It is generally assumed that the primary role of phloem loading is to drive long-distance transport by elevating hydrostatic pressure in sieve elements. This concept is consistent with the fact that, in many plants, energy is used to increase the concentrations of photoassimilates in the leaf phloem to levels well above those in mesophyll cells. However, on the basis of recent data, these fundamental assumptions need reevaluation. The data indicate that a large number of woody species—plants with the longest transport distances—load solute passively by maintaining high concentrations of Suc, and in some cases sugar alcohol, in mesophyll cells. Here I suggest that the adaptive advantage of active phloem loading, in the evolutionary sense, is to permit plants to maintain low foliar concentrations of nonstructural carbohydrates (NSCs), i.e. sugars, sugar alcohols, and starch. Economic considerations of inventory costs indicate that maintaining low NSC levels in leaves improves return on investment in carbohydrate synthesis, leading to a substantial increase in growth potential.
ACTIVE PHLOEM LOADING IS NOT UNIVERSAL
Münch (1930) did not invoke active phloem loading in his model of long-distance transport. He assumed that the solute produced by photosynthesis generates sufficient hydrostatic pressure in mesophyll cells to drive transport from leaves to sink organs through the symplast. However, subsequent research indicated that the sugar concentration in the phloem is much higher than in mesophyll cells (for an early review, see Crafts, 1961). As the Münch hypothesis gained acceptance, so too did the notion that the role of loading is to energetically concentrate sugars in the phloem, increasing turgor and thereby providing the motivating force for pressure flow.
This interpretation of the role of phloem loading has persisted. However, when one considers the primary finding—that there is a disparity in photoassimilate concentrations between the phloem and photosynthetic cells—it is equally reasonable to postulate that the adaptive advantage is derived from keeping the concentrations of these compounds low in the mesophyll. Viewed this way, active phloem loading allows plants to maintain low photoassimilate concentrations in leaves and at the same time elevate pressure in the phloem sufficiently to enable long-distance transport.
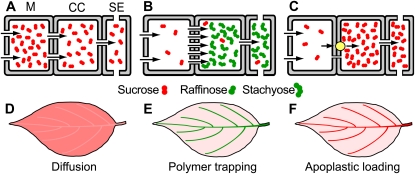
To understand the difference between these two views, it is important to make the distinction between active and passive loading. In active loading, metabolic energy is used to pump photoassimilate into the phloem, against a concentration gradient. Two species-specific, active mechanisms are known (Fig. 1). In apoplastic loading, the proton motive force is used to carry Suc, and in some cases sugar alcohols, into the phloem via transporters (Lalonde et al., 2004; Sauer, 2007; Braun and Slewinski, 2009). The second mechanism, polymer trapping (Schulz, 2005; Turgeon and Wolf, 2009), is also active although the initial step is not. In the initial step, Suc diffuses from the mesophyll into the minor vein companion cells through plasmodesmata. The Suc in the companion cells is then used to synthesize raffinose and stachyose, a process that increases the concentrations of these sugars in the sieve element-companion cell complex. Although polymer trapping does not involve active transport in the formal sense of moving ions or molecules across a membrane, it is thermodynamically active overall; energy is used to create a concentration difference between the mesophyll and the phloem. There is now convincing evidence, by four independent protocols—histochemistry (Pristupa, 1983), microdissection of minor vein phloem (Haritatos et al., 1996), plasmolysis (Turgeon and Hepler, 1989), and analysis of aphid stylet sap (Voitsekhovskaja et al., 2006)—that the solute concentration in the phloem of polymer trap species is close to, or as high as, that found in plants that load via the apoplast. The polymer trap hypothesis is supported by recent evidence that loading is insensitive to down-regulation of the Suc transporter (Zhang and Turgeon, 2009), but highly sensitive to down-regulation of raffinose and stachyose synthesis (McCaskill and Turgeon, 2007).
Figure 1.
Schematic diagrams of putative phloem-loading strategies at the minor vein (A–C) and whole leaf (D–F) levels. In A and D, Suc diffuses through plasmodesmata (gaps in walls) into the minor vein companion cells (CC) and sieve elements (SE), a passive process. The Suc concentration in mesophyll cells (M), and therefore the entire leaf, is high; the Suc concentration in the veins is slightly lower. In B and E, Suc diffuses through plasmodesmata into the minor vein CCs (intermediary cells) and is converted to raffinose and stachyose, thus actively elevating the transport sugar concentration in the phloem by polymer trapping; this allows the mesophyll cells to maintain low Suc levels. In C and F, Suc is pumped from the apoplast into the minor vein phloem by transporters (yellow circle), which also enables the leaf to minimize the overall Suc concentration in the leaf blade. Phloem parenchyma cells, which may constitute part of the transport pathway in A and C, are not shown. For a diagram of sugar alcohol loading strategies see Rennie and Turgeon (2009).
