Abstract
Amantadine and dextromethorphan suppress levodopa (L-DOPA)-induced dyskinesia in Parkinson's disease patients and abnormal involuntary movements (AIM) in the 6-hydroxydopamine (6-OHDA) rat model. These medications have been hypothesized to exert their therapeutic effects by a noncompetitive N-methyl-D-aspartate (NMDA) antagonist mechanism, but they also have known serotonin (5-HT) indirect agonist effects that could suppress AIM. This raised the possibility that NMDA antagonists lacking 5-HTergic effects would not have the antidyskinetic action predicted by previous investigators. To test this hypothesis, we investigated MK-801, the most widely-studied NMDA antagonist. We found that chronic low-dose MK-801 (0.1 mg/kg) had no effect on development of AIM or contraversive rotation. In addition, in L-DOPA primed rats, low-dose MK-801 (0.1 mg/kg) had no effect on expression of AIM, contraversive rotation, or sensorimotor function. Conversely, higher doses of MK-801 (0.2–0.3 mg/kg) suppressed expression of AIM. However, as we show for the first time, anti-dyskinetic doses of MK-801 also suppressed L-DOPA-induced contralateral rotation and impaired sensorimotor function, likely due to non-specific interference of MK-801 with L-DOPA-induced behavior. We conclude that noncompetitive NMDA antagonists are unlikely to suppress dyskinesia clinically without worsening parkinsonism.
Keywords: Dyskinesia, 6-hydroxydopamine, NMDA, Parkinson's disease, Rats
Introduction
Levodopa (L-DOPA) suppresses the cardinal symptoms of Parkinson's disease, but also causes debilitating L-DOPA-induced dyskinesia (LID) in 30–80% of patients after 5–10 years of treatment (Bhidayasiri and Truong, 2008; Chan et al., 2008; Fabbrini et al., 2007). In the 6-hydroxydopamine (6-OHDA) rat model of Parkinson's disease, L-DOPA evokes similar side effects, which have been termed abnormal involuntary movements (AIMs). L-DOPA-induced AIMs in the 6-OHDA rat have predictive validity as a model of clinical LID: amantadine, dextromethorphan, and buspirone have been shown to suppress both AIMs (amantadine, Blanchet et al., 1998; Lundblad et al., 2002, 2005; buspirone, Dekundy et al., 2007; Eskow et al., 2007; Lundblad et al., 2002; dextromethorphan, Paquette et al., 2008) and LID (amantadine, Del Dotto et al., 2001; Metman et al., 1999; Verhagen-Metman et al., 1998b,c; dextromethorphan, Verhagen-Metman et al., 1998a,b,d; buspirone, Bonifati et al., 1994). While direct DA receptor agonists have also been shown to induce AIMs (Delfino et al., 2007; Dupre et al., 2007, 2008a; Jaunarajs et al., 2009; Taylor et al., 2006), the current study focused specifically on AIMs induced by L-DOPA.
Understanding the mechanisms underlying L-DOPA-induced AIMs and LID should lead to the development of better pharmacotherapies. Amantadine and dextromethorphan have long been thought to suppress AIMs and LID via their known noncompetitive N-methyl-D-aspartate (NMDA) antagonist effects (Chase et al., 2000; Del Dotto et al., 2001; Marin et al., 2000; Verhagen-Metman, 1998a,b,c,d). However, these drugs are also thought to cause serotonin (5-HT) overflow (Baptista et al., 1997; Gaikwad et al., 2005), which would stimulate 5-HT1A autoreceptors, a mechanism known to suppress AIMs and LID (Bishop et al., 2009; Paquette et al., 2009a). We therefore questioned whether selective NMDA antagonism could reduce L-DOPA-induced AIMs at doses below those that produce competing behaviors (e.g., locomotor stimulation or ataxia; Frantz and Van Hartesveldt, 1999; Haggerty and Brown, 1996). We also evaluated whether higher doses would interfere with L-DOPA's therapeutic effects on sensorimotor function, as agents that inhibit dyskinesia at the expense of blocking therapeutic effects would be of little use clinically.
The noncompetitive NMDA antagonist MK-801 has previously been shown to suppress expression of L-DOPA-induced AIMs at 0.3 mg/kg (Dupre et al., 2008b), but not 0.1 mg/kg (Dupre et al., 2008b; Paquette et al., 2008). Whether the anti-dyskinesia effect of 0.3 mg/kg MK-801 is due to a general motor suppressant effect is unknown, as conflicting data show this dose to suppress L-DOPA-induced contraversive rotation (Spooren et al., 2000) or to have no effect on rotation (Dupre et al., 2008b). Furthermore, no known studies have explored the effect of MK-801 on L-DOPA-mediated sensorimotor improvement or on the development of AIM. Therefore, we conducted a dose-response study of the effects of MK-801 (0.1, 0.2, or 0.3 mg/kg) in L-DOPA primed rats on L-DOPA-induced expression of AIMs, contraversive rotational behavior, and sensorimotor improvement, using the Cylinder, Vibrissae-Stimulated Forelimb Placement (VSFP), and Grip Release tests. We also tested the effects of chronic low-dose MK-801 (0.1 mg/kg) on the development of L-DOPA-induced AIMs.
Methods
Animals
A total of 29 male Sprague-Dawley rats (n = 9–10/group; Harlan, Indianapolis, IN, USA) weighing 300 gm at surgery were used. Rats were housed on a 12-h light:dark cycle (lights on at 6:00) and were given food and water ad libitum. Testing was conducted between 9:00–15:00. Procedures were conducted in accordance with the Institutional Animal Care and Use Committee, and the Portland VA Medical Center is certified by the Association for Assessment and Accreditation of Laboratory Animal Care.
6-OHDA Lesions
6-OHDA lesions were conducted as previously described (Paquette et al., 2008, 2009a). Briefly, during stereotaxic surgery under isoflurane anesthesia, a 30 gauge infusion cannula, attached via PE20 tubing to a 25 μl Hamilton gastight syringe and driven by a microinfusion pump (Harvard Apparatus, Holliston, MA, USA) was used to infuse 6-OHDA hydrobromide (22.8 μg/2 μl of 0.9% saline with 0.2% ascorbic acid, 0.5 μl/min; Sigma-Aldrich, St. Louis, MO, USA) into each of 2 sites within the right medial forebrain bundle (AP −4.3 and −4.8, ML ±1.2, DV −8.6 in mm relative to Bregma; Paxions and Watson, 1998) at least 30 min after desipramine hydrochloride (25 mg/kg, i.p.; Sigma-Aldrich). After surgery, rats received one dose of buprenorphen analgesic (0.05 mg/kg, s.c.; Reckitt Benckiser Pharmaceuticals, Richmond, VA, USA) and supplemental soft food until regaining their pre-surgery weight.
