuries, the association with H. pylori infections has been recognized for only the past few decades (Censini et al., 1996; Shmuely et al., 2001). Although the disease has declined in most industrialized countries, it remains the second most common cause of cancer death worldwide and is, in theory, largely preventable (Censini et al., 1996). Since gastric cancer is a malignancy with a high morbidity and mortality worldwide, there has been an increased interest in the development of alternative therapies that inhibit or reduce the growth of H. pylori and its carcinogenic processes.
Previously, we have reported that extracts of ginger (Zingiber officinale Roscoe, Zingiberaceae) rhizomes (root) inhibited the growth of 19 strains of H. pylori in vitro with a minimum inhibitory concentration range 0.78 to 12.5 μg/mL, with significant activity against the cagA+ strains (Mahady et al., 2003). These data suggested that specific ginger extracts containing the gingerols 6–10 may be of use for the treatment or prevention of H. pylori cagA+ strains in vivo. In the present work, we have assessed the effect of a standardized ginger extract on the prevention and treatment of H. pylori-induced infection and inflammation in Mongolian gerbils. In addition, further investigations were performed to elucidate the in vitro mechanism of action of the ginger extract.
Materials and methods
Plant materials and extract preparation
Dried ginger rhizomes (Z. officinale) were obtained from Frontier Natural Products (Norway, IA, USA). The plant materials were identified by macroscopic and microscopic analysis and by analytical HPLC. The dried and milled whole rhizomes (5 kg) were extracted as previously described (Mahady et al., 2003). The extracts were continuously fractionated over a silica gel column using a gradient solvent of chloroform (100–0%)–methanol (0–100%) until an active fraction containing 20±5% gingerols was obtained (Mahady et al., 2003). Analytical HPLC was performed on the active fraction and the gingerols (6-, 8-, 10-) and 6-shogoal (Figure 1) were present in a ratio of 7.5:1:13:2 (%), respectively. All four compounds had in vitro activity against H. pylori (Mahady et al., 2003). No one compound could explain the in vitro activity of active fraction; thus the active extract was standardized to contain 23.9% gingerols (6-, 8-, 10-) and 6-shogoal, with an enrichment for 10-gingerol.
Figure 1.
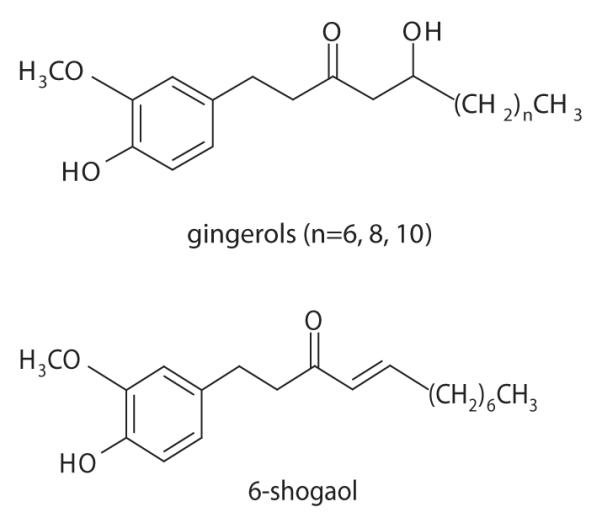
Structures of the gingerols and shogoal from ginger rhizome extract.
H. pylori assay
The cagA+ H. pylori strain B128 was maintained as previously described (Israel et al., 2001). Gram stain appearance and a positive urease test confirmed the identification of the organism. The bacterium was stored frozen at −70°C in skimmed milk plus 17% glycerol. For susceptibility testing, the organisms were inoculated on to 5% sheep blood agar plates, and incubated at 37°C in a 10% CO2 atmosphere for 72 h as described (Mahady et al., 2003). The organisms were inoculated on to agar plates containing consecutive dilutions of the plant extracts via a 32-prong inoculating device. The device delivers 8 μL per spot, resulting in a final inoculum of approximately 1×106 cfu/spot. After the spots dried, the plates were incubated at 37°C in 10% CO2 and examined for growth after 3 days. All procedures were performed in duplicate. The minimum inhibitory concentration (MIC), defined as the lowest concentration of the compound at which there was no visible growth or only a faint haze, was determined for each plant extract and pure compound. Assessment of bactericidal activity was performed as previously described (NCCLS, 2008).