In contrast to these active mechanisms, passive loading is energetically downhill (Fig. 1). In the leaves of these plants, sugar levels are higher in the mesophyll than in the phloem. Ions and molecules diffuse through plasmodesmata at each interface, without a concentrating step (Turgeon and Medville, 1998; Reidel et al., 2009; Rennie and Turgeon, 2009). (For simplicity, passive transport through plasmodesmata is referred to here as diffusion, although bulk flow may also be possible in plasmodesmata with sufficiently large radii [Fisher and Cash-Clark, 2000; Voitsekhovskaja et al., 2006]).
How can a passive transport process motivate long-distance flow in sieve elements? Recall that this is the hypothesis put forward by Münch (1930). The driving force for transport comes from the creation of high concentrations of solutes in the photosynthetic cells. The solutes diffuse into the phloem through plasmodesmata and in the sieve elements the hydrostatic pressure engendered by the solute molecules motivates mass flow (not diffusion) toward the sinks. According to Münch, mass flow in sieve elements occurs as long as the hydrostatic pressure in the phloem is higher at the source than in the sinks, irrespective of how this pressure is developed. It is interesting in this regard that Arabidopsis (Arabidopsis thaliana), though an apoplastic loader with reduced plasmodesmatal numbers in the phloem, is nonetheless able to complete its life cycle when the Suc transporter gene AtSUC2 has been genetically ablated (Srivastava et al., 2009).
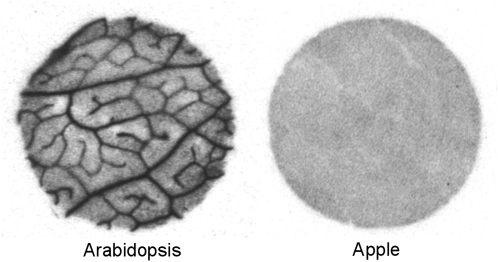
We recently analyzed loading strategies in 45 herbaceous and woody species (Rennie and Turgeon, 2009). Many of these plants, especially trees, exhibited the characteristics of passive loading. First, vein images were absent in autoradiographs when leaf discs were incubated in 14C-labeled Suc or 14C-labeled sugar alcohol. Autoradiographic vein images are expected if plants load actively, but if loading is passive radiolabeled compounds will pass freely between all symplastically connected cell types in the discs (Fig. 2). Second, concentrations of foliar transport sugars were high—up to 50-fold higher than those in the leaves of many herbs—as required if transport is due to diffusion. Third, all of the species in the survey with the two characteristics discussed above—lack of autoradiographic vein images and elevated sugar concentrations—have abundant minor vein plasmodesmata, a prerequisite for diffusion of large quantities of photoassimilate into the phloem (Gamalei, 1989).
Figure 2.
Autoradiographs of leaf discs from Arabidopsis and apple (Malus domestica). Abraded discs were incubated in [14C]Suc, washed, freeze dried, and pressed against x-ray film. Minor veins are apparent in Arabidopsis, but not apple, discs. Discs = 8 mm diameter.
The data strongly suggest that many plants transport photoassimilate from source leaves to sinks without the need for active phloem loading, in agreement with Münch's original hypothesis. The fact that these plants are almost all trees makes it difficult to argue that energy expenditure at the loading step is needed for efficient phloem transport. If a willow (Salix spp.) or oak (Quercus spp.) tree can transport sugars over tens of meters without active loading, can it really be necessary in an herbaceous plant that is orders of magnitude smaller? This is not to say that active loading has no function in other parts of these plants. Even if the initial loading step from the mesophyll into the minor veins is passive, it is possible, indeed likely, that transporters introduce Suc and other materials into the phloem in other regions of the vasculature to compensate for leakage or to remobilize stored nutrients. It is also possible that active loading in leaves is initiated in certain developmental contexts, or under stress conditions.
Phylogenetic analysis also suggests that active phloem loading is not necessary for long-distance transport. Although more work needs to be done on basal angiosperms, available evidence indicates that extensive plasmodesmatal continuity between the mesophyll and the minor vein phloem, an indicator of passive loading, is ancestral in the angiosperms (Gamalei, 1989; Turgeon et al., 2001). Active loading, either by transporters or polymer trapping, appears to be a derived trait. Again, it is difficult to argue that active loading is necessary if early angiosperms, predominately trees, thrived without it.