Amphetamine-Evoked Rotational Behavior
An a priori criterion of an average of ≥ 5 turns/min over 10 consecutive min in response to amphetamine (5 mg/kg, i.p.) was used to select rats with significant hemi-parkinsonism (Paquette et al., 2008, 2009a). Lesion size was later confirmed by quantification of tyrosine hydroxylase protein levels via Western blotting.
Drugs
L-DOPA methyl ester hydrochloride, benserazide hydrochloride, L-ascorbic acid, d-amphetamine sulfate, and (+) MK-801 hydrogen maleate were obtained from Sigma-Aldrich. All drugs were dissolved in 0.9% saline and administered i.p. at 1 ml/kg of body weight. MK-801 was administered 30 min prior to L-DOPA. The range of MK-801 doses tested (0.1, 0.2, and 0.3 mg/kg) was selected based on previous data in the chronic L-DOPA-treated 6-OHDA rat (Dupre et al., 2008b).
L-DOPA Treatment
At least 3 days after rotational screening, all rats received L-DOPA (7.5 mg/kg, i.p.), combined with benserazide (15 mg/kg, i.p.) and ascorbic acid (2.6 mg/kg, i.p.), once daily for 21 consecutive days (chronic treatment) to induce AIMs development. Thereafter, L-DOPA-experienced (or “primed”) rats received two injections/week of the L-DOPA cocktail to maintain AIMs expression. With this treatment regimen, all animals that pass AMPH rotational screening develop stable dyskinesias, albeit with some variability in their severity. Thus, no animals are excluded due to failure to develop AIMs. Furthermore, dose failures due to misplaced i.p. L-DOPA injections are exceedingly rare (~1 in 50 injections) and have only a minor impact on variability, thus these data are not discarded.
Experimental Design
AIMs Development
Rats in the chronic MK-801 study were tested in a between-subjects design in which they received MK-801 (0 or 0.1 mg/kg) 30 min prior to L-DOPA on days 1–14 and 16–21. Days 0, 15, and 22 of L-DOPA treatment were L-DOPA challenge days when all rats received vehicle + L-DOPA so that AIMs could be compared across time in the absence of MK-801. A similar regimen of daily L-DOPA for 21 days has previously been shown to sensitize or “prime” rats to LDOPA (Lundblad et al., 2002).
AIMs Expression in L-DOPA-primed rats
A separate cohort of rats were primed with L-DOPA/benserazide/ascorbic acid (7.5/15/2.6 mg/kg, i.p., once daily for 21 days). After priming, rats were tested in a within-subjects design on four doses of MK-801 (0, 0.1, 0.2, and 0.3 mg/kg), administered 30 min prior to L-DOPA with at least 72 h between test days. Doses were tested in a randomized order to control for sensitization to repeated MK-801 (Carey et al., 1995; Gaytan et al., 2000).
AIMs Assessment
AIMs were assessed by an investigator blind to treatment, using an adaptation of the Abnormal Involuntary Movement Scale described by Cenci et al. (1998). Briefly, each rat is rated on a scale of 0 (absent) to 4 (most severe) on each of four subscales (limb dyskinesia, axial dystonia, oral dyskinesia, and contraversive rotation) based on 1-min observations conducted every 20 min. Specifically, for limb dyskinesia, a rating of 1 indicated 1–2 bouts of abnormal movements, 2 indicated 3 or more bouts of abnormal movements, and 3–4 indicated continuous abnormal movements, with 4 indicating that these could not be interrupted by a loud tap on the test cage. For axial dystonia, a rating of 1 indicated a contralateral bias in head orientation, 2 indicated a contralateral bias in head and upper body orientation, and 3–4 indicated a severe contralateral bias in head and upper body orientation (i.e. head above the tail in a four-paw stance), with 4 indicating loss of balance (i.e. falling). For oral dyskinesia, a rating of 1 indicated 2–3 bouts of chewing, 2 indicated more than 3 bouts of chewing, and 3–4 indicated continuous chewing, with 4 indicating the presence tongue protrusions. For L-DOPA-induced contraversive rotation, a rating of 1 indicated 2–3 contraversive turns, 2 indicated more than 3 contraversive turns, and 3–4 indicated continuous contraversive rotation, with a 4 indicating that these could not be interrupted by a loud tap on the cage. When ipsiversive rotation was observed, this was rated identically to contraversive rotation, but as a negative score. Limb + axial + oral subscale scores were summed to create an AIMs score for each time point. Total scores for AIMs, as well as for each subscale, were summed over the first hour after L-DOPA and over the 3-h session. To assess sensitization in the chronic MK-801 study, difference scores were computed for the L-DOPA challenge days (Day 15 – Day 0, Day 22 – Day 0, Day 22 – Day 15). Rats in the chronic MK-801 study were assessed 2–3 times weekly, while primed rats in the dose-response study were assessed on each test day.
Sensorimotor Assessment
Primed rats in the dose-response study were subjected to the sensorimotor battery at baseline (prior to the lesion), at least two weeks after the 6-OHDA lesion, and on days on which AIMs assessment was performed. Sensorimotor testing was completed from 10–80 min after L-DOPA treatment, while dyskinesia was apparent. Two tests were carefully selected based on previous research demonstrating their validity to assess parkinsonian deficits: Vibrissae-Stimulated Forepaw Placement (VSFP; Lindner et al., 1996; Monville et al., 2005) and the Cylinder test (Lundblad et al., 2002). A third test was also used that was recently developed in our laboratory, the Grip Release test. As described below, our test is a simplified form of the grip strength test described by Dunnett et al. (1998).
VSFP
In the VSFP test, rats were held around the torso with their bodies parallel to the floor, their hindpaws supported, and one forelimb restrained, then moved downward until the vibrissae ipsilateral to the unrestrained forelimb touched a tabletop. In an intact animal, this elicits a placing response of the unrestrained forelimb onto the tabletop. Each forelimb was tested for ten trials between 10–30 min after L-DOPA, and the percentage of successful placements (“hits”) was calculated for each forelimb.
Cylinder
Rats were placed in a clear Plexiglas cylinder (19.5 cm ID × 30 cm h), and all weight-bearing forepaw placements on the walls of the cylinder were scored as ipsilateral, contralateral, or simultaneous placements, then the percentage of the total was calculated for each type of placement. Each test session lasted for 1 minute from the time of the first forepaw placement on the cylinder walls. When necessary, animals were encouraged to place forepaws on the cylinder walls by covering the cylinder with a dark cloth. The Cylinder test was conducted between 30–70 min after L-DOPA.
