Abstract
We have previously shown that 17beta-estradiol (E2) increases vascular endothelial growth factor A (Vegfa) gene expression in the rat uterus, resulting in increased microvascular permeability, and that this involves the simultaneous recruitment of hypoxia-inducible factor 1 (HIF1) and estrogen receptor alpha (ESR1) to the Vegfa gene promoter. Both events require the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway. However, those studies were carried out using whole uterine tissue, and while most evidence indicates that the likely site of E2-induced Vegfa expression is luminal epithelial (LE) cells, other studies have identified stromal cells as the site of that expression. To address this question, the pathway regulating Vegfa expression was reexamined using LE cells rapidly isolated after E2 treatment. In addition, we further characterized the nature of the receptor through which E2 triggers the signaling events that lead to Vegfa expression using the specific ESR1 antagonist ICI 182,780. In agreement with previous results in the whole uterus, E2 stimulated Vegfa mRNA expression in LE cells, peaking at 1 h (4- to 14-fold) and returning to basal levels by 4 h. Treatment with E2 also increased phosphorylation of AKT in LE cells, as well as of the downstream mediators FRAP1 (mTOR), GSK3B, and MDM2. The alpha subunit of HIF1 (HIF1A) was present in LE cells before E2 treatment, was unchanged 1 h after E2, but was >2-fold higher by 4 h. Chromatin immunoprecipitation analysis showed that HIF1A was recruited to the Vegfa promoter by 1 h and was absent again by 4 h. The E2 activation of the PI3K/AKT pathway, HIF1A recruitment to the Vegfa promoter, and Vegfa expression were all blocked by ICI 182,780. In summary, the rapid E2-induced signaling events that lead to the expression of Vegfa observed previously using the whole uterus occur in LE cells and appear to be initiated via a membrane form of ESR1.
Keywords: AKT, ChIP, chromatin immunoprecipitation, endometrium, ERα, ESR1, estradiol, 17β-estradiol, estrogen, estrogen receptor α, HIF-1α, HIF1A, hypoxia-inducible factor 1 alpha, PI3K, PI3K/AKT pathway, rat, signal transduction, uterus, vascular endothelial growth factor, vascular endothelial growth factor A, VEGF, VEGFA, Vegfa
Estradiol rapidly induces Vegfa expression in rat endometrial epithelial cells via an ICI 182,780-inhibited receptor, activation of the PI3K/AKT signaling pathway, and recruitment of the transcription factor HIF1 to the Vegfa gene promoter.
INTRODUCTION
During the reproductive cycle, the uterine endometrium undergoes a precisely timed complex sequence of physiological and morphological changes in preparation for implantation. These changes are controlled primarily by the ovarian steroid hormones 17β-estradiol (E2) and progesterone. The E2 regulates the immediate preovulatory phase (proestrus in rodents), which is characterized by endometrial stromal edema, and is followed ∼16 h later by a wave of luminal epithelial (LE) cell proliferation. This proliferation is considered one of the critical effects of E2 on the endometrium, but the mechanisms involved are still incompletely understood. A primary mediator of the effects of E2 in the endometrium is vascular endothelial growth factor A (VEGFA) [1–4]. E2 rapidly (within ∼1 h) and robustly increases Vegfa gene expression in the uterus [1, 4]. While this increase is transient, consistent with its being an immediate early gene response [1], it causes the increased stromal microvascular permeability and plasma efflux that are the hallmarks of the initial action of E2 in the uterus [2]. Evidence indicates that this acute exudation of plasma is essential for subsequent LE cell proliferation, angiogenesis, and other growth and remodeling events [5–8]. We recently showed that E2 induction of Vegfa expression in the uterus requires the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway and involves the recruitment of 1) the heterodimeric transcription factor hypoxia-inducible factor 1 (HIF1), which is made up of HIF1A (also known as HIF-1α) and the aryl hydrocarbon receptor nuclear translocator (ARNT [also known as HIF-1β]) to the hypoxia response element (HRE), and 2) estrogen receptor alpha (ESR1) to proximal transacting transcription factor 1 (SP1)-binding sites on the Vegfa gene promoter [4, 9]. This represents the first demonstration of a specific role for HIF1—which is increasingly recognized to be a central regulator of normal development, postnatal physiology, and cancer and other pathologies [10–12]—in a biological action of E2.
These findings were made using the whole uterus, which is a complex multilayered organ. Previous in situ hybridization (ISH) studies [13–15] in intact rodents indicated that the site of the rapid E2-induced Vegfa expression in the uterus is the LE cell layer of the endometrium. Other studies [16, 17] performed in ovariectomized animals, however, identified sub-LE stromal cells as the initial site of E2-induced Vegfa expression. Establishing the exact site of Vegfa expression in the endometrium is essential because many effects of E2 on the uterus, as well as the mammary gland, appear to require interactions between epithelial cells and the stroma [18, 19], although the nature of those interactions is controversial. VEGFA could be the key to that interaction. Identifying the cell type in which Vegfa expression initially occurs could help to explain inconsistencies in the literature about both the uterus and mammary gland concerning 1) the relative roles of ESR1 in epithelial cells and stromal cells in the proliferation of the former, 2) the nature of the stromal contribution to that proliferation, and 3) the apparent additional requirement for systemic factors [7, 18, 20–23]. Therefore, to clarify whether LE cells express the Vegfa gene in response to E2 in the normal uterus and to confirm that the associated signaling events previously identified using the whole uterus occur in this cell type, we reexamined those events in LE cells rapidly isolated after E2 treatment. We also extended the analysis of the role of the PI3K/AKT pathway in E2 action by examining E2-induced phosphorylation of the downstream PI3K/AKT mediators FRAP1 (also known as molecular target of rapamycin or mTOR), GSK3B (glycogen synthase kinase 3 beta), and MDM2 (mouse double minute protein).
Given that PI3K is a plasma membrane-localized enzyme, its activation by E2 most likely is initiated through a membrane form of ESR1 [24]. In most cases, E2 activation of cytoplasmic signaling pathways is blocked by the specific ESR1 antagonist ICI 182,780 [25–29], indicating that a form of ESR1 is involved; however, exceptions to this have been reported [30–33]. The second major objective of these experiments, therefore, was to determine whether the rapid activation of the PI3K pathway by E2 that leads to HIF1A activation in the uterus is initiated via an ICI 182,780-sensitive receptor.
MATERIALS AND METHODS
Animals, Treatments, and Tissue Collection
Animal studies were conducted in accord with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and approved by the Institutional Animal Care and Use Committee, University of Maryland School of Medicine. Immature (21-day-old) female Sprague-Dawley rats (Charles River, Wilmington, MA) were injected (s.c.) with either E2 (0.05 μg/g body weight) or vehicle (ethanol:PBS ratio, 1:500). In some experiments, ICI 182,780 (3 μg/g body weight [i.p.]; Sigma-Aldrich, St. Louis, MO) or vehicle (dimethylsulfoxide [DMSO]) was administered 1.5 h before E2 treatment. For the experiment on the role of FRAP1 in E2-induced VEGF expression, rapamycin (5 μg/g body weight [i.p.]; LC Laboratories, Woburn, MA) or vehicle (DMSO) was injected 1 h before E2 treatment.
Animals were killed by cervical dislocation 1 or 4 h after E2 treatment, the reproductive tract was exposed through a midline incision, and the uterus and ovaries were excised together and placed on a moistened paper towel on top of a frozen gel pack. The ovaries and oviducts, fat, and mesometrial membranes were quickly trimmed away, and the uterus was weighed.
Processing of Whole Uterine Samples
For experiments involving whole uterine tissue, one horn was stored in RNAlater (Qiagen, Valencia, CA) at 4°C. The other horn was flash frozen in liquid nitrogen and stored at −80°C for protein extraction. For chromatin immunoprecipitation (ChIP), uterine horns were slit open longitudinally and fixed in 1.5% formaldehyde/Dulbecco modified Eagle medium-F12 for 15 min at room temperature [9]. Fixation was stopped by adding 1 M glycine to a final concentration of 0.25 M and incubation for 10 min. The fixed horns were rinsed in ice-cold PBS (Quality Biologicals, Gaithersburg, MD) plus 1× protease and 1× phosphatase inhibitor cocktails (Roche Applied Sciences, Indianapolis, IN), hereafter referred to as PBS+, and stored at −80°C for later processing (described herein).