COMPOSITION OF PHLOEM SAP
Early experiments in transport physiology indicated that Glc and Fru are not translocated in the phloem (Arnold, 1968), suggesting that the leaf regulates the composition of export sap, presumably at the loading step. However, in symplastic loaders the opportunities for selecting particular substances for export are limited because flux of molecules through plasmodesmata is essentially nonspecific, except on the basis of size (Roberts and Oparka, 2003). How is regulation reconciled with an open, symplastic pathway into the phloem?
First, there is actually little evidence that selectivity must be imposed at the loading step. Glc and Fru need not be specifically excluded from the phloem because they are sequestered almost entirely in the vacuoles of leaf cells (Heineke et al., 1994; Voitsekhovskaja et al., 2006; Nadwodnik and Lohaus, 2008). Therefore, they do not have access to the phloem, no matter the loading strategy. Furthermore, phloem sap is not a simple mixture of sugar and selected metabolites. It is a complex broth of organic and inorganic materials including, but not restricted to, ions, amino acids, amides, ureides, organic acids, nucleotides, phosphorylated metabolites of glycolysis, hormones, macromolecules, and several classes of secondary compounds, in addition to nonreducing sugars and sugar alcohols (Ziegler, 1975; Geigenberger et al., 1993; Turgeon and Wolf, 2009). The complexity of the sap indicates that a broad range of materials finds its way into the phloem. There are undoubtedly differences in composition between the cytosol of mesophyll cells and phloem sap, but these differences do not have to be explained by selective loading. The phloem sap could be, and probably is, modified all along the transport route by metabolism in companion cells, leakage from sieve elements, and retrieval of materials from the transpiration stream.
Another concern raised by the concept of symplastic loading, either active (polymer trapping) or passive, is that unrestricted access to the phloem could result in an excessive and debilitating drain of essential metabolites from mesophyll cells toward the sinks. However, this seems unlikely when it is considered that minor vein companion cells in all plants cope successfully with such loss. In the minor vein phloem of leaves, net flux of ions and small molecules between companion cells and sieve elements is in the direction of the latter because the contents of the sieve elements are constantly being flushed away. Since the plasmodesmata-pore complex between these cell types is large enough to accommodate GFP (see Turgeon and Wolf, 2009), the companion cells must be constantly losing essential metabolites, even small proteins, to the river of sieve tube sap in the loading zone. Some small molecules are retrieved and returned to the leaf (Ayre et al., 2003), but many others must be resynthesized. This may be a partial explanation for the high metabolic activity of companion cells, especially in minor veins: Not only do they keep the sieve elements alive, they must persistently expend metabolic energy to restore the metabolites needed for their own survival. If companion cells are able to maintain their integrity under these conditions, mesophyll cells should be able to do the same considering that they are far more numerous than companion cells and will therefore suffer proportionately less metabolite loss.
Viewed from this perspective, there is an advantage to symplastic loading: Plasmodesmata provide an uninterrupted and relatively unrestricted pathway for materials to replenish the companion cells and to provide essential nutrients of many types to the sinks. It is also possible that certain types of secondary compounds—for example, defensive molecules—have better access to the phloem through plasmodesmata than through the apoplast because there is no requirement for specialized transporters.
If apoplastic loading imposes a burden on the plant by requiring the costly and selective use of transporters to maintain what in symplastic loaders is the free and largely unrestricted supply of photoassimilate and other substances to the phloem and sinks, it must confer some compensatory advantage. It does not seem likely that the advantage accrues simply from elevating hydrostatic pressure to motivate export, as discussed above. Another explanation must be sought.
ACTIVE PHLOEM LOADING INCREASES GROWTH POTENTIAL
The growth of herbaceous plants roughly follows the dictates of compound interest laws; i.e. growth is proportional to present biomass. New leaves create photosynthetic potential, which fuels the growth of yet more leaves. The result is exponential growth. For the same reason, overall plant productivity is most favored when rapid growth occurs early (Harper, 1989; Dohleman and Long, 2009). Not surprisingly, plants with high relative growth rates (RGRs) allocate a large fraction of plant carbon to leaves, i.e. they have a high leaf weight fraction (Poorter and Remkes, 1990). It follows, therefore, that diversion of carbon away from building new photosynthetic machinery, whether by respiration, construction of other organs, or storage, has a negative impact on RGR (Bloom et al., 1985; Lambers and Poorter, 1992).
