Abstract
Thyroid cancer, and its most common type, papillary carcinoma, frequently have chromosomal rearrangements and therefore represents a good model for the understanding of mechanisms of chromosomal rearrangements in solid tumors. Several types of rearrangement known to occur in thyroid cancer, including RET/PTC, NTRK1 and BRAF/AKAP9, are more common in radiation-associated thyroid tumors and RET/PTC can be induced experimentally by exposing human thyroid cells to ionizing radiation. In this review, the molecular mechanisms of generation of RET/PTC and other chromosomal rearrangements are discussed, with the emphasis on the role of nuclear architecture and interphase gene proximity in the generation of intrachromosomal rearrangements in thyroid cells.
Thyroid cancer and chromosomal rearrangements
Thyroid cancer is the most common malignant tumor of the endocrine system and accounts for approximately 1% of all newly diagnosed cancer cases [1]. Papillary thyroid carcinoma is the most prevalent type of thyroid malignancy and constitutes ~80% of all thyroid cancers. More than 70% of papillary carcinomas have known genetic alterations all of which lead to the activation of the mitogen-activated protein kinase (MAPK) signaling pathway [2–4]. These abnormalities include chromosomal rearrangements (intrachromosomal inversions and interchromosomal translocations) and point mutations. Most common point mutations involve the BRAF gene as well as RAS genes [5,6]. The most common chromosomal rearrangement involves the RET gene and is called RET/PTC [7,8]. In addition to RET/PTC, chromosomal rearrangements involving the NTRK1 and BRAF genes also occur in papillary thyroid carcinomas, although with a significantly lower prevalence [9,10]. As a result, papillary thyroid carcinoma represents a good model to study the mechanisms of chromosomal rearrangements in solid tumors.
Radiation-induced thyroid cancer involves chromosomal rearrangements rather than point mutations
Exposure to ionizing radiation is a well known risk factor for thyroid cancer, especially for papillary carcinoma. An increased incidence of thyroid cancer has been documented after therapeutic use of ionizing radiation during childhood [11,12] as well as after accidental environmental exposure. The latter includes survivors of the atomic bomb explosion in Hiroshima and Nagasaki in 1945 [13,14] and of the nuclear test fallout in the Marshall Islands in 1954 [15,16] and those exposed to radiation after the Chernobyl nuclear disaster in 1986 [17–19]. Studied of thyroid cancer in various populations revealed a sharply different prevalence of chromosomal rearrangements and point mutations in tumors from individuals exposed to ionizing radiation as compared to sporadic tumors, i.e. arising in patients with no radiation history [10,20] (Table 1). Indeed, the prevalence of RET/PTC is very high in individuals with a history of radiation exposure. This includes those subjected to either accidental (mostly radioiodine) irradiation or therapeutic (mostly external beam) irradiation, as 50–80% of those papillary carcinomas harbor RET/PTC [21–23]. In contrast, in the general population clonal RET/PTC rearrangements are seen in 10–40% of papillary carcinomas in most studies, although the reported prevalence varies dramatically [24,25], largely due to different sensitivities of the techniques used for their detection [26,27]. Higher prevalence of RET/PTC is seen in pediatric tumors [23,28,29], a significant portion of which may be associated with radiation exposure. Another chromosomal rearrangement, BRAF/AKAP9 is also found predominantly in papillary carcinomas associated with radiation exposure [10]. The opposite is true for point mutations, such as those involving the BRAF gene. BRAF V600E point mutation represents the most common genetic alteration in sporadic papillary carcinomas, being found in 40–45% of those tumors [30,31], but it is rarely found in radiation-related tumors [32]. Moreover, among papillary carcinomas in atomic bomb survivors in Japan, the presence of RET/PTC directly correlated with the dose of radiation, whereas the inverse correlation was found between the dose and BRAF point mutations [33,34]. These findings provide evidence that generation of chromosomal rearrangements in human thyroid carcinomas is closely linked to radiation exposure.
Table 1.
Prevalence of chromosomal rearrangements and point mutation in sporadic and radiation-induced papillary thyroid carcinomas
Types of RET/PTC rearrangement in sporadic and radiation-induced cancers
RET/PTC rearrangement is formed by fusion between the 3’ portion of the RET gene, coding for the receptor tyrosine kinase, and the 5’ portion of various unrelated genes. The two most common rearrangement types, RET/PTC1 and RET/PTC3, are paracentric inversions since both RET and its respective fusion partner, H4 or NCOA4 (ELE1; RFG, ARA70), reside on the long arm of chromosome 10 [8,35,36] RET/PTC2 and nine more recently identified types of RET/PTC are all interchromosomal translocations [37,38].
In most populations, RET/PTC1 is the most common type of RET/PTC as it comprises 60–70% of positive cases, whereas RET/PTC3 accounts for 20–30%, and RET/PTC2 and other novel rearrangement types for less than 5–10% [24,25]. In individuals exposed to accidental or therapeutic radiation, RET/PTC1 remained to be the most common rearrangement type except for the tumors that developed less than 10 years after radiation exposure at Chernobyl, where RET/PTC3 was the predominant rearrangement type [21,22,39,40].
Experimental evidence for the association between RET/PTC rearrangements and radiation exposure
The association between RET/PTC rearrangement and ionizing radiation is supported by several studies demonstrating the induction of RET/PTC by irradiation of cultured human thyroid cells [41,42] and of human fetal thyroid tissue xenografts in SCID mice [43,44]. It has been shown that exposure of HTori-3 human thyroid cells to physiologically relevant doses of gamma-radiation (0.1–10 Gy) resulted in a dose-dependent generation of both RET/PTC1 and RET/PTC3 rearrangements [42]. In this study, RET/PTC1 was more common than RET/PTC3 after each dose, comprising 80% of all rearrangements.
Although the dose of exposure were significantly higher (50–100 Gy) in two studies that employed human fetal thyroid tissue xenografts, they demonstrated that X-ray irradiation led to the generation of both RET/PTC1 and RET/PTC3 rearrangements, with RET/PTC1 type being the most common [43,44]. These studies provide evidence for the direct link between exposure to ionizing radiation and generation of RET/PTC rearrangement in human thyroid cells.
Molecular mechanisms of chromosomal aberrations induced by radiation
Ionizing radiation damages DNA in a variety of ways as a result of either direct energy deposition along the radiation track or by secondary reactive oxygen species produced by ionization of water. It is known that 1 Gy of X-ray radiation produces 500–1000 single-strand DNA breaks, 20–40 double-strand breaks (DSBs), >3000 damaged bases, and ~150 DNA-protein crosslinks per cell [45,46]. Of these types of DNA damage, DSBs are considered to be a crucial primary lesion for a variety of biological end points, including cell killing, chromosomal aberrations, and cell transformation [47,48]. However, how exactly radiogenic DSBs lead to chromosomal rearrangements remains not fully understood. Several basic theories have been proposed [49–51]. The most widely accepted is the Breakage-and-Reunion theory. It postulates that chromosomal aberrations arise mainly as a result of rejoining of two DSBs located closely in space and time (two-hit event) [49,50]. Presumably, most rejoining events occur via non-homologous end joining (NHEJ) [52,53]. The initial distribution of primary breaks is assumed to be random, although the rejoining efficiency is expected to be influenced by their proximity. An alternative, one-hit mechanism is suggested by the Molecular theory, which postulates that one radiation-induced DSB is sufficient to initiate an exchange that occurs with an undamaged DNA molecule [54,55]. The only plausible mechanism for such a series of events is homologous recombination initiated by one DSB. The Exchange theory, suggests that the initiation lesions are not DNA breaks induced by radiation but rather “unstable lesions” that do not disrupt the continuity of chromosomes but can initiate exchange between two lesions [56].