Effect of ginger on H. pylori infection in Mongolian gerbils
Mongolian gerbils 4–8 weeks of age were purchased from either Harlan Sprague Dawley Inc. (Indianapolis, IN, USA) or Charles River Laboratories (Wilmington, MA, USA). All procedures used in this investigation were approved by the Institutional Animal Care Committees of Vanderbilt University and the University of Illinois at Chicago. Gerbil-passed cagA+ vacA strains B128 were used. Gerbils were inoculated with sterile broth or H. pylori by gastric lavage, as described previously (Israel et al., 2001). After treatment, the gerbils were sacrificed; one half of the glandular stomach was fixed in 10% neutral buffered formalin for histological examination, and the other half was homogenized in sterile phosphate-buffered saline (PBS) (pH 7.4), plated on selective Trypticase soy agar plates containing vancomycin (20 μg/mL), nalidixic acid (10 μg/mL), bacitracin (30 μg/mL), and amphotericin B (2 μg/mL), and grown for 4–5 days, as described previously (Mahady et al., 2003). Colonies were identified as H. pylori based on their characteristic morphology, and by urease and oxidase activities; colony counts were expressed as log cfu per stomach.
For prevention studies, specific pathogen-free, 6-week-old male gerbils were fed either a standard diet chow (Purina 5100, Nestlé Puina PetCase Company, St. Louis, MO) or the same chow supplemented with the botanical extract (providing 100 mg extract/kg body weight) for 3 weeks prior to challenge with H. pyori. Gerbils (n=14) were then inoculated with the rodent-adapted H. pylori cagA+ strain B128 (5×109 cfu). The animals were maintained on control or supplemented chow until sacrifice 6 weeks after inoculation. H. pylori strain B128 for each challenge was grown from freezer stocks for 24–36 h, harvested in Brucella broth (BBL, Becton, Dickenson and Company, Sparks, MA), and administered by gastric lavage to the animals immediately after harvest, as described previously (Israel et al., 2001). For the prevention study, at 6 weeks after the inoculation, all animals in the prevention study were sacrificed and their stomachs were resected along the greater curvature. For the treatment studies, gerbils (n=16) were inoculated with rodent-adapted H. pylori cagA+ strain B128 (5×109 cfu) and fed normal chow for 6 weeks; then one group (n=8) was fed chow supplemented with ginger extract (dose 100 mg/kg body weight per day) for 4 weeks and then returned to normal chow for a further 4 weeks. The control group (n=8) was maintained on normal chow. After 14 weeks, the animals were then sacrificed and assessed using the protocols described above. An outline of the prevention and treatment studies is given in Figure 2.
Figure 2.
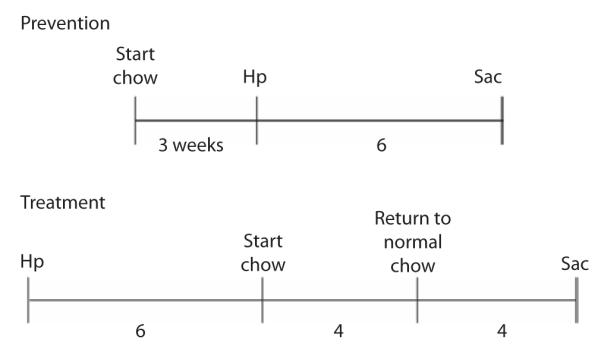
Outline of prevention and treatment studies in Mongolian gerbils infected with Helicobacter pylori (Hp) strain B128. In the prevention study, treatment chow was started 3 weeks prior to infection and lasted a total of 9 weeks prior to sacrifice (Sac). In the treatment study, treatment was started 6 weeks post-infection and lasted for 4 weeks, with a return to normal chow for 4 weeks following treatment, prior to sacrifice.