Foliar NSC that is not needed for metabolism or transport can be considered excess inventory in the sense that it is not part of the photosynthetic machinery and is therefore not productive. Some NSC is essential, of course. Leaves accumulate carbohydrate during the day to accommodate respiration and export at night (Smith and Stitt, 2007). However, they do not need substantial reserves once night draws to a close; in a changeable world, at least dawn is predictable. Therefore, predawn NSC levels provide a reasonable measure of excess inventory. Literature values indicate that predawn NSC concentrations are low in many herbaceous plants. Sugar beet (Beta vulgaris) leaves retain only 42 μg carbon cm−2 in NSC at the end of the night (Fondy and Geiger, 1982). Since they export approximately 440 μg carbon cm−2 over a 24-h period, the predawn level is only enough to support respiration and export for a few additional hours. Arabidopsis leaves retain only 10% of carbon fixed during the day at the end of the night and experience gene expression changes associated with carbon starvation when the dark period is extended by just 2 to 4 h (Usadel et al., 2008). Why do herbaceous plants maintain such minimal leaf reserves, requiring finely tuned mechanisms to adjust diurnal assimilation and storage patterns (Smith and Stitt, 2007)? One possibility is that storing carbon reduces growth potential.
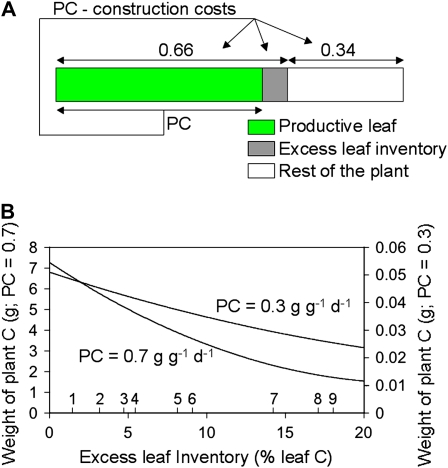
We modeled the impact of maintaining predawn inventory in the leaves of herbaceous plants using the strategy of Körner (1991; Fig. 3A) and data on growth and carbon balance characteristics of 24 nonwoody species (Poorter and Remkes, 1990; Poorter et al., 1990). The modeling strategy is explained in more detail in Supplemental Information S1. The initial state of the virtual plant is ideal in the sense that the leaves use up all NSC during the night, have no predawn reserves, and all foliar carbon is assumed to be necessary, either directly or indirectly, to the photosynthetic function. The initial weight of plant carbon is 10 mg. Initial leaf weight is 6.6 mg carbon, since the maximum leaf weight fraction for rapidly growing plants is 0.66 g g−1 (Poorter and Remkes, 1990). In the model, each g of leaf carbon produces 0.72 g carbon d−1 by net photosynthesis (Poorter et al., 1990) and this carbon is allocated to new leaves and the rest of the plant in the original plant proportions (66% and 34%, respectively). Construction costs (1.4 g carbon required to construct 1.0 g biomass carbon; Poorter and de Jong, 1999) are subtracted from the produced carbon prior to allocation. Daily respiratory losses of 8% leaf carbon (the amount respired at night; Thomas and Griffin, 1994) and 8% root carbon (the amount respired in 24 h; Bloom et al., 1992) are also subtracted.
Figure 3.
Modeling the effect of predawn NSCs on plant growth. A, Modeling strategy. In the virtual plant, 66% of the carbon is in leaves, 34% in the rest of the plant. Zero percent to 20% of leaf carbon is excess inventory (predawn, NSC), and the rest of the leaf carbon is photosynthetically productive (green). Photosynthetically fixed carbon (PC), from the productive portion of the leaf only, is allocated to the plant in its original proportions, after construction costs are subtracted. Respiratory losses from the roots, and from the leaves at night, are also subtracted. Interest is compounded daily. B, Final weight of plant carbon after 30 d growth, plotted against excess leaf inventory, from 0% to 20% of leaf carbon. The initial weight of plant is 10 mg carbon. The photosynthetic portion of leaf carbon produces either 0.3 or 0.7 g carbon g−1 carbon d−1 (net photosynthesis). Literature values for the percentage of leaf carbon in NSCs at predawn are included on the abscissa for (1) Arabidopsis (Zeeman and ap Rees, 1999), (2) Nicotiana tabacum (Camacho-Cristóbal and González-Fontes, 1999), (3) sugar beet (Fondy and Geiger, 1982), (4) Glycine max (Qiu and Israel, 1992), (5) Acer saccharum (Topa et al., 2001), (6) Ricinus communis (Grimmer et al., 1999), (7) apple (Klages et al., 2001), (8) Populus deltoides (Dickson, 1987), and (9) celery (Davis and Loescher, 1991).