LE Cell Isolation
The LE cells were isolated as previously described by Fagg et al. [34] and by Chen et al. [19]. Briefly, uterine horns were slit open longitudinally and rinsed in 1 ml of ice-cold PBS+. Four to six horns were placed in a round-bottom 14-ml (17 × 100 mm) polypropylene tube containing 1 ml of ice-cold PBS+ and five 3-mm polytetrafluoroethylene solid balls (Fisher Scientific, Fair Lawn, NJ). The horns were then vortexed for six 30-sec cycles with 30 sec on ice between cycles. Residual tissue was removed with forceps, flash frozen in liquid nitrogen, and stored at −80°C. The supernatant containing the LE cells was aspirated and divided into aliquots appropriate for RNA (100 μl) and protein (100 μl) extraction or ChIP analysis (800 μl).
For ChIP, an equal volume (800 μl) of 2% formaldehyde in PBS was added to LE cell aliquots to give a final formaldehyde concentration of 1%, and the cells were fixed for 10 min at room temperature. Fixed cells were pelleted by centrifugation at 300 × g for 5 min at 4°C. They were then rinsed by resuspension in 1 ml of ice-cold PBS+ and centrifuged again. The supernatant was removed, and the fixed cell pellet was flash frozen in liquid nitrogen and stored at −80°C.
RNA Extraction
The LE cell aliquots for RNA were pelleted by centrifugation (300 × g for 5 min) at 4°C, and the supernatant was discarded. The LE cell pellets were resuspended and homogenized in Buffer RLT (Qiagen) by centrifugation through QIAshredder spin columns (Qiagen). Frozen whole uterine tissue or residual tissue was homogenized in ice-cold Buffer RLT using a Mini Beadbeater and 1.0-mm zirconia/silica beads (BioSpec Products, Bartlesville, OK). The beads were removed, and the samples were further homogenized and sheared by centrifugation through QIAshredder spin columns. Total RNA was extracted and purified from homogenates using the RNeasy Mini Kit (Qiagen). The RNA concentration and purity were determined based on 260/280-nm absorbances.
RT-PCR Analysis
Vegfa and progesterone receptor (Pgr) mRNA expression in LE cells and whole or residual uterine tissue was measured using both conventional and real-time RT-PCR as described previously [4, 9]. The following PCR primers were used in both cases: rat Vegfa +12 to +644: forward 5′-GCTCTCTTGGGTGCACTGGA-3′ and reverse 5′-CACCGCCTTGGCTTGTCACA-3′ (GenBank accession No. NM031836); rat Pgr +1910 to +2359: forward 5′-TTATGAGAGCCCTCGATGGT-3′ and reverse 5′-GCGAGTAGAATGACAACTC-3′ (GenBank accession No. L16922); and Rn18s (18S rRNA) +364 to +647: forward 5′-CAACTTTCGATGGTAGTCGC-3′ and reverse 5′-CGCTATTGGAGCTGGAATTAC-3′ (GenBank accession Nos. X01117 and H01593). Rat Hif1a primers were +681 to +890: forward 5′-TGCTTGGTGCTGATTTGTGA-3′ and reverse 5′-GGTCAGATGATCAGAGTCCA-3′ (GenBank accession Nos. AF057308 and NM024359).
Protein Extraction
The LE cell aliquots were centrifuged at 300 × g for 5 min at 4°C, and the supernatant was discarded. The cell pellets were resuspended and lysed in 150 μl of radioimmunoprecipitation (RIPA) buffer (50 mM Tris-HCl [pH 7.4], 1% Nonidet P-40, 0.25% deoxycholic acid, 1 mM edetic acid [EDTA], and protease and phosphatase inhibitors as already described). The samples were then cleared by centrifugation at 3000 rpm for 30 min at 4°C, and the supernatant was collected and stored at −80°C.
Whole uterine tissue was homogenized on ice in 200 μl of RIPA buffer using a Tissue-Tearor rotor-stator homogenizer (Biospec Products) at power setting 1.5. The homogenate was then cleared by centrifugation as already described, and the supernatant was collected and stored at −80°C.
Western Blot Analysis
Protein concentration was determined using the BCA Protein Assay (Pierce Chemical, Rockford, IL). Six to ten micrograms of protein was added to 15 μl of 2× SDS loading buffer (0.125 M Tris [pH 6.8], 4% SDS, 20% glycerol, 10% β-mercaptoethanol, and 0.004% bromophenol blue) and incubated at 100°C for 5 min. Protein samples and 10 μl of Precision Plus Protein dual-color standards (Biorad Laboratories, Hercules, CA) were loaded onto 4%–12% gradient Bis-Tris NuPAGE gels (Invitrogen, Carlsbad, CA) and run at ∼200 V for 1 h. Proteins were then transferred overnight onto polyvinylidene fluoride membranes (Fisher Scientific). Membranes were blocked with 5% nonfat dry milk (NFD)/1× Tris-buffered saline (TBS) with 0.1% Tween-20 (TTBS) for 1 h at 37°C before being incubated with primary antibodies (Supplemental Table 1 available at www.biolreprod.org) in 1% NFD/1× TBS with 0.05% Tween-20 for either 2 h at room temperature or overnight at 4°C. After four 5-min washes in TTBS, the membranes were incubated for 1 h with either goat anti-mouse horseradish peroxidase (HRP)-conjugated antibody (1:10 000; Jackson Immunoresearch Laboratories, West Grove, PA) or goat anti-rabbit HRP-conjugated antibody (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA) in 1% NFD/1× TBS with 0.05% Tween-20. The membranes then received four 5-min washes in TTBS. Western Lightning Chemiluminescence Reagent Plus (PerkinElmer, Boston, MA) was used for visualization of protein bands according to the manufacturer's instructions. Whenever blots were reprobed, they were first stripped with Restore Western Blot Stripping Buffer (Pierce Chemical) for 30 min at 37°C.
ChIP Analysis
ChIP analyses of LE cells and whole uterine tissue were done as previously described [4]. Frozen LE cell pellets were resuspended in nuclear lysis buffer (50 mM Tris-HCl [pH 8.0], 5 mM EDTA, 1% SDS, and protease inhibitors as already described) and incubated on ice for 15 min. Frozen fixed whole uterine tissue was homogenized on ice in 200 μl of RIPA buffer. The homogenate was then centrifuged at 5000 rpm for 5 min at 4°C. The supernatant was discarded, and the nuclear pellet was resuspended in nuclear lysis buffer (as already described) and incubated on ice for 15 min. Nuclear samples from both LE cells and whole uterine tissue were sonicated on ice for ten 10-sec cycles with 20-sec pauses between each cycle using a Microson Ultrasonic cell disruptor (Misonix, Farmingdale, NY) at power level 2.5. After sonication, the samples were centrifuged at 14 000 × g for 10 min at 4°C. The supernatants were then collected and stored at −80°C.
Primers that encompassed the rat Vegfa promoter region −944 to −611 (HRE-containing region) were used for PCR [9]. Theses were forward 5′-TCTGCCAGACTCCACAGTG-3′ and reverse 5′-TGCGTGTTTCTAACACCCAC-3′ (Genbank accession No. U22373).
Statistical Analysis
Statistical analyses were done using factorial analysis of variance and appropriate post hoc tests. An exception was the uterine weight data, which were analyzed using Kruskal-Wallis test (StatView version 4.5; Abacus Concepts, Berkeley, CA).
RESULTS
E2 Activates the PI3K/AKT Pathway in LE Cells
The mechanical method used to rapidly isolate LE cells from the uterus has been reported to yield an LE cell fraction of >95% purity [19]. To confirm that cells isolated in this way do not contain a significant level of stromal cell contamination, we examined a functional end point that distinguishes LE cells from stroma, namely, E2 induction of Pgr mRNA expression. E2 significantly increases Pgr expression in the rodent uterus [9, 35], and several studies [36–39] have shown that this occurs exclusively in stromal cells. Pgr mRNA levels in the stroma-containing residual tissue were increased significantly (2.6-fold [P < 0.05]) at 1 h after E2 treatment, whereas Pgr expression in isolated LE cells went in the opposite direction, with message becoming almost undetectable by 4 h. This confirmed that the LE cell fraction was not contaminated to a significant degree by stromal cells.
