Abstract
Complexation of arsenite [As(III)] with phytochelatins (PCs) is an important mechanism employed by plants to detoxify As; how this complexation affects As mobility was little known. We used high-resolution inductively coupled plasma-mass spectrometry and accurate mass electrospray ionization-mass spectrometry coupled to HPLC to identify and quantify As(III)-thiol complexes and free thiol compounds in Arabidopsis (Arabidopsis thaliana) exposed to arsenate [As(V)]. As(V) was efficiently reduced to As(III) in roots. In wild-type roots, 69% of As was complexed as As(III)-PC4, As(III)-PC3, and As(III)-(PC2)2. Both the glutathione (GSH)-deficient mutant cad2-1 and the PC-deficient mutant cad1-3 were approximately 20 times more sensitive to As(V) than the wild type. In cad1-3 roots, only 8% of As was complexed with GSH as As(III)-(GS)3 and no As(III)-PCs were detected, while in cad2-1 roots, As(III)-PCs accounted for only 25% of the total As. The two mutants had a greater As mobility, with a significantly higher accumulation of As(III) in shoots and 4.5 to 12 times higher shoot-to-root As concentration ratio than the wild type. Roots also effluxed a substantial proportion of the As(V) taken up as As(III) to the external medium, and this efflux was larger in the two mutants. Furthermore, when wild-type plants were exposed to l-buthionine sulfoximine or deprived of sulfur, both As(III) efflux and root-to-shoot translocation were enhanced. The results indicate that complexation of As(III) with PCs in Arabidopsis roots decreases its mobility for both efflux to the external medium and for root-to-shoot translocation. Enhancing PC synthesis in roots may be an effective strategy to reduce As translocation to the edible organs of food crops.
Arsenic (As) contamination in the environment is caused by both geogenically and/or anthropogenically derived activities. This problem is the most serious in South and Southeast Asia where As-contaminated groundwater has been extracted for drinking and for irrigating rice (Oryza sativa) crops (Brammer and Ravenscroft, 2009). As contamination in soil can cause phytotoxicity and consequently yield losses (Panaullah et al., 2009) and elevated levels of As in rice grain that may pose a significant risk to human health (Meharg and Rahman, 2003; Zhu et al., 2008; Meharg et al., 2009). To develop mitigation strategies to reduce the transfer of As to the food chain requires a better understanding of the mechanisms of As uptake, translocation, and detoxification. It is known that As accumulation varies greatly among different plant species (e.g. Raab et al., 2007) and also among different genotypes within a species (e.g. Norton et al., 2009). Since root-to-shoot translocation is often the bottleneck for the accumulation of metal(loid)s in the shoots (Zhao and McGrath, 2009), understanding what controls As translocation within plants is important for designing strategies to decrease As concentrations in the edible parts of food crops.
With the exception of As hyperaccumulating plants, translocation of As from roots to shoots is generally restricted in most plant species (for review, see Zhao et al., 2009). An explanation for this limited translocation is that arsenate [As(V)] is rapidly reduced to arsenite [As(III)] in roots, followed by complexation of As(III) with phytochelatins (PCs) and subsequent sequestration in root vacuoles (Dhankher et al., 2006; Raab et al., 2007; Zhao et al., 2009). The extent of As(III) complexation may therefore determine its mobility in roots. To test this hypothesis, we used the model plant Arabidopsis (Arabidopsis thaliana) mutants defective in glutathione (GSH) or PC synthesis, as well as manipulation of thiol synthesis in wild-type plants by the use of the specific inhibitor l-buthionine sulfoximine (BSO) and sulfur (S) deprivation. Both the PC-deficient mutant cad1-3 and the GSH-deficient mutant cad2-1 were isolated by their phenotype of cadmium (Cd) sensitivity (Howden et al., 1995a, 1995b). cad1-3 is a recessive loss-of-function mutant with a mutation in the PC synthase gene (AtPCS1; Ha et al., 1999) and is unable to synthesize PCs in response to Cd exposure (Howden et al., 1995b). cad2-1 has a deletion in the gene encoding the γ-glutamylcysteine synthetase, resulting in 60% to 85% lower levels of GSH compared with the wild type and little production of PCs in response to Cd exposure (Howden et al., 1995a; Cobbett et al., 1998).
As(III) has a high affinity to thiol groups, and there is strong evidence that PCs play a constitutive role in the detoxification of As through complexation of As(III) in As nonhyperaccumulator plants. As strongly induces PC synthesis (Grill et al., 1987; Sneller et al., 1999; Schmöger et al., 2000). Both cad1-3 and cad2-1 are hypersensitive to As(V) (Ha et al., 1999; Li et al., 2006). Inhibition of GSH and PC synthesis by BSO results in As hypersensitivity in a number of plant species (Schmöger et al., 2000; Hartley-Whitaker et al., 2002; Schat et al., 2002). It has been shown that overexpression of PCS enhances As tolerance in transgenic plants, but interestingly not As accumulation (Li et al., 2004; Gasic and Korban, 2007). Furthermore, a range of intact As(III)-PC complexes has been identified in sunflower (Helianthus annuus) and Thunbergia alata plants after exposure to As(V) or As(III) (Raab et al., 2005; Bluemlein et al., 2008). In contrast, As hyperaccumulators, such as Pteris vittata, appear not to rely mainly on PC-dependent strategies for As detoxification, as very small proportions of As in roots and fronds are complexed with thiols (Webb et al., 2003; Zhao et al., 2003; Raab et al., 2004; Pickering et al., 2006). Lack of As(III)-PC complexation in P. vittata may be one of the important reasons for the highly efficient translocation of As from roots to fronds (Su et al., 2008; Zhao et al., 2009).
While the role of PCs in As sensitivity is well established, how they influence As mobility in plants is not clear. Gong et al. (2003) showed that PCs may be transported from roots to shoots in a study involving root-specific expression of the wheat (Triticum aestivum) PCS gene (TaPCS1) in the Arabidopsis cad3-1 mutant. Furthermore, both root-specific and ectopic expression of TaPCS1 was found to enhance long-distance transport of Cd from roots to stems and rosette leaves, suggesting that PCs may be carriers of Cd in xylem transport. However, direct measurements of the xylem sap collected from As-exposed sunflower showed only traces of nonreactive oxidized PC2 and oxidized glutathione (GSSG) with no evidence of As-PC complexation (Raab et al., 2005). Similarly, only trace levels of PCs were detected in the xylem sap from Cd-exposed Brassica napus (Mendoza-Cózatl et al., 2008). The role of PCs in the xylem mobility of As has not been investigated in detail. Interestingly, recent studies have shown that GSH, PCs, and other thiol peptides can be transported from shoots to roots via phloem (Chen et al., 2006; Li et al., 2006). High levels of PCs, GSH, and Cd were found in the phloem sap of B. napus, suggesting that thiol peptides may be carriers of Cd in the long-distance phloem transport (Mendoza-Cózatl et al., 2008).
Here, we present evidence that decreasing As(III)-PC complexation in Arabidopsis roots led to greater As mobility, manifested by enhanced As(III) efflux to the external medium and enhanced As translocation from roots to shoots.
