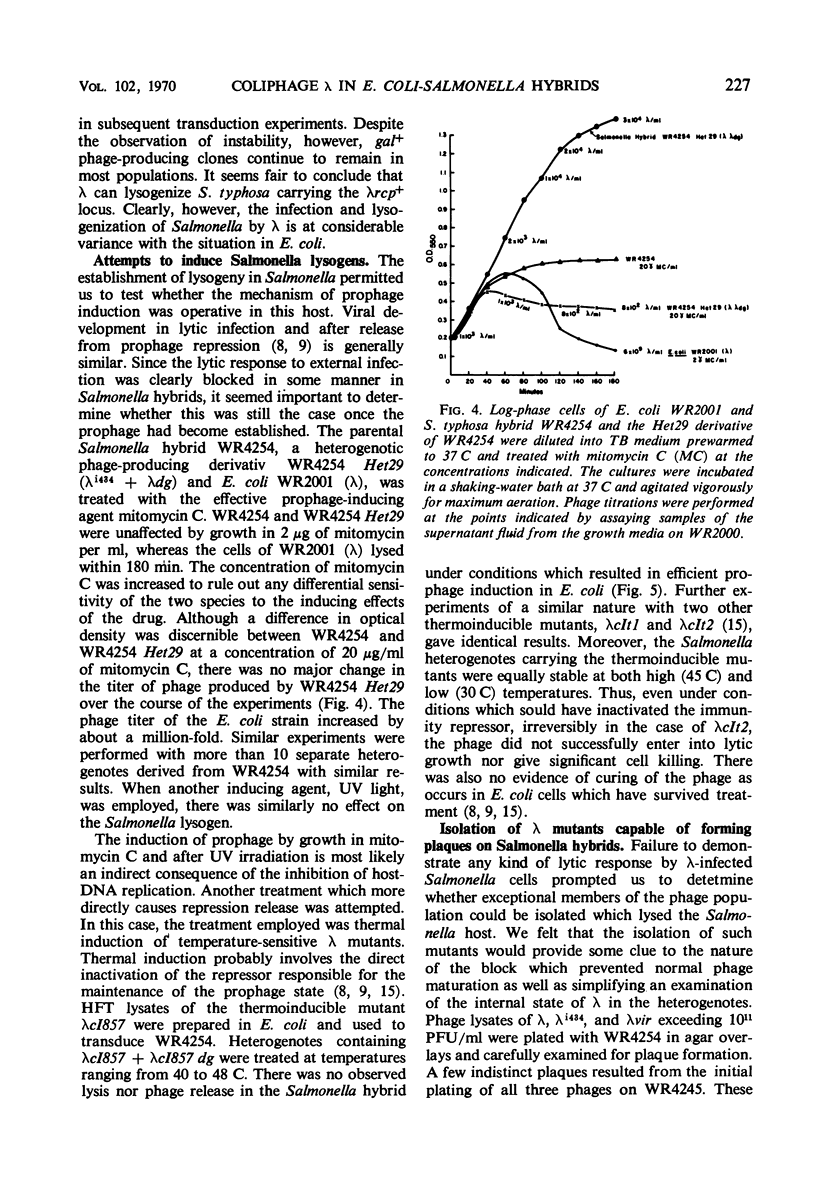
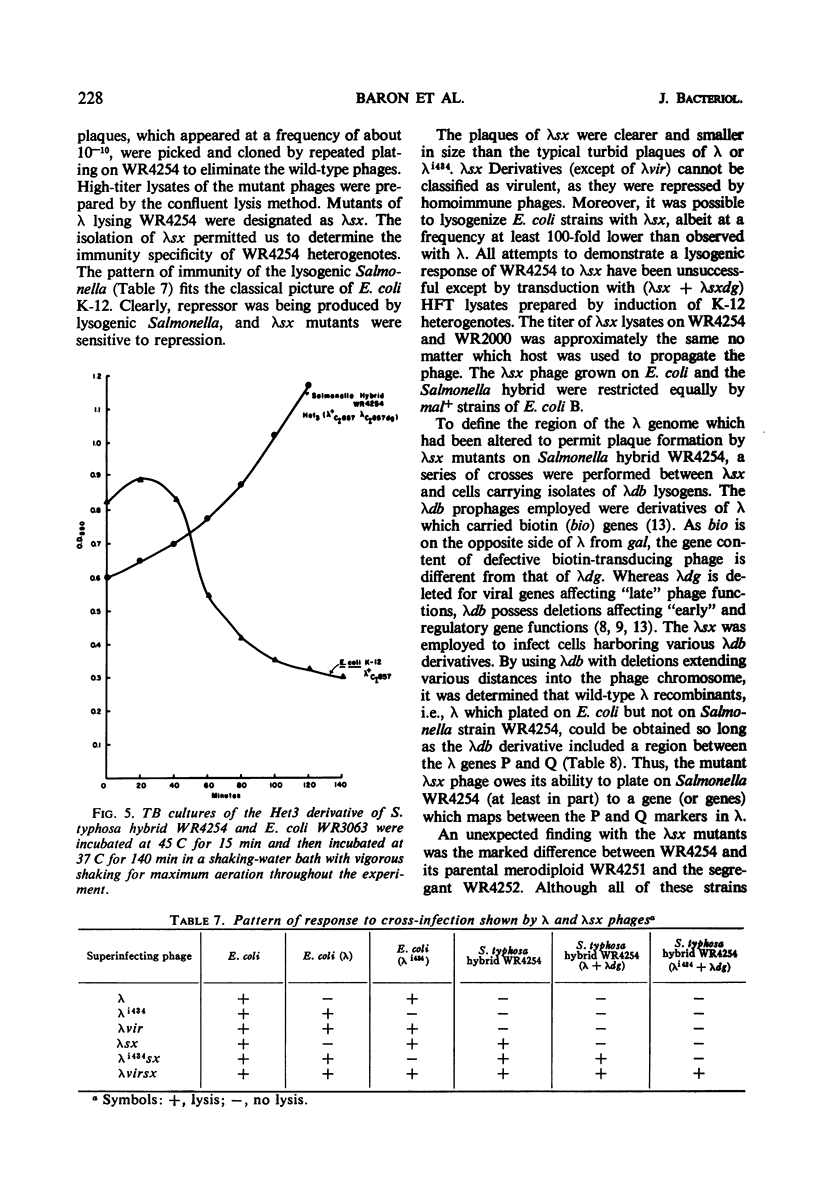
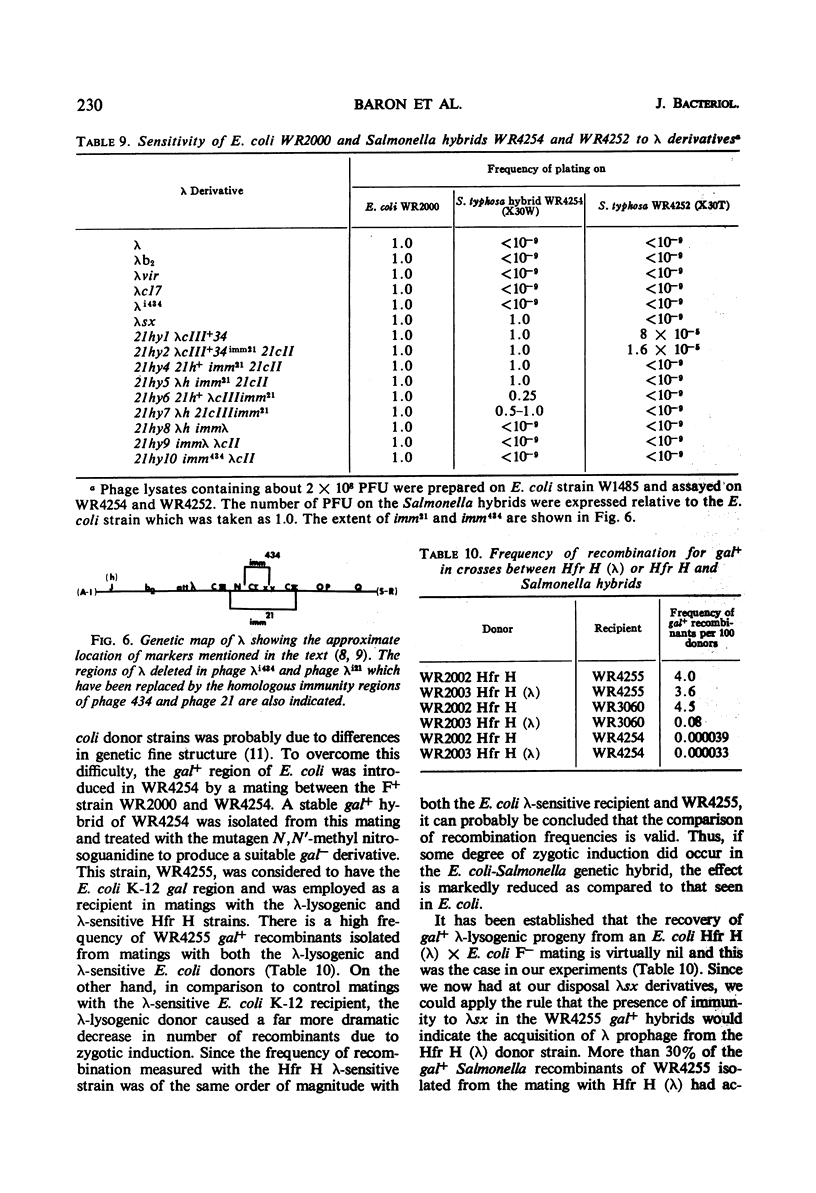
Abstract
Salmonella typhosa hybrids able to adsorb lambda were obtained by mating S. typhosa recipients with Escherichia coli K-12 donors. After adsorption of wild-type λ to these S. typhosa hybrids, no plaques or infective centers could be detected. E. coli K-12 gal+ genes carried by the defective phage λdg were transduced to S. typhosa hybrids with HFT lysates derived from E. coli heterogenotes. The lysogenic state which resulted in the S. typhosa hybrids after gal+ transduction differed from that of E. coli. Ability to produce λ, initially present, was permanently segregated by transductants of the S. typhosa hybrid. S. typhosa lysogens did not lyse upon treatment for phage induction with mitomycin C, ultraviolet light, or heat in the case of thermoinducible λ. A further difference in the behavior of λ in Salmonella hybrids was the absence of zygotic induction of the prophage when transferred from E. coli K-12 donors to S. typhosa. A new λ mutant class, capable of forming plaques on S. typhosa hybrids refractory to wild-type λ, was isolated at low frequency by plating λ on S. typhosa hybrid WR4254. Such mutants have been designated as λsx, and a mutant allele of λsx was located between the P and Q genes of the λ chromosome. Plaques were formed also on the S. typhosa hybrid host with a series of λi21 hybrid phages which contain the N gene of phage 21. The significance of these results in terms of Salmonella species as hosts for λ is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W. Host-controlled modification of bacteriophage. Annu Rev Microbiol. 1965;19:365–378. doi: 10.1146/annurev.mi.19.100165.002053. [DOI] [PubMed] [Google Scholar]