One half of the resected stomachs was fixed in 10% neutral buffered formalin for histological examination, and the other half was transferred to 1.0 mL of sterile 0.1 M PBS (pH 7.4), homogenized, plated on to selective Trypticase soy agar/5% sheep blood plates containing vancomycin (20 μg/mL), nalidixic acid (10 μg/mL), bacitracin (30 μg/mL), and amphotericin B (2 μg/mL) (Sigma Chemical Company, St. Louis, MO, USA), and grown for 3–5 days, as previously described (Israel et al., 2001). Colonies were identified as H. pylori based on their resistance to the antibiotics listed above, characteristic morphology, and by urease and oxidase activity, as previously described (Israel et al., 2001). The other half was washed with saline and then macroscopic gastric lesions (edema and hemorrhage) were recorded, followed by measurement of the wet weight of the whole stomach, including fore-stomach and glandular stomach. Half of the glandular mucosa was scraped for detection of colonizing H. pylori, and the residual part was formalin-fixed and embedded in paraffin for histological observation.
Histological examination and serum antibodies to H. pylori
Hematoxylin and eosin sections were examined by a single experienced pathologist, Dr K. Tham, Vanderbilt University, Nashville, TN, USA. For semi-quantitative estimates, the following parameters were graded from 0 to 3: acute and chronic inflammation, epithelial cell degeneration, and erosions (Table 1). Colonization density was determined by quantitative culture and acute inflammation and chronic inflammation (mononuclear cell infiltration independent from lymphoid follicles) were each graded from 0 to 3 in the gastric antrum.
Table 1.
Grading criteria for chronic inflammation and activity of gastritis.
| Grade | Chronic inflammation | Activity |
|---|---|---|
| 0 | no increase in number of inflammatory cells |
same |
| 1 | uniform infiltration of lamina propria by lymphocytes, plasma cells, and some eosinophils |
scattered neutrophils in the lamina propria with no leukopedesis in the region of the gastric pits |
| 2 | moderately dense infiltra- tion of the lamina propria by lymphocytes and plasma cells |
moderate number of neutrophils in the lamina propria with microabscess in the region of gastric pits |
| 3 | very dense lymphoplasma cell infiltration in the lamina propria |
extensive neutrophils in the lamina propria with obvious cryptitis |
Effect on cytokine release, COX-2, and NF-κβ transcriptional response in vitro
Cytokine release
Modulation of cytokine release was performed using the protocol of Welker et al. (1996). Briefly, peripheral blood mononuclear cells (MDS Pharma Services, Bothell, WA, USA) were treated with lipopolysaccharide (LPS) (25 ng/mL; Sigma Chemical Company) to stimulate the release of interleukin (IL)-1β and IL-6, and released cytokines in the cell supernatant were measured by ELISA. Cells (106/mL) with LPS were cultured for 16 h at 37°C in RPMI medium (Gibco, North Andoves, MA) without serum, alone, or with 0, 6.25, 12.5, 25, 50, or 100 μg ginger extract/mL. Positive control was dexamethasone. All experiments were performed in triplicate.
Assay for tumor necrosis factor (TNF)-α release from human peripheral blood mononuclear cells (5×105 cells; MDS Pharma Services) was performed in cells pre-incubated at 37°C for 1 h in 0.5 mL of medium containing 0, 6.25, 12.5, 25, 50, or 100 μg extract/mL. After three washes with PBS, the cells were further incubated at 37°C for 6 h in 0.5 mL of EMEM (Eagle’s Minimum Essential Medium) containing 10% fetal bovine serum in the presence of LPS (25 ng/mL) and the corresponding concentration of each drug (Welker et al., 1996). After 6 h incubation, TNF-α level in the conditioned medium was determined using a TNF-α ELISA kit (BioSource, Camarillo, CA) according to the manufacturer’s instructions.
COX-2 assay
Inhibition assays were performed by assessing human recombinant cyclooxygenase-2 (COX-2) catalytic activity as described previously by measuring prostaglandin E2 (PGE2) production (Warner et al., 1999). Reaction mixtures were prepared in 100 mM Tris–HCl buffer, pH 8.0, containing 1 μM heme, 500 μM phenol, 300 μM epinephrine, sufficient amounts of COX-2 to generate 150 ng PGE2/mL, and various concentrations of test samples. A standard curve for PGE2 (Cayman Chemical, Ann Arbor, MI, USA) was generated from the same plate, which was used to quantify the PGE2 levels produced in the presence of test samples. Results were expressed as a percentage relative to a control (solvent-treated samples). All determinations were performed in duplicate, and values generally agreed within 10%. Concentration–response curves were generated for the calculation of 50% inhibitory concentration (IC50) values.