Although the Breakage-and-Reunion theory remains most widely accepted, none of the three theories can adequately explain all available experimental data on the dose-effect relationship and complexity of radiation-induced aberrations [57]. Moreover, these theories are based on the assumption that primary DNA lesions, either DSBs or less well-defined “unstable lesions,” are directly induced by radiation (direct mechanism). However, there is at least a theoretical possibility that radiation can lead to chromosomal exchanges entirely by the indirect mechanism, i.e. mediated by radiation-induced genomic instability and not involving the actual breaks induced by radiation. This possibility is supported by studies showing the occurrence of new chromosomal aberrations in subsequent generations of a cell exposed to radiation [58,59], and by a bystander effect, where aberrations are found in cells plated close to, but not in, the field of irradiation or partial irradiation of a cell cytoplasm [60–62].
Interphase gene proximity provides structural basis for the generation of RET/PTC rearrangement
It appears that nuclear architecture contributes to the generation of RET/PTC and other recurrent chromosomal rearrangements found in cancer cells by placing potentially recombinogenic chromosomal loci in close proximity in the interphase nuclei of human cells (Fig 1). For RET/PTC, this was initially demonstrated for the RET and H4 genes in a study that utilized fluorescence in situ hybridization (FISH) and three-dimensional (3D) confocal microscopy and showed that these genes were non-randomly located with respect to each other in the interphase nuclei of human thyroid cells and were much closer than expected based on their genomic separation [63]. In fact, at least one pair of RET and H4 were found juxtaposed in more than one third of adult thyroid cells. This study also showed that the proximity between potentially recombinogenic genes was cell-type specific and was not present in some non-thyroid cells such as mammary epithelial cells. More recently, similar finding were provided for RET and NCOA4, the partners of RET/PTC3 rearrangement [64]. Using FISH and high-resolution 3D confocal microscopy, it was shown that NCOA4 was located closer to RET than expected based on their genomic separation. In addition, spatial proximity was found to exist between the partners of another rearrangement occurring in papillary thyroid cancer, TRK [65]. Utilizing both 2D distance measurements and 3D mathematical projection, NTRK1 was shown to be closer to its translocation partner, TPR, in thyroid cells but not in lymphocytes.
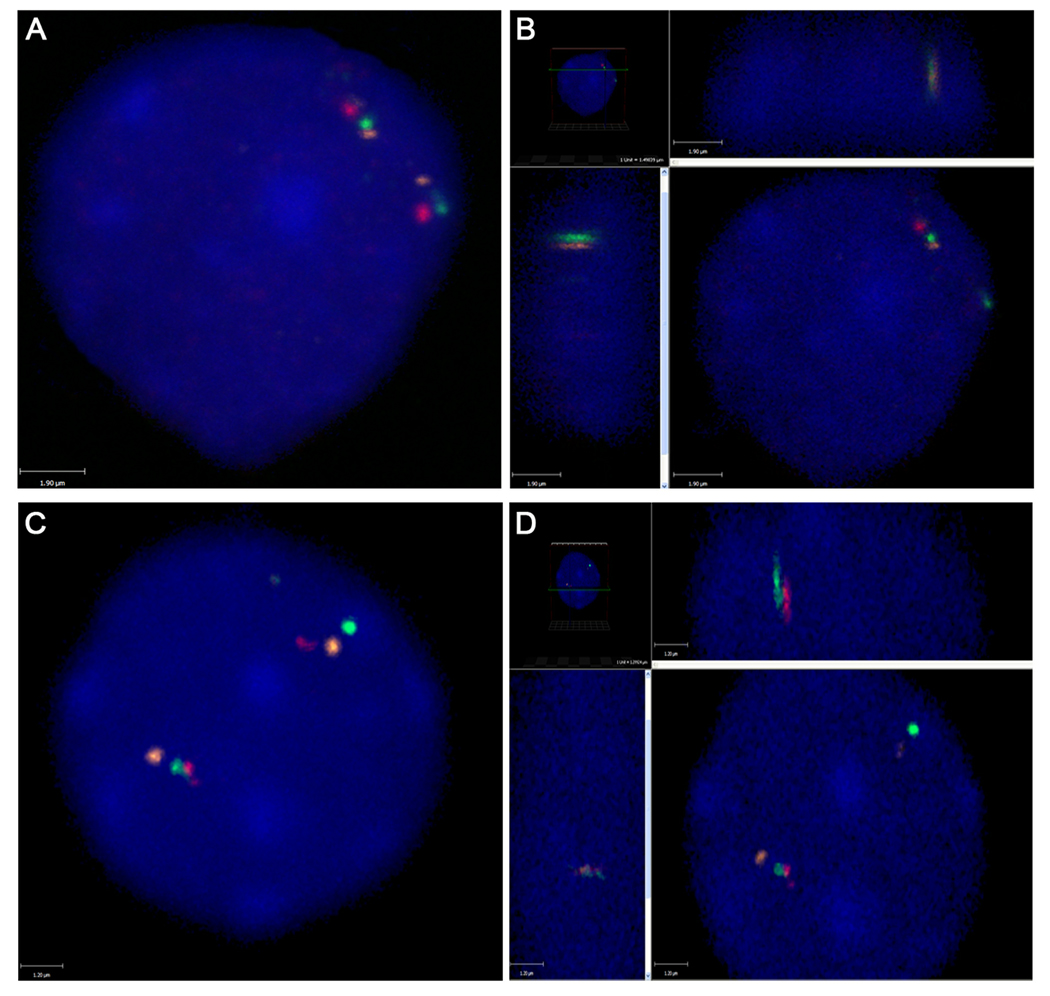
Figure 1.
Three-color fluorescence in situ hybridization (FISH) showing positioning of RET (green), NCOA4 (orange) and H4 (red) in interphase nuclei of thyroid cells. A. 2D image of a nucleus showing two sets of RET, NCOA4 and H4 with one pair of RET and NCOA4 positioned close to each other. B. 3D image showing that RET and NCOA4 are juxtaposed to each other in the same z plane. C. 2D image of a nucleus showing one pair of RET and H4 positioned close to each other. D. 3D image showing that RET and H4 are juxtaposed to each other in the same z plane.
It is likely that spatial proximity represent a pre-requisite for most rearrangements in human tumors, including intrachromosomal and interchromosomal exchanges. Thus, BCR and ABL genes, which are located on different chromosomes and frequently rearranged in leukemias, were located close to each other in normal human lymphocytes [66]. Likewise, MYC, BCL and immunoglobulin loci, which are located on different chromosomes and recombined in various types of B-cell lymphoma, were shown to be preferentially positioned in close spatial proximity relative to each other in normal B cells [67].
Irrespective of the specific DNA repair mechanism involved in recombination, spatial proximity is likely to predispose to specific rearrangements by making the neighboring regions prone to simultaneous damage by radiation or other DNA-damaging agents, and/or by facilitating mis-rejoining of free DNA ends located immediately adjacent to each other. Since the nuclear architecture is cell type specific, it may also provide an explanation why, in contrast to point mutations, almost all cancer-related chromosomal rearrangements are specific for particular cell/tumor types.