RESULTS
As(V) Sensitivity
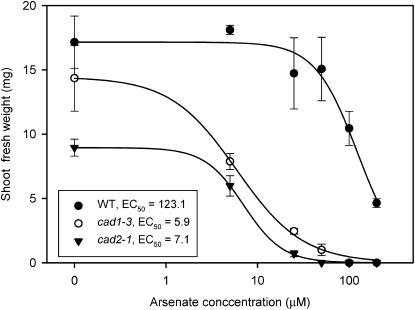
Ha et al. (1999) reported that the PC-deficient mutant cad1-3 was 10- to 20-fold more sensitive to As(V) than the wild type. The GSH-deficient mutant cad2-1 was also found to be qualitatively more sensitive to As(V) (Li et al., 2006). To quantify their relative sensitivity to As(V), these two mutants and their wild type (Columbia-0) were grown in agar plates containing 0 to 200 μm As(V) for 7 d. Growth of both cad1-3 and cad2-1 seedlings was much more sensitive to As(V) than the wild-type plants (Fig. 1). The effect concentrations of As(V) causing 50% inhibition of growth (EC50), calculated from the fitted dose-response curve between shoot biomass and As(V) concentration, were 123.1 ± 14.2, 5.9 ± 0.5, and 7.1 ± 0.2 μm, respectively, for the wild type, cad1-3, and cad2-1, indicating that both mutants were about 20-fold more sensitive to As(V) than the wild type. In this experiment, cad2-1 grew less well in the control treatment than the other two.
Figure 1.
Sensitivity of Arabidopsis wild-type (WT) and mutant seedlings to As(V) exposure. FWs of wild-type, cad1-3, and cad2-1 mutant seedlings after growth in the medium with different concentrations of As(V) for 1 week. Data are means ± se (n = 12). Lines are fitted log-logistic curves. A small value (0.1) was added to the zero As(V) treatment to allow log transformation. EC50, Effect concentration of As(V) (μm) causing 50% inhibition of growth.
As(V) Reduction
Seedlings of cad1-3, cad2-1, and the wild type were exposed to 5 μm As(V) for 24 h, and As speciation in root and shoot tissues was measured by anion-exchange HPLC-ICP-MS (inductively coupled plasma-mass spectrometry). Wild-type Arabidopsis plants had a high capacity for reducing As(V), with 96% and 100% of the total As in roots and shoots, respectively, being in the form of As(III) (Table I). The capacity for As(V) reduction decreased in both mutants, especially cad2-1; the As(III)% was significantly lower than the wild type in cad2-1 roots and shoots and in cad1-3 shoots. Still, most of the As in roots (85%–92%) and shoots (95%–97%) of the two mutants had been reduced to As(III) (Table I). The proportion of As(III) was higher in shoots than in roots in all three lines of Arabidopsis.
Table I. As(V) reduction capacity of the Arabidopsis wild type and cad1-3 and cad2-1 mutants.
Plants were exposed to 5 μm As(V) for 24 h and As speciation determined. Data are means ± se (n = 4). Means followed by different letters are significantly (P < 0.05, lsd test) from each other.
| % As(III) |
||
| Roots | Shoots | |
| Wild type | 96.3 ± 0.6 a | 100.0 ± 0.0 a |
| cad1-3 | 91.7 ± 1.4 a | 96.7 ± 1.2 b |
| cad2-1 | 84.5 ± 2.3 b | 95.3 ± 0.3 b |
As(III) Complexation with PCs and GSH
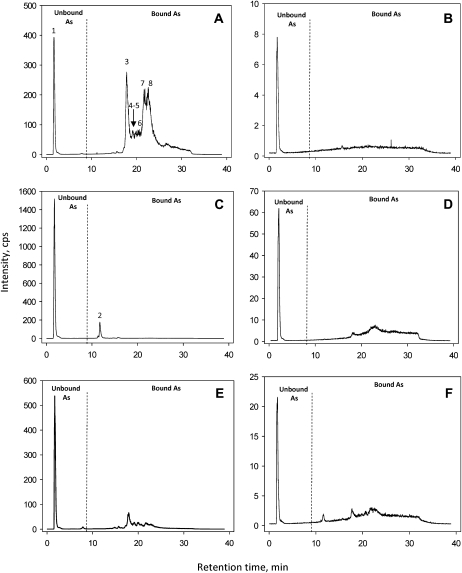
Seedlings of cad1-3, cad2-1, and the wild type were pregrown in hydroponic culture for 3 weeks and then exposed to 10 μm As(V) for 3 d. In this experiment, the exposure concentration and duration were increased in order to enhance the As signal for molecular analysis of As complexes. Also, reasonably uniform seedlings were used for As(V) exposure so that the plant biomass was similar among the three lines (data not shown). As speciation in root and shoot extracts was quantified by reverse-phase HPLC coupled to high-resolution ICP-MS and accurate mass high-resolution electrospray ionization (ESI)-MS. Using this analytical method, which limits the degradation of labile As(III)-GSH and As(III)-PC complexes in plant extracts during chromatography (Bluemlein et al., 2009b), it is possible to simultaneously collect elemental and molecular data from a single sample. Figure 2 shows the As signal (normalized by the internal standard 103Rh) from the root and shoot extracts of the wild type and the mutants. The initial peak (peak 1 in Fig. 2A) eluted in the void volume around 2 min was uncomplexed (unbound) inorganic As, being predominantly As(III) as determined by the assay using anion-exchange HPLC-ICP-MS (Table I). The peaks eluted after 10 min represent complexed (bound) As(III). Two features are apparent in Figure 2. First, most of the As in the cad1-3 and cad2-1 roots was unbound, compared with the dominance of complexed As(III) in the wild-type roots. Second, most of the As in the shoots of both the wild type and mutants was uncomplexed, although the concentrations of As in shoots were much lower than those in roots.
Figure 2.
ICP-MS m/z 75 (As) chromatograms for Arabidopsis wild-type root (A) and shoot (B), cad1-3 root (C) and shoot (D), and cad2-1 root (E) and shoot (F) extracts, respectively. The dashed line represents the arbitrary border between unbound and GSH/PC-bound As. The peaks are as follows: 1, inorganic As; 2, As(III)-(GS)3 complex; 3, As(III)-PC3 complex; 4 and 5, As(III)-(PC2)2 complexes (two isoforms); 6, unknown; 7 and 8, As(III)-PC4 complexes (at least two isoforms). See Figure 3 for their identification.
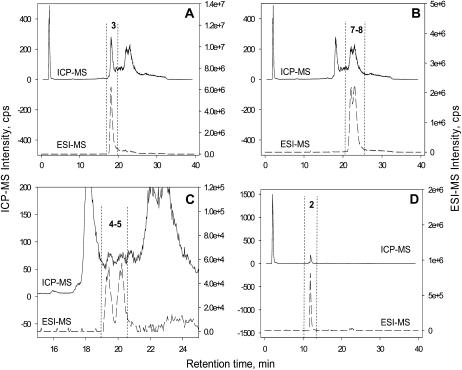
There were seven peaks of complexed As species (Fig. 2, A and C). Overlaying of ICP-MS chromatograms for As and ESI-MS chromatograms (Fig. 3) allows for the unequivocal identification and quantification of all but one peak (peak 6). Peak 2, which appeared only in the cad1-3 root extract, corresponds to the As(III)-GSH complex [As(III)-(GS)3]. Three As(III)-PC complexes were identified: As(III)-PC3, As(III)-(PC2)2, and As(III)-PC4 (Fig. 3). The ESI-MS signal for As(III)-PC3 appears as a single peak (peak 3), whereas that of As(III)-(PC2)2 appears as two distinct peaks (peaks 4 and 5) because it has two structural isomers that can be separated by C18 reverse phase chromatography and the eluent conditions used in this study. The As(III)-PC4 complex appears as two unresolved broad peaks (peaks 7 and 8) in both ICP-MS and ESI-MS traces; these represent four structural isomers of the As(III)-PC4 complex that are not fully separated at the baseline by the HPLC method used. For the quantification of the total amount of As in each PC complex, it is not necessary to resolve a baseline separation of different isomers. Peak 6 could not be identified (U1), although this was small compared with other As species.