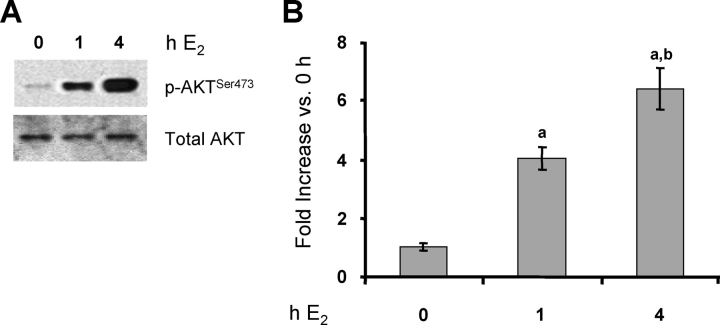
The rapid E2 induction of Vegfa expression in the uterus involves activation of the PI3K/AKT pathway and subsequent recruitment of both nuclear ESR1 and HIF1A to the Vegfa gene promoter [4, 9]. Therefore, we first determined whether activation of this signaling pathway can be demonstrated in LE cells. As shown in Figure 1, there were ∼4-fold and ∼6-fold increases in AKT phosphorylation on serine473 at 1 h and 4 h after E2 treatment, respectively. The total AKT level was unaffected by E2 at either time point.
FIG. 1.
E2 activates the PI3K/AKT pathway in endometrial LE cells. Rats were treated with E2 for the indicated times or with vehicle (0 h). Total protein was extracted from LE cells, and phosphorylated and total AKT was analyzed by Western blot analysis (representative gels [A] and densitometry [B]). Densitometry results are expressed as the fold increase in phosphorylated (p)-AKTSer473 compared with control cells (0-h E2) after normalization to total AKT (mean ± SEM, n = 3 independent LE cell samples/group; each sample contained cells pooled from two uteri). a, vs. 0-h E2 (P < 0.01); b, vs. 1-h E2 (P < 0.05).
Phosphorylation of AKT leads in turn to activation of a wide range of downstream mediators (including FRAP1 and MDM2) and to inactivation of others (such as GSK3B), but there have been few studies of E2 regulation of the activity of these proteins in the normal uterus. To determine whether these mediators are phosphorylated in LE cells in response to E2, Western blot analyses using phosphospecific antibodies were carried out. Values were normalized to the value of total ESR1 protein, which was unchanged after E2 treatment at these time points. As shown in Figure 2, E2 progressively increased phosphorylation of FRAP1, MDM2, and GSK3B after 1 h (2- to 4-fold) and 4 h (5- to 9-fold). In addition, total levels of all three proteins were significantly elevated by 4 h (>2.5-fold in each case). Thus, all of these factors are potential mediators of E2-induced effects on uterine LE cells.
FIG. 2.
E2 induces phosphorylation of FRAP1, MDM2, and GSK3B in LE cells. Rats were treated as described in Figure 1. Phosphorylated (p) and total FRAP1, MDM2, and GSK3B and total ESR1 were analyzed by Western blot analysis (representative blots [A] and densitometry [B]). Densitometry results are expressed as the fold increase in phosphorylated protein compared with that in control cells (0-h E2) after normalization to total ESR1 protein (mean ± SEM, n = 3 LE cell samples/group). a, vs. 0-h E2 (P < 0.01); b, vs. 0-h E2 (P < 0.05); c, vs. 1-h E2 (P < 0.05).
Activation of the PI3K/AKT Pathway in the Uterus Is Blocked by ICI 182,780
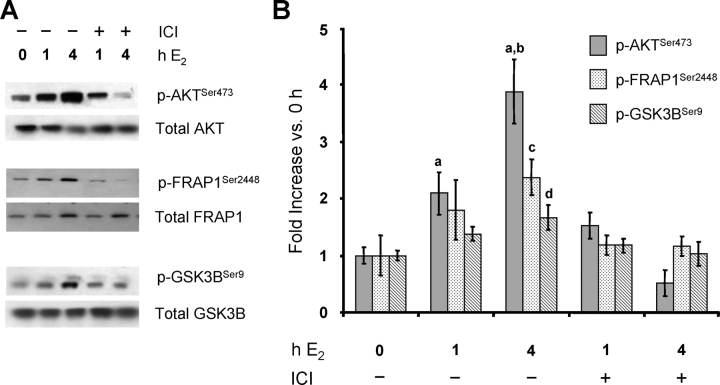
The rapid activation of PI3K, an event that occurs at the plasma membrane, by E2 suggests that it is mediated by a membrane-associated form of ESR1 [24]. Several studies [27, 28, 40–44] have shown that the effects of membrane ESR1, like those of the nuclear form, are blocked by the specific ESR1 antagonist ICI 182,780. Therefore, we determined whether the rapid activation of the PI3K/AKT pathway in the rat uterus is blocked by this antagonist. Having demonstrated that PI3K/AKT activation is similar regardless of whether it is measured in isolated LE cells (Figs. 1 and 2) or in whole uterus [9], this analysis was conducted using the latter. As shown in Figure 3, the E2-induced phosphorylation of AKT, as well as that of FRAP1 and GSK3B, is completely blocked by pretreatment of rats with ICI 182,780, indicating that activation of this pathway originates with a membrane form of ESR1.
FIG. 3.
The E2 activation of the PI3K/AKT pathway in the uterus is blocked by ICI 182,780. Rats were treated with either vehicle or the ESR1 antagonist ICI 182,780 (ICI) 1.5 h before treatment with either vehicle or E2 for the indicated times. Total protein was extracted from whole uteri, and phosphorylated (p) and total AKT, FRAP1, and GSK3B were analyzed by Western blot analysis (representative blots [A] and densitometry [B]). Densitometry results are expressed as the fold increase in phosphorylated protein expression compared with that of uteri not exposed to either ICI or E2 (0 h) after normalization to the total level of each protein (mean ± SEM, n = 3–4 uteri/group). a, vs. 0 h and ICI 4-h E2 (P < 0.01); b, vs. 1-h E2 and ICI 1-h E2 (P < 0.01); c, vs. 0 h, ICI 1-h E2, and ICI 4-h E2 (P < 0.05); d, vs. 0 h, ICI 1-h E2, and ICI 4-h E2 (P < 0.05).
HIF1A Is Present in LE Cells and Further Induced by E2
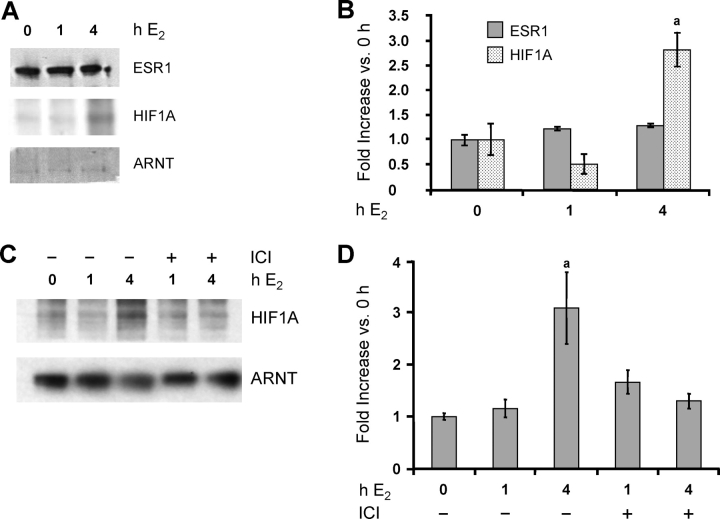
We have previously shown that HIF1A is present in the immature rat uterus before E2 treatment and that E2 further induces its expression, but not that of ARNT or ESR1, by 4 h [4]. Isolated LE cells also expressed detectable HIF1A, ARNT, and ESR1 protein under basal conditions (Fig. 4A). As in the whole uterus, both ARNT and ESR1 levels were unchanged after E2 treatment, as was the level of HIF1A at 1 h. By 4 h after E2, however, the level of HIF1A protein had doubled (Fig. 4, A and B). Hif1a mRNA was also increased ∼2.5-fold by 4 h (P < 0.05 [data not shown]). This increase in HIF1A expression suggests that it has a sustained role in E2 action on LE cells. As shown in Figure 4, C and D, the stimulation of HIF1A expression by E2 at 4 h is also blocked by ICI 182,780. To our knowledge, this is the first time that expression of the HIF1A gene has been demonstrated to be mediated by ESR1; whether this involves both membrane and nuclear forms of ESR1 is still unknown.