Grip Release
To quantify rigidity in the unilateral 6-OHDA rat model, Dunnett et al. (1998) described a grip strength test that shows increased resistance to release in the contralateral limb. Based on our prior experience with this test (Paquette et al., 2009b), we developed a novel apparatus that uses the rat's own body weight to encourage grip release. Rats were held by the tail above a plastic mesh grid (15 mm openings), supported by an oval-shaped metal frame (33 × 26 mm), which results in a response to grasp the mesh with both forepaws. The frame was then rapidly inverted so that rats hung vertically by their forepaws 30 cm above a cushioned surface. To prevent rats from grasping the mesh with their hindpaws and climbing onto the apparatus, rats' tails were held firmly in place, but no downward pressure was exerted to force release. When rats released their grip with one or both paws, resulting in a fall, the release was scored as ipsilateral, contralateral, or both, depending on which forepaw(s) initiated the release immediately prior to the fall. Thus, when rats released their grip, but replaced the paw without falling, the release was not scored; the trial continued until the rat initiated a grip release that caused a fall. When animals failed to grasp the grid with either paw prior to falling, this was scored as a failure. The percentage of grip release initiations for each limb, as well as the percentage of failures, were calculated over six trials, which were conducted between 60–80 min after L-DOPA.
Lesion Size Quantification
The average loss of tyrosine hydroxylase protein ipsilateral to the 6-OHDA-infused side, relative to the contralateral side, was calculated to reflect the extent of the lesion. Lesion sizes for animals in the chronic MK-801 study (n = 20) were 90.81 ± 2.62% in neostriatum and 72.52 ± 4.94% in ventral midbrain. Lesion sizes for animals in the dose-response study (n = 9) were 91.44 ± 0.87% in neostriatum and 76.11 ± 5.24% in ventral midbrain. Tyrosine hydroxylase was assessed by Western blotting, as previously described (Paquette et al., 2008, 2009a). Briefly, samples (15 μg) were separated on a 10% Criterion Tris-hydrochloride polyacrylamide gel (Bio-Rad, Hercules, CA, USA; 200 V, 1 hr), transferred to polyvinylidene difluoride membranes (30 V for 13 h or 60 V for 1 h), then blocked in 5% milk in Tris-buffered saline with 0.05% Tween-20 for 1 h. With washes in Tris-buffered saline with Tween-20 (3 × 10 min) between steps, membranes were exposed to monoclonal primary antibody (2 h; 1:10,000 anti-tyrosine hydroxylase, catalog #22941; Immunostar, Hudson, WI, USA) and goat anti-mouse antibody (1 h; 1:3,000; Bio-Rad) in 1% milk, visualized using ECF (15 min; Amersham, Piscataway, NJ, USA), and quantified using the Typhoon fluorescence detection system (Typhoon 9410 Variable Mode Imager, GE Healthcare, Piscataway, NJ, USA; Emission filter: 520 BP40, Laser: 488 Blue2).
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences (Chicago, IL, USA) with p ≤ 0.05 considered significant and p < 0.10 considered to be a trend. Results with p > 0.10 were considered non-significant and are not reported. A two-way ANOVA (time × treatment) was used to analyze AIMs over the 21-day chronic treatment period and over each 3-h test session. Independent t-tests were used to analyze effects of treatment on behavioral sensitization to L-DOPA across L-DOPA challenge days (i.e., change scores). One-way ANOVAs or t-tests were used to analyze total AIMs scores in the first hour after L-DOPA and in the full 3-h sessions, as well as to analyze sensorimotor data. When data violated homogeneity of variance assumptions, Greenhouse-Geisser corrections or t-tests in which equal variance was not assumed were used for repeated or between-subjects data, respectively, yielding fractional degrees of freedom.
Results
Abnormal Involuntary Movements (AIMs)
Development of AIMs
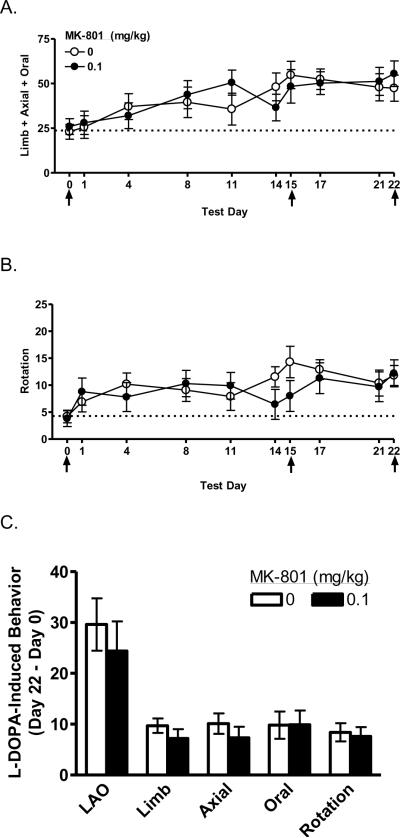
Rats showed increasing AIMs in response to repeated L-DOPA [F (3.49, 683.68) = 221.73, p < 0.001], as shown in Figure 1A. The increasing scores reflect increases in all three subscales (limb, axial, and oral, data not shown) [F's ≥ 77.93, p's < 0.001]. There were no effects of MK-801 on AIMs or the subscales. Contraversive rotation also increased in response to repeated L-DOPA [F (2.79, 547.07) = 176.44, p < 0.001], as shown in Figure 1B. There was no effect of MK-801 on rotation.
Figure 1.
Effect of chronic MK-801 on development of L-DOPA-induced AIMs. Control and MK-801-treated animals developed AIMs that worsened over repeated L-DOPA injections, as indicated by increasing scores on limb + axial + oral AIMs (A), limb AIMs (B), axial AIMs (C), oral AIMs (D), and contraversive rotation (E). There was no effect of MK-801 treatment on the development of sensitized responses to L-DOPA (F). Animals were treated with 0.1 mg/kg MK-801, i.p., 30 min prior to L-DOPA, on Days 1–14 and 16–21. Days 0, 15, and 22 (see arrows) were L-DOPA challenge days when all animals received vehicle + L-DOPA. LAO, limb + axial + oral AIMs.
Difference scores were computed to assess development of sensitization across L-DOPA challenge days in the absence of MK-801 (Days 0, 15, and 22, marked with arrows). These scores showed no effect of MK-801 from Day 0 to Day 15 (data not shown), Day 15 to Day 22 (data not shown), or Day 0 to Day 22, as shown in Figure 1C. Thus, data were collapsed across MK-801 doses to assess animals' responsiveness to repeated L-DOPA challenges. Across the three challenge days, significant increases were observed in AIMs (see Figure 1A, Days 0, 15, and 22), as well as the limb, axial, and oral subscales (data not shown) [F's (2, 36) ≥ 16.28, p's < 0.001]. L-DOPA-induced contraversive rotation also increased across the three challenge days [F (2, 36) = 13.61, p < 0.001] (see Figure 1B, Days 0, 15, and 22). Planned comparisons revealed that scores on all variables (i.e., AIMs, limb, axial, and oral subscales, and contraversive rotation) increased from Day 0 to Day 15, as well as from Day 0 to Day 22 [t's (9) ≥ 4.03, p's ≤ 0.001]. However, no increases were observed in any variables from Day 15 to Day 22, indicating that it is unnecessary to continue daily L-DOPA administration beyond Day 15 when AIMs reach their zenith.