Figure 3.
Overlaid ICP-MS m/z 75 (As, top) and ESI-MS (bottom) [M+H]+ chromatograms for As(III)-GS/As-PC complex identification taken from the wild-type root extract (A–C) and cad1-3 root extract (D). Peaks are labeled as in Figure 2. Left-hand axes for all graphs are the ICP-MS intensities; right-hand axes for all graphs are the ESI-MS intensities. A, ICP-MS and ESI-MS m/z 844.0897 [As(III)-PC3]. B, ICP-MS and ESI-MS m/z 1076.1414 [As(III)-PC4]. C, ICP-MS and ESI-MS m/z 1151.1723 [As(III)-(PC2)2]. D, ICP-MS and ESI-MS m/z 994.1525 [As(III)-(GS)3].
Table II presents the concentrations of As-containing species. In the wild-type roots, 69% As was complexed with PCs, with the most abundant complex being As(III)-PC4, followed by As(III)-PC3 and As(III)-(PC2)2. In contrast, only 8% and 25% of As in the roots of cad1-3 and cad2-1, respectively, were complexed. In the case of cad1-3 roots, this complexation was only with GSH, with no evidence of As(III)-PC complexation. In the cad2-1 roots, there were small amounts of As(III)-PC3, As(III)-(PC2)2, As(III)-PC4, and U1. Shoots contained much smaller levels of As, all of which was unbound in the wild type and 13% was PC complexed in the two mutants. The ratio of shoot-to-root As concentration in the cad1-3 and cad2-1 mutants was 10- and 5.4-fold higher, respectively, than that in the wild type (Table II), suggesting an enhanced root-to-shoot translocation when little As is complexed in roots. This was investigated further in experiments described later.
Table II. Quantities (nmol As g−1 FW) of unbound inorganic As and As(III)-PC complexes in wild-type Arabidopsis and cad1-3 and cad2-1 mutants.
Plants were exposed to 10 μm As(V) for 3 d. Data are means ± se (n = 3).
| Sample | Unbound |
As(III)-GS/As(III)-PC Complexes |
Total As | Percentage |
Shoot-to-Root As Concentration Ratio | |||||
| Inorganic As | As(III)-(GS)3 | As(III)-PC3 | As(III)-(PC2)2 | U1a | As(III)-PC4 | Unbound | Bound | |||
| Wild-type shoots | 2 ± 0.01 | 2 | 100 | 0 | 0.007 | |||||
| Wild-type roots | 97 ± 2 | 60 ± 0.16 | 23 ± 2 | 14 ± 2 | 121 ± 3 | 316 | 30.8 | 69.2 | ||
| cad1-3 shoots | 20 ± 0.36 | 0.6 ± 0.28 | 2 ± 1 | 23 | 86.8 | 13.2 | 0.070 | |||
| cad1-3 roots | 303 ± 3 | 27 ± 1 | 330 | 91.9 | 8.1 | |||||
| cad2-1 shoots | 5 ± 0.13 | 0.1 ± 0.04 | 0.2 ± 0.01 | 0.5 ± 0.06 | 6 | 87.4 | 12.6 | 0.038 | ||
| cad2-1 roots | 115 ± 1 | 16 ± 1 | 10 ± 0.24 | 3 ± 1 | 10 ± 4 | 153 | 75.4 | 24.6 | ||
An unidentified species eluting around 20.9 min (U1), between the As(III)-(PC2)2 and As(III)-PC4 complexes, was also quantified. This As species is included as bound As.
With a high-resolution ICP-MS, it is possible to measure sulfur on mass-to-charge ratio (m/z) 32 without the polyatomic interference from O2. A number of S-containing compounds were identified and quantified when ICP-MS chromatograms for S and ESI-MS chromatograms were aligned using elemental rather than compound specific calibration as described previously (Bluemlein et al., 2008, 2009a, 2009b; Supplemental Fig. S1; Supplemental Table S1). These include a number of free thiol compounds, GSSG, oxidized PC2, reduced PC2, oxidized PC3, and oxidized PC4, as well as the As(III)-thiol complexes As(III)-PC3 and As(III)-(GS)3. All these compounds were identified using both the elemental signals of S and As on the ICP-MS and at the same time the protonated molecular mass, which were measured with a high-resolution ESI-MS with an accuracy better than 5 ppm (Supplemental Table S2). The level of GSSG was higher in cad1-3 than the wild type and was not detectable in cad2-1 (Supplemental Table S1). Free GSH was eluted close to the void volume of the C18 column and was swamped by the coelution of other thiol species, except in the cad1-3 roots in which there were no other thiols to interfere with the detection of GSH. The concentration of As(III)-PC3 in the wild-type roots quantified from the S signal (68 nmol g−1 fresh weight [FW]) was comparable to that measured from the As signal (60 nmol g−1 FW), whereas for As(III)-(GS)3, there was a larger discrepancy between the two quantification approaches; 16 and 26 nmol g−1 FW for S and As signals, respectively.
As(III) Efflux
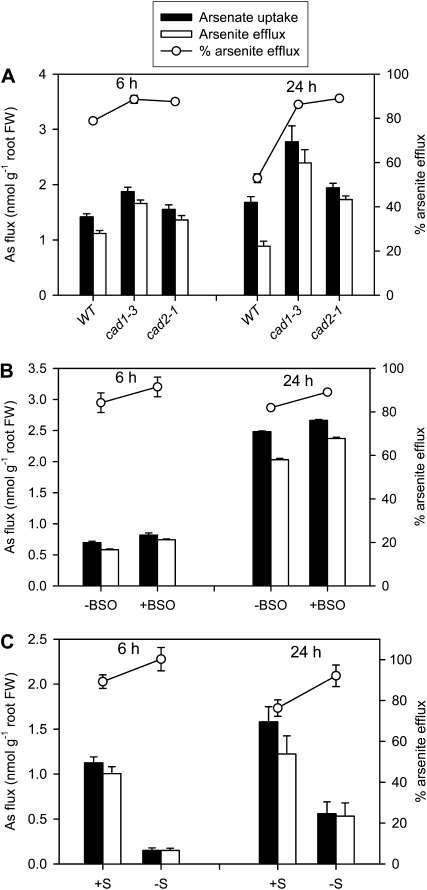
Previous studies have shown that roots of a number of plant species take up As(V) and efflux substantial amounts of As(III) to the external solution (Xu et al., 2007; Logoteta et al., 2009; Zhang et al., 2009). Here, we investigated if As(III) efflux is influenced by PC complexation in Arabidopsis roots. As(III) efflux was determined by monitoring As speciation in the nutrient solution to which 5 μm As(V) was initially added. After 6 h, 65% to 80% of the As in the nutrient solution was detected as As(III), and by 24 h, 98% to 99% was As(III). Figure 4A shows As(V) uptake (measured as the decrease of As(V) from the solution), As(III) efflux [measured as the production of As(III) in the solution], and As(III) efflux as a percentage of As(V) uptake in the cad1-3, cad2-1, and wild-type plants. It should be pointed out that the As(V) uptake data were strongly influenced by the degree of As(V) depletion in the nutrient solution; smaller As(V) uptake in the wild type at 24 h was mainly due to a faster depletion of As(V) owing to the larger root biomass in wild-type plants (root FW: wild type, 0.149 g; cad2-1, 0.127 g; and cad1-3, 0.091 g; P < 0.01; also significant difference in shoot FW, P < 0.05; Supplemental Fig. S2). However, As(III) efflux as a proportion of As(V) uptake should be independent of this effect. At both time points, the two mutants effluxed significantly (P < 0.001) more As(III) than wild-type plants.