FIG. 4.
A and B) E2 induces HIF1A expression in LE cells. Rats were treated as described in Figure 1. Total protein was extracted from LE cells, and ESR1, HIF1A, and ARNT proteins were analyzed by Western blot analysis (representative gels [A] and densitometry [B]). Densitometry results are expressed as the fold increase in each protein compared with cells not exposed to E2 (0-h E2) after normalization to ARNT (mean ± SEM, n = 3 LE cell samples/group). a, vs. 0 h and 1-h E2 (P < 0.01). C and D) The E2 induction of HIF1A expression in the uterus is blocked by ICI 182,780. Rats were treated as described in Figure 3. Total protein was extracted from whole uteri, and HIF1A and ARNT proteins were analyzed by Western blot analysis (representative gels [C] and densitometry [D]). Densitometry results are expressed as the fold increase in HIF1A compared with uteri not exposed to either ICI or E2 (0 h) after normalization to ARNT (mean ± SEM, n = 3–4 uteri/group). a, vs. 0 h, 1-h E2, ICI 1-h E2, and ICI 4-h E2 (P < 0.01).
E2 Stimulates Vegfa Expression in LE Cells
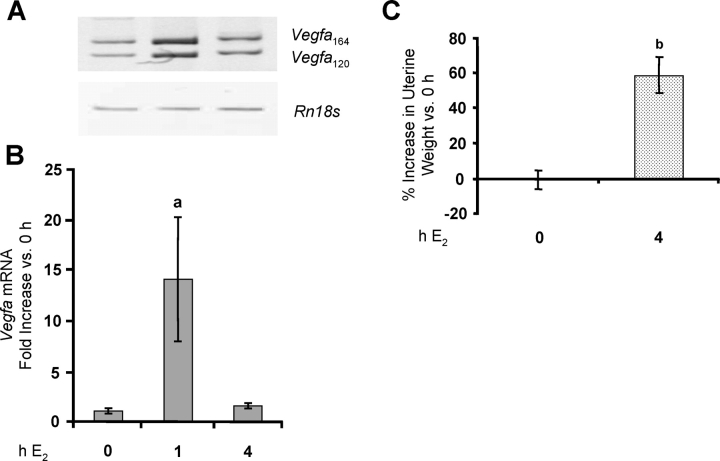
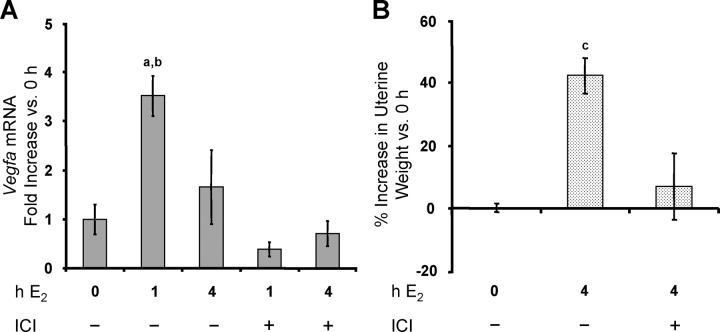
In the whole uterus, E2 induces a large increase in Vegfa mRNA levels by 1–2 h, with a return to basal levels by 4 h [1, 4, 9]. As shown in Figure 5, Vegfa mRNA expression in isolated LE cells increased strongly by 1 h after E2 treatment, before declining to basal levels by 4 h. In the animals from which the cells were isolated 4 h after E2 treatment, there was a >50% increase in uterine wet weight (Fig. 5C), which is dependent on VEGFA [2]. Thus, isolated epithelial cells show exactly the same pattern of Vegfa expression that is observed when RNA is prepared from the whole uterus, consistent with their being the primary site of that increase. The E2 induction of Vegfa expression in the endometrium and the increase in uterine weight at 4 h were both blocked by ICI 182,780 (Fig. 6, A and B). Because our work indicates that both membrane and nuclear ESR1 mediate E2-induced Vegfa expression, the effect of ICI 182,780 is likely due to inhibition of both.
FIG. 5.
E2 induces Vegfa expression in endometrial LE cells. Rats were treated as described in Figure 1. Total RNA was extracted from LE cells, and Vegfa mRNA was analyzed by conventional (A) and real-time (B) RT-PCR. Real-time results are expressed as the fold increase in Vegfa mRNA levels compared with untreated controls (0 h) after normalization to Rn18s (mean ± SEM, n = 3 LE cell samples/group). a, vs. 0 h and 4 h E2 (P < 0.05). C) Uteri were weighed before LE cell isolation, and the percentage increase in wet weight (normalized to body weight) vs. 0 h is given (mean ± SEM, n = 6 uteri/group). b, vs. 0 h (P < 0.001).
FIG. 6.
The E2 induction of Vegfa expression in the uterus is blocked by ICI 182,780. Rats were treated as described in Figure 3. A) Total RNA was extracted from whole uteri, and Vegfa mRNA was analyzed using quantitative real-time RT-PCR (mean ± SEM, n = 3–4 uteri/group). a, vs. 0 h, ICI 1-h E2, ICI 4-h E2 (P < 0.001); b, vs. 4-h E2 (P < 0.01). B) Uteri were weighed, and the percentage increase in wet weight (normalized to body weight) is compared vs. uteri not exposed to either ICI or E2 (0 h; mean ± SEM, n = 3–4 uteri/group). c, vs. 0 h and ICI 4-h E2 (P < 0.01).
E2-Induced Vegfa Gene Expression in LE Cells Does Not Involve FRAP1
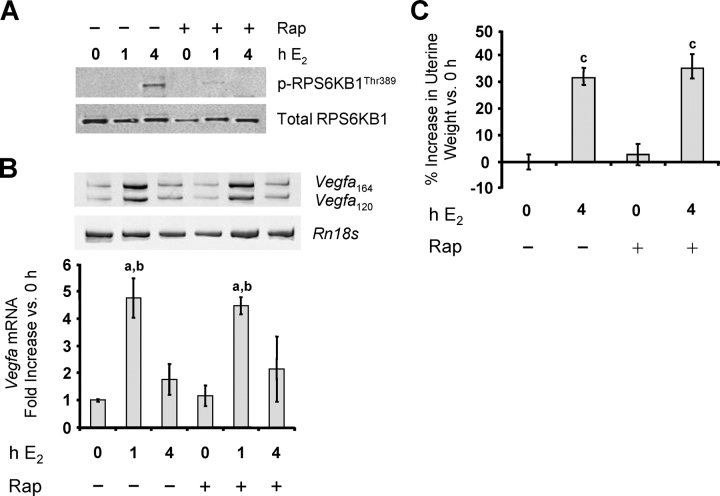
FRAP1 can be effectively inhibited in vivo by rapamycin. To determine whether FRAP1 has a role in E2 activation of the Vegfa gene in the uterus, the effect of this inhibitor was examined. To confirm the effectiveness of rapamycin in blocking E2-induced FRAP1 activity, the phosphorylation state of RPS6KB1 (ribosomal protein S6 kinase, 70 kDa, polypeptide 1), a downstream target of FRAP1, was examined. The E2 caused a marked increase in its phosphorylation by 4 h, without affecting the total RPS6KB1 levels (Fig. 7A). Pretreatment of rats with rapamycin effectively blocked this effect, indicating that the dose of rapamycin was sufficient to inhibit FRAP1 activity in vivo. Blocking FRAP1 activity, however, had no effect on E2-induced increases in Vegfa expression or uterine weight (Fig. 7, B and C). Thus, FRAP1 is not required for E2 induction of Vegfa expression. This lack of connection of FRAP1 to Vegfa expression is also suggested by the absence of phosphorylation of RPS6KB1 at 1 h, when Vegfa mRNA expression is already maximal.
FIG. 7.
The E2-induced Vegfa expression in LE cells is not mediated by FRAP1. Rats were treated with either vehicle or rapamycin (Rap) 1 h before treatment with either vehicle or E2 as described previously, and LE cells were isolated. A) Expression of the FRAP1 target ribosomal protein S6 kinase, polypeptide 1 (RPS6KB1, also known as p70S6 kinase) and phosphorylated (p) RPS6KB1Thr389 was analyzed by Western blot analysis (representative gels; n = 3 cell samples/group). B) Vegfa mRNA was analyzed as described previously (mean ± SEM, n = 3 cell samples/group. a, vs. 0 h and Rap 0 h (P < 0.01); b, vs. 4-h E2 and Rap 4-h E2 (P < 0.05). C) Uteri were weighed at 4 h, and the percentage increase in wet weight (normalized to body weight) is compared vs. uteri not exposed to either E2 or rapamycin (0 h; mean ± SEM, n = 6 uteri/group). c, vs. 0 h and Rap 0 h (P < 0.0001).
