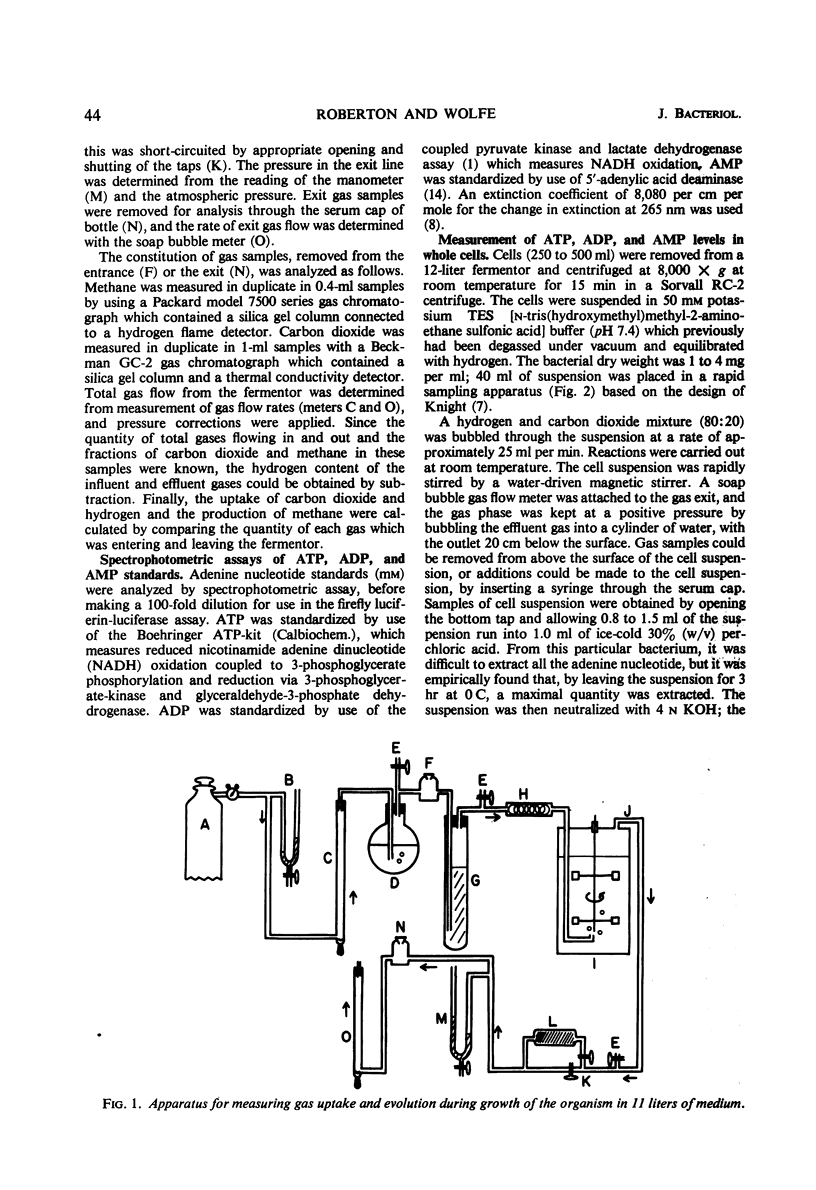
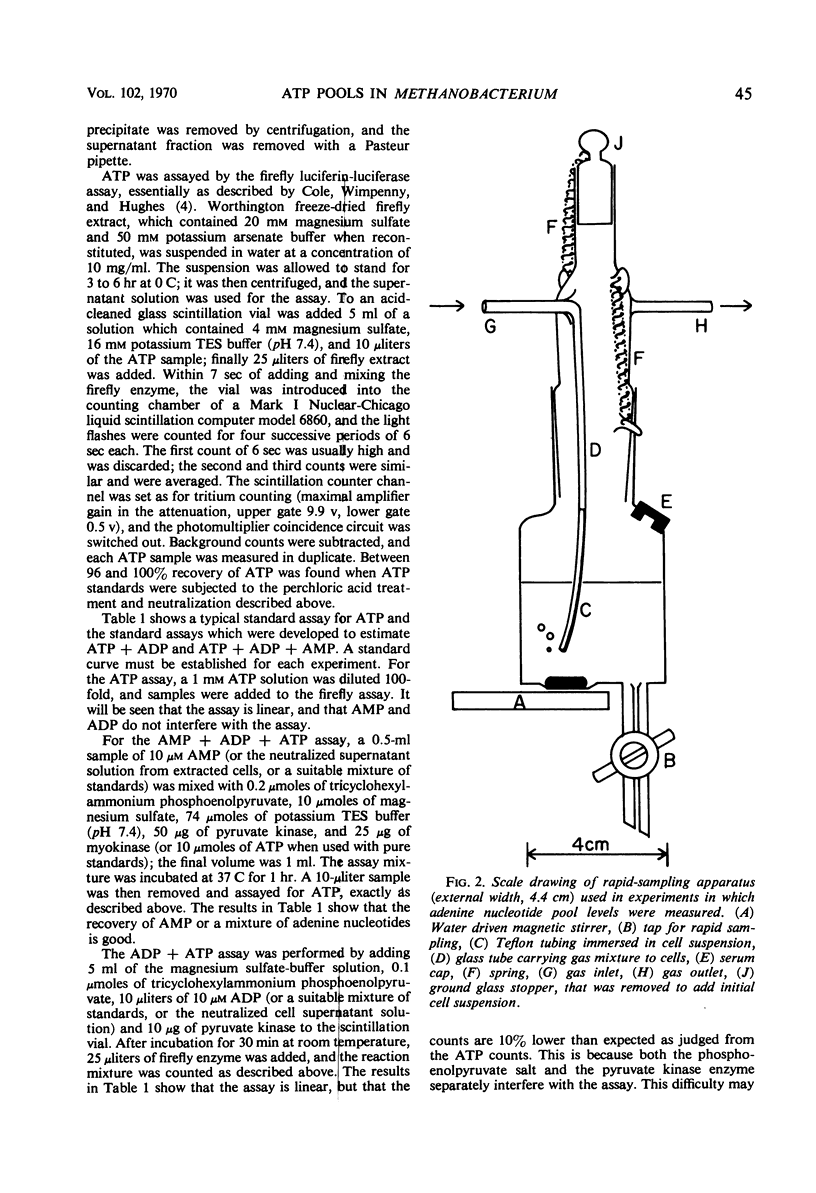
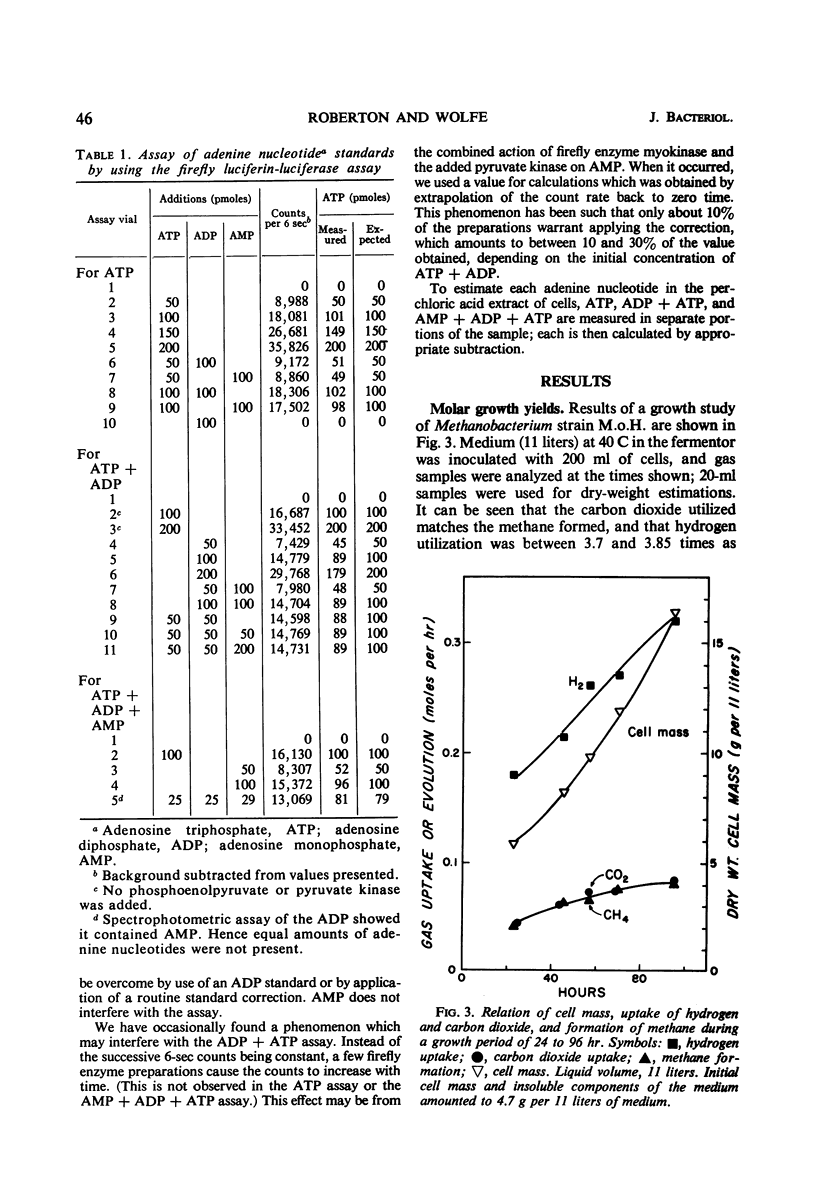
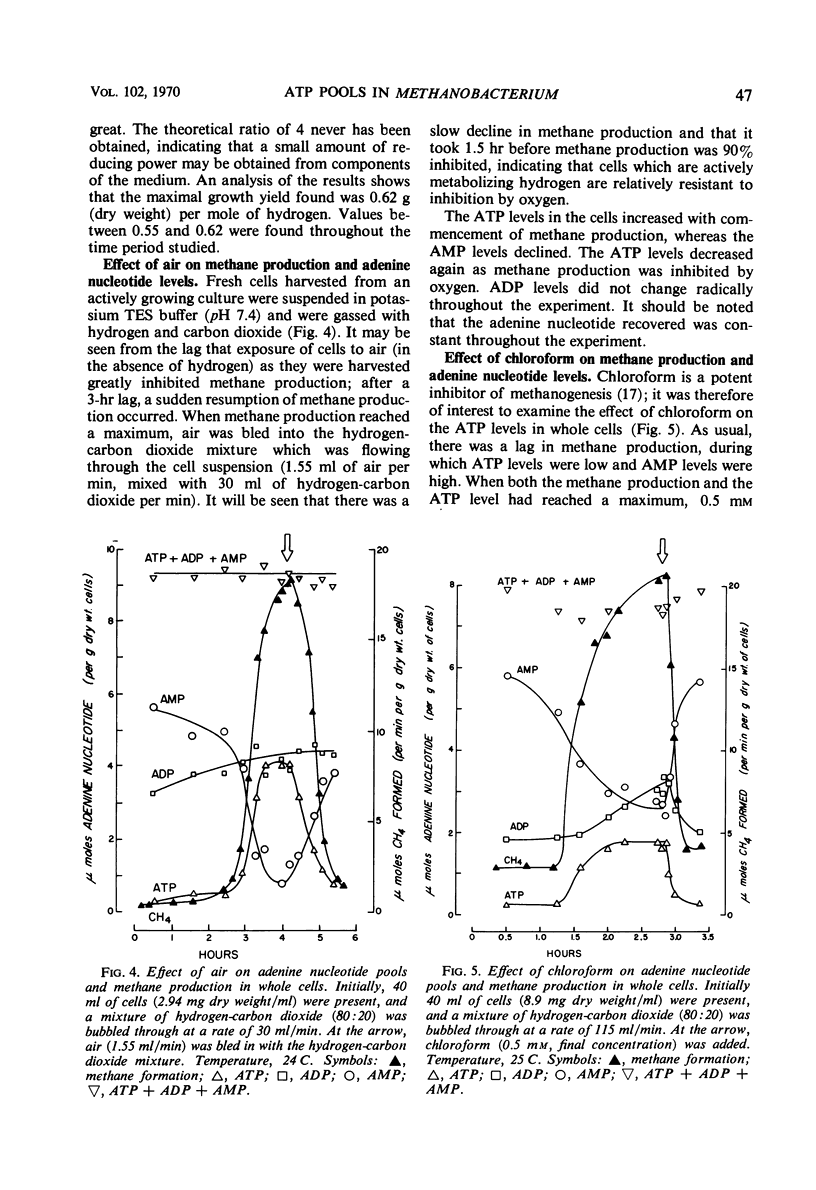
Abstract
Certain aspects of adenosine triphosphate (ATP) metabolism in the strict anaerobe Methanobacterium strain M.o.H. have been investigated. Results of growth yield studies suggest that ATP conservation is very inefficient (0.06 mole of ATP per mole of hydrogen) under the conditions used to grow the bacterium in a fermentor. Experiments designed to demonstrate net ATP formation in cell-free extracts were negative. In whole-cell studies, substances which decreased ATP pool levels and increased adenosine monophosphate (AMP) pool levels were air, chloroform, 2,4-dinitrophenol, carbonylcyanide-m-chlorophenylhydrazone, and pentachlorophenol. The results suggest that the latter compounds act either as inhibitors of electron transport or as uncouplers of an energy-linked process. All the above compounds also inhibit methane formation in cell-free extracts, an ATP-requiring process. Methods are