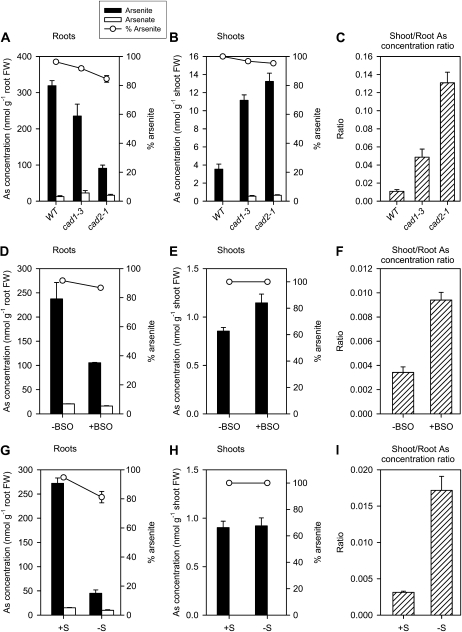
Figure 4.
As(V) uptake, As(III) efflux, and As(III) efflux as a percentage of As(V) uptake in the wild type (WT) and cad1-3 and cad2-1 mutants of Arabidopsis (A), the wild type with or without BSO (B), and the wild type pretreated with or without S deprivation (C). Data are means ± se (n = 3 for the BSO experiment and 4 for the other experiments).
In further experiments, we tested whether manipulation of GSH and PC synthesis by the use of BSO or manipulation of plant S status influences As(III) efflux from wild-type Arabidopsis roots. BSO did not significantly influence As(V) uptake but increased As(III) efflux as a percentage of As(V) uptake (Fig. 4B). This effect was significant (P < 0.01) at the 24-h time point. No significant differences in root or shoot FW were found between the −/+BSO treatments (Supplemental Fig. S2).
In the S nutrition experiment, deprivation of S for 1 week prior to As(V) exposure also increased As(III) efflux as a percentage of As(V) uptake; this effect was significant (P < 0.05) at the 24 h time point (Fig. 4C). However, the S-deprived plants took up much less As(V): only 14% and 35% of the S-sufficient plants (P < 0.001) at 6 and 24 h, respectively. This difference was not attributable to different root biomass in the –S and +S treatments causing different degrees of As(V) depletion from the solution, as there was no significant difference in root or shoot biomass (Supplemental Fig. S2).
As Accumulation and Distribution in Plants
As accumulation and distribution between roots and shoots were determined in plants from the three experiments described above. After exposure to 5 μm As(V) for 24 h, the cad1-3 and cad2-1 roots contained significantly (P < 0.001) smaller amounts of As [mainly As(III)] than the wild type (Fig. 5A). In contrast, the pattern for shoot As was the opposite: the total As concentration in cad1-3 and cad2-1 was 3.4 and 3.9 times that of the wild type, respectively (P < 0.001). Consequently, the shoot-to-root As concentration ratio was 4.5- and 12-fold higher in cad1-3 and cad2-1 than in the wild type (P < 0.001; Fig. 5A). Similarly, the percentage of As distributed to shoots was much higher in the two mutants (12.5% ± 2.1% and 27.8% ± 1.9% in cad1-3 and cad2-1, respectively) than in the wild type (3.2% ± 0.7%).
Figure 5.
As accumulation and speciation in roots and shoots of the wild type (WT) and cad1-3 and cad2-1 mutants of Arabidopsis (A–C), the wild type with or without BSO (D–F), and the wild type pretreated with or without S deprivation (G–I). Data are means ± se (n = 3 for the BSO experiment and 4 for the other experiments).
In the BSO experiment, +BSO decreased As accumulation in the roots but increased As accumulation in the shoots of the wild type; this had the effect of increasing the shoot-to-root As concentration ratio by 2.7-fold (Fig. 5B). +BSO also decreased the As(III)% in the roots from 92% to 87%, suggesting a small inhibition of As(V) reduction. In the shoots, only As(III) was found in both −/+ BSO treatments.
In the S nutrition experiment, S deprivation decreased As accumulation in the wild-type roots to 18% of the S-sufficient plants but had little effect on As concentration in the shoots; consequently, the shoot-to-root As concentration ratio was increased by 5.5-fold in the S-deprived plants (Fig. 5C). The As(III)% in the roots was also decreased by S deprivation: from 95% in the S-sufficient plants to 82% in the S-deprived plants. Shoots of both −/+ S treatments contained only As(III).
DISCUSSION
Both the cad1-3 and cad2-1 mutants were similarly hypersensitive to As(V). As(V) was reduced to As(III) rapidly in Arabidopsis roots, as indicated by the predominance of As(III) after 24-h exposure to As(V) (Table I). This result corroborates the finding of Dhankher et al. (2006), who found that most of As in Arabidopsis was As(III) in a study using x-ray absorption spectrometry. The ability to reduce As(V) was decreased in the two mutants, most noticeably in the GSH-deficient cad2-1 mutant. This is consistent with the GSH-dependent As(V) reduction pathway mediated by a yeast ACR2-like As(V) reductase in plants (Bleeker et al., 2006; Dhankher et al., 2006; Ellis et al., 2006; Duan et al., 2007). The lower reduction capacity in the +BSO or S-deprived wild-type plants (Fig. 5) may also be attributed to a decrease in GSH. It is noteworthy that cad2-1 was still able to reduce the majority (>80%) of As(V) to As(III). This ability may be attributed to the residual level of GSH in the cad2-1 mutant (15%–40% of the wild type) (Howden et al., 1995a; Cobbett et al., 1998), which can be utilized for As(V) reduction, or that GSH-independent pathways of As(V) reduction exist. There is circumstantial evidence that multiple As(V) reductase enzymes or multiple reduction pathways may contribute to As(V) reduction in plants (Zhao et al., 2009). The reason why the PC-deficient cad1-3 mutant had a slightly decreased As(III) proportion is not clear, as this mutant contains more, not less, GSH than wild-type plants (Howden et al., 1995b). A possible explanation is that the lack of As(III) complexation by PCs in cad1-3 (discussed below) may make reoxidation to As(V) more likely.