E2 Rapidly Induces Recruitment of HIF1A to the Vegfa Gene Promoter in LE Cells
The E2-induced PI3K/AKT activation leads to the rapid recruitment of HIF1A to the HRE of the Vegfa promoter [9]. ChIP analyses to detect E2-induced transcription factor binding to the Vegfa promoter showed that E2 increased HIF1A binding to the Vegfa promoter in rat LE cells at 1 h, which was lost again by 4 h (Fig. 8, B and C). This pattern correlated with E2-induced Vegfa mRNA expression (Figs. 5 and 6). This recruitment of HIF1A to the Vegfa gene promoter was blocked by ICI 182,780 (Fig. 8D), again indicating the involvement of membrane ESR1.
FIG. 8.
E2 induces recruitment of HIF1A to the Vegfa gene promoter in LE cells. Rats were treated as described in Figure 1. Protein-chromatin complexes were extracted from isolated LE cells, and HIF1A binding to the HRE on the rat Vegfa gene promoter was analyzed by ChIP as described in Materials and Methods. A) Schematic of Vegfa promoter region. AP1, activator protein 1. B) Representative PCR gel. IP, immunoprecipitation. C) Densitometry results expressed as fold increase compared with control cells (0 h; mean ± SEM, n = 3 pools/group). a, vs. 0 h and 4-h E2 (P < 0.05). D) E2-induced recruitment of HIF1A is blocked by ICI 182,780. Rats were treated as described in Figure 3. HIF1A binding to Vegfa promoter in the whole uterus was analyzed by ChIP. Densitometry results are expressed as fold increase vs. uteri not exposed to either ICI or E2 (0 h; mean ± SEM, n = 3 uteri/group). b, vs. 0 h, ICI 1-h E2, and ICI 4-h E2 (P < 0.05); c, vs. 4-h E2 (P < 0.01).
DISCUSSION
The rapid but transient increase in Vegfa expression in LE cells after E2 treatment is identical to that previously observed using the whole uterus [1, 4, 9]. This is consistent with several ISH studies [13–15] that identified the LE cells as the only site of E2-induced Vegfa expression in the rodent endometrium. In the mouse, Vegfa expression was also restricted to LE cells on Day 1 of pregnancy, when (according to the authors) the uterus is primarily under the influence of preovulatory E2 [45–47]. However, two other studies [16, 17] reported that E2 induction of Vegfa mRNA occurs in stromal cells. In both cases, the animals used were ovariectomized, in contrast to the previously mentioned studies, which were all done using intact animals. This suggests that removal of the ovaries may cause a shift in E2-induced Vegfa expression from LE cells to sub-LE stromal cells. In one of the two studies in ovariectomized animals [17], Vegfa expression reverted to LE cells by 24 h after E2 treatment, by which time stromal expression was very low, confirming that shifts in the cellular site of Vegfa expression do occur. The mechanical approach we used for the isolation of LE cells does not yield a pure population of stromal cells; after vortexing, the horns still contain residual LE cells and glandular epithelial cells. Thus, we cannot rule out the possibility that E2 also affects Vegfa expression by stromal cells. As discussed previously, however, all ISH studies conducted to date in intact rodents have shown expression only in LE cells [13–15, 46–48], while stromal expression has been reported only in ovariectomized animals [16, 17]. Thus, regardless of the model used, E2-induced expression has been found to occur either in one compartment or the other. The fact that the pattern of E2-induced Vegfa expression in LE cells, as well as the signaling events previously linked to that expression, is identical to that seen when the whole uterus was used is further evidence that the LE cells are the site of that expression.
Establishing the exact spatial and temporal patterns of Vegfa expression in the uterus is important because of the critical role VEGFA may have in the initiation of E2-induced endometrial growth. VEGFA mediates the early effects of E2 on the endometrial microvasculature [2], namely, markedly increased permeability and plasma exudation, which are thought to be essential for normal LE cell proliferation [5–7]. Most important, VEGFA from the mammary epithelium has also recently been shown to be essential for normal alveolar expansion and microvascular function in the mouse mammary gland [48, 49]. It follows that VEGFA produced in epithelial cells must be secreted and diffused to nearby subepithelial capillaries. The primary isoforms of VEGFA expressed in the uterus are the more soluble forms [1]. Thus, synthesis in LE cells is compatible with a paracrine action on the sub-LE capillary bed. We propose, therefore, that the key events in LE cell proliferation are 1) E2-induced LE cell Vegfa expression, 2) VEGFA-induced increased microvascular permeability, and 3) increased delivery of growth factors to LE cells from plasma (Fig. 9). This model is entirely consistent with a recent study [22] that attributes the growth of ESR1– mammary epithelial cells to a direct effect of E2 on a subpopulation of ESR1+ epithelial cells. Zhu and Pollard [50] have proposed that the E2-induced factor that causes endometrial LE cell proliferation is insulin-like growth factor 1 (IGF1), as IGF1 receptors are required for that effect. Sato et al. [7] reached a similar conclusion in investigations using IGF1-null mice but found that the involved IGF1 originated from the circulation. These findings are compatible with the model proposed herein (Fig. 9) in which E2 acts on LE cells to induce Vegfa expression, which in turn enhances delivery of circulating IGF1 to LE cells via induction of interendothelial cell gaps. VEGFA is a potent inducer of such gaps [51], which appear in endometrial microvessels following E2 treatment [52, 53]. Furthermore, circulating IGF1 enters tissues from the blood primarily via the interendothelial cell route [54, 55].
FIG. 9.
The proposed role of VEGFA in endometrial epithelial cell proliferation and stromal remodeling. As E2 levels rise during proestrus (or in response to exogenous E2 in immature or ovariectomized animals), Vegfa is expressed by LE cells (Phase 1). VEGFA induces a large increase in the permeability of stromal microvessels, flooding the stroma with plasma, the edema that is the sine qua non of the early effects of E2 on the uterus (Phase 2). The plasma is rich in growth factors such as IGF1 and carries additional E2 bound to serum proteins. Growth factors and E2 act in concert to induce maximal LE cell proliferation (Phase 3). The plasma also contains plasminogen that triggers the collagenolytic cascade and the degradation of the stromal extracellular matrix (ECM), as well as fibrinogen, which is converted to fibrin, creating a temporary matrix that promotes cell proliferation, cell migration, and angiogenesis [8]. The E2 also primes stromal fibroblasts for later proliferation in response to progesterone.
We also show that key signaling events that we have previously linked to E2-induced Vegfa expression using the whole uterus, namely, activation of the PI3K/AKT pathway and recruitment of HIF1A to the Vegfa promoter within 1 h, occur in LE cells. Thus, LE cells possess all of the signaling machinery necessary for activating Vegfa expression in response to E2, and, conversely, stromal cell-derived factors are not involved in Vegfa expression. This is consistent with the rapid nature of the induction and with previous findings that E2 regulation of the Vegfa gene occurs at the transcriptional level and does not require the synthesis of new proteins [1, 56]. As when the whole uterus was used [4], we also found that E2 induces a further increase in HIF1A levels in LE cells by 4 h. We previously showed that this increase in HIF1A protein is matched by an increase in Hif1a mRNA [4], and this was again confirmed in LE cells. Thus, E2 induces activation of HIF1A from the basal pool by 1 h and a further increase in the level of HIF1A by 4 h. The role of the latter increase in the effects of E2 on the uterus remains to be determined.
Although we postulate that the activation of PI3K/AKT is mediated by a membrane-associated form of ESR1 (described herein), others have attributed it to IGF1 secreted by stromal cells in response to E2 [50]. As already discussed, however, systemic rather than locally produced IGF1 appears to mediate E2-induced LE cell proliferation [7]. We propose that VEGFA is the primary E2-induced paracrine factor, which in turn causes the delivery of IGF1 via the endocrine route.