Inhibition of NF-κB transcriptional response
For the detection of nuclear factor (NF)-κB, a non-radioactive method, NF-κB p50 Transcription Factor Assay (Chemicon International, Temecula, CA), was employed according to the instructions of the manufacturer. The ginger extract in concentration of 0, 6.25, 12.5, 25, 50, or 100 μg/mL or vehicle was incubated with the Jurkat T cells (1.5×106/mL) in the presence of A23187 (0.5 mM) and PMA (phorbol 12-myristate 13-acetate) (50 ng/mL) in RPMI-1640, pH 7.4, at 37°C for 4 h. The cells were harvested and the isolated nuclear extract was added to the capture probe in solution. The capture probe was a double-stranded biotinylated oligonucleotide containing the consensus sequence for NF-κB binding (5′-GGGACTTTCC-3′). The active form of NF-κB bound to its consensus sequence. After incubation, the extract/probe/buffer mixture was transferred to the streptavidin-coated 96-well plate. The biotinylated capture probe, which was bound by active protein, NF-κB was immobilized and any inactive, unbound material was washed away. The bound NF-κB transcription factor subunit was detected with a specific primary antibody for the NF-κB p50 subunit. The horseradish peroxidase-conjugated secondary antibody binds to the specific primary antibody. A chromogenic substrate was added and the relative amount of DNA bound was determined by measuring the absorbance of the samples. Several controls were used including an NF-κB specific competitor control, a negative control probe, and a positive control (TNF-α-treated HeLa whole cell extract). The NF-κB competitor control was an unlabeled oligonucleotide containing the identical consensus sequence as the NF-κB capture probe. The negative control probe is a non-specific oligonucleotide, which provides a background control for nonspecific DNA binding.
Statistical analysis
The Mann–Whitney U test was used to compare scores between samples, whereas scores for inflammation and proliferation within the same animals were compared by linear regression analysis. Proliferation and inflammation data are presented as the mean, and significance was defined as P≤0.05.
Results
Prevention of H. pylori infection in Mongolian gerbils
Ten of the 14 H. pylori-challenged gerbils were successfully colonized, indicating that these animals can be reproducibly infected with H. pylori strain B128. Of the gerbils that were successfully colonized, there were no differences in colonization density. The gerbils pretreated with the ginger extract for 3 weeks prior to infection showed a reduced H. pylori load. In addition, the parameters of acute and chronic inflammation induced by H. pylori were significantly decreased in treated animals as compared with the control group (Table 2). Both chronic (mean: 2.2 vs. 1.0; control vs. extract, respectively) and acute mucosal (mean: 2.0 vs. 0.6; control vs. extract, respectively, P<0.05) inflammation scores, as well as acute and chronic submucosal inflammatory parameters (mean: 1.3 vs. 0 and 1.6 vs. 0.25; control vs. extract respectively, P<0.01), were decreased in gerbils treated with extracts. These changes were paralleled by significant reductions in the severity of epithelial cell degeneration (1.8 control vs. 0.25 extract, P<0.01), cryptitis (0.8 control vs. 0.25 extract, P<0.05), and erosions (1.8 control vs. 0 extract, P<0.01). In general, acute submucosal inflammation and erosion were completely suppressed in animals maintained on a chow containing the standardized ginger extract. Importantly, this extract did not increase morbidity or mortality of the animals.
Table 2.
Bacterial load and inflammation induced by Helicobacter pyrlori infection in Mongolian gerbils fed a normal diet or a diet supplemented with standardized ginger extract: prevention study.
| CI |
AI |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Expt. | Food | Colonies | M | S | Lymph | M | S | C | ED | E |
| 1 | Control | 77.83 | 2.20 | 1.60 | 0.80 | 2.00 | 1.30 | 0.80 | 1.80 | 1.80 |
| 1 | Ginger | 56.71 | 1.00* | 0.25** | 0.25 | 0.60* | 0.00** | 0.25* | 0.25** | 0.00** |
Seven animals per arm; gerbils were fed a normal diet throughout (Purina 5001 chow; control) or a normal diet supplemented with 100 mg ginger extract /kg body weight in daily rations for 3 weeks prior to inoculation and 6 weeks after (9 weeks total); inflammation was graded using the criteria of Table 1. Colonies = bacterial load in log cfu per stomach; CI = chronic inflammation; Lymph = Lymphocyte infiltration; AI = acute inflammation; M = mucosal; S = submucosal; C = cryptitis; ED = epithelial cell degeneration; E = erosion. Mean value was significantly different from that of the control group:
P<0.05
P<0.01.