Expression of AIMs
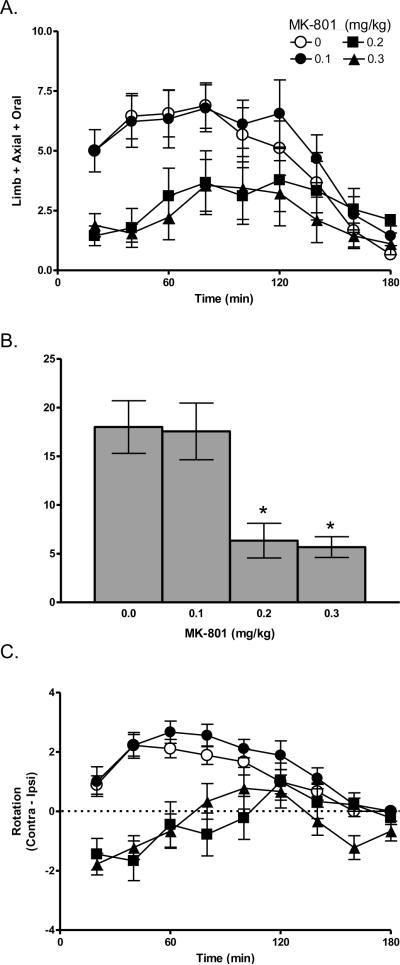
Within the 3-h test session, rats expressed AIMs in response to L-DOPA [F (3.54, 113.12) = 14.85, p < 0.001], as shown in Figure 2A. There was a dose-dependent effect of MK-801 [F (3, 32) = 3.43, p < 0.05]. During the first hour after L-DOPA, when maximal anti-dyskinesia effects are typically observed (Paquette et al., 2008, 2009a), 0.1 mg/kg MK-801 had no effect, but 0.2–0.3 mg/kg MK-801 suppressed AIMs relative to vehicle [t's (8) ≥ 3.80, p's < 0.01], as shown in Figure 2B. Similar effects were observed over the full 3-h session [t's (8) ≥ 2.66, p's < 0.05] (data not shown).
Figure 2.
Effect of acute MK-801 on expression of L-DOPA-induced AIM. MK-801 dose-dependently suppressed limb + axial + oral AIMs (A), especially during the first hour after L-DOPA (B). Effects on the limb AIMs (C), axial AIMs (D), and oral AIMs subscales (E), as well as on L-DOPA-induced rotation (F), were similar to the summed AIMs score (compare to panel A) and are described in detail in the text. * p < 0.05 relative to vehicle.
Similar effects were also observed on the subscales (data not shown). Specifically, for the limb subscale, there were significant effects of L-DOPA and MK-801 [F's ≥ 3.57, p's < 0.01]. MK-801 (0.2–0.3 mg/kg) suppressed limb AIMs relative to vehicle during the first hour and over the 3-h test [t's (8) ≥ 2.67, p's < 0.05]. For the axial subscale, there was a significant effect of L-DOPA [F (3.44, 110.11) = 13.03, p < 0.001] and a trend for MK-801 [F (3, 32) = 2.60, p = 0.069]. MK-801 (0.2–0.3 mg/kg) suppressed axial AIMs relative to vehicle during the first hour, while only the highest dose (0.3 mg/kg) suppressed axial AIMs over the 3-h test [t's (8) ≥ 2.50, p < 0.05]. For the oral subscale, the was a significant effect of L-DOPA only [F (5.31, 169.75) = 2.54, p < 0.05]. MK-801 (0.2 mg/kg) suppressed oral AIMs relative to vehicle during the first hour and over the 3-h test [t's (8) ≥ 3.15, p ≤ 0.01].
Contralateral rotation showed significant effects of L-DOPA [F (4.08, 130.43) = 11.09, p < 0.001] and MK-801 [F (3, 32) = 10.78, p < 0.001], as shown in Figure 2C. Relative to vehicle, 0.1 mg/kg had no effect over the first hour, but increased contralateral rotation over the 3-h test [t (8) = −2.75, p < 0.05]. Conversely, 0.2–0.3 mg/kg MK-801 decreased contralateral rotation scores during the first hour and over the 3-h test [t's (8) ≥ 3.31, p's ≤ 0.01]. In fact, 0.2–0.3 mg/kg MK-801 caused a shift from contraversive to ipsiversive rotation, which was especially striking during the first 40 min of the test.
Sensorimotor Assessment
VSFP
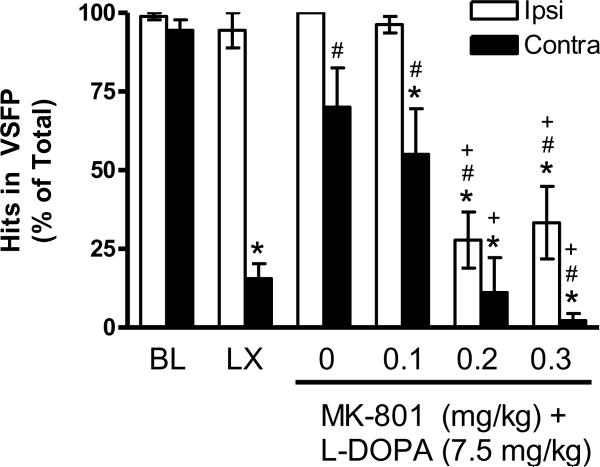
At baseline, rats responded equally well with the contralateral and ipsilateral limbs, as shown in Figure 3. After the 6-OHDA lesion, impairment was observed relative to baseline as a decrease in successful contralateral placements (“hits”) relative to baseline [t (8) = 11.33, p < 0.001] and to the ipsilateral limb [t (8) = 13.99, p < 0.001]. After L-DOPA priming + vehicle for MK-801 (see 0 mg/kg), contralateral performance recovered to baseline levels, which represented an improvement relative to the lesion [t (8) = −4.41, p ≤ 0.01]. However, rats remained less successful with the contralateral relative to the ipsilateral limb [t (8) = 2.38, p < 0.05].
Figure 3.
Intact rats show no limb use asymmetry in the VSFP test at baseline (BL). The unilateral 6-OHDA lesion (LX) impairs contralateral performance, and this impairment is partially restored by chronic L-DOPA + vehicle for MK-801 (see 0 mg/kg). MK-801 dose-dependently prevents L-DOPA's therapeutic effects. * p < 0.05 relative to BL, # p < 0.05 relative to LX, + p < 0.05 relative to vehicle for MK-801 (0 mg/kg).