By using high-resolution ICP-MS and high-resolution ESI-MS coupled in tandem to HPLC, we were able to identify and quantify most of the As(III)-thiol complexes, as well as a number of free thiol compounds in reduced or oxidized states. Thus, the method allows a simultaneous and extensive profiling of thiol and As(III)-thiol compounds in plant extracts. The close agreement between measured and theoretical molecular masses of the thiol peptides as well as the As-thiol complexes (Supplemental Table S2) is unequivocal evidence of the identified molecules in the plant extracts. It has previously been shown that no degradation or de novo synthesis of those complexes took place during the extraction and chromatography method used (Bluemlein et al., 2008, 2009b). For some of the As(III)-thiol complexes, quantification can be made using both As and S signals, which was made possible with the high-resolution ICP-MS. Similar concentrations of As(III)-PC3 were determined by this novel quantification approach using the element-specific (As or S) and compound-independent calibration. The mismatch of the concentration of As(III)-(GS)3 using the S signal (16 nmol g−1 FW) and the As signal (26 nmol g−1 FW) was described before (Bluemlein et al., 2009a) and was interpreted as being due to coeluting As hydroxyl GSH compounds, which altered the difference in the As-to-S elemental ratio leading to the higher amount of As complex using the As signal.
Hence, these results provide direct evidence that the majority of As(III) in the wild-type roots of Arabidopsis was complexed by PCs. This complexation was absent in the cad1-3 roots and was much decreased in the cad2-1 roots, consistent with the absence or the greatly diminished level of PCs in the two respective mutants (Howden et al., 1995a, 1995b). Interestingly, As(III)-(GS)3 was found to exist in an appreciable amount only in the cad1-3 roots. This mutant accumulates more GSH/GSSG than the wild type because of the block in PC synthesis (Howden et al., 1995b; Supplemental Table S1). It may be that the higher GSH concentration explains the occurrence of As(III)-(GS)3 in cad1-3. However, a more plausible explanation is that As(III)-(GS)3 is less stable than As(III)-PCs due to the entropy effect (Bluemlein et al., 2009b); therefore, the former complex forms only when there is little competition from PCs for As(III) binding, as was the case in cad1-3.
Compared with the wild-type roots, the proportion of thiol-complexed As(III) was much smaller in the two mutants (Table II). This lack of complexation in roots, especially with PCs, is most likely to be the primary reason for the As(V) hypersensitivity exhibited by the two mutants because uncomplexed As(III) is disruptive to cell metabolism through its binding to critical thiols in proteins and generation of oxidative stress (Hughes, 2002; Requejo and Tena, 2005). Presumably As(III)-thiol complexes are sequestered in root vacuoles, although plant transporters responsible for the uptake of these complexes into vacuoles have not been identified (Verbruggen et al., 2009; Zhao et al., 2009). It has been shown in yeast that the vacuolar transporter Ycf1p, a member of the ATP-binding cassette superfamily, transports the glutathione-S-conjugated As(III) [As(III)-(GS)3] into the vacuole (Ghosh et al., 1999).
In contrast to roots, As(III) in the wild-type shoots was uncomplexed, whereas, surprisingly, small proportions (approximately 13%) of As(III) in the shoots of the two mutants were complexed to PCs or GSH. This difference may be due to the low level of As accumulation in the wild-type shoots, which did not trigger PC synthesis. A previous study showed the presence of As(III)-PC complexes in sunflower leaves after exposure to a high level (66 μm) of As(V) or As(III) (Raab et al., 2005).
In this study, we observed two consequences of a markedly decreased As(III)-thiol complexation in roots: enhanced As(III) efflux to the external medium and enhanced As translocation from roots to shoots, resulting in decreased As accumulation in roots and increased As accumulation in shoots. The results were consistent among the experiments with the cad1-3 and cad2-1 mutants and with wild-type plants manipulated by the use of BSO or S nutrition. Although not determined in this study, the BSO or S deprivation treatment was likely to lead to less complexation of As(III) with thiols. In addition, S deprivation had a marked effect on As(V) uptake, presumably through an effect on the expression or regulation of phosphate/As(V) transporters, which warrants further investigation.
Similar to previous studies with tomato (Solanum lycopersicum), rice, and Holcus lanatus (Xu et al., 2007; Logoteta et al., 2009), As(V) supplied to Arabidopsis roots was rapidly converted to As(III) in the external solution. This transformation of As species is mediated by roots, not microorganisms or root exudates in the medium (Xu et al., 2007). As(III) efflux was most rapid during the phase of, almost concurrent with, As(V) uptake; efflux was much smaller when As-preloaded roots of tomato were transferred to an As-free solution to collect further release of As(III) (Xu et al., 2007). This can be explained by increased As(III)-PC complexation with time, resulting in less free As(III) for efflux. This interpretation is supported by the inverse relationship between As(III)-thiol complexation and As(III) efflux observed in this study. The scale of As(III) efflux as a proportion of As(V) uptake was very large in Arabidopsis and other As nonhyperaccumulating species tested (Xu et al., 2007; Logoteta et al., 2009; Zhang et al., 2009), suggesting that it may represent a constitutive tolerance mechanism in plants (Logoteta et al., 2009), as has been demonstrated in microorganisms (Bhattacharjee and Rosen, 2007). Without As(III) efflux, the cellular burden of As in Arabidopsis roots would have escalated dramatically.
Typical of As nonhyperaccumulating plants, Arabidopsis translocated only a small proportion of As to the shoots. This translocation was enhanced substantially in the cad1-3 and cad2-1 mutants, as well as in the BSO-treated or S-deprived wild-type plants. Previously, Li et al. (2006) also found approximately 2-fold higher shoot As concentration in cad2-1 than wild-type plants after a 3-week exposure to 25 μm As(V). These results supports a model in which free As(III) is the predominant form of As loaded into the xylem and transported to the shoots and that As(III) complexation with PCs contributes to the sequestration of As in roots. As speciation measurements in a number of plant species show the predominance of As(III) in the xylem sap (Mihucz et al., 2005; Xu et al., 2007; Su et al., 2008; Zhao et al., 2009). This As(III) is not likely to be complexed with strong ligands such as GSH or PCs because no As(III)-thiol complexes were detected in the xylem sap collected from sunflower (Raab et al., 2005), and there was also no evidence from x-ray absorption spectrometry of an As-S coordination for the As species found in the xylem sap of Brassica juncea (Pickering et al., 2000). However, the possibility of labile complexes with weaker ligands cannot be excluded due to the analytical methodology used (Feldmann et al., 2009). Some As(V) may be transported from roots and shoots in Arabidopsis, especially the two mutants because of the slight decrease in the As(V) reduction capacity in roots, but this is likely to account for a small proportion of the total As translocated to shoots considering the predominance of As(III) in both roots and shoots. Furthermore, studies with the Arabidopsis phosphate mutant pho1, which is defective in xylem loading of phosphate, showed no effect on As distribution to the shoots (Quaghebeur and Rengel, 2004); a decrease in As transport to the shoots would be expected if As(V) were the main form translocated. When As(V) reduction in roots is suppressed in the Arabidopsis RNA interference knockdown lines of AtACR2, As accumulation in shoots was enhanced, presumably through enhanced As(V) translocation (Dhankher et al., 2006). The model of As(III)-PC complexation in roots restricting As(III) mobility to shoots is also in accord with the observations in the As hyperaccumulator P. vittata, which has very little As(III)-PC complexation in roots (Zhao et al., 2003; Huang et al., 2008) and an exceedingly efficient xylem transport of As(III) (Su et al., 2008). However, unlike P. vittata, cad1-3 and cad2-1 did not exhibit the phenotype of As hyperaccumulation in shoots possibly owing to a combinations of reasons: (1) much of the uncomplexed As(III) was effluxed to the external medium in cad1-3 and cad2-1 but not in P. vittata (Su et al., 2008); (2) P. vittata may possess a highly efficient xylem loading system for As(III) not found in Arabidopsis; and (3) P. vittata is able to detoxify large concentrations of As(III) in the frond tissues (for review, see Zhao et al., 2009).