It remains unclear why specific chromosomal regions are located close to each other. For genetic loci located on the same chromosome, this is likely to involve high order chromosome folding that would allow the genes to be positioned non-randomly with respect to each other. It is known that double stranded DNA is wrapped around histones forming nucleosomes which are then arranged in a 30 nm fiber, solenoid structure [68]. Diverse models varying from irregularly folded chromatin fibers [69], radial loops [70,71], giant loops [72] to the random walk/giant loop model [73] have been proposed for higher order interphase chromatin compaction with the eventual packaging of interphase chromosomes into well defined chromosomal territories (CTs) [74]. With respect to the 18 Mb region on 10q containing RET, NCOA4, and H4, evidence for the large-scale helical folding of this chromosomal region in the interphase nuclei of human thyroid cells was provided [64]. This pattern of chromatin folding can offer the basis for proximity between RET and NCOA4 and H4. Whether or not such folding represents a unique structure of this chromosomal region or is a universal feature of interphase chromosome organization remains unknown.
Location of genes within chromosomal territories may influence the type of recombination
A peculiar feature of rearrangements found in papillary thyroid cancers is that almost all of them are intrachromosomal inversions rather then interchromosomal translocations. Indeed, in addition to RET/PTC1 and RET/PTC3 that involve genes on 10q11.2–q21, the TRK rearrangements most commonly involve the NTRK1 (1q21–q22) fusion to either TPR (1q25) or TPM3 (1q25) [9] and recently indentified BRAF/AKAP9 rearrangement involve two genes located on 7q [10]. A recent study provides experimental evidence suggest that the predominance of intrachromosomal recombination in thyroid cells may also be in part due to the nuclear architecture [75]. In this study, the location of specific chromosomal loci involved in intrachromosomal and interchromosomal exchanges in thyroid cells were analyzed. Simultaneous hybridization with gene-specific probes and their respective whole chromosome paints was used to establish the positioning of specific recombinogenic loci within their chromosome territories (CTs). It was found that genes involved in intrachromosomal rearrangements were positioned at significantly greater distances away from the CT edge and internally within their CTs as compared to genes involved in translocations that were positioned closer to the CT edge [75]. The frequent location of RET and its recombinogenic partners within the interior of the chromosomal territory, surrounded by its own chromosomal material and with limited availability to interact with neighboring chromosomal territories, is likely to predispose it to intrachromosomal exchange, such as seen in most cases of RET/PTC (Fig 2). Similar findings have been obtained in another study that demonstrated a significant correlation between the extent of intermingling between different CTs and frequency of translocation involving specific chromosome pairs [76].
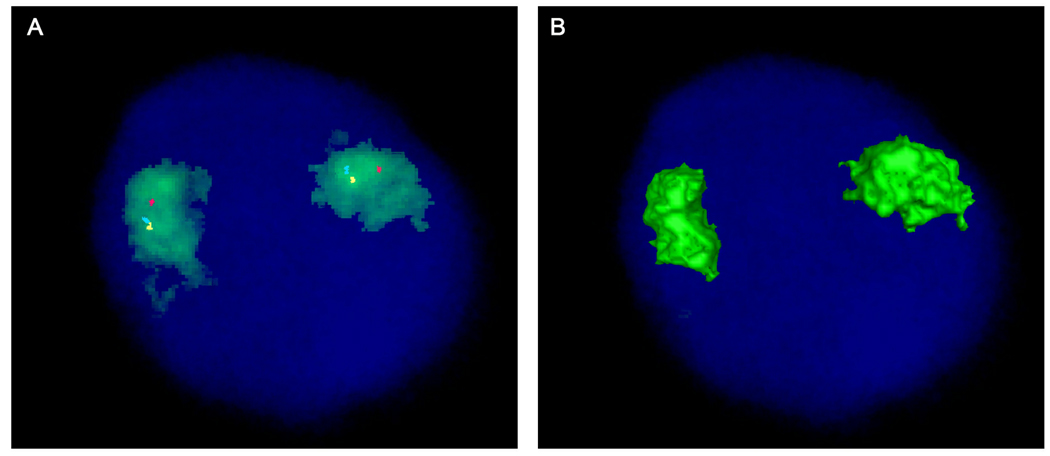
Figure 2.
Four-color FISH showing chromosome 10 territory (green) and location of RET (blue pseudocolor), NCOA4 (yellow pseudocolor) and H4 (red). A. All three genes, RET, NCOA4 and H4, are positioned within the chromosome 10 territory and away from the CT edge. B. 3D rendered image showing no signals on the surface of the CT due to the gene positioning inside the CT.
Potential DNA repair mechanisms involved in RET/PTC rearrangement
In mammalian cells, DSBs are repaired by two general pathways that are based on homology-dependent or nonhomologous recombination. The homology-dependant mechanism encompasses several pathways such as homologous recombination repair (HRR), single strand annealing (SSA), and non-allelic homologous recombination (NAHR). Nonhomologous mechanism is known as nonhomologous end joining (NHEJ). Another recently described repair pathway, microhomology mediated end joining (MMEJ), combines features of the two major pathways as it joins DNA ends after preliminary aligning them using short homology DNA sequences located distant to the break. These repair pathways utilize common enzymatic factors as well as those distinct to specific repair mechanisms. Usage of ATM/ATR and NBS1 kinases as the primary DSB sensors is common for homology based and non-homologous repair [77]. However, DNA ends are hold together and initially processed by different enzymes, DNA-PKs (Ku70/Ku80) in NHEJ [78] and Rad52 in HHR and SSA [79]. In all pathways, the processing of DNA ends and trimming is carried by conserved multiprotein MRE11/Rad50/NBS1 (MRN) complex, which plays an important role in DSB repair, meiotic recombination and telomere maintenance [80,81]. SSA and MMEJ additionally require the use of ERCC1-XPF (Rad10-Rad1) complex to incise double-stranded DNA at the junction with single-stranded DNA, nicking bubble structures and 3’ single-strand overhangs [82]. After homology search, strand annealing and end processing DNA integrity is restored. NHEJ employs XPCC4 and Lig4 to ligate the DNA ends [78,83,84].
Several mechanisms have been proposed for the formation of RET/PTC rearrangement. They include HRR, NHEJ, SSA, and MMEJ [85–87]. While the genomic sequence of RET/PTC1 fusion point is difficult to obtain due to a very large size of intron 1, which is a breakpoint cluster region of the H4 gene, the genomic sequences of 31 RET/PTC3 fusions from post-Chernobyl thyroid tumors have been reported [85–87] and can be used for the analysis (Table 2).
Table 2.