MK-801 had dose-dependent effects on performance. At 0.1 mg/kg MK-801, contralateral performance remained partially improved relative to the lesion [t (8) = −3.50, p < 0.01], but not to baseline levels [t (8) = 2.28, p ≤ 0.05], and rats were less successful with the contralateral limb relative to the ipsilateral limb, similar to vehicle treatment (compare to 0 mg/kg) [t (8) = 2.75, p < 0.05]. At 0.2–0.3 mg/kg MK-801, both limbs were impaired [t's (8) ≥ 3.02, p's < 0.05], and at 0.3 mg/kg, contralateral use was more impaired than ipsilateral [t (8) = 2.74, p < 0.05].
Cylinder
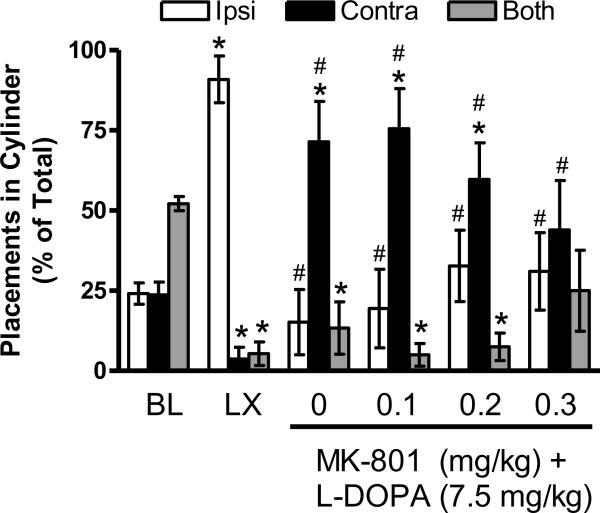
At baseline, rats used both limbs together more often than using the ipsilatateral or contralateral limb alone [F (2, 16) = 16.51, p < 0.001; t's (8) ≥ 5.16, p's ≤ 0.001], as shown in Figure 4. After the 6-OHDA lesion, impairment was observed relative to baseline [t's (8) ≥ 3.48, p's < 0.01] as an increase in ipsilateral limb use relative to use of the contralateral limb or both limbs together [F (1.02, 8.13) = 61.96, p < 0.001; t's (8) ≥ 7.83, p's < 0.001]. Chronic L-DOPA + vehicle for MK-801 (see 0 mg/kg) led to greater use of the contralateral limb relative to the ipsilateral limb or both limbs together [F (2, 16) = 6.63, p ≤ 0.01; t's (8) ≥ 2.63, p's < 0.05]. However, it is important to note that these contralateral limb placements in dyskinetic rats were rapid, repetitive movements that appeared to be an extension of contralateral limb AIMs, unlike the spontaneous, purposeful placements observed at baseline or after the lesion.
Figure 4.
Intact rats show no limb use asymmetry in the Cylinder test at baseline (BL). The unilateral 6-OHDA lesion (LX) impairs contralateral limb use. Chronic L-DOPA + vehicle for MK-801 causes a shift to contralateral limb use (see 0 mg/kg), but placements are qualitatively different than those observed when animals are not expressing AIMs, as described in the text. MK-801 dose-dependently restores behavior toward control levels. * p < 0.05 relative to BL, # p < 0.05 relative to LX.
MK-801 had dose-dependent effects on performance. At 0.1 mg/kg, rats continued to use the contralateral limb more than the ipsilateral limb or both limbs together [F (1.12, 8.95) = 11.97, p < 0.01; t's (8) ≥ 2.75, p's < 0.05]. At 0.2 mg/kg MK-801, contralateral limb use was still increased relative to use of both limbs together [F (1.22, 8.56) = 5.00, p < 0.05; t (7) = 3.98, p < 0.01], but animals used the ipsilateral and contralateral limbs equally. At 0.3 mg/kg, animals used the ipsilateral limb, contralateral limb, or both limbs together with equal frequency.
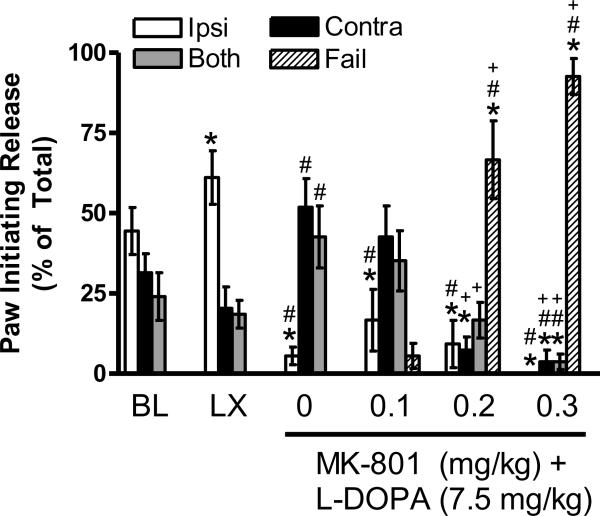
Grip Release
At baseline, rats were equally likely to initiate grip release with the ipsilateral, contralateral, or both limbs together, as shown in Figure 5. The 6-OHDA lesion caused a deficit relative to baseline [t (8) = 2.45, p < 0.05], such that rats initiated grip release with the ipsilateral limb more than the contralateral limb or both limbs together [F (2, 16) = 8.72, p < 0.01; t's (8) ≥ 2.82, p's < 0.05]. L-DOPA treatment + vehicle for MK-801 (see 0 mg/kg) altered behavior relative to the lesion [t's (8) ≥ 2.71, p's ≤ 0.05], as rats initiated release with the contralateral limb or both limbs together more than the ipsilateral limb [F (1.13, 9.01) = 6.60, p < 0.05; t's (8) ≥ 3.36, p ≤ 0.01].
Figure 5.
Intact rats show no limb use asymmetry in the Grip Release test at baseline (BL). The unilateral 6-OHDA lesion (LX) increases use of the ipsilateral limb to initiate grip release. Chronic L-DOPA + vehicle for MK-801 causes a shift to initiating release with the contralateral limb or both limbs together (see 0 mg/kg). MK-801 dose-dependently impairs performance by causing inability to grasp the grid (“fail”), as described in the text. * p < 0.05 relative to BL, # p < 0.05 relative to LX, + p < 0.05 relative to vehicle for MK-801 (0 mg/kg).