Treatment of H. pylori infection in Mongolian gerbils
The data from gerbils treated with the ginger extract for 4 weeks starting at 6 weeks post-infection are presented in Table 3. No significant reduction in bacterial load or suppression of any inflammatory parameters was observed. Chronic (mean: 2.25 vs. 2.66; control vs. ginger extract, respectively) and acute mucosal (mean: 3.0 vs. 2.66; control vs. extract, respectively) inflammation scores remained relatively unchanged. In addition, no reduction in the severity of epithelial cell degeneration, cryptitis, or erosions was observed in any of the treated animals.
Table 3.
Bacterial load and inflammation induced by Helicobacter pyrlori infection in Mongolian gerbils fed a normal diet or a diet supplemented with standardized ginger extract: treatment study.
| CI |
AI |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Expt. | Food | Colonies | M | S | Lymph | M | S | C | ED | E |
| 2 | Control | 70.40 | 2.25 | 3.00 | 1.50 | 3.00 | 2.00 | 1.00 | 2.00 | 2.00 |
| 2 | Ginger | 75.00 | 2.66 | 2.50 | 1.00 | 2.66 | 2.16 | 0.83 | 1.66 | 1.83 |
Eight per arm; gerbils were fed a normal diet throughout (Purina 5001 chow; control) or a normal diet supplemented with 100 mg ginger extract /kg body weight in daily rations for 4 weeks (6 weeks after inoculation) and then returned to normal chow for another 4 weeks; inflammation was graded using the criteria of Table 1. Colonies = bacterial load in log cfu per stomach; CI = chronic inflammation; Lymph = Lymphocyte infiltration; AI = acute inflammation; M = mucosal; S = submucosal; C = cryptitis; ED = epithelial cell degeneration; E = erosion.
In vitro, the ginger extract inhibited the activity of COX-2, with an IC50 of 8.5 μg/mL; inhibited the NF-κB transcriptional response in kBZ Jurkat cells (human T lymphocytes) with an IC50 of 24.6 μg/mL; and significantly inhibited the release of IL-1β, IL-6, IL-8, and TNF-α from LPS-stimulated human peripheral blood mononuclear cells with IC50 values of 3.89, 7.7, 8.5, and 8.37 μg/mL (P<0.05), respectively.
Discussion
For thousands of years, ginger has been used in traditional medicine to treat a wide range of disorders, including dyspepsia, peptic ulcer, motion sickness, and inflammatory diseases (Tjendraputra et al., 2001; Mahady et al., 2003). More recent investigations have demonstrated that ginger extracts and the gingerols have potent chemopreventive activities as well (Surh, 2002; Park & Pezzuto, 2002). For example, 6-gingerol inhibits tumor promotion in mouse skin; inhibits neoplastic transformation and activation of AP-1 in mouse epidermal JB6 cells treated with epidermal growth factor; suppresses the proliferation of human cancer cells through the induction of apoptosis; and abrogates pulmonary metastasis in mice implanted with B16F10 melanoma cells (Huang et al., 1996; Katiyar et al., 1996; Lee & Surh, 1998; Lee et al., 1998; Bode et al., 2001). Furthermore, dietary administration of gingerol to rodents ameliorated azoxymethane-induced intestinal tumorigenesis (Yoshimi et al., 1992).