In the model, the weight of carbon in the ideal plant increases exponentially, rising to 7.3 g on day 30 (Fig. 3B). It must be emphasized that actual plant growth rate is subject to numerous developmental and environmental constraints and is far more complex than the model implies. However, the model does provide a framework for comparing the growth potentials of plants with different amounts of stored carbon in similar conditions.
To estimate the effect of carrying excess inventory in leaves, the percentage of leaf carbon allocated to predawn NSC is increased in the model from 0% to 20% without changing the total carbon allocation (66%) to leaves (Fig. 3A). The percentage of carbon in excess inventory is kept constant during the 30-d growth period, i.e. predawn NSC mimics physiological steady state. The growth penalty for maintaining stored, nonproductive NSC is severe. On day 30, a plant with 20% excess inventory contains only 1.47 g carbon, 20% of that in the ideal plant (Fig. 3B).
The results indicate, in agreement with other analyses of the effects of storage on growth (Harper, 1989), that maintaining nonproductive carbon in leaves has a pronounced effect on growth potential. Published values of predawn NSC in mature leaves of several species are included in Figure 3B. These values are not intended to predict or simulate the growth characteristics of these plants, only to illustrate how actual concentrations of predawn NSC found in different species affect modeled growth potential.
Although the highest inventories are generally found in the leaves of woody species, there are exceptions. NSC concentrations are very high in celery (Apium graveolens), an herbaceous species that loads Suc and mannitol from the apoplast (Rennie and Turgeon, 2009). Foliar solute protects celery against salt stress (Everard et al., 1994) and the model suggests that the cost of this defense on RGR is high. Also note that NSC levels vary in different environmental and developmental situations. For example, growth usually slows as plants age and it is therefore unlikely that the RGR is ever constant over a 30-d period, as in the model. These cautions aside, measured predawn foliar inventories clearly have an effect on instantaneous RGR, a vital element of plant performance. Interestingly, Cross et al. (2006) found that, in 24 Arabidopsis accessions, fast growth correlates with low starch and sugar levels in leaves at the end of the night.
Do low inventory levels benefit slow growing, as well as fast growing, plants? If productivity in the model is reduced from 0.72 g carbon d−1 to 0.30 g carbon d−1, as might occur in the shade, or by self shading, growth is considerably reduced (Fig. 3B). The proportionate advantage enjoyed by plants without excess inventory in these situations is not as great as when productivity is higher; nonetheless, it is still substantial (Fig. 3B). Therefore, there is a clear advantage to reducing foliar inventory in plants that are competing with one another, even in a slow-growth environment.
If active phloem loading is so advantageous, why do trees not employ it more generally? Here it is important to recall that most trees produce leaves in flushes in the spring rather than by progressive addition of new leaves throughout the growing season, as is the norm with herbaceous plants (Borchert, 1991). Much of the carbon used to fuel episodic growth flushes comes from storage (Dickson, 1989). Therefore, the advantage accrued by keeping NSC low—to fuel successive leaf development—does not apply to most woody species to the same extent as it does to herbaceous plants. In general, the RGR of trees is well below that of herbs (Lambers and Poorter, 1992).
There may be other reasons for maintaining low NSC in leaves. Herbivory results in the loss of more carbon from leaves with greater inventory. Herbivores are attracted to nitrogenous compounds more than carbohydrates, but the amount of carbon lost is nonetheless greater if more carbon is vulnerable (Schwachtje et al., 2006).
Another reason for maintaining low Suc concentrations could be to avoid feedback inhibition of photosynthesis. Although the processes and signaling pathways involved are not well understood, there is convincing evidence that photosynthesis, carbon metabolism, and allocation are feedback regulated (Paul and Pellny, 2003; Rolland et al., 2006). Perhaps active phloem loading benefits plants by allowing leaves to maintain noninhibiting concentrations of NSC. We have previously suggested that apple—which loads passively—synthesizes and transports sorbitol to reduce reliance on Suc, thus maximizing diffusion of photoassimilate into the phloem without inducing feedback inhibition of photosynthesis (Reidel et al., 2009).