Interestingly, the model discussed above for As is opposite that proposed for Cd. Gong et al. (2003) showed that cad1-3 accumulated more Cd in the roots and less Cd in the shoots than the wild type and that transgenic expression of the wheat PC synthase gene (TaPCS1) in cad1-3 reduced Cd accumulation in roots and increased Cd distribution to shoots compared with untransformed cad1-3. They suggest that PCs can be transported from roots to shoots, implying PCs as possible carriers for Cd in the xylem transport, although recent studies reported only trace levels of PCs in the xylem sap of sunflower (Raab et al., 2005) and B. napus (Mendoza-Cózatl et al., 2008). The exact reasons underlying the contrasting effects of PCs on Cd and As mobility remain to be investigated. An important difference between PC-Cd and PC-As(III) complexes is that the former are stable in neutral and alkaline conditions but not in acidic environments (Johanning and Strasdeit, 1998), whereas the opposite is true for the latter (Schmöger et al., 2000). Therefore, PC-Cd complexes may be less stable in root vacuoles (pH approximately 5.5) than PC-As(III) complexes, allowing Cd to be more easily effluxed from the vacuole to the cytoplasm for radial transport to the xylem than As(III). Incorporation of sulfide into the PC-Cd complex can increase the stability of the complex in the vacuole (Cobbett and Goldsbrough, 2002). However, it has been observed that the degradation of Cd-induced PCs after Cd exposure ceases was much faster than that of As-induced PCs in roots of Silene vulgaris (de Knecht et al., 1995; Sneller et al., 1999), consistent with a weaker sequestration and higher mobility of Cd in roots than As(III). Given the contrasting stability at different pHs, PC-Cd complexes are unlikely to be stable in the acidic xylem sap, whereas PC-As(III) would be unstable in the alkaline phloem sap.
In conclusion, this study has shown that the majority of As in wild-type Arabidopsis roots is complexed by PCs and that decreasing As(III)-PC complexation led to enhanced As(III) efflux to the external medium and enhanced As translocation to the shoots. Enhancing PC synthesis in roots may be an effective strategy to decrease As accumulation in shoots or grain of food crops.
MATERIALS AND METHODS
Plant Culture
The Arabidopsis (Arabidopsis thaliana) lines used in this study were wild type (Columbia-0), cad1-3 (PC-deficient mutant), and cad2-1 (GSH-deficient mutant; Howden et al., 1995a, 1995b). Seeds were germinated on the 0.5% agar medium supplemented with one-tenth-strength Hoagland nutrient solution in 0.5-mL Eppendorf vials that were cut about 0.8 cm from the bottom to allow root growth into the nutrient solution. The vials were inserted in holes of plastic boxes containing 600 mL one-tenth-strength Hoagland solution. The composition of the nutrient solution was as follows: 0.6 mm KNO3, 0.4 mm (NH4)2HPO4, 0.1 mm MgSO4, 0.4 mm Ca(NO3)2, 2 μm H3BO3, 0.06 μm CuSO4, 0.36 μm MnCl2, 0.1 μm ZnSO4, 0.04 μm NaMoO4, and 20 μm FeNaEDTA. The solution pH was buffered at 5.5 with 2 mm MES (pH adjusted with KOH). Nutrient solution was renewed every 3 d after germination. All the experiments were performed in a growth room (20°C/20°C day/night temperature, light intensity 120 μmol m−2 s−1, 16-h photon period per day, and relative humidity 70%).
As Toxicity Test
Seeds of the wild type, cad1-3, and cad2-1 were surface sterilized by immersion in 70% ethanol and then sown on agar plates in petri dishes containing 2.2 g L−1 Murashige and Skoog salts, 2% (w/v) Suc, and 1.2% agar (pH 5.8 using MES buffer). Petri dishes were placed in the dark at 4°C for 3 d and then transferred to a growth room. Three days after germination, when roots were 1.5 to 2 cm long, seedlings were transferred to petri dishes of the same medium amended with 0, 5, 25, 50, 100, or 200 μm As(V). Each As concentration was replicated in four dishes. Four seedlings of each of the three lines were placed in each dish. Plants were allowed to grow vertically on the agar plates for 7 d. Total fresh weight was determined.
As(V) Uptake, As(III) Efflux, As Speciation, and Translocation in Arabidopsis
Three-week-old seedlings of the wild-type, cad1-3, and cad2-1 were transferred into 50-mL pots (one seedling per pot) containing one-tenth-strength Hoagland solution with 5 μm As(V). Phosphate was omitted from the solution to allow a faster uptake of As(V). Each line was replicated in four pots, and the experiment was repeated twice with reproducible results. At 6 and 24 h, aliquots of 0.5 mL nutrient solution were removed from each pot, diluted with 4.5 mL of phosphate-buffered solution (PBS) containing 2 mm NaH2PO4 and 0.2 mm Na2-EDTA (pH 5.5), and filtered through 0.45 μm before analysis of As speciation. At 24 h, the volume of nutrient solution was recorded. Plant shoots were rinsed with deionized water, blotted dry, weighed, and then frozen in liquid nitrogen. Plant roots were rinsed briefly in an ice-cold desorption solution containing 1 mm K2HPO4, 0.5 mm Ca(NO3)2, and 5 mm MES (pH 5.5) and immersed in 1 liter of the same solution for 10 min to remove apoplastic As. Roots were blotted dry, weighed, and frozen in liquid nitrogen. Shoots and roots were ground in liquid nitrogen to fine powder in a mortar and pestle for As analysis. The finely ground materials were extracted with 10 mL PBS solution for 1 h under sonication in a 4°C cool room. The extract was filtered through Whatman number 42 filter paper and then a 0.2 μm filter before analysis. The efficiency of As extraction by PBS was 50% to 70% (Xu et al., 2007).
Wild-type seedlings (18 d old) were transferred to boxes (nine seedlings per box) containing 600 mL of nutrient solution with or without 0.5 mm BSO for 3 d. Thereafter, seedlings were exposed to 5 μm As(V) without phosphate for 24 h. There were three replicates for each treatment. Nutrient solution and root and shoot samples were collected for As speciation analysis as described above.
Wild-type seedlings (2 weeks old) were transferred to nutrient solutions containing no or normal level of S and grown for another week. In the −S treatment, all sulfate salts used in the nutrient solution were replaced with chloride salts. Thereafter, seedlings were used for the experiment to determine As speciation in nutrient solution and in root and shoots as described above. There were four replicates for each treatment.
For the determination of As(III)-PC complexation, 3-week-old seedlings of the wild type, cad1-3, and cad2-1 were exposed to 10 μm As(V) in one-tenth-strength Hoagland solution with the phosphate concentration reduced to 10 μm. After 3 d, roots were washed first with tap water and then soaked in 10 mm KH2PO4 solution for 10 min to remove apoplastic As, followed by final rinses with tap water and deionized water. Roots and shoots were blotted dry using a paper towel.