In the present work, we have demonstrated that the administration of a standardized ginger extract (100 mg/kg body weight) three weeks prior to H. pylori challenge reduced bacterial load in Mongolian gerbils. Pretreatment also significantly reduced the acute and chronic inflammation, epithelial cell degeneration, erosion, and cryptitis induced by H. pylori infection. The Mongolian gerbil model is an exceptional model to investigate management of H. pylori infections for a number of reasons. First, Mongolian gerbils rarely have gastritis. Gastritis occurs in only 2% of animals, unless they are infected with H. pylori. Second, the CFU of H. pylori needed to colonize Mongolian gerbils is much less than in mice, but the histological changes found are typical and similar to changes that occur in H. pylori-infected humans, including gastric atrophy, ulcers, intestinal metaplasia, and adenocarcinomas. Third, the average lifespan of Mongolian gerbils is longer than that of mice, and so they are suitable for long-term studies (Yao et al., 2002). Furthermore, the histopathological and histochemical alterations found in the inflamed mucosa of experimentally infected Mongolian gerbils closely resemble those found in the H. pylori-infected human stomach. The infection in Mongolian gerbil induces chronic active gastritis, in which a marked mucosal infiltration of neutrophils on a background of chronic inflammation are detectable at 4 weeks after inoculation and may continue up to 52 weeks (Ikeno et al., 1999). Chronic inflammation is known to accelerate the development of neoplasms in the gastrointestinal tract, and H. pylori interacts with host cells to induce pro-inflammatory cytokines and free radicals. Free radicals cause mutations in target cells so that neoplastic clones are established. Accumulation of such genetic alterations may induce malignant transformation of some cell lines (Tsuji et al., 2003). In addition, inflammatory alterations may promote the growth, expansion, and invasion of gastrointestinal epithelial cells. The latter changes caused by inflammation may occur even without further genetic mutations or epigenetic alterations, and therefore may be categorized as ‘perigenetic alterations’ of neoplastic cells (Tsuji et al., 2003). Thus, reduction of bacterial load and prevention of H. pylori-induced chronic inflammatory processes in Mongolian gerbils pretreated with ginger may be one mechanism by which ginger acts as a chemopreventive agent.
The mechanism by which ginger reduces inflammation in response to bacterial infection is not well understood. However, cytokines are known to play an important role in H. pylori-associated gastrointestinal diseases, and infection induces a characteristic local inflammatory response in the gastric mucosa causing acute gastritis, which later develops into chronic gastritis (Wallace, 1991). Both forms of gastritis are characterized by a considerable neutrophil infiltration, which contributes to the induction of gastritis by releasing pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α, as well as free radicals (Kozol et al., 1991; Wallace, 1991; Suzuki et al. 1993). H. pylori and its products can recruit and activate neutrophils by directly stimulating these inflammatory cells or by stimulating the release of epithelial chemokines (Ernst et al., 1997). Other H. pylori components such as LPS and proteins can attract and activate neutrophils and other inflammatory cells, thereby stimulating the production of TNF-α, IL-1, IL-6, and IL-8 (Bliss et al., 1998; Bhattacharyya et al., 2000; Fujiwara et al., 2001). A significant increase in COX-2 expression and PGE2 synthesis has been observed in rats and Mongolian gerbils treated with H. pylori (Takahashi et al., 2000; Sakai et al., 2003). The cox-2 gene was upregulated during the acute phase of inflammation (1–3 months after infection) in Mongolian gerbils (Sakai et al., 2003), thus suggesting that pretreatment or treatment of the animals with COX-2 inhibitors at the time of infection may reduce COX-2 expression and inflammation induced by H. pylori. Our in vitro data indicate that ginger extracts inhibit the activity of COX-2, the NF-κB transcriptional response, and the release of IL-1β, IL-6, and IL-8. Thus, ginger may suppress H. pylori-induced acute and chronic inflammation through the inhibition of a number of components of this pro-inflammatory signaling pathway. This mechanism needs further elucidation in vivo.