The E2 activation of PI3K/AKT led to phosphorylation of the downstream effectors FRAP1, GSK3B, and MDM2 in LE cells. This indicates that they could be involved in the uterotrophic effects of E2. All three have been linked to E2-induced cell proliferation [19, 50, 57, 58]. They have also been linked to HIF1A activation and/or Vegfa expression in other systems [59–67]. We did not find any linkage, however, between FRAP1 activation and Vegfa expression. There are other reports of increased HIF1A activity and/or Vegfa induction independent of FRAP1 [68, 69]. FRAP1 may be involved in other later E2 effects in the uterus, as has been shown for GSK3B in E2-induced cell proliferation [19]. Indeed, phosphorylation and total expression of FRAP1 and GSK3B in LE cells were highest after 4 h of E2 treatment, when E2-induced Vegfa expression has already returned to basal levels. Additional work is needed to determine the specific roles of these downstream effectors in mediating the effects of E2 in the uterus. Although GSK3B can be inhibited by LiCl, the design of a study on its role in E2 action is not straightforward because its activity is inhibited by E2 itself [50]. MDM2 can be inhibited by Nutlin3 [65], but this compound has not yet been shown to be effective in vivo.
PI3K/AKT could also activate HIF1A independent of any of these factors. First, AKT can be translocated to the nucleus, where it could directly phosphorylate the coactivator EP300 (E1A-binding protein p300 [commonly known as just p300]) [70], which is a key regulator of HIF1A activity [71–76]. A recent study [77] found that E2 induces nuclear localization of activated AKT in myocardial cells, and in preliminary studies we have confirmed that this is also true for LE cells (Kazi and Koos, unpublished results), although we have not yet connected this to phosphorylation of EP300. Another recent study [78] found that PI3K/AKT stimulation of HIF1A transcriptional activity on the promoter for the gene for glucose transporter, type 1 (which, like Vegfa, is induced by E2 in the endometrium) involved dissociation of the transcriptional inhibitor FOXO3 from a complex with HIF1A and EP300 and its translocation to the cytoplasm.
We have now linked activation of PI3K/AKT to E2-induced Vegfa expression using both the whole uterus and isolated LE cells. PI3K activation of AKT occurs at the plasma membrane. Thus, a membrane E2 receptor is likely involved, and there is now strong evidence that a subpopulation of ESR1 resides at the plasma membrane [28] and can activate PI3K [79]. Most recently, Pedram and colleagues [80] showed that the E domain of ESR1 localized to the membrane was all that was needed for E2 activation of PI3K. Numerous studies [27, 28, 40–44, 81] indicate that ICI 182,780 blocks both nuclear and membrane forms of ESR1, including ESR1 targeted specifically to the plasma membrane [82]. Effects of ICI 182,780 specifically on PI3K/AKT activation by E2, however, vary among studies. In some cases, ICI 182,780 did not block E2-stimulated AKT activation [30–33]. On the other hand, it did inhibit E2-induced AKT phosphorylation in endothelial cells [25, 26, 28], MCF-7 cells [28], cerebral blood vessels [27], and rat uterus in vivo [29]. Our finding that it inhibits E2-induced activation of PI3K/AKT and HIF1A in the uterus provides further evidence that those effects are mediated by a form of ESR1 localized at the cell membrane. Finally, the phytosterol diosgenin has been reported to induce Vegfa expression in osteoblasts via the PI3K/AKT pathway and HIF1A [83], and, as herein, these effects were completely blocked by ICI 182,780. The lack of effect of ICI 182,780 in some studies may reflect differences in methods such as time of treatment. In one study [84], for example, ICI 182,780 inhibited E2-induced activation of AKT at 30 min but not at 3 min.
As proposed previously [4, 9], E2 induction of Vegfa expression appears to involve both membrane and nuclear forms of ESR1, with the former mediating activation of the PI3K/AKT signaling pathway and the latter being recruited to proximal SP1 sites on the Vegfa promoter. Because the amount of material obtained from LE cells was limited, we were unable to repeat the ChIP studies of ESR1 recruitment carried out previously in the whole uterus. As we clearly show, however, LE cells contain abundant ESR1 (Figs. 2 and 4); in other studies, we have found that there is a marked translocation of ESR1 from the cytoplasm to the nuclei of LE cells after E2 treatment (Kazi et al., unpublished results). We are examining the relationships between PI3K/AKT activation, HIF1 binding, and nuclear ESR1 recruitment to the Vegfa promoter, events that appear to be closely linked [9] (Kazi et al., unpublished results). We have not yet fully examined possible roles of the β form of E2 receptor (ESR2) in any of the effects of E2 or ICI 182,780 on Vegfa expression or related signaling events. Studies [85–87] in ESR1- and ESR2-null animals or using ESR1- or ESR2-specific ligands, however, have clearly shown that ESR1 has the major role in the effects of E2 on the endometrium, although ESR2 may modulate its activity [88].
In conclusion, linking the PI3K/AKT pathway and HIF1 to E2 induction of Vegfa in LE cells advances our understanding of the molecular mechanisms that underlie the effects of E2 in the uterus and probably other tissues as well. It also suggests novel targets and strategies for the treatment of uterine disorders that are exacerbated by E2. Excessive HIF1 and VEGFA are associated with both endometriosis [89–91] and endometrial cancer [92, 93], which is also strongly linked to high PI3K/AKT activity [94].
Supplementary Material
Footnotes
1Supported by the NICHD/ NIH through cooperative agreement U54 HD36207 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, NIH R21 ES013061, and R01 HD047275. K.H.M. was supported by NHLBI/ NIH Institutional Training Grant HL72751.
REFERENCES
- Cullinan-Bove K, Koos RD.Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology 1993; 133: 829–837. [DOI] [PubMed] [Google Scholar]
- Rockwell LC, Pillai S, Olson CE, Koos RD.Inhibition of vascular endothelial growth factor/vascular permeability factor action blocks estrogen-induced uterine edema and implantation in rodents. Biol Reprod 2002; 67: 1804–1810. [DOI] [PubMed] [Google Scholar]
- Koos RD, Rockwell LC.The microvasculature of the endometrium. Shepro D.Microvascular Research: Biology and Pathology San Diego:Academic Press/Elsevier;2006: 587–594. [Google Scholar]
- Kazi AA, Jones JM, Koos RD.Chromatin immunoprecipitation analysis of gene expression in the rat uterus in vivo: estrogen-induced recruitment of both estrogen receptor alpha and hypoxia-inducible factor 1 to the vascular endothelial growth factor promoter. Mol Endocrinol 2005; 19: 2006–2019. [DOI] [PubMed] [Google Scholar]
- Spaziani E.Relationship between early vascular responses and growth in the rat uterus: stimulation of cell division by estradiol and vasodilating amines. Endocrinology 1963; 72: 180–191. [DOI] [PubMed] [Google Scholar]
- Hastings JM, Licence DR, Burton GJ, Charnock-Jones DS, Smith SK.Soluble vascular endothelial growth factor receptor 1 inhibits edema and epithelial proliferation induced by 17beta-estradiol in the mouse uterus. Endocrinology 2003; 144: 326–334. [DOI] [PubMed] [Google Scholar]
- Sato T, Wang G, Hardy MP, Kurita T, Cunha GR, Cooke PS.Role of systemic and local IGF-I in the effects of estrogen on growth and epithelial proliferation of mouse uterus. Endocrinology 2002; 143: 2673–2679. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF.Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 2008; 11: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazi AA, Koos RD.Estrogen-induced activation of hypoxia-inducible factor-1α, vascular endothelial growth factor expression, and edema in the uterus are mediated by the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology 2007; 148: 2363–2374. [DOI] [PubMed] [Google Scholar]
- Semenza GL.Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol 2006; 91: 803–806. [DOI] [PubMed] [Google Scholar]
- Boutin AT, Johnson RS.Waiting to inhale: HIF1 modulates aerobic respiration. Cell 2007; 129: 29–30. [DOI] [PubMed] [Google Scholar]