CONCLUSION
Carbon partitioning is dauntingly complex, involving growth and storage responses in source and sink tissues, diurnal and feedback regulation of photosynthesis, and integration of carbon and nitrogen metabolism, all mediated by hormones and poorly understood signaling pathways. We will not understand how phloem loading is integrated into this complex scheme unless we understand its primary role.
There is little doubt that high turgor is needed in source phloem to drive long-distance nutrient transport. High concentrations of solute in the phloem also allow the sieve elements to maintain turgor, and to continue to function, when the plant is under severe water stress (Sung and Krieg, 1979; Smith and Milburn, 1980). However, there is reason to doubt that active loading is needed to establish and maintain this pressure since many plants with the longest transport distances function without it. In turn, this suggests that active phloem loading evolved for another purpose. Economic considerations suggest that the adaptive advantage accrues from maintaining low foliar NSC levels, thus making additional carbon available for growth.
It could be argued that the reverse is true, that active loading evolved to drive phloem transport and that over time plants took advantage of this mechanism to lower NSC levels in mesophyll cells, thus increasing growth potential. However, there are two difficulties with this scenario. First, phylogenetic analysis indicates that active loading, either by transporters or polymer trapping, is a derived trait in the angiosperms. Second, many extant trees transport nutrients efficiently over exceptionally long distances by passively transferring photoassimilate into the phloem. Both observations are difficult to reconcile with the hypothesis that active loading is essential for efficient pressure flow and evolved for that purpose. On the other hand, the hypothesis that active loading evolved not to motivate transport but to allow plants to draw down foliar carbon reserves is compatible with the phylogenetic data and helps explain the distribution of phloem-loading mechanisms in different families and in different life forms. According to this view, the evolution of herbaceous plants, characterized by iterative leaf production and rapid growth, was made possible in part by active loading, resulting in more efficient use of photoassimilate. In apoplastic loaders there may have been a price to pay in terms of restricted access to the phloem for nutrients and specialized compounds, but benefits clearly outweighed the costs for species competing with each other on the basis of rapid growth.
From available evidence it appears that predawn NSC levels vary in herbaceous crop plants, though as a general rule they are lower than those of woody plants. It may be that some herbs are conservative in this respect, compromising growth potential by storing extra foliar reserves as a safety factor. If this is true, and extra reserves are no longer needed in cultivated conditions, manipulation of storage in leaves could result in significant increases in the growth potential of crop plants. The fact that relatively minor differences in predawn carbohydrate levels in Arabidopsis, a species with very low constitutive NSC levels, correlate with measurable differences in growth (Cross et al., 2006), suggests that these gains could be substantial.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Information S1. Modeling strategy.
Supplementary Material
Acknowledgments
I thank Hendrik Poorter for helpful discussions and also thank Lailiang Cheng, Donald Geiger, Andre Jagendorf, Carl Leopold, Kevin Nixon, Edwin Reidel, Emilie Rennie, Shmuel Wolf, and Cankui Zhang for constructive reviews of the manuscript.
References
- Arnold WN. (1968) The selection of sucrose as the translocate of higher plants. J Theor Biol 21: 13–20 [DOI] [PubMed] [Google Scholar]
- Ayre BG, Keller F, Turgeon R. (2003) Symplastic continuity between companion cells and the translocation stream: long distance transport is controlled by retention and retrieval mechanisms in the phloem. Plant Physiol 131: 1518–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Chapin FS, III, Mooney HA. (1985) Resource limitation in plants—an economic analogy. Annu Rev Ecol Syst 16: 363–392 [Google Scholar]
- Bloom AJ, Sukrapanna SS, Warner RL. (1992) Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiol 99: 1294–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchert R. (1991) Growth periodicity and dormancy. Raghavendra AS, , Physiology of Trees. John Wiley & Sons, New York, pp 221–245 [Google Scholar]