DNA sequence features of RET/PTC3 breakpoints in post-Chernobyl tumors and their correspondence to specific DNA repair pathways
| NHEJ | ||||||
|---|---|---|---|---|---|---|
| MMEJ | SSA | |||||
| case ID* |
microhomology at breakpoint |
distant microhomology (less than 5 nt) |
distant microhomology (5 or more nt) |
Deletions at breakpoint |
repeats in both genes |
deleted repeats |
| C22 | yes | yes | yes | yes | yes | 2 copies |
| C82 | no | yes | no | yes | yes | none |
| C102 | yes | yes | yes | yes | yes | none |
| C112 | no | yes | yes | no | yes | none |
| C142 | yes | yes | yes | yes | yes | none |
| C152 | yes | yes | yes | no | none | none |
| C172 | no | yes | yes | no | none | none |
| C202 | no | yes | no | yes | none | none |
| C242 | yes | yes | no | yes | none | none |
| C272 | yes | yes | no | yes | yes | 2 copies |
| C282 | yes | yes | yes | yes | none | none |
| C302 | no | yes | no | yes | none | none |
| M2T1 | yes | yes | no | yes | yes | 1 copy |
| M12T1 | yes | yes | no | yes | yes | 1 copy |
| M80T1 | yes | yes | yes | yes | yes | 2 copies |
| M81T1 | no | yes | yes | yes | yes | 3 copies |
| M89T1 | no | no | no | yes | yes | 3 copies |
| M122T1 | yes | yes | yes | yes | yes | 1 copy |
| M129T1 | no | yes | yes | yes | yes | none |
| M153T1 | yes | yes | yes | yes | yes | 1 copy |
| M161T1 | yes | yes | yes | yes | yes | 2 copies |
| M162T1 | yes | yes | yes | yes | yes | 1 copy |
| M190T1 | yes | yes | yes | yes | none | none |
| M216T1 | no | yes | yes | yes | yes | 1 copy |
| M219T1 | no | yes | yes | yes | none | none |
| M225T1 | yes | yes | yes | yes | yes | 1 copy |
| M259T1 | yes | yes | yes | yes | yes | 2 copies |
| M263T1 | no | yes | yes | yes | yes | 1 copy |
| CH43 | no | yes | yes | yes | yes | 1 copy |
| CH83 | no | yes | yes | yes | none | none |
| CH103 | no | yes | yes | no | yes | none |
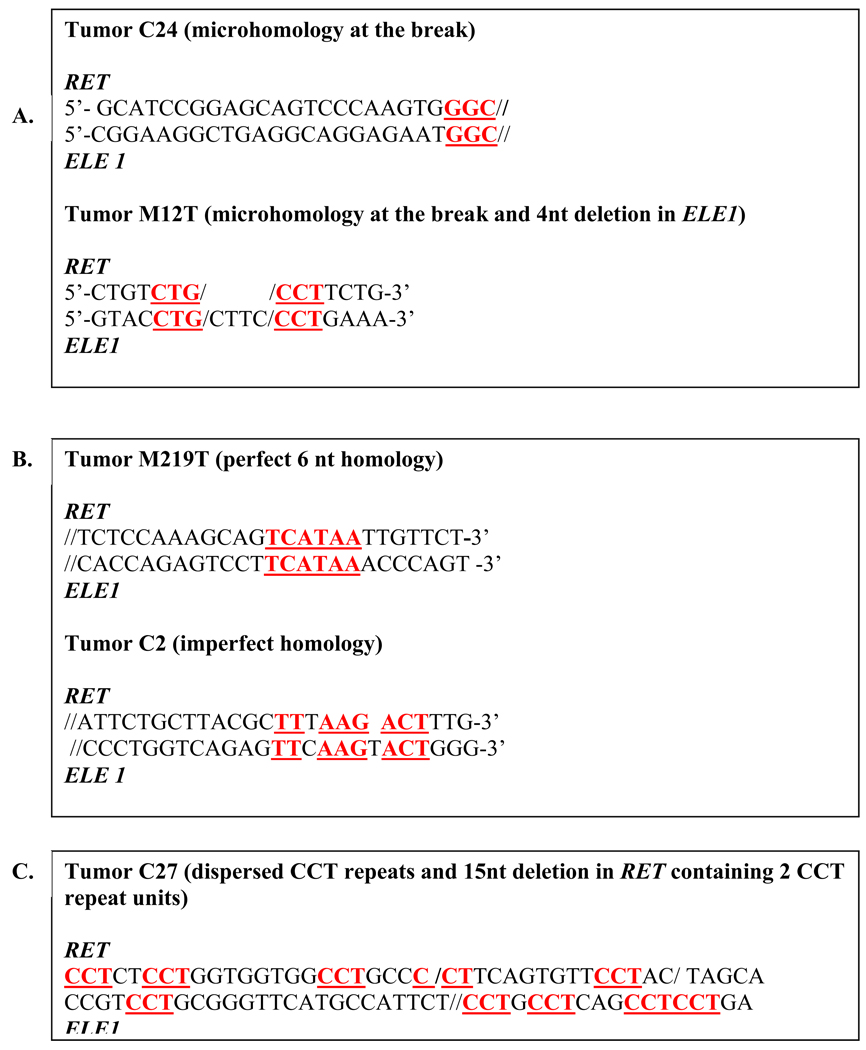
NHEJ utilizes microhomology (2–4 nt) at DNA ends, and frequently produces microdeletions/insertions at the breakpoints, usually joining the corresponding ends by fast end processing [88]. The nucleotide sequence feature of NHEJ is the presence of microhomology regions located immediately at the fusion points. In addition, sequence modifications, including small deletions and insertions, are common at the fusion point. Among 31 post-Chernobyl tumors with reported RET/PTC3 genomic sequence [85–87], 55% of cases had 3–5 nucleotide homology located at the break (Fig. 3A). Modifications at breakpoints, typically small deletions, were present in 26 (84%) of post-Chernobyl tumors with RET/PTC3. In addition to microhomology located immediately at breakpoints, NHEJ may utilize short homology regions located up to 60–300 nt away from the break, as it has been shown in prokaryotic cells [89,90]. In post-Chernobyl tumors with RET/PTC3, microhomology regions located within 50 nt from breakpoint were seen perfectly aligned relatively to the breakpoint in 58% of cases and with 1–2 nucleotide shift in 68% (Fig. 3B). Overall, microhomology regions were present at the breaks or on adjacent to the breaks in 97% of RET/PTC3 fusions, making the NHEJ pathway a strong candidate in the formation of RET/PTC products.
Figure 3.
Representative examples of sequences at RET/PTC3 breakpoints (//) with DNA-based characteristics for NHEJ (A), MMEJ (B) and SSA (C).
MMEJ is another repair pathway that utilizes short homology sequences. It has been reported that nuclear extracts from urothelial cancers repair DSBs preferentially by MMEJ compared to normal urothelial cell extracts [91]. Characteristic attributes of MMEJ are the utilization of 5–25 nt homology stretches and the presence of deletions flanking the breaks [92]. It has been suggested that high levels of DNA damage can induce MMEJ over typically predominant NHEJ [93]. Among RET/PTC fusions, 19% (6 out of 31) had 5 or more nucleotides in homologous regions, and another 49% (15 from 31) had 5–10 nucleotides imperfect homology regions with inserted base(s) between short homologous sequences (Fig. 3C). Overall, 61% of fusions had 5 nt homology stretches and deletions at the fusion point, suggesting that MMEJ may also serve as a mechanism for RET/PTC rearrangement in many cases.
SSA and NAHR utilize the repeatable DNA elements for alignment of broken DNA strand(s) and in quiescent cells have typically a limited participation in DSB repair. However, the loss of NHEJ due to down-regulation of its key factors leads to higher incidence of SSA and NAHR [79,90]. Of these two repair pathways, NAHR is unlikely to play a significant role in the generation of RET/PTC rearrangements because of the requirement for non-canonical DNA structures (Z-DNA in CG reach DNA regions) at the site of recombination, which are not present in the RET/PTC breakpoint cluster regions [94]. SSA utilizes homology regions larger than 15 nt and induces recombination between direct repeats with concomitant loss of one or more repeat units [95]. In model systems, tandem direct repeats serve the best for SSA, but in living cell SSA may use not only direct tandem repeats but also mirror and inverted repeats and repeats dispersed throughout flanking regions of the breaks [85,95]. The available RET/PTC3 fusion sequences revealed no 15 nt stretches of tandem repeat homology in any of the cases. However, dispersed homologous di-, tri- or tetranucleotide repeats in both fusion partners could be found in 22 (71%) cases. In addition, 16 of those 22 sequences had a deletion involving at least one repeat copy (Fig. 3D). Thus, SSA may be an additional potential repair mechanism for RET/PTC rearrangement.