Acknowledgements
This publication was made possible by Grant Number AT00412-02 (Mahady) from the National Center for Complementary and Alternative Medicine (NCCAM) of the National Institutes of Health (NIH). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or NIH.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bhattacharyya A, Pathak S, Datta S, Chattopadhyay S, Basu J, Kundu M. Mitogen-activated protein kinases and nuclear factor-κB regulate Helicobacter pylori-mediated interleukin-8 release from macrophages. Biochem J. 2000;368(Pt 1):121–129. doi: 10.1042/BJ20020555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss CM, Jr, Golenbock DT, Keates S, Linevsky JK, Kelly CP. Helicobacter pylori lipopolysaccharide binds to CD14 and stimulates release of interleukin-8, epithelial neutrophil-activating peptide 78, and monocyte chemotactic protein 1 by human monocytes. Infect Immun. 1998;66:5357–5363. doi: 10.1128/iai.66.11.5357-5363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer-Katschinski B, Nemes K, Marr A, Rump B, Leiendecker B, Breuer N, Goebell H. Helicobacter pylori and the risk of colonic adenomas. Colorectal Adenoma Study Group. Digestion. 1999;60:210–215. doi: 10.1159/000007661. [DOI] [PubMed] [Google Scholar]
- Bode AM, Wei-Ya Ma, Surh YJ, Dong ZG. Inhibition of epidermal growth factor-induced cell transformation and activator protein 1 activation by [6]-gingerol. Cancer Res. 2001;61:850–853. [PubMed] [Google Scholar]
- Censini S, Lange C, Xiang Z. Cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease associated virulence factors. Proc Natl Acad Sci U S A. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst PB, Crowe SE, Reyes V. How does Helicobacter pylori cause mucosal damage? The inflammatory response. Gastroenterology. 1997;113:S35–S42. doi: 10.1016/s0016-5085(97)80009-1. [DOI] [PubMed] [Google Scholar]
- Figueiredo C, Machado JC, Pharoah P. Helicobacter pylori and interleukin 1 genotyping: An opportunity to identify highrisk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680–1687. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- Forman D, Graham DY. Impact of Helicobacter pylori on society – role for a strategy of ‘search and eradicate’. Aliment Pharmacol Ther. 1994;19(Suppl 1):17–21. doi: 10.1111/j.0953-0673.2004.01831.x. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Higuchi K, Fukuda T, Watanabe T, Tominaga K, Arakawa T. Inhibitory effect of sofalcone on tumor necrosis factor-α and interleukin-1β production in human monocytes stimulated by Helicobacter pylori water extract. Drugs Exp Clin Res. 2001;27:103–106. [PubMed] [Google Scholar]
- Huang C, Ma WY, Dong Z. Requirement for phosphatidylinositol 3-kinase in epidermal growth factor-induced AP-1 transactivation and transformation in JB6 P+ cells. Mol Cell Biol. 1996;16:6427–6435. doi: 10.1128/mcb.16.11.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Schistomsomes, Liver Flukes and Helicobacter pylori. Infections with Helicobacter pylori. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 61. International Agency for Research on Cancer; Lyon: 1994. pp. 177–201. [Google Scholar]
- Ikeno T, Ota H, Sugiyama A. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, and gastric ulcer in Mongolian gerbils. Am J Pathol. 1999;154:951–960. doi: 10.1016/S0002-9440(10)65343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel DA, Salama N, Arnold CN, Peek R., Jr Helicobacter pylori-strain specific differences in genetic content, identified by microarray, influence host inflammatory response. J. Clin Invest. 2001;107:611–620. doi: 10.1172/JCI11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar SK, Agarwal R, Mukhtar H. Inhibition of tumor promotion in SENCAR mouse skin by ethanol extract of Zingiber officinale rhizome. Cancer Res. 1996;56:1023–1030. [PubMed] [Google Scholar]
- Kozol RA, Domanowski R, Jaszewski R, Czanko B, McCurdy M, Prasad B, Fromm R. Neutrophil chemotaxis in gastric mucosa. A signal-to-response comparison. Dig Dis Sci. 1991;36:1277–1280. doi: 10.1007/BF01307522. [DOI] [PubMed] [Google Scholar]