described for estimation of ATP, adenosine diphosphate (ADP), and AMP in whole cells, with a sensitivity in the range of 10 to 200 pmoles. An apparatus for quick sampling from an anaerobic suspension of whole cells also is described.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., McBride B. C., Wolfe R. S. Hydrogen-oxidizing methane bacteria. I. Cultivation and methanogenesis. J Bacteriol. 1968 Mar;95(3):1118–1123. doi: 10.1128/jb.95.3.1118-1123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIESOW L. ON THE ASSIMILATION OF ENERGY FROM INORGANIC SOURCES IN AUTOTROPHIC FORMS OF LIFE. Proc Natl Acad Sci U S A. 1964 Oct;52:980–988. doi: 10.1073/pnas.52.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIGHT M. The photometabolism of propionate by Rhodospirillum rubrum. Biochem J. 1962 Jul;84:170–185. doi: 10.1042/bj0840170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHNISHI T. Oxidative phosphorylation coupled with nitrate respiration with cell free extract of Pseudomonas denitrificans. J Biochem. 1963 Jan;53:71–79. doi: 10.1093/oxfordjournals.jbchem.a127660. [DOI] [PubMed] [Google Scholar]
- Ota A. Oxidative phosphorylation coupled with nitrate respiration. 3. Coupling factors and mechanism of oxidative phosphorylation. J Biochem. 1965 Aug;58(2):137–144. doi: 10.1093/oxfordjournals.jbchem.a128175. [DOI] [PubMed] [Google Scholar]
- Peck H. D., Jr Energy-coupling mechanisms in chemolithotrophic bacteria. Annu Rev Microbiol. 1968;22:489–518. doi: 10.1146/annurev.mi.22.100168.002421. [DOI] [PubMed] [Google Scholar]
- Peck H. D., Jr Phosphorylation coupled with electron transfer in extracts of the sulfate reducing bacterium, Desulfovibrio gigas. Biochem Biophys Res Commun. 1966 Jan 4;22(1):112–118. doi: 10.1016/0006-291x(66)90611-5. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Methane fermentation. Annu Rev Microbiol. 1967;21:121–142. doi: 10.1146/annurev.mi.21.100167.001005. [DOI] [PubMed] [Google Scholar]
- Switzer R. L. Regulation and mechanism of phosphoribosylpyrophosphate synthetase. I. Purification and properties of the enzyme from Salmonella typhimurium. J Biol Chem. 1969 Jun 10;244(11):2854–2863. [PubMed] [Google Scholar]
- Wolin E. A., Wolfe R. S., Wolin M. J. Viologen dye inhibition of methane formation by Methanobacillus omelianskii. J Bacteriol. 1964 May;87(5):993–998. doi: 10.1128/jb.87.5.993-998.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M., Kennedy F. S., Wolfe R. S. The reaction of multihalogenated hydrocarbons with free and bound reduced vitamin B 12. Biochemistry. 1968 May;7(5):1707–1713. doi: 10.1021/bi00845a013. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Wolfe R. S. Components required for the formation of CH-4 from methylcobalamin by extracts of Methanobacillus omelianskii. J Bacteriol. 1966 Sep;92(3):696–700. doi: 10.1128/jb.92.3.696-700.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMANAKA T., OTA A., OKUNUKI K. Oxidative phosphorylation coupled with nitrate respiration. I. Evidence for phosphorylation coupled with nitrate reduciton in a cell-free extract of Pseudomonas aeruginosa. J Biochem. 1962 Apr;51:253–258. doi: 10.1093/oxfordjournals.jbchem.a127529. [DOI] [PubMed] [Google Scholar]