These data, which are based on the analysis of DNA sequences at the fusion points, suggest that the generation of RET/PTC rearrangement may involve several possible DNA repair mechanisms, particularly NHEJ and MMEJ, and to lesser extent SSA. It remains unknown whether all of these mechanisms contribute to the generation of RET/PTC with similar frequency and if the choice is determined by specific conditions and/or individual genetic background.
Acknowledgments
This work was supported by the NIH grant R01 CA88041 to Y.E.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [see commetns]. [DOI] [PubMed] [Google Scholar]
- 2.Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21 Suppl 2:S37–S43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fagin JA. Genetics of papillary thyroid cancer initiation: implications for therapy. Trans Am Clin Climatol Assoc. 2005;116:259–269. discussion 269-71. [PMC free article] [PubMed] [Google Scholar]
- 4.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicularcell neoplasia. Nat Rev Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 5.Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, Beller U, Westra WH, Ladenson PW, Sidransky D. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003;120:71–77. doi: 10.1309/ND8D-9LAJ-TRCT-G6QD. [DOI] [PubMed] [Google Scholar]
- 7.Fusco A, Grieco M, Santoro M, Berlingieri MT, Pilotti S, Pierotti MA, Della Porta G, Vecchio G. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature. 1987;328:170–172. doi: 10.1038/328170a0. [DOI] [PubMed] [Google Scholar]
- 8.Grieco M, Santoro M, Berlingieri MT, Melillo RM, Donghi R, Bongarzone I, Pierotti MA, Della Porta G, Fusco A, Vecchio G. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990;60:557–563. doi: 10.1016/0092-8674(90)90659-3. [DOI] [PubMed] [Google Scholar]
- 9.Pierotti MA, Bongarzone I, Borrello MG, Mariani C, Miranda C, Sozzi G, Greco A. Rearrangements of TRK proto-oncogene in papillary thyroid carcinomas. J Endocrinol Invest. 1995;18:130–133. doi: 10.1007/BF03349721. [DOI] [PubMed] [Google Scholar]
- 10.Ciampi R, Knauf JA, Kerler R, Gandhi M, Zhu Z, Nikiforova MN, Rabes HM, Fagin JA, Nikiforov YE. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. 2005;115:94–101. doi: 10.1172/JCI23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, Schneider AB, Tucker MA, Boice JD., Jr Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 12.Winship T, Rosvoll RV. Cancer of the thyroid in children. Proc Natl Cancer Conf. 1970;6:677–681. [PubMed] [Google Scholar]
- 13.Parker LN, Belsky JL, Yamamoto T, Kawamoto S, Keehn RJ. Thyroid carcinoma after exposure to atomic radiation. A continuing survey of a fixed population, Hiroshima and Nagasaki, 1958–1971. Ann Intern Med. 1974;80:600–604. doi: 10.7326/0003-4819-80-5-600. [DOI] [PubMed] [Google Scholar]
- 14.Prentice RL, Kato H, Yoshimoto K, Mason M. Radiation exposure and thyroid cancer incidence among Hiroshima and Nagasaki residents. Natl Cancer Inst Monogr. 1982;62:207–212. [PubMed] [Google Scholar]
- 15.Conrad R. Late radiation effects in Marshall Islanders exposed to fallout 28 years ago. In: Boice JJ, Fraumeni JJ, editors. Radiation Carcinogenesis: Epidemiology and Biological Significance. New York: raven Press; 1984. pp. 57–65. [Google Scholar]
- 16.Hamilton TE, van Belle G, LoGerfo JP. Thyroid neoplasia in Marshall Islanders exposed to nuclear fallout. Jama. 1987;258:629–635. [PubMed] [Google Scholar]
- 17.Kazakov VS, Demidchik EP, Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992;359:21. doi: 10.1038/359021a0. [DOI] [PubMed] [Google Scholar]
- 18.Nikiforov Y, Gnepp DR. Pediatric thyroid cancer after the Chernobyl disaster. Pathomorphologic study of 84 cases (1991–1992) from the Republic of Belarus. Cancer. 1994;74:748–766. doi: 10.1002/1097-0142(19940715)74:2<748::aid-cncr2820740231>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Cardis E, Howe G, Ron E, Bebeshko V, Bogdanova T, Bouville A, Carr Z, Chumak V, Davis S, Demidchik Y, Drozdovitch V, Gentner N, Gudzenko N, Hatch M, Ivanov V, Jacob P, Kapitonova E, Kenigsberg Y, Kesminiene A, Kopecky KJ, Kryuchkov V, Loos A, Pinchera A, Reiners C, Repacholi M, Shibata Y, Shore RE, Thomas G, Tirmarche M, Yamashita S, Zvonova I. Cancer consequences of the Chernobyl accident: 20 years on. J Radiol Prot. 2006;26:127–140. doi: 10.1088/0952-4746/26/2/001. [DOI] [PubMed] [Google Scholar]
- 20.Nikiforov YE. Radiation-induced thyroid cancer: what we have learned from chernobyl. Endocr Pathol. 2006;17:307–317. doi: 10.1007/s12022-006-0001-5. [DOI] [PubMed] [Google Scholar]
- 21.Rabes HM, Demidchik EP, Sidorow JD, Lengfelder E, Beimfohr C, Hoelzel D, Klugbauer S. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin Cancer Res. 2000;6:1093–1103. [PubMed] [Google Scholar]
- 22.Bounacer A, Wicker R, Caillou B, Cailleux AF, Sarasin A, Schlumberger M, Suarez HG. High prevalence of activating ret proto-oncogene rearrangements, in thyroid tumors from patients who had received external radiation. Oncogene. 1997;15:1263–1273. doi: 10.1038/sj.onc.1200206. [DOI] [PubMed] [Google Scholar]
- 23.Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H, Fagin JA. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57:1690–1694. [PubMed] [Google Scholar]
- 24.Nikiforov YE. RET/PTC Rearrangement in Thyroid Tumors. Endocr Pathol. 2002;13:3–16. doi: 10.1385/ep:13:1:03. [DOI] [PubMed] [Google Scholar]
- 25.Tallini G, Asa SL. RET oncogene activation in papillary thyroid carcinoma. Adv Anat Pathol. 2001;8:345–354. doi: 10.1097/00125480-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Z, Ciampi R, Nikiforova MN, Gandhi M, Nikiforov YE. Prevalence of Ret/Ptc Rearrangements in Thyroid Papillary Carcinomas: Effects of the Detection Methods and Genetic Heterogeneity. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2006-1006. [DOI] [PubMed] [Google Scholar]
- 27.Unger K, Zitzelsberger H, Salvatore G, Santoro M, Bogdanova T, Braselmann H, Kastner P, Zurnadzhy L, Tronko N, Hutzler P, Thomas G. Heterogeneity in the distribution of RET/PTC rearrangements within individual post-Chernobyl papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:4272–4279. doi: 10.1210/jc.2003-031870. [DOI] [PubMed] [Google Scholar]