After MK-801 at any dose (0.1, 0.2, or 0.3 mg/kg), animals were equally likely to initiate grip release with the ipsilateral, contralateral, or both limbs. At 0.1 mg/kg, rats initiated grip release with the ipsilateral limb less frequently than at baseline or lesion, [t's (8) ≥ 2.45, p's ≤ 0.05], similar to vehicle-treated rats (compare to 0 mg/kg). However, at 0.2–0.3 mg/kg MK-801, rats showed fewer grip release initiations with the ipsilateral limb [t's (8) ≥ 2.50, p's < 0.01], contralateral limb [t's (8) ≥ 3.00, p's < 0.05], and both limbs [t (8) = 2.26, p ≤ 0.05]. The dramatic reductions in grip release after MK-801 are at least partially explained by a striking dose-dependent impairment of grasping induced by MK-801, which we attribute to ataxia. Specifically, the percentage of trials on which rats failed to grasp the grid after 0.1, 0.2, and 0.3 mg/kg MK-801 was 6%, 67%, and 92%, respectively, and this profile after 0.2–0.3 mg/kg MK-801 was significantly different than at baseline, after lesion, or after chronic L-DOPA treatment [t's (8) ≥ 5.51, p's ≤ 0.001].
Discussion
In the current study, we show for the first time that MK-801 suppressed L-DOPA-induced AIMs in the 6-OHDA rat model only at doses that reversed the direction of L-DOPA-induced rotation and prevented L-DOPA's therapeutic effects. Specifically, 0.1 mg/kg MK-801 had no effect on any of the L-DOPA-mediated behaviors studied, while 0.2–0.3 mg/kg MK-801 suppressed L-DOPA-induced AIMs, contraversive rotation, and sensorimotor improvement in the VSFP and Grip Release tests. Based on these data, we conclude that noncompetitive NMDA antagonism is unlikely to be a clinically effective treatment for dyskinesia and may in fact worsen parkinsonism.
MK-801 is known to increase spontaneous and burst firing of dopaminergic neurons (0.01–3.2 mg/kg, i.v.; Murase et al., 1993; Zhang et al., 1992), to cause dopamine release after systemic (0.1 mg/kg; Mathe et al., 1998) or intracerebral administration (10–100 μM, i.c.; Hondo et al., 1994), and to increase dopamine turnover (0.2 mg/kg; Reith et al., 1998). In the 6-OHDA rat, MK-801 causes ipsiversive rotation (0.05–0.25 mg/kg MK-801; Goto et al., 1993), and reduces contraversive turning in response to an acute challenge with 25 mg/kg L-DOPA (0.3 mg/kg MK-801; Spooren et al., 2000) or 0.05 mg/kg apomorphine (0.1 mg/kg MK-801; Robinson et al., 2001). Our data support and extend these findings by showing for the first time that MK-801 (0.2–0.3 mg/kg) shifts rotation from contralateral to ipsilateral in the L-DOPA-treated 6-OHDA rat.
The current rotational data are in direct contrast to a recent study by Dupre et al. (2008), which found no effect of 0.3 mg/kg MK-801 on L-DOPA-induced contraversive rotation. No obvious methodological differences explain these disparate findings, though variations in 6-OHDA delivery coordinates, amount of neurotoxin, and volume of infusion are different. However, both the current study and Dupre et al. (2008b) report large dopaminergic lesions and use well-established L-DOPA regimens previously been shown to induce AIMs (Bishop et al., 2006; Eskow et al., 2007; Taylor et al., 2005; Paquette et al., 2008, 2009a). Given recent interest in the role of serotonin in dyskinesia (c.f. Bishop et al., 2009; Dupre et al., 2008; Eskow et al., 2007; Jaunarajs et al., 2009; Marin et al., 2009; Muñoz et al., 2008; Paquette et al., 2009a), it is interesting to speculate whether the serotonin system was differentially affected in the current study versus in Dupre et al. (2008b), and this question is worthy of pursuit in future studies.
Research using other models of Parkinson's disease with predictive validity, including the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse and reserpine rat, suggest that low doses of MK-801 may enhance L-DOPA's motor-activating effects, but higher doses (0.3–0.5 mg/kg) may reduce these effects. Specifically, in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse, amantadine augments the behavioral activating effects of L-DOPA on locomotion and rearing (Fredriksson et al., 2001). A low doses of MK-801 (0.1 mg/kg) partially mimics amantadine by increasing locomotion, but a high dose (0.3 mg/kg) has the opposite effect, reducing rearing (Fredriksson et al., 2001). In the reserprine rat model, the antidyskinesia drugs amantadine and idazoxan (Rascol et al., 2000) selectively suppress rearing without affecting locomotion, while the D2 antagonist haloperidol causes general motor suppressant effects on rearing and locomotion (Johnston et al., 2005). Low doses of MK-801 (0.01–0.1 mg/kg) act like amantadine in this model, while a very high dose (0.5 mg/kg) acts like haloperidol (Johnston et al., 2005). While 0.2–0.3 mg/kg MK-801 have not been tested in the reserpined rat, these data support the idea that high doses of MK-801 may have general motor suppressant effects.
In summary, the current study demonstrates that the selective noncompetitive NMDA antagonist MK-801 suppresses AIMs only at doses that decrease L-DOPA-induced contraversive rotation and exacerbate sensorimotor deficits. These data suggest that noncompetitive NMDA antagonists are unlikely to be clinically useful, as any benefit would be outweighed by the risk of worsening parkinsonism.
Acknowledgements
This work was funded by NINDS NS38715 (SWJ) and Veterans Affairs Merit Review grants (CKM and SPB).