- Braun DM, Slewinski TL. (2009) Genetic control of carbon partitioning in grasses: roles of Sucrose Transporters and Tie-dyed loci in phloem loading. Plant Physiol 149: 71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Cristóbal JJ, González-Fontes A. (1999) Boron deficiency causes a drastic decrease in nitrate content and nitrate reductase activity, and increases the content of carbohydrates in leaves from tobacco plants. Planta 209: 528–536 [DOI] [PubMed] [Google Scholar]
- Crafts AS. (1961) Translocation in Plants. Holt, Rinehart and Winston, New York [Google Scholar]
- Cross JM, von Korff M, Altmann T, Bartzetko L, Sulpice R, Gibon Y, Palacios N, Stitt M. (2006) Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in C-limited conditions. Plant Physiol 142: 1574–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Loescher WH. (1991) Diurnal pattern of carbohydrates in celery leaves of various ages. HortScience 26: 1404–1406 [Google Scholar]
- Dickson RE. (1987) Diurnal changes in leaf chemical constituents and 14C partitioning in cottonwood. Tree Physiol 3: 157–171 [DOI] [PubMed] [Google Scholar]
- Dickson RE. (1989) Carbon and nitrogen allocation in trees. Ann Sci For (Suppl) 46: 680s–683s [Google Scholar]
- Dohleman FG, Long SP. (2009) More productive than maize in the midwest: how does Miscanthus do it? Plant Physiol 150: 2104–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard JD, Gucci R, Kann SC, Flore JA, Loescher WH. (1994) Gas exchange and carbon partitioning in the leaves of celery (Apium graveolens L.) at various levels of root zone salinity. Plant Physiol 106: 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB, Cash-Clark CE. (2000) Sieve tube unloading and post-phloem transport of fluorescent tracers and proteins injected into sieve tubes via severed aphid stylets. Plant Physiol 123: 125–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy BR, Geiger DR. (1982) Diurnal pattern of translocation and carbohydrate metabolism in source leaves of Beta vulgaris L. Plant Physiol 70: 671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamalei Y. (1989) Structure and function of leaf minor veins in trees and herbs. Trees (Berl) 3: 96–110 [Google Scholar]
- Geigenberger P, Langenberger S, Wilke I, Heineke D, Heldt HW, Stitt M. (1993) Sucrose is metabolized by sucrose synthase and glycolysis within the phloem complex of Ricinus communis L. seedlings. Planta 190: 446–453 [Google Scholar]
- Grimmer C, Bachfischer T, Komor E. (1999) Carbohydrate partitioning into starch in leaves of Ricinus communis L. grown under elevated CO2 is controlled by sucrose. Plant Cell Environ 22: 1275–1280 [Google Scholar]
- Haritatos E, Keller F, Turgeon R. (1996) Raffinose oligosaccharide concentrations measured in individual cell and tissue types in Cucumis melo L. leaves: implications for phloem loading. Planta 198: 614–622 [DOI] [PubMed] [Google Scholar]
- Harper JL. (1989) The value of a leaf. Oecologia 80: 53–58 [DOI] [PubMed] [Google Scholar]
- Heineke D, Wildenberger K, Sonnewald U, Willmitzer L, Heldt HW. (1994) Accumulation of hexoses in leaf vacuoles: studies with transgenic tobacco plants expressing yeast-derived invertase in the cytosol, vacuole or apoplasm. Planta 194: 29–33 [Google Scholar]
- Klages K, Donnison H, Wünsche J, Boldingh H. (2001) Diurnal changes in non-structural carbohydrates in leaves, phloem exudate and fruit in ‘Braeburn’ apple. Aust J Plant Physiol 28: 131–139 [Google Scholar]
- Körner C. (1991) Some often overlooked plant characteristics as determinants of plant growth: a reconsideration. Funct Ecol 5: 162–173 [Google Scholar]
- Lalonde S, Wipf D, Frommer WB. (2004) Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu Rev Plant Biol 55: 341–372 [DOI] [PubMed] [Google Scholar]
- Lambers H, Poorter H. (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Adv Ecol Res 23: 187–261 [Google Scholar]
- McCaskill A, Turgeon R. (2007) Phloem loading in Verbascum phoeniceum L. depends on the synthesis of raffinose-family oligosaccharides. Proc Natl Acad Sci USA 104: 19619–19624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch E. (1930) Die Stoffbewegungen in Der Pflanze. Gustav Fischer, Jena, Germany [Google Scholar]
- Nadwodnik J, Lohaus G. (2008) Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens. Planta 227: 1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Pellny TK. (2003) Carbon metabolite feedback regulation of leaf photosynthesis and development. J Exp Bot 54: 539–547 [DOI] [PubMed] [Google Scholar]
- Poorter H, de Jong R. (1999) A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytol 143: 163–176 [Google Scholar]
- Poorter H, Remkes C. (1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83: 553–559 [DOI] [PubMed] [Google Scholar]