- 28.Bongarzone I, Fugazzola L, Vigneri P, Mariani L, Mondellini P, Pacini F, Basolo F, Pinchera A, Pilotti S, Pierotti MA. Age-related activation of the tyrosine kinase receptor protooncogenes RET and NTRK1 in papillary thyroid carcinoma. J Clin Endocrinol Metab. 1996;81:2006–2009. doi: 10.1210/jcem.81.5.8626874. [DOI] [PubMed] [Google Scholar]
- 29.Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab. 2000;85:1170–1175. doi: 10.1210/jcem.85.3.6472. [DOI] [PubMed] [Google Scholar]
- 30.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 31.Ciampi R, Nikiforov YE. Alterations of the BRAF gene in thyroid tumors. Endocr Pathol. 2005;16:163–172. doi: 10.1385/ep:16:3:163. [DOI] [PubMed] [Google Scholar]
- 32.Nikiforova MN, Ciampi R, Salvatore G, Santoro M, Gandhi M, Knauf JA, Thomas GA, Jeremiah S, Bogdanova TI, Tronko MD, Fagin JA, Nikiforov YE. Low prevalence of BRAF mutations in radiation-induced thyroid tumors in contrast to sporadic papillary carcinomas. Cancer Lett. 2004;209:1–6. doi: 10.1016/j.canlet.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Hamatani K, Eguchi H, Ito R, Mukai M, Takahashi K, Taga M, Imai K, Cologne J, Soda M, Arihiro K, Fujihara M, Abe K, Hayashi T, Nakashima M, Sekine I, Yasui W, Hayashi Y, Nakachi K. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 2008;68:7176–7182. doi: 10.1158/0008-5472.CAN-08-0293. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi K, Eguchi H, Arihiro K, Ito R, Koyama K, Soda M, Cologne J, Hayashi Y, Nakata Y, Nakachi K, Hamatani K. The presence of BRAF point mutation in adult papillary thyroid carcinomas from atomic bomb survivors correlates with radiation dose. Mol Carcinog. 2007;46:242–248. doi: 10.1002/mc.20277. [DOI] [PubMed] [Google Scholar]
- 35.Santoro M, Dathan NA, Berlingieri MT, Bongarzone I, Paulin C, Grieco M, Pierotti MA, Vecchio G, Fusco A. Molecular characterization of RET/PTC3; a novel rearranged version of the RET proto-oncogene in a human thyroid papillary carcinoma. Oncogene. 1994;9:509–516. [PubMed] [Google Scholar]
- 36.Bongarzone I, Butti MG, Coronelli S, Borrello MG, Santoro M, Mondellini P, Pilotti S, Fusco A, Della Porta G, Pierotti MA. Frequent activation of ret protooncogene by fusion with a new activating gene in papillary thyroid carcinomas. Cancer Res. 1994;54:2979–2985. [PubMed] [Google Scholar]
- 37.Nikiforov YE. Papillary carcinoma. In: Nikiforov YE, et al., editors. Diagnostic pathology and molecular genetics of the thyroid. Baltimore: Lippincott Williams & Wilkins; 2009. pp. 160–213. [Google Scholar]
- 38.Ciampi R, Giordano TJ, Wikenheiser-Brokamp K, Koenig RJ, Nikiforov YE. HOOK3-RET: a novel type of RET/PTC rearrangement in papillary thyroid carcinoma. Endocr Relat Cancer. 2007;14:445–452. doi: 10.1677/ERC-07-0039. [DOI] [PubMed] [Google Scholar]
- 39.Smida J, Salassidis K, Hieber L, Zitzelsberger H, Kellerer AM, Demidchik EP, Negele T, Spelsberg F, Lengfelder E, Werner M, Bauchinger M. Distinct frequency of ret rearrangements in papillary thyroid carcinomas of children and adults from Belarus. Int J Cancer. 1999;80:32–38. doi: 10.1002/(sici)1097-0215(19990105)80:1<32::aid-ijc7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Elisei R, Romei C, Vorontsova T, Cosci B, Veremeychik V, Kuchinskaya E, Basolo F, Demidchik EP, Miccoli P, Pinchera A, Pacini F. RET/PTC rearrangements in thyroid nodules: studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J Clin Endocrinol Metab. 2001;86:3211–3216. doi: 10.1210/jcem.86.7.7678. [DOI] [PubMed] [Google Scholar]
- 41.Ito T, Seyama T, Iwamoto KS, Hayashi T, Mizuno T, Tsuyama N, Dohi K, Nakamura N, Akiyama M. In vitro irradiation is able to cause RET oncogene rearrangement. Cancer Res. 1993;53:2940–2943. [PubMed] [Google Scholar]
- 42.Caudill CM, Zhu Z, Ciampi R, Stringer JR, Nikiforov YE. Dose-dependent generation of RET/PTC in human thyroid cells after in vitro exposure to gamma-radiation: a model of carcinogenic chromosomal rearrangement induced by ionizing radiation. J Clin Endocrinol Metab. 2005;90:2364–2369. doi: 10.1210/jc.2004-1811. [DOI] [PubMed] [Google Scholar]
- 43.Mizuno T, Kyoizumi S, Suzuki T, Iwamoto KS, Seyama T. Continued expression of a tissue specific activated oncogene in the early steps of radiation-induced human thyroid carcinogenesis. Oncogene. 1997;15:1455–1460. doi: 10.1038/sj.onc.1201313. [DOI] [PubMed] [Google Scholar]
- 44.Mizuno T, Iwamoto KS, Kyoizumi S, Nagamura H, Shinohara T, Koyama K, Seyama T, Hamatani K. Preferential induction of RET/PTC1 rearrangement by X-ray irradiation. Oncogene. 2000;19:438–443. doi: 10.1038/sj.onc.1203343. [DOI] [PubMed] [Google Scholar]
- 45.Goodhead DT. The initial physical damage produced by ionizing radiations. Int J Radiat Biol. 1989;56:623–634. doi: 10.1080/09553008914551841. [DOI] [PubMed] [Google Scholar]
- 46.Ward JF. DNA damage as the cause of ionizing radiation-induced gene activation. Radiat Res. 1994;138:S85–S88. [PubMed] [Google Scholar]
- 47.Bryant PE, Riches AC. Oncogenic transformation of murine C3H 10T1/2 cells resulting from DNA double-strand breaks induced by a restriction end onuclease. Br J Cancer. 1989;60:852–854. doi: 10.1038/bjc.1989.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winegar RA, Lutze LH, Rufer JT, Morgan WF. Spectrum of mutations produced by specific types of restriction enzyme-induced double-strand breaks. Mutagenesis. 1992;7:439–445. doi: 10.1093/mutage/7.6.439. [DOI] [PubMed] [Google Scholar]
- 49.Hlatky L, Sachs RK, Vazquez M, Cornforth MN. Radiation-induced chromosome aberrations: insights gained from biophysical modeling. Bioessays. 2002;24:714–723. doi: 10.1002/bies.10126. [DOI] [PubMed] [Google Scholar]
- 50.Savage JR. A brief survey of aberration origin theories. Mutat Res. 1998;404:139–147. doi: 10.1016/s0027-5107(98)00107-9. [DOI] [PubMed] [Google Scholar]
- 51.Pfeiffer P, Goedecke W, Obe G. Mechanisms of DNA double-strand break repair and their potential to induce chromosomal aberrations. Mutagenesis. 2000;15:289–302. doi: 10.1093/mutage/15.4.289. [DOI] [PubMed] [Google Scholar]
- 52.Rothkamm K, Kuhne M, Jeggo PA, Lobrich M. Radiation-induced genomic rearrangements formed by nonhomologous end-joining of DNA double-strand breaks. Cancer Res. 2001;61:3886–3893. [PubMed] [Google Scholar]