- Gordan JD, Simon MC.Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev 2007; 17: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Neufeld G, Gitay-Goren H, Keshet E.Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J Clin Invest 1993; 91: 2235–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuri AR, Kumar AM, Mukhopadhyay D.Differential expression and selective localization of vascular permeability factor/vascular endothelial growth factor in the rat uterus during the estrous cycle. J Endocrinol 1998; 159: 489–499. [DOI] [PubMed] [Google Scholar]
- Yi XJ, Jiang HY, Lee KK, O WS, Tang PL, Chow PH.Expression of vascular endothelial growth factor (VEGF) and its receptors during embryonic implantation in the golden hamster (Mesocricetus auratus). Cell Tissue Res 1999; 296: 339–349. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Stancel GM, Chiappetta C, Murthy L, Boettger-Tong HL, Makela S.Uterine expression of vascular endothelial growth factor is increased by estradiol and tamoxifen. Cancer Res 1996; 56: 3954–3960. [PubMed] [Google Scholar]
- Ma W, Tan J, Matsumoto H, Robert B, Abrahamson DR, Das SK, Dey SK.Adult tissue angiogenesis: evidence for negative regulation by estrogen in the uterus. Mol Endocrinol 2001; 15: 1983–1992. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Cooke PS, Kurita T.Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol 2004; 67: 417–434. [DOI] [PubMed] [Google Scholar]
- Chen B, Pan H, Zhu L, Deng Y, Pollard JW.Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase–>AKT–>GSK-3beta–>cyclin D1–>pRB pathway to block uterine epithelial cell proliferation. Mol Endocrinol 2005; 19: 1978–1990. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR.Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A 1997; 94: 6535–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigsby RM, Caperell-Grant A, Berry N, Nephew K, Lubahn D.Estrogen induces a systemic growth factor through an estrogen receptor-alpha-dependent mechanism. Biol Reprod 2004; 70: 178–183. [DOI] [PubMed] [Google Scholar]
- Mallepell S, Krust A, Chambon P, Brisken C.Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci U S A 2006; 103: 2196–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Manka D, Wagner K, Khan SA.Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci U S A 2007; 104: 14718–14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER.Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 2005; 19: 1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y.Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 2001; 276: 3459–3467. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Rabkin E, Liao JK.Molecular basis of cell membrane estrogen receptor interaction with phosphatidylinositol 3-kinase in endothelial cells. Arterioscler Thromb Vasc Biol 2003; 23: 198–203. [DOI] [PubMed] [Google Scholar]
- Stirone C, Boroujerdi A, Duckles SP, Krause DN.Estrogen receptor activation of phosphoinositide-3 kinase, akt, and nitric oxide signaling in cerebral blood vessels: rapid and long-term effects. Mol Pharmacol 2005; 67: 105–113. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER.Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 2006; 20: 1996–2009. [DOI] [PubMed] [Google Scholar]
- Lengyel F, Vértes Z, Kovács KA, Környei JL, Sümegi B, Vértes M.Effect of estrogen and inhibition of phosphatidylinositol-3 kinase on Akt and FOXO1 in rat uterus. Steroids 2007; 72: 422–428. [DOI] [PubMed] [Google Scholar]
- Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S.Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J Neurosci Res 2000; 60: 321–327. [DOI] [PubMed] [Google Scholar]
- Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, Maggi A, DiAugustine RP, Korach KS.Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem 2002; 277: 8531–8537. [DOI] [PubMed] [Google Scholar]
- Ivanova T, Mendez P, Garcia-Segura LM, Beyer C.Rapid stimulation of the PI3-kinase/Akt signalling pathway in developing midbrain neurones by oestrogen. J Neuroendocrinol 2002; 14: 73–79. [DOI] [PubMed] [Google Scholar]
- Guzeloglu Kayisli O, Kayisli UA, Luleci G, Arici A.In vivo and in vitro regulation of Akt activation in human endometrial cells is estrogen dependent. Biol Reprod 2004; 71: 714–721. [DOI] [PubMed] [Google Scholar]
- Fagg B, Martin L, Rogers L, Clark B, Quarmby VE.A simple method for removing the luminal epithelium of the mouse uterus for biochemical studies. J Reprod Fertil 1979; 57: 335–339. [DOI] [PubMed] [Google Scholar]
- Kraus WL, Katzenellenbogen BS.Regulation of progesterone receptor gene expression and growth in the rat uterus: modulation of estrogen actions by progesterone and sex steroid hormone antagonists. Endocrinology 1993; 132: 2371–2379. [DOI] [PubMed] [Google Scholar]
- Parczyk K, Madjno R, Michna H, Nishino Y, Schneider MR.Progesterone receptor repression by estrogens in rat uterine epithelial cells. J Steroid Biochem Mol Biol 1997; 63: 309–316. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Saji S, Mäkinen S, Cheng G, Jensen EV, Warner M, Gustafsson JA.Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci U S A 2000; 97: 5936–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR.Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod 2000; 62: 821–830. [DOI] [PubMed] [Google Scholar]
- Sahlin L, Masironi B, Akerberg S, Eriksson H.Tissue- and hormone-dependent progesterone receptor distribution in the rat uterus. Reprod Biol Endocrinol 2006; 4: e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW.Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res 2000; 87: E44–E52. [DOI] [PubMed] [Google Scholar]
- Russell KS, Haynes MP, Sinha D, Clerisme E, Bender JR.Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc Natl Acad Sci U S A 2000; 97: 5930–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Park ST, Levin ER.Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem 2003; 278: 2701–2712. [DOI] [PubMed] [Google Scholar]
- Taguchi Y, Koslowski M, Bodenner D.Binding of estrogen receptor with estrogen conjugated to bovine serum albumin (BSA). Nucl Recept 2004; 2: e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS.Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem 2005; 280: 7460–7468. [DOI] [PubMed] [Google Scholar]
- Chakraborty I, Das SK, Dey SK.Differential expression of vascular endothelial growth factor and its receptor mRNAs in the mouse uterus around the time of implantation. J Endocrinol 1995; 147: 339–352. [DOI] [PubMed] [Google Scholar]
- Halder JB, Zhao X, Soker S, Paria BC, Klagsbrun M, Das SK, Dey SK.Differential expression of VEGF isoforms and VEGF(164)-specific receptor neuropilin-1 in the mouse uterus suggests a role for VEGF(164) in vascular permeability and angiogenesis during implantation. Genesis 2000; 26: 213–224. [PubMed] [Google Scholar]
- Daikoku T, Matsumoto H, Gupta RA, Das SK, Gassmann M, DuBois RN, Dey SK.Expression of hypoxia-inducible factors in the peri-implantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner: evidence for differential function of HIFs during early pregnancy. J Biol Chem 2003; 278: 7683–7691. [DOI] [PubMed] [Google Scholar]
- Rossiter H, Barresi C, Ghannadan M, Gruber F, Mildner M, Födinger D, Tschachler E.Inactivation of VEGF in mammary gland epithelium severely compromises mammary gland development and function. FASEB J 2007; 21: 3994–4004. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Bevan H, Weeraperuma S, Wratting D, Murphy D, Neal CR, Bates DO, Harper SJ.Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. FASEB J 2008; 22: 1104–1112. [DOI] [PubMed] [Google Scholar]
- Zhu L, Pollard JW.Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci U S A 2007; 104: 15847–15851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WG, Palade GE.Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci 1995; 108: 2369–2379. [DOI] [PubMed] [Google Scholar]
- Friederici HH.The early response of uterine capillaries to estrogen stimulation: an electron microscopic study. Lab Invest 1967; 17: 322–333. [PubMed] [Google Scholar]
- Ham KN, Hurley JV, Lopata A, Ryan GB.A combined isotopic and electron microscopic study of the response of the rat uterus to exogenous oestradiol. J Endocrinol 1970; 46: 71–81. [DOI] [PubMed] [Google Scholar]
- Bastian SE, Walton PE, Belford DA.Paracellular transport of insulin-like growth factor-I (IGF-I) across human umbilical vein endothelial cell monolayers. J Cell Physiol 1997; 170: 290–298. [DOI] [PubMed] [Google Scholar]
- Paye JMD, Forsten-Williams K.Regulation of insulin-like growth factor-I (IGF-I) delivery by IGF binding proteins and receptors. Ann Biomed Eng 2006; 34: 618–632. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Nawaz Z, Chiappetta C, Stancel GM.Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res 2000; 60: 3183–3190. [PubMed] [Google Scholar]