Footnotes
Address where the work was carried out: Department of Veterans Affairs Medical Center, Portland, OR 97239
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baptista T, López ME, Teneud L, Contreras Q, Alastre T, de Quijada M, Araujo de Baptista E, Alternus M, Weiss SR, Musseo E, Páez X, Hernández L. Amantadine in the treatment of neuroleptic-induced obesity in rats: behavioral endocrine and neurochemical correlates. Pharmacopsychiatry. 1997;30:43–54. doi: 10.1055/s-2007-979482. [DOI] [PubMed] [Google Scholar]
- Bhidayasiri R, Truong DD. Motor complications in Parkinson disease: clinical manifestations and management. Journal of the Neurological Sciences. 2008;266:204–215. doi: 10.1016/j.jns.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Bishop C, Taylor JL, Kuhn DM, Eskow KL, Park JY, Walker PD. MDMA and fenfluramine reduce L-DOPA-induced dyskinesia via indirect 5-HT1A receptor stimulation. European Journal of Neuroscience. 2006;23:2669–2676. doi: 10.1111/j.1460-9568.2006.04790.x. [DOI] [PubMed] [Google Scholar]
- Bishop C, Krolewski DM, Eskow KL, Barnum CJ, Dupre KB, Deak T, Walker PD. Contribution of the striatum to the effects of 5-HT1A receptor stimulation in L-DOPA-treated hemiparkinsonian rats. Journal of Neuroscience Research. 2009;87:1645–1658. doi: 10.1002/jnr.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet PJ, Konitsiotis S, Chase TN. Amantadine reduces levodopa-induced dyskinesias in parkinsonian monkeys. Movement Disorders. 1998;13:798–802. doi: 10.1002/mds.870130507. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Fabrizio E, Cipriani R, Vanacore N, Meco G. Buspirone in levodopa-induced dyskinesias. Clinical Neuropharmacology. 1994;17:73–82. doi: 10.1097/00002826-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Dai H, Krost M, Huston JP. The NMDA receptor and cocaine: evidence that MK-801 can induce behavioral sensitization effects. Pharmacology Biochemistry & Behavior. 1995;51:901–908. doi: 10.1016/0091-3057(95)00074-7. [DOI] [PubMed] [Google Scholar]
- Chan DK, Cordato DJ, O'Rourke F. Management for motor and non-motor complications in late Parkinson's disease. Geriatrics. 2008;63:22–27. [PubMed] [Google Scholar]
- Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88:612–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- Chase TN, Oh JD, Konitsiotis S. Antiparkinsonian and antidyskinetic activity of drugs targeting central glutamatergic mechanisms. Journal of Neurology. 2000;247(Suppl 2):II36–II42. doi: 10.1007/pl00007759. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds. Behavioural Brain Research. 2007;179:76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Del Dotto P, Pavese N, Gambaccini G, Bernardini S, Metman LV, Chase TN, Bonuccelli U. Intravenous amantadine improves levadopa-induced dyskinesias: an acute double-blind placebo-controlled study. Movement Disorders. 2001;16:515–520. doi: 10.1002/mds.1112. [DOI] [PubMed] [Google Scholar]
- Delfino M, Kalisch R, Czisch M, Larramendy C, Ricatti J, Taravini I, Trenkwalder C, Murer MG, Auer DP, Gershanik OS. Mapping the effects of three dopamine agonists with different dyskinetogenic potential and receptor selectivity using pharmacological functional magnetic resonance imaging. Neuropsychopharmacology. 2007;32:1911–1921. doi: 10.1038/sj.npp.1301329. [DOI] [PubMed] [Google Scholar]
- Dupre KB, Eskow KL, Barnum CJ, Bishop C. Striatal 5-HT1A receptor stimulation reduces D1 receptor-induced dyskinesia and improves movement in the hemiparkinsonian rat. Neuropharmacology. 2008a;55:1321–1328. doi: 10.1016/j.neuropharm.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre KB, Eskow KL, Negron G, Bishop C. The differential effects of 5-HT(1A) receptor stimulation on dopamine receptor-mediated abnormal involuntary movements and rotations in the primed hemiparkinsonian rat. Brain Research. 2007;1158:135–143. doi: 10.1016/j.brainres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Dupre KB, Eskow KL, Steiniger A, Klioueva A, Negron GE, Lormand L, Park JY, Bishop C. Effects of coincident 5-HT (1A) receptor stimulation and NMDA receptor antagonism on L-DOPA-induced dyskinesia and rotational behaviors in the hemi-parkinsonian rat. Psychopharmacology (Berlin) 2008b;199:99–108. doi: 10.1007/s00213-008-1135-6. [DOI] [PubMed] [Google Scholar]
- Eskow KL, Gupta V, Alam S, Park JY, Bishop C. The partial 5-HT (1A) agonist buspirone reduces the expression and development of L-DOPA-induced dyskinesia in rats and improves L-DOPA efficacy. Pharmacology Biochemistry & Behavior. 2007;87:306–314. doi: 10.1016/j.pbb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG. Levodopa-induced dyskinesias. Movement Disorders. 2007;22:1379–1389. doi: 10.1002/mds.21475. [DOI] [PubMed] [Google Scholar]
- Frantz K, Van Hartesveldt C. Locomotion elicited by MK-801 in developing and adult rats: temporal environmental and gender effects. European Journal of Pharmacology. 1999;369:145–157. doi: 10.1016/s0014-2999(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Danysz W, Quack G, Archer T. Co-administration of memantine and amantadine with sub/suprathreshold doses of L-Dopa restores motor behaviour of MPTP-treated mice. Journal of Neural Transmission. 2001;108:167–187. doi: 10.1007/s007020170086. [DOI] [PubMed] [Google Scholar]
- Gaikwad RV, Gaonkar RK, Jadhav SA, Thorat VM, Jadhav JH, Balsara JJ. Involvement of central serotonergic systems in dextromethorphan-induced behavioural syndrome in rats. Indian Journal of Experimental Biology. 2005;43:620–625. [PubMed] [Google Scholar]
- Gaytan O, Nason R, Alagugurusamy R, Swann A, Dafny N. MK-801 blocks the development of sensitization to the locomotor effects of methylphenidate. Brain Research Bulletin. 2000;51:485–492. doi: 10.1016/s0361-9230(99)00268-3. [DOI] [PubMed] [Google Scholar]
- Goto S, Korematsu K, Inoue N, Yamada K, Oyama T, Nagahiro S, Ushio Y. N-methyl-D-aspartate receptor antagonist MK-801 induced circling behavior in rats with unilateral striatal ischemic lesions or nigral 6-hydroxydopamine lesions. Acta Neuropathologica. 1993;86:480–483. doi: 10.1007/BF00228583. [DOI] [PubMed] [Google Scholar]
- Haggerty GC, Brown G. Neurobehavioral profile of subcutaneously administered MK-801 in the rat. Neurotoxicology. 1996;17:913–921. [PubMed] [Google Scholar]
- Hondo H, Yonezawa Y, Nakahara T, Nakamura K, Hirano M, Uchimura H, Tashiro N. Effect of phencyclidine on dopamine release in the rat prefrontal cortex; an in vivo microdialysis study. Brain Research. 1994;633:337–342. doi: 10.1016/0006-8993(94)91558-x. [DOI] [PubMed] [Google Scholar]
- Jaunarajs KL, Dupre KB, Steiniger A, Klioueva A, Moore A, Kelly C, Bishop C. Serotonin 1B receptor stimulation reduces D1 receptor agonist-induced dyskinesia. NeuroReport. 2009;20:1265–1269. doi: 10.1097/WNR.0b013e3283300fd7. [DOI] [PubMed] [Google Scholar]