- 53.Yates BL, Morgan WF. Nonhomologous DNA end rejoining in chromosomal aberration formation. Mutat Res. 1993;285:53–60. doi: 10.1016/0027-5107(93)90051-g. [DOI] [PubMed] [Google Scholar]
- 54.Chadwick KH, Leenhouts HP. The rejoining of DNA double-strand breaks and a model for the formation of chromosomal rearrangements. Int J Radiat Biol Relat Stud Phys Chem Med. 1978;33:517–529. doi: 10.1080/09553007814550431. [DOI] [PubMed] [Google Scholar]
- 55.Goodhead DT, Thacker J, Cox R. Weiss Lecture. Effects of radiations of different qualities on cells: molecular mechanisms of damage and repair. Int J Radiat Biol. 1993;63:543–556. doi: 10.1080/09553009314450721. [DOI] [PubMed] [Google Scholar]
- 56.Revell SH. Proceedings: A speculation about observed differences in X-ray sensitivities of euploid and aneuploid mammalian cells. Br J Radiol. 1975;48:416–417. [PubMed] [Google Scholar]
- 57.Edwards AA. Modelling radiation-induced chromosome aberrations. Int J Radiat Biol. 2002;78:551–558. doi: 10.1080/09553000210132315. [DOI] [PubMed] [Google Scholar]
- 58.Huang L, Snyder AR, Morgan WF. Radiation-induced genomic instability and its implications for radiation carcinogenesis. Oncogene. 2003;22:5848–5854. doi: 10.1038/sj.onc.1206697. [DOI] [PubMed] [Google Scholar]
- 59.Little JB. Genomic instability and radiation. J Radiol Prot. 2003;23:173–181. doi: 10.1088/0952-4746/23/2/304. [DOI] [PubMed] [Google Scholar]
- 60.Ludwikow G, Xiao Y, Hoebe RA, Franken NA, Darroudi F, Stap J, Van Oven CH, Van Noorden CJ, Aten JA. Induction of chromosome aberrations in unirradiated chromatin after partial irradiation of a cell nucleus. Int J Radiat Biol. 2002;78:239–247. doi: 10.1080/09553000110110086. [DOI] [PubMed] [Google Scholar]
- 61.Little JB, Nagasawa H, Li GC, Chen DJ. Involvement of the nonhomologous end joining DNA repair pathway in the bystander effect for chromosomal aberrations. Radiat Res. 2003;159:262–267. doi: 10.1667/0033-7587(2003)159[0262:iotnej]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 62.Morgan WF, Hartmann A, Limoli CL, Nagar S, Ponnaiya B. Bystander effects in radiation-induced genomic instability. Mutat Res. 2002;504:91–100. doi: 10.1016/s0027-5107(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 63.Nikiforova MN, Stringer JR, Blough R, Medvedovic M, Fagin JA, Nikiforov YE. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science. 2000;290:138–141. doi: 10.1126/science.290.5489.138. [DOI] [PubMed] [Google Scholar]
- 64.Gandhi M, Medvedovic M, Stringer JR, Nikiforov YE. Interphase chromosome folding determines spatial proximity of genes participating in carcinogenic RET/PTC rearrangements. Oncogene. 2006;25:2360–2366. doi: 10.1038/sj.onc.1209268. [DOI] [PubMed] [Google Scholar]
- 65.Roccato E, Bressan P, Sabatella G, Rumio C, Vizzotto L, Pierotti MA, Greco A. Proximity of TPR and NTRK1 rearranging loci in human thyrocytes. Cancer Res. 2005;65:2572–2576. doi: 10.1158/0008-5472.CAN-04-4294. [DOI] [PubMed] [Google Scholar]
- 66.Kozubek S, Lukasova E, Ryznar L, Kozubek M, Liskova A, Govorun RD, Krasavin EA, Horneck G. Distribution of ABL and BCR genes in cell nuclei of normal and irradiated lymphocytes. Blood. 1997;89:4537–4545. [PubMed] [Google Scholar]
- 67.Roix JJ, McQueen PG, Munson PJ, Parada LA, Misteli T. Spatial proximity of translocation-prone gene loci in human lymphomas. Nat Genet. 2003;34:287–291. doi: 10.1038/ng1177. [DOI] [PubMed] [Google Scholar]
- 68.Lodish H, Berk A, Zipursky LS, Matsudaira P, Baltimore D, Darnell J. Molecular Cell Biology. New York: W.H. Freeman & Co.; 1999. [Google Scholar]
- 69.DuPraw EJ. Macromolecular organization of nuclei and chromosomes: a folded fibre model based on whole-mount electron microscopy. Nature. 1965;206:338–343. doi: 10.1038/206338a0. [DOI] [PubMed] [Google Scholar]
- 70.Rattner JB, Lin CC. Radial loops and helical coils coexist in metaphase chromosomes. Cell. 1985;42:291–296. doi: 10.1016/s0092-8674(85)80124-0. [DOI] [PubMed] [Google Scholar]
- 71.Manuelidis L. A view of interphase chromosomes. Science. 1990;250:1533–1540. doi: 10.1126/science.2274784. [DOI] [PubMed] [Google Scholar]
- 72.Ostashevsky JY, Lange CS. The 30 nm chromatin fiber as a flexible polymer. J Biomol Struct Dyn. 1994;11:813–820. doi: 10.1080/07391102.1994.10508034. [DOI] [PubMed] [Google Scholar]
- 73.Yokota H, van den Engh G, Hearst JE, Sachs RK, Trask BJ. Evidence for the organization of chromatin in megabase pair-sized loops arranged along a random walk path in the human G0/G1 interphase nucleus. J Cell Biol. 1995;130:1239–1249. doi: 10.1083/jcb.130.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2:292–301. doi: 10.1038/35066075. [DOI] [PubMed] [Google Scholar]
- 75.Gandhi MS, Stringer JR, Nikiforova MN, Medvedovic M, Nikiforov YE. Gene position within chromosome territories correlates with their involvement in distinct rearrangement types in thyroid cancer cells. Genes Chromosomes Cancer. 2009;48:222–228. doi: 10.1002/gcc.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Branco MR, Pombo A. Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol. 2006;4:e138. doi: 10.1371/journal.pbio.0040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahnesorg P, Smith P, Jackson SP. XLF interacts with the XRCC4-DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell. 2006;124:301–313. doi: 10.1016/j.cell.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 78.Mari PO, Florea BI, Persengiev SP, Verkaik NS, Bruggenwirth HT, Modesti M, Giglia-Mari G, Bezstarosti K, Demmers JA, Luider TM, Houtsmuller AB, van Gent DC. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc Natl Acad Sci U S A. 2006;103:18597–18602. doi: 10.1073/pnas.0609061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mansour WY, Schumacher S, Rosskopf R, Rhein T, Schmidt-Petersen F, Gatzemeier F, Haag F, Borgmann K, Willers H, Dahm-Daphi J. Hierarchy of nonhomologous end-joining, single-strand annealing and gene conversion at site-directed DNA double-strand breaks. Nucleic Acids Res. 2008;36:4088–4098. doi: 10.1093/nar/gkn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paull TT, Gellert M. The 3' to 5' exonuclease activity of Mre 11 facilitates repair of DNA double-strand breaks. Mol Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- 81.Trujillo KM, Yuan SS, Lee EY, Sung P. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J Biol Chem. 1998;273:21447–21450. doi: 10.1074/jbc.273.34.21447. [DOI] [PubMed] [Google Scholar]