Analytical Methods
As speciation in nutrient solutions and in root and shoot tissues was determined by anion-exchange HPLC-ICP-MS (Agilent LC1100 series and Agilent ICP-MS 7500ce; Agilent Technologies). As species [As(III), As(V), DMA, and MMA] were separated by an anion-exchange column (Hamilton PRP X-100) with a mobile phase of 6.6 mm NH4H2PO4 and 6.6 mm NH4NO3 (pH 6.3), run isocratically at 0.6 mL min−1. The outlet of the separation column was connected to a concentric nebulizer and a water-jacketed cyclonic spray chamber of the ICP-MS. An internal standard (germanium [Ge]) was mixed continuously with the postcolumn solution through a peristaltic pump. Signals at m/z 75 (As) and 72 (Ge) were collected with a dwell time of 500 ms. Possible polyatomic interference of 40Ar35Cl on m/z 75 was removed by the Agilent Octopole Reaction System operating in the helium gas mode. The As signal was normalized using the Ge signal to correct any signal drift during the analysis. Peaks were identified by comparisons with the retention times of standard compounds. As species in the samples were quantified by external calibration curves with peak areas. Analysis of As species was carried out immediately following sample collection or extraction. For each batch of samples, the analysis was completed within 12 h; no changes in As speciation were observed during this period of time. No MMA or DMA was detected in any of the samples.
For the analysis of As(III)-thiol complexes, subsamples of roots and shoots were taken and ground with a mortar and pestle in liquid nitrogen to a fine powder. Samples were then transferred to 15-mL Grenier tubes, followed by the addition of 1% formic acid, such that an approximate ratio of 1 g plant material to 2 g 1% formic acid was achieved. Samples were then extracted at 4°C for 1 h, with intermittent shaking by hand. Samples were subsequently centrifuged at 3500 rpm for 3 min after extraction. Approximately 1 mL of supernatant was then transferred to an Eppendorf vial, where the supernatant was further centrifuged at 13,000 rpm for 3 min. From this vial, the supernatant was then sampled for speciation analysis, carried out using HPLC coupled with high-resolution ICP-MS (Element 2; Thermo Fisher Scientific) and high resolution ESI-MS (LTQ Orbitrap Discovery; Thermo Fisher Scientific). Separation was performed on a C18 reverse-phase column, using a water-methanol gradient. Starting from 100% water, methanol was added to the eluent at the rate of 1% per min over the first 20 min. The eluent was held at 80% water/20% methanol for a further 10 min, followed by decreasing the methanol concentration to 0% over the subsequent 5 min and allowing the column to flush 100% water for a further 5 min. Total chromatographic run time was 40 min. For the ICP-MS analysis, As and S were measured on m/z 75 and 32, respectively. Rhodium was added postcolumn as internal standard for the ICP-MS, and its signal was measured on m/z 103. The molecular masses of the eluted compounds were determined with high resolution during a chromatographic run using GSH as an internal standard for the mass determination. The molecular masses of As(III)-thiol complexes and free thiols measured by ESI-MS agreed with the actual molecular masses with deviations of <5 ppm (Supplemental Table S2). Supplemental Figure S3 shows the molecular structures of As(III)-thiol complexes and free thiols measured in this study. The efficiency of As extraction by 1% formic acid was around 90% (Raab et al., 2005).
Data Analysis
Data were analyzed by ANOVA, followed by comparisons between means using the lsd.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Overlaid ICP-MS m/z 32 (S, top) and ESI-MS (bottom) [M+H]+ chromatograms for quantification of sulfur species, taken from the cad1-3 root extract (a–c) and wild-type root extract (d–h).
Supplemental Figure S2. Fresh weights of Arabidopsis plants.
Supplemental Figure S3. Molecular structures of unbound thiol compounds and As(III)-thiol complexes detected in Arabidopsis.
Supplemental Table S1. Quantities (ng S g−1 plant fresh weight) of GSH and PC species in the wild-type Arabidopsis and the cad1-3 and cad2-1 mutants.
Supplemental Table S2. Measured and actual masses for unbound thiol compounds and As(III)-PC complexes in Arabidopsis samples, detected via high-resolution ESI-MS.
Supplementary Material
Acknowledgments
We thank Chris Cobbett (University of Melbourne) and Henk Schat (Free University of Amsterdam) for providing seeds of Arabidopsis mutants.
References
- Bhattacharjee H, Rosen BP. (2007) Arsenic metabolism in prokaryotic and eukaryotic microbes. Nies DH, Silver S, , Molecular Microbiology of Heavy Metals, Vol Microbiol Monograph 6. Springer-Verlag, Berlin, pp 371–406 [Google Scholar]
- Bleeker PM, Hakvoort HWJ, Bliek M, Souer E, Schat H. (2006) Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate-tolerant Holcus lanatus. Plant J 45: 917–929 [DOI] [PubMed] [Google Scholar]
- Bluemlein K, Krupp EM, Feldmann J. (2009a) Advantages and limitations of a desolvation system coupled online to HPLC-ICPqMS/ES-MS for the quantitative determination of sulfur and arsenic in arseno-peptide complexes. J Anal At Spectrom 24: 108–113 [Google Scholar]
- Bluemlein K, Raab A, Feldmann J. (2009b) Stability of arsenic peptides in plant extracts: off-line versus on-line parallel elemental and molecular mass spectrometric detection for liquid chromatographic separation. Anal Bioanal Chem 393: 357–366 [DOI] [PubMed] [Google Scholar]
- Bluemlein K, Raab A, Meharg AA, Charnock JM, Feldmann J. (2008) Can we trust mass spectrometry for determination of arsenic peptides in plants: comparison of LC-ICP-MS and LC-ES-MS/ICP-MS with XANES/EXAFS in analysis of Thunbergia alata. Anal Bioanal Chem 390: 1739–1751 [DOI] [PubMed] [Google Scholar]
- Brammer H, Ravenscroft P. (2009) Arsenic in groundwater: a threat to sustainable agriculture in South and South-east Asia. Environ Int 35: 647–654 [DOI] [PubMed] [Google Scholar]
- Chen A, Komives EA, Schroeder JI. (2006) An improved grafting technique for mature Arabidopsis plants demonstrates long-distance shoot-to-root transport of phytochelatins in Arabidopsis. Plant Physiol 141: 108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P. (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B. (1998) The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in gamma-glutamylcysteine synthetase. Plant J 16: 73–78 [DOI] [PubMed] [Google Scholar]
- de Knecht JA, Vanbaren N, Ten Bookum WM, Sang HWWF, Koevoets PLM, Schat H, Verkleij JAC. (1995) Synthesis and degradation of phytochelatins in cadmium-sensitive and cadmium-tolerant Silene vulgaris. Plant Sci 106: 9–18 [Google Scholar]
- Dhankher OP, Rosen BP, McKinney EC, Meagher RB. (2006) Hyperaccumulation of arsenic in the shoots of Arabidopsis silenced for arsenate reductase (ACR2). Proc Natl Acad Sci USA 103: 5413–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan GL, Zhou Y, Tong YP, Mukhopadhyay R, Rosen BP, Zhu YG. (2007) A CDC25 homologue from rice functions as an arsenate reductase. New Phytol 174: 311–321 [DOI] [PubMed] [Google Scholar]