- Poorter H, Remkes C, Lambers H. (1990) Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiol 94: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pristupa NA. (1983) Distribution of ketosugars among cells of conducting bundles of the Cucurbita pepo leaf. Soviet Plant Physiology 30: 372–378 [Google Scholar]
- Qiu J, Israel DW. (1992) Diurnal starch accumulation and utilization in phosphorus-deficient soybean plants. Plant Physiol 98: 316–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidel EJ, Rennie EA, Amiard V, Cheng L, Turgeon R. (2009) Phloem loading strategies in three plant species that transport sugar alcohols. Plant Physiol 149: 1601–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie EA, Turgeon R. (2009) A comprehensive picture of phloem loading strategies. Proc Natl Acad Sci USA 106: 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AG, Oparka KJ. (2003) Plasmodesmata and the control of symplastic transport. Plant Cell Environ 26: 103–124 [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. (2006) Sugar sensing and singling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57: 675–709 [DOI] [PubMed] [Google Scholar]
- Sauer N. (2007) Molecular physiology of higher plant sucrose transporters. FEBS Lett 581: 2309–2317 [DOI] [PubMed] [Google Scholar]
- Schulz A. (2005) Role of plasmodesmata in solute loading and unloading. Oparka KJ, , Plasmodesmata, Annual Plant Reviews, Vol 18. Blackwell, Oxford, pp 135–161 [Google Scholar]
- Schwachtje J, Minchin PEH, Jahnke S, van Dongen JT, Schittko U, Baldwin IT. (2006) SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proc Natl Acad Sci USA 103: 12935–12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Stitt M. (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126–1149 [DOI] [PubMed] [Google Scholar]
- Smith JAC, Milburn JA. (1980) Phloem turgor and the regulation of sucrose loading in Ricinus communis L. Planta 148: 42–48 [DOI] [PubMed] [Google Scholar]
- Srivastava AC, Dasgupta K, Ajieren E, Costilla G, McGarry RC, Ayre BG. (2009) Arabidopsis plants harboring a mutation in AtSUC2, encoding the predominant sucrose/proton symporter necessary for efficient phloem transport, are able to complete their life cycle and produce viable seed. Ann Bot (Lond) 104: 1121–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung FJM, Krieg DR. (1979) Relative sensitivity of photosynthetic assimilation and translocation of 14Carbon to water stress. Plant Physiol 64: 852–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RB, Griffin KL. (1994) Direct and indirect effects of atmospheric carbon dioxide enrichment on leaf respiration of Glycine max (L.) Merr. Plant Physiol 104: 355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topa MA, Vanderklein DW, Corbin A. (2001) Effects of elevated ozone and low light on diurnal and seasonal carbon gain in sugar maple. Plant Cell Environ 24: 663–677 [Google Scholar]
- Turgeon R, Hepler PK. (1989) Symplastic continuity between mesophyll and companion cells in minor veins of mature Cucurbita pepo L. leaves. Planta 179: 24–31 [DOI] [PubMed] [Google Scholar]
- Turgeon R, Medville R. (1998) The absence of phloem loading in willow leaves. Proc Natl Acad Sci USA 95: 12055–12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Medville R, Nixon KC. (2001) The evolution of minor vein phloem and phloem loading. Am J Bot 88: 1331–1339 [PubMed] [Google Scholar]
- Turgeon R, Wolf S. (2009) Phloem transport: cellular pathways and molecular trafficking. Annu Rev Plant Biol 60: 207–221 [DOI] [PubMed] [Google Scholar]
- Usadel B, Bläsing OE, Gibon Y, Retzlaff K, Höhne M, Günther M, Stitt M. (2008) Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol 146: 1834–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voitsekhovskaja OV, Koroleva OA, Batashev DR, Knop C, Tomos AD, Gamalei V, Heldt HW, Lohaus G. (2006) Phloem loading in two Scrophulariaceae species: what can drive symplastic flow via plasmodesmata? Plant Physiol 140: 383–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, ap Rees T. (1999) Changes in carbohydrate metabolism and assimilate export in starch-excess mutants of Arabidopsis. Plant Cell Environ 22: 1445–1453 [Google Scholar]
- Zhang C, Turgeon R. (2009) Downregulating the sucrose transporter VpSUT1 in Verbascum phoeniceum does not inhibit phloem loading. Proc Natl Acad Sci USA 106: 18849–18854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H. (1975) Nature of substances translocated in the phloem. Zimmermann MH, Milburn JA, , Transport in Plants 1: Phloem Transport, Encyclopedia of Plant Physiology, New Ser, Vol 1. Springer-Verlag, New York, pp 59–100 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.