- 82.Ahmad A, Robinson AR, Duensing A, van Drunen E, Beverloo HB, Weisberg DB, Hasty P, Hoeijmakers JH, Niedernhofer LJ. ERCC1-XPF endonuclease facilitates DNA double-strand break repair. Mol Cell Biol. 2008;28:5082–5092. doi: 10.1128/MCB.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teo SH, Jackson SP. Identification of Saccharomyces cerevisiae DNA ligase IV: involvement in DNA double-strand break repair. Embo J. 1997;16:4788–4795. doi: 10.1093/emboj/16.15.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Z, Otevrel T, Gao Y, Cheng HL, Seed B, Stamato TD, Taccioli GE, Alt FW. The XRCC4 gene encodes a novel protein involved in DNA double-strand break repair and V(D)J recombination. Cell. 1995;83:1079–1089. doi: 10.1016/0092-8674(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 85.Klugbauer S, Pfeiffer P, Gassenhuber H, Beimfohr C, Rabes HM. RET rearrangements in radiation-induced papillary thyroid carcinomas: high prevalence of topoisomerase I sites at breakpoints and microhomology-mediated end joining in ELE1 and RET chimeric genes. Genomics. 2001;73:149–160. doi: 10.1006/geno.2000.6434. [DOI] [PubMed] [Google Scholar]
- 86.Nikiforov YE, Koshoffer A, Nikiforova M, Stringer J, Fagin JA. Chromosomal breakpoint positions suggest a direct role for radiation in inducing illegitimate recombination between the ELE1 and RET genes in radiation-induced thyroid carcinomas. Oncogene. 1999;18:6330–6334. doi: 10.1038/sj.onc.1203019. [DOI] [PubMed] [Google Scholar]
- 87.Bongarzone I, Butti MG, Fugazzola L, Pacini F, Pinchera A, Vorontsova TV, Demidchik EP, Pierotti MA. Comparison of the breakpoint regions of ELE1 and RET genes involved in the generation of RET/PTC3 oncogene in sporadic and in radiation-associated papillary thyroid carcinomas. Genomics. 1997;42:252–259. doi: 10.1006/geno.1997.4685. [DOI] [PubMed] [Google Scholar]
- 88.Lobrich M, Rydberg B, Cooper PK. Repair of x-ray-induced DNA double-strand breaks in specific Not I restriction fragments in human fibroblasts: joining of correct and incorrect ends. Proc Natl Acad Sci U S A. 1995;92:12050–12054. doi: 10.1073/pnas.92.26.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brissett NC, Doherty AJ. Repairing DNA double-strand breaks by the prokaryotic non-homologous end-joining pathway. Biochem Soc Trans. 2009;37:539–545. doi: 10.1042/BST0370539. [DOI] [PubMed] [Google Scholar]
- 90.Lee JA, Carvalho CM, Lupski JR. A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell. 2007;131:1235–1247. doi: 10.1016/j.cell.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 91.Windhofer F, Krause S, Hader C, Schulz WA, Florl AR. Distinctive differences in DNA double-strand break repair between normal urothelial and urothelial carcinoma cells. Mutat Res. 2008;638:56–65. doi: 10.1016/j.mrfmmm.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 92.McVey M, Lee SE. MMEJ repair of double-strand breaks (director's cut): deleted sequences and alternative endings. Trends Genet. 2008;24:529–538. doi: 10.1016/j.tig.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katsura Y, Sasaki S, Sato M, Yamaoka K, Suzukawa K, Nagasawa T, Yokota J, Kohno T. Involvement of Ku80 in microhomology-mediated end joining for DNA double-strand breaks in vivo. DNA Repair (Amst) 2007;6:639–648. doi: 10.1016/j.dnarep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Gu W, Zhang F, Lupski JR. Mechanisms for human genomic rearrangements. Pathogenetics. 2008;1:4. doi: 10.1186/1755-8417-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Odom OW, Baek KH, Dani RN, Herrin DL. Chlamydomonas chloroplasts can use short dispersed repeats and multiple pathways to repair a double-strand break in the genome. Plant J. 2008;53:842–853. doi: 10.1111/j.1365-313X.2007.03376.x. [DOI] [PubMed] [Google Scholar]
- 96.Beimfohr C, Klugbauer S, Demidchik EP, Lengfelder E, Rabes HM. NTRK1 re-arrangement in papillary thyroid carcinomas of children after the Chernobyl reactor accident. Int J Cancer. 1999;80:842–847. doi: 10.1002/(sici)1097-0215(19990315)80:6<842::aid-ijc7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 97.Kumagai A, Namba H, Saenko VA, Ashizawa K, Ohtsuru A, Ito M, Ishikawa N, Sugino K, Ito K, Jeremiah S, Thomas GA, Bogdanova TI, Tronko MD, Nagayasu T, Shibata Y, Yamashita S. Low frequency of BRAFT1796A mutations in childhood thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:4280–4284. doi: 10.1210/jc.2004-0172. [DOI] [PubMed] [Google Scholar]
- 98.Lima J, Trovisco V, Soares P, Maximo V, Magalhaes J, Salvatore G, Santoro M, Bogdanova T, Tronko M, Abrosimov A, Jeremiah S, Thomas G, Williams D, Sobrinho-Simoes M. BRAF mutations are not a major event in post-Chernobyl childhood thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:4267–4271. doi: 10.1210/jc.2003-032224. [DOI] [PubMed] [Google Scholar]
- 99.Powell N, Jeremiah S, Morishita M, Dudley E, Bethel J, Bogdanova T, Tronko M, Thomas G. Frequency of BRAF T1796A mutation in papillary thyroid carcinoma relates to age of patient at diagnosis and not to radiation exposure. J Pathol. 2005;205:558–564. doi: 10.1002/path.1736. [DOI] [PubMed] [Google Scholar]
- 100.Nikiforov YE, Nikiforova MN, Gnepp DR, Fagin JA. Prevalence of mutations of ras and p53 in benign and malignant thyroid tumors from children exposed to radiation after the Chernobyl nuclear accident. Oncogene. 1996;13:687–693. [PubMed] [Google Scholar]
- 101.Santoro M, Thomas GA, Vecchio G, Williams GH, Fusco A, Chiappetta G, Pozcharskaya V, Bogdanova TI, Demidchik EP, Cherstvoy ED, Voscoboinik L, Tronko ND, Carss A, Bunnell H, Tonnachera M, Parma J, Dumont JE, Keller G, Hofler H, Williams ED. Gene rearrangement and Chernobyl related thyroid cancers. Br J Cancer. 2000;82:315–322. doi: 10.1054/bjoc.1999.0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suchy B, Waldmann V, Klugbauer S, Rabes HM. Absence of RAS and p53 mutations in thyroid carcinomas of children after Chernobyl in contrast to adult thyroid tumours. Br J Cancer. 1998;77:952–955. doi: 10.1038/bjc.1998.157. [DOI] [PMC free article] [PubMed] [Google Scholar]