- BARON L. S., SPILMAN W. M., CAREY W. F. Diploid heterozygous hybrids from matings between Escherichia coli and Salmonella typhosa. J Exp Med. 1960 Aug 1;112:361–372. doi: 10.1084/jem.112.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron L. S., Carey W. F., Spilman W. M. GENETIC RECOMBINATION BETWEEN ESCHERICHIA COLI AND SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1959 Jul;45(7):976–984. doi: 10.1073/pnas.45.7.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron L. S., Gemski P., Johnson E. M., Wohlhieter J. A. Intergeneric bacterial matings. Bacteriol Rev. 1968 Dec;32(4 Pt 1):362–369. [PMC free article] [PubMed] [Google Scholar]
- Bases R. E., King A. S. Inhibition of Rauscher murine leukemia virus growth in vitro by actinomycin D. Virology. 1967 Jun;32(2):175–183. doi: 10.1016/0042-6822(67)90268-1. [DOI] [PubMed] [Google Scholar]
- Court D., Sato K. Studies of novel transducing variants of lambda: dispensability of genes N and Q. Virology. 1969 Oct;39(2):348–352. doi: 10.1016/0042-6822(69)90060-9. [DOI] [PubMed] [Google Scholar]
- Falkow S., Baron L. S. Plasmid formation after lambda bacteriophage infection of Escherichia coli-Salmonella typhosa hybrids. J Bacteriol. 1970 Apr;102(1):288–290. doi: 10.1128/jb.102.1.288-290.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Rownd R., Baron L. S. GENETIC HOMOLOGY BETWEEN ESCHERICHIA COLI K-12 AND SALMONELLA. J Bacteriol. 1962 Dec;84(6):1303–1312. doi: 10.1128/jb.84.6.1303-1312.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. D. Phage lambda mutants deficient in r-II exclusion. Science. 1967 Dec 22;158(3808):1588–1589. doi: 10.1126/science.158.3808.1588. [DOI] [PubMed] [Google Scholar]