- Lee E, Surh YJ. Induction of apoptosis in HL-60 cells by pungent vanilloids, [6]-gingerol, and [6]-paradol. Cancer Lett. 1998;134:163–168. doi: 10.1016/s0304-3835(98)00253-5. [DOI] [PubMed] [Google Scholar]
- Lee E, Park KK, Lee JM, Chun KS, Kang JY, Lee SS, Surh YJ. Suppression of mouse skin tumor promotion and induction of apoptosis in HL-60 cells by Alpinia oxyphylla Miquel (Zingiberaceae) Carcinogenesis (Lond) 1998;19:1377–1381. doi: 10.1093/carcin/19.8.1377. [DOI] [PubMed] [Google Scholar]
- Mahady GB, Pendland SL, Yun GS, Lu ZZ, Stoia A. Ginger (Zingiber officinale Roscoe) and the gingerols inhibit the growth of CagA+ strains of Helicobacter pylori. Anticancer Res. 2003;23:3699–3702. [PMC free article] [PubMed] [Google Scholar]
- NCCLS . Performance Standards for Antimicrobial Susceptibility Testing; Ninth Informational Supplement. NCCLS document M100-S9. National Committee for Clinical Laboratory Standards; Wayne, PA: 2008. pp. 120–126. [Google Scholar]
- Park EJ, Pezzuto JM. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:231–255. doi: 10.1023/a:1021254725842. [DOI] [PubMed] [Google Scholar]
- Sakai T, Fukui H, Franceschi F, Penland R, Sepulveda AR, Fujimori T, Terano A, Genta RM, Graham DY, Yamaoka Y. Cyclooxygenase expression during Helicobacter pylori infection in Mongolian gerbils. Digest Dis Sci. 2003;48:2139–2146. doi: 10.1023/b:ddas.0000004517.83166.26. [DOI] [PubMed] [Google Scholar]
- Scheiman JM, Cutler AF. Helicobacter pylori and gastric cancer. Am J Med. 1999;106:222–226. doi: 10.1016/s0002-9343(98)00393-3. [DOI] [PubMed] [Google Scholar]
- Shmuely H, Passaro D, Figer A, Niv Y, Pitlik S, Samra Z, Koren R, Yahav J. Relationship between Helicobacter pylori CagA status and colorectal cancer. Am J Gastroenterol. 2001;96:3406–3410. doi: 10.1111/j.1572-0241.2001.05342.x. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review. Food Chem Toxicol. 2002;40:1091–1097. doi: 10.1016/s0278-6915(02)00037-6. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Miura S, Suematsu M. Helicobacter pylori elicits gastric mucosal cell damage associated with neutrophil-derived toxic oxidants. Eur J Gastroenterol Hepatol. 1993;5:S35–S39. [Google Scholar]
- Takahashi S, Fujita T, Yamamoto A. Role of cyclooxygenase-2 in Helicobacter pylori-induced gastritis in Mongolian gerbils. Am J Physiol Gastrointest Liver Physiol. 2000;279:G791–G798. doi: 10.1152/ajpgi.2000.279.4.G791. [DOI] [PubMed] [Google Scholar]
- Tjendraputra E, Tran VH, Liu-Brennan D, Roufogalis BD, Duke CC. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg Chem. 2001;29:156–163. doi: 10.1006/bioo.2001.1208. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Kawai N, Tsujii M, Kawano S, Hori M. Inflammation-related promotion of gastrointestinal carcinogenesis – a perigenetic pathway. Aliment Pharmacol Ther. 2003;18(Suppl 1):82–89. doi: 10.1046/j.1365-2036.18.s1.22.x. [DOI] [PubMed] [Google Scholar]
- Wallace JL. Possible mechanisms and mediators of gastritis associated with Helicobacter pylori infection. Scand J Gastroenterol. 1991;187:65–70. [PubMed] [Google Scholar]
- Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. Proc Natl Acad Sci U S A. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker P, Lippert U, Nurnberg W, Kruger-Krasagakes S, Moller A, Czarnetzki BM. Glucocorticoid-induced modulation of cytokine secretion from normal and leukemic human myelo-monocytic cells. Int Arch Allergy Immunol. 1996;109:110–115. doi: 10.1159/000237208. [DOI] [PubMed] [Google Scholar]
- Yao YL, Zhang WD, Xu B. Long-term infection with Helicobacter pylori in Mongolian gerbils. Chin J Digest Dis. 2002;3:115–119. [Google Scholar]
- Yoshimi N, Wang A, Morishita Y, Tanaka T, Sugie S, Kawai K, Yamahara J, Mori H. Modifying effects of fungal and herb metabolites on azoxymethane-induced intestinal carcinogenesis in rats. Jpn J Cancer Res. 1992;83:1273–1278. doi: 10.1111/j.1349-7006.1992.tb02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