- Ellis DR, Gumaelius L, Indriolo E, Pickering IJ, Banks JA, Salt DE. (2006) A novel arsenate reductase from the arsenic hyperaccumulating fern Pteris vittata. Plant Physiol 141: 1544–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann J, Salaun P, Lombi E. (2009) Critical review perspective: elemental speciation analysis methods in environmental chemistry—moving towards methodological integration. Environ Chem 6: 275–289 [Google Scholar]
- Gasic K, Korban SS. (2007) Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Mol Biol 64: 361–369 [DOI] [PubMed] [Google Scholar]
- Ghosh M, Shen J, Rosen BP. (1999) Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 96: 5001–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JM, Lee DA, Schroeder JI. (2003) Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc Natl Acad Sci USA 100: 10118–10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E, Winnacker EL, Zenk MH. (1987) Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci USA 84: 439–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O'Connell MJ, Goldsbrough PB, Cobbett CS. (1999) Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 11: 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley-Whitaker J, Woods C, Meharg AA. (2002) Is differential phytochelatin production related to decreased arsenate influx in arsenate tolerant Holcus lanatus? New Phytol 155: 219–225 [Google Scholar]
- Howden R, Andersen CR, Goldsbrough PB, Cobbett CS. (1995a) A cadmium-sensitive, glutathione deficient mutant of Arabidopsis thaliana. Plant Physiol 107: 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Goldsbrough PB, Andersen CR, Cobbett CS. (1995b) Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol 107: 1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZC, Chen TB, Lei M, Liu YR, Hu TD. (2008) Difference of toxicity and accumulation of methylated and inorganic arsenic in arsenic-hyperaccumulating and -hypertolerant plants. Environ Sci Technol 42: 5106–5111 [DOI] [PubMed] [Google Scholar]
- Hughes MF. (2002) Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133: 1–16 [DOI] [PubMed] [Google Scholar]
- Johanning J, Strasdeit H. (1998) A coordination-chemical basis for the biological function of the phytochelatins. Angew Chem Int Ed 37: 2464–2466 [DOI] [PubMed] [Google Scholar]
- Li YJ, Dhankher OP, Carreira L, Lee D, Chen A, Schroeder JI, Balish RS, Meagher RB. (2004) Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol 45: 1787–1797 [DOI] [PubMed] [Google Scholar]
- Li YJ, Dankher OP, Carreira L, Smith AP, Meagher RB. (2006) The shoot-specific expression of gamma-glutamylcysteine synthetase directs the long-distance transport of thiol-peptides to roots conferring tolerance to mercury and arsenic. Plant Physiol 141: 288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logoteta B, Xu XY, Macnair MR, McGrath SP, Zhao FJ. (2009) Arsenite efflux is not enhanced in the arsenate-tolerant phenotype of Holcus lanatus. New Phytol 183: 340–348 [DOI] [PubMed] [Google Scholar]
- Meharg AA, Rahman M. (2003) Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environ Sci Technol 37: 229–234 [DOI] [PubMed] [Google Scholar]
- Meharg AA, Williams PN, Adomako E, Lawgali YY, Deacon C, Villada A, Cambell RCJ, Sun G, Zhu YG, Feldmann J, et al. (2009) Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ Sci Technol 43: 1612–1617 [DOI] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Butko E, Springer F, Torpey JW, Komives EA, Kehr J, Schroeder JI. (2008) Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J 54: 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihucz VG, Tatar E, Virag I, Cseh E, Fodor F, Zaray G. (2005) Arsenic speciation in xylem sap of cucumber (Cucumis sativus L.). Anal Bioanal Chem 383: 461–466 [DOI] [PubMed] [Google Scholar]
- Norton GJ, Islam MR, Deacon CM, Zhao FJ, Stroud JL, McGrath SP, Islam S, Jahiruddin M, Feldmann J, Price AH, et al. (2009) Identification of low inorganic and total grain arsenic rice cultivars from Bangladesh. Environ Sci Technol 43: 6070–6075 [DOI] [PubMed] [Google Scholar]
- Panaullah GM, Alam T, Hossain MB, Loeppert RH, Lauren JG, Meisner CA, Ahmed ZU, Duxbury JM. (2009) Arsenic toxicity to rice (Oryza sativa L.) in Bangladesh. Plant Soil 317: 31–39 [Google Scholar]
- Pickering IJ, Gumaelius L, Harris HH, Prince RC, Hirsch G, Banks JA, Salt DE, George GN. (2006) Localizing the biochemical transformations of arsenate in a hyperaccumulating fern. Environ Sci Technol 40: 5010–5014 [DOI] [PubMed] [Google Scholar]
- Pickering IJ, Prince RC, George MJ, Smith RD, George GN, Salt DE. (2000) Reduction and coordination of arsenic in Indian mustard. Plant Physiol 122: 1171–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaghebeur M, Rengel Z. (2004) Arsenic uptake, translocation and speciation in pho1 and pho2 mutants of Arabidopsis thaliana. Physiol Plant 120: 280–286 [DOI] [PubMed] [Google Scholar]
- Raab A, Feldmann J, Meharg AA. (2004) The nature of arsenic-phytochelatin complexes in Holcus lanatus and Pteris cretica. Plant Physiol 134: 1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab A, Schat H, Meharg AA, Feldmann J. (2005) Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): formation of arsenic-phytochelatin complexes during exposure to high arsenic concentrations. New Phytol 168: 551–558 [DOI] [PubMed] [Google Scholar]
- Raab A, Williams PN, Meharg A, Feldmann J. (2007) Uptake and translocation of inorganic and methylated arsenic species by plants. Environ Chem 4: 197–203 [Google Scholar]
- Requejo R, Tena M. (2005) Proteome analysis of maize roots reveals that oxidative stress is a main contributing factor to plant arsenic toxicity. Phytochemistry 66: 1519–1528 [DOI] [PubMed] [Google Scholar]
- Schat H, Llugany M, Vooijs R, Hartley-Whitaker J, Bleeker PM. (2002) The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and non-hyperaccumulator metallophytes. J Exp Bot 53: 2381–2392 [DOI] [PubMed] [Google Scholar]
- Schmöger MEV, Oven M, Grill E. (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122: 793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller FEC, Van Heerwaarden LM, Kraaijeveld-Smit FJL, Ten Bookum WM, Koevoets PLM, Schat H, Verkleij JAC. (1999) Toxicity of arsenate in Silene vulgaris, accumulation and degradation of arsenate-induced phytochelatins. New Phytol 144: 223–232 [Google Scholar]
- Su YH, McGrath SP, Zhu YG, Zhao FJ. (2008) Highly efficient xylem transport of arsenite in the arsenic hyperaccumulator Pteris vittata. New Phytol 180: 434–441 [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C, Schat H. (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12: 364–372 [DOI] [PubMed] [Google Scholar]
- Webb SM, Gaillard JF, Ma LQ, Tu C. (2003) XAS speciation of arsenic in a hyper-accumulating fern. Environ Sci Technol 37: 754–760 [DOI] [PubMed] [Google Scholar]
- Xu XY, McGrath SP, Zhao FJ. (2007) Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol 176: 590–599 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhao FJ, Huang Q, Williams PN, Sun GX, Zhu YG. (2009) Arsenic uptake and speciation in the rootless duckweed Wolffia globosa