- Johnston TH, Lee J, Gomez-Ramirez J, Fox SH, Brotchie JM. A simple rodent assay for the in vivo identification of agents with potential to reduce levodopa-induced dyskinesia in Parkinson's disease. Experimental Neurology. 2005;191:243–250. doi: 10.1016/j.expneurol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Plone MA, Francis JM, Emerich DF. Validation of a rodent model of Parkinson's Disease: evidence of a therapeutic window for oral Sinemet. Brain Research Bulletin. 1996;39:367–372. doi: 10.1016/0361-9230(96)00027-5. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. European Journal of Neuroscience. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Usiello A, Carta M, Hkanssond K, Fisone G, Cenci MA. Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Experimental Neurology. 2005;194:66–75. doi: 10.1016/j.expneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Marin C, Jimenez A, Bonastre M, Chase TN, Tolosa E. Non-NMDA receptor-mediated mechanisms are involved in levodopa-induced motor response alterations in Parkinsonian rats. Synapse. 2000;36:267–274. doi: 10.1002/(SICI)1098-2396(20000615)36:4<267::AID-SYN3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Marin C, Aguilar E, Rodríguez-Oroz MC, Bartoszyk GD, Obeso JA. Local administration of sarizotan into the subthalamic nucleus attenuates levodopa-induced dyskinesias in 6-OHDA-lesioned rats. Psychopharmacology (Berl) 2009;204:241–250. doi: 10.1007/s00213-008-1452-9. [DOI] [PubMed] [Google Scholar]
- Mathé JM, Nomikos GG, Schilström B, Svensson TH. Non-NMDA excitatory amino acid receptors in the ventral tegmental area mediate systemic dizocilpine (MK-801) induced hyperlocomotion and dopamine release in the nucleus accumbens. Journal of Neuroscience Research. 1998;51:583–592. doi: 10.1002/(SICI)1097-4547(19980301)51:5<583::AID-JNR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Metman LV, Del Dotto P, LePoole K, Konitsiotis S, Fang J, Chase TN. Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Archives of Neurology. 1999;56:1383–1386. doi: 10.1001/archneur.56.11.1383. [DOI] [PubMed] [Google Scholar]
- Monville C, Torres EM, Dunnett SB. Validation of the l-dopa-induced dyskinesia in the 6-OHDA model and evaluation of the effects of selective dopamine receptor agonists and antagonists. Brain Research Bulletin. 2005;68:16–23. doi: 10.1016/j.brainresbull.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Muñoz A, Li Q, Gardoni F, Marcello E, Qin C, Carlsson T, Kirik D, Di Luca M, Björklund A, Bezard E, Carta M. Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of L-DOPA-induced dyskinesia. Brain. 2008;131(Pt 12):3380–3394. doi: 10.1093/brain/awn235. [DOI] [PubMed] [Google Scholar]
- Murase S, Mathé JM, Grenhoff J, Svensson TH. Effects of dizocilpine (MK-801) on rat midbrain dopamine cell activity: differential actions on firing pattern related to anatomical localization. Journal of Neural Transmission General Section. 1993;91:13–25. doi: 10.1007/BF01244915. [DOI] [PubMed] [Google Scholar]
- Paquette MA, Brudney EG, Putterman DB, Meshul CK, Johnson SW, Berger SP. Sigma ligands but not N-methyl-D-aspartate antagonists reduce levodopa-induced dyskinesias. NeuroReport. 2008;19:111–115. doi: 10.1097/WNR.0b013e3282f3b0d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette MA, Foley K, Brudney EG, Meshul CK, Johnson SW, Berger SP. The sigma-1 antagonist BMY-14802 inhibits L-DOPA-induced abnormal involuntary movements by a WAY-100635-sensitive mechanism. Psychopharmacology. 2009a;204:743–754. doi: 10.1007/s00213-009-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette MA, Marsh ST, Hutchings JE, Castañeda E. Amphetamine-evoked rotation requires newly synthesized dopamine at 14 days but not 1 day after intranigral 6-OHDA and is consistently dissociated from sensorimotor behavior. Behavioural Brain Research. 2009b;200:197–207. doi: 10.1016/j.bbr.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Rascol O, Arnulf I, Peyro-Saint Paul H, Brefel-Courbon C, Vidailhet M, Thalamas C, Bonnet AM, Descombes S, Bejjani B, Fabre N, Montastruc JL, Agid Y. Idazoxan, an alpha-2 antagonist, and L-DOPA-induced dyskinesias in patients with Parkinson's disease. Movement Disorders. 2001;16:708–713. doi: 10.1002/mds.1143. [DOI] [PubMed] [Google Scholar]
- Reith J, Cumming P, Gjedde A. Enhanced [3H]DOPA and [3H]dopamine turnover in striatum and frontal cortex in vivo linked to glutamate receptor antagonism. Journal of Neurochemistry. 1998;70:1979–1985. doi: 10.1046/j.1471-4159.1998.70051979.x. [DOI] [PubMed] [Google Scholar]
- Robinson S, Krentz L, Moore C, Meshul CK. Blockade of NMDA receptors by MK-801 reverses the changes in striatal glutamate immunolabeling in 6-OHDA-lesioned rats. Synapse. 2001;42:54–61. doi: 10.1002/syn.1099. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Gasparini F, Bergmann R, Kuhn R. Effects of the prototypical mGlu(5) receptor antagonist 2-methyl-6-(phenylethynyl)-pyridine on rotarod., locomotor activity and rotational responses in unilateral 6-OHDA-lesioned rats. European Journal of Pharmacology. 2000;406:403–410. doi: 10.1016/s0014-2999(00)00697-x. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Bishop C, Ullrich T, Rice KC, Walker PD. Serotonin 2A receptor antagonist treatment reduces dopamine D1 receptor-mediated rotational behavior but not L-DOPA-induced abnormal involuntary movements in the unilateral dopamine-depleted rat. Neuropharmacology. 2006;50:761–768. doi: 10.1016/j.neuropharm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Bishop C, Walker PD. Dopamine D1 and D2 receptor contributions to L-DOPA-induced dyskinesia in the dopamine-depleted rat. Pharmacology Biochemistry & Behavior. 2005;81:887–893. doi: 10.1016/j.pbb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Verhagen Metman L, Blanchet PJ, van den Munckhof P, Del Dotto P, Natté R, Chase TN. A trial of dextromethorphan in parkinsonian patients with motor response complications. Movement Disorders. 1998a;13:414–417. doi: 10.1002/mds.870130307. [DOI] [PubMed] [Google Scholar]
- Verhagen Metman L, Del Dotto P, Blanchet PJ, van den Munckhof P, Chase TN. Blockade of glutamatergic transmission as treatment for dyskinesias and motor fluctuations in PD. Amino Acids. 1998b;14:75–82. doi: 10.1007/BF01345246. [DOI] [PubMed] [Google Scholar]
- Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian MM, Chase TN. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson's disease. Neurology. 1998c;50:1323–1326. doi: 10.1212/wnl.50.5.1323. [DOI] [PubMed] [Google Scholar]
- Verhagen Metman L, Del Dotto P, Natté R, van den Munckhof P, Chase TN. Dextromethorphan improves levodopa-induced dyskinesias in Parkinson's disease. Neurology. 1998d;51:203–206. doi: 10.1212/wnl.51.1.203. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chiodo LA, Freeman AS. Electrophysiological effects of MK-801 on rat nigrostriatal and mesoaccumbal dopaminergic neurons. Brain Research. 1992;590:153–163. doi: 10.1016/0006-8993(92)91091-r. [DOI] [PubMed] [Google Scholar]