- KORN D., WEISSBACH A. Thymineless induction in Escherichia coli K12 (lambda). Biochim Biophys Acta. 1962 Nov 26;61:775–790. doi: 10.1016/0926-6550(62)90060-9. [DOI] [PubMed] [Google Scholar]
- Kayajanian G. Studies on the genetics of biotin-transducing, defective variants of bacteriophage lambda. Virology. 1968 Sep;36(1):30–41. doi: 10.1016/0042-6822(68)90113-x. [DOI] [PubMed] [Google Scholar]
- Lieb M. Studies of heat-inducible lambda bacteriophage. I. Order of genetic sites and properties of mutant prophages. J Mol Biol. 1966 Mar;16(1):149–163. doi: 10.1016/s0022-2836(66)80269-3. [DOI] [PubMed] [Google Scholar]
- Morse M L, Lederberg E M, Lederberg J. Transductional Heterogenotes in Escherichia Coli. Genetics. 1956 Sep;41(5):758–779. doi: 10.1093/genetics/41.5.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Tomizawa J. Replication of bacteriophage DNA. I. Replication of DNA of lambda phage defective in early functions. J Mol Biol. 1968 Dec 14;38(2):217–225. doi: 10.1016/0022-2836(68)90407-5. [DOI] [PubMed] [Google Scholar]
- Packman S., Sly W. S. Constitutive lambda DNA replication by lambda-C17, a regulatory mutant related to virulence. Virology. 1968 Apr;34(4):778–789. doi: 10.1016/0042-6822(68)90099-8. [DOI] [PubMed] [Google Scholar]
- Signer E. R. Plasmid formation: a new mode of lysogeny by phase lambda. Nature. 1969 Jul 12;223(5202):158–160. doi: 10.1038/223158a0. [DOI] [PubMed] [Google Scholar]
- WOLLMAN E. L., JACOB F. Sur les processus de conjugaison et de recombinaison chez Escherichia coli. II. La localisation chromosomique du prophage lambda et les conséquences génétiques de l'induction zygotique. Ann Inst Pasteur (Paris) 1957 Sep;93(3):323–339. [PubMed] [Google Scholar]