- Suga S, Kato K, Ohgami T, Yamayoshi A, Adachi S, Asanoma K, Yamaguchi S, Arima T, Kinoshita K, Wake N.An inhibitory effect on cell proliferation by blockage of the MAPK/estrogen receptor/MDM2 signal pathway in gynecologic cancer. Gynecol Oncol 2007; 105: 341–350. [DOI] [PubMed] [Google Scholar]
- Yin XJ, Wang G, Khan-Dawood FS.Requirements of phosphatidylinositol-3 kinase and mammalian target of rapamycin for estrogen-induced proliferation in uterine leiomyoma- and myometrium-derived cell lines. Am J Obstet Gynecol 2007; 196: 176.e1–176.e5. [DOI] [PubMed] [Google Scholar]
- Mayerhofer M, Valent P, Sperr WR, Griffin JD, Sillaber C.BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood 2002; 100: 3767–3775. [DOI] [PubMed] [Google Scholar]
- Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, Michiels C.Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem 2003; 278: 31277–31285. [DOI] [PubMed] [Google Scholar]
- Bárdos JI, Chau N, Ashcroft M.Growth factor-mediated induction of HDM2 positively regulates hypoxia-inducible factor 1alpha expression. Mol Cell Biol 2004; 24: 2905–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HD, Zhong X, Gao N, Shi X, Jiang B.Arsenite induces p70S6K1 activation and HIF-1alpha expression in prostate cancer cells. Mol Cell Biochem 2004; 255: 19–23. [DOI] [PubMed] [Google Scholar]
- Nieminen A, Qanungo S, Schneider EA, Jiang B, Agani FH.Mdm2 and HIF-1alpha interaction in tumor cells during hypoxia. J Cell Physiol 2005; 204: 364–369. [DOI] [PubMed] [Google Scholar]
- Lau CK, Yang ZF, Lam CT, Tam KH, Poon RT, Fan ST.Suppression of hypoxia inducible factor-1alpha (HIF-1alpha) by YC-1 is dependent on murine double minute 2 (Mdm2). Biochem Biophys Res Commun 2006; 348: 1443–1448. [DOI] [PubMed] [Google Scholar]
- LaRusch GA, Jackson MW, Dunbar JD, Warren RS, Donner DB, Mayo LD.Nutlin3 blocks vascular endothelial growth factor induction by preventing the interaction between hypoxia inducible factor 1alpha and Hdm2. Cancer Res 2007; 67: 450–454. [DOI] [PubMed] [Google Scholar]
- Jiang B, Liu L.PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta 2008; 1784: 150–158. [DOI] [PubMed] [Google Scholar]
- Yao Y, Yin H, Shen B, Smith RS, Jr, Liu Y, Gao L, Chao L, Chao J.Tissue kallikrein promotes neovascularization and improves cardiac function by the Akt-glycogen synthase kinase-3beta pathway. Cardiovasc Res 2008; 80: 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pore N, Jiang Z, Shu H, Bernhard E, Kao GD, Maity A.Akt1 activation can augment hypoxia-inducible factor-1alpha expression by increasing protein translation through a mammalian target of rapamycin-independent pathway. Mol Cancer Res 2006; 4: 471–479. [DOI] [PubMed] [Google Scholar]
- Kurmasheva RT, Harwood FC, Houghton PJ.Differential regulation of vascular endothelial growth factor by Akt and mammalian target of rapamycin inhibitors in cell lines derived from childhood solid tumors. Mol Cancer Res 2007; 6: 1620–1628. [DOI] [PubMed] [Google Scholar]
- Huang WC, Chen CC.Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol Cell Biol 2005; 25: 6592–6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM.An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci U S A 1996; 93: 12969–12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert BL, Bunn HF.Regulation of transcription by hypoxia requires a multiprotein complex that includes hypoxia-inducible factor 1, an adjacent transcription factor, and p300/CREB binding protein. Mol Cell Biol 1998; 18: 4089–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas JL, Poellinger L, Pereira T.Functional analysis of hypoxia-inducible factor-1 alpha-mediated transactivation: identification of amino acid residues critical for transcriptional activation and/or interaction with CREB-binding protein. J Biol Chem 2002; 277: 38723–38730. [DOI] [PubMed] [Google Scholar]
- Sang N, Fang J, Srinivas V, Leshchinsky I, Caro J.Carboxyl-terminal transactivation activity of hypoxia-inducible factor 1 alpha is governed by a von Hippel-Lindau protein-independent, hydroxylation-regulated association with p300/CBP. Mol Cell Biol 2002; 22: 2984–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid T, Zhou J, Köhl R, Brüne B.p300 Relieves p53-evoked transcriptional repression of hypoxia-inducible factor-1 (HIF-1). Biochem J 2004; 380: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LH, Boussouar F, Boyd K, Xu W, Biesen M, Rehg J, Baudino TA, Cleveland JL, Brindle PK.Two transactivation mechanisms cooperate for the bulk of HIF-1-responsive gene expression. EMBO J 2005; 24: 3846–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA.Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res 2001; 88: 1020–1027. [DOI] [PubMed] [Google Scholar]
- Emerling BM, Weinberg F, Liu J, Mak TW, Chandel NS.PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Forkhead transcription factor 3a (FOXO3a). Proc Natl Acad Sci U S A 2008; 105: 2622–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Simoncini T.Extra-nuclear signaling of estrogen receptors. IUBMB Life 2008; 60: 502–510. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Kim JK, O'Mahony F, Lee EY, Luderer U, Levin ER.Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem 2009; 284: 3488–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos EG, Dieudonne MN, Pecquery R, Le Moal V, Giudicelli Y, Lacasa D.Rapid nongenomic E2 effects on p42/p44 MAPK, activator protein-1, and cAMP response element binding protein in rat white adipocytes. Endocrinology 2002; 143: 930–940. [DOI] [PubMed] [Google Scholar]
- Rai D, Frolova A, Frasor J, Carpenter AE, Katzenellenbogen BS.Distinctive actions of membrane-targeted versus nuclear localized estrogen receptors in breast cancer cells. Mol Endocrinol 2005; 19: 1606–1617. [DOI] [PubMed] [Google Scholar]
- Yen ML, Su JL, Chien CL, Tseng KW, Yang CY, Chen WF, Chang CC, Kuo ML.Diosgenin induces hypoxia-inducible factor-1 activation and angiogenesis through estrogen receptor-related phosphatidylinositol 3-kinase/Akt and p38 mitogen-activated protein kinase pathways in osteoblasts. Mol Pharmacol 2005; 68: 1061–1073. [DOI] [PubMed] [Google Scholar]
- Marino M, Acconcia F, Trentalance A.Biphasic estradiol-induced AKT phosphorylation is modulated by PTEN via MAP kinase in HepG2 cells. Mol Biol Cell 2003; 14: 2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS.Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology 2002; 143: 4172–4177. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS.Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol 2003; 17: 2070–2083. [DOI] [PubMed] [Google Scholar]
- Hillisch A, Peters O, Kosemund D, Müller G, Walter A, Schneider B, Reddersen G, Elger W, Fritzemeier KH.Dissecting physiological roles of estrogen receptor alpha and beta with potent selective ligands from structure-based design. Mol Endocrinol 2004; 18: 1599–1609. [DOI] [PubMed] [Google Scholar]
- Wada-Hiraike O, Hiraike H, Okinaga H, Imamov O, Barros RP, Morani A, Omoto Y, Warner M, Gustafsson JA.Role of estrogen receptor beta in uterine stroma and epithelium: Insights from estrogen receptor beta−/− mice. Proc Natl Acad Sci U S A 2006; 103: 18350–18355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren J.Vascular endothelial growth factor and endometriotic angiogenesis. Hum Reprod Update 2000; 6: 45–55. [DOI] [PubMed] [Google Scholar]
- Wu M, Chen K, Lin S, Lgu C, Tsai S.Aberrant expression of leptin in human endometriotic stromal cells is induced by elevated levels of hypoxia inducible factor-1alpha. Am J Pathol 2007; 170: 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CM, Rohwer N, Funakoshi T, Cramer T, Bernhardt W, Birsner A, Folkman J, D'Amato RJ.2-Methoxyestradiol inhibits hypoxia-inducible factor-1{alpha} and suppresses growth of lesions in a mouse model of endometriosis. Am J Pathol 2008; 172: 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrée N, van Diest PJ, van der Groep P, Sie-Go DMDS, Heintz APM.Hypoxia and angiogenesis in endometrioid endometrial carcinogenesis. Cell Oncol 2007; 29: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat AA, Merritt WM, Coffey D, Lin YG, Patel PR, Broaddus R, Nugent E, Han LY, Landen CN, Jr, Spannuth WA, Lu C, Coleman RL, et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin Cancer Res 2007; 13: 7487–7495. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Ellenson LH.Endometrial carcinoma. Annu Rev Pathol 2007; 2: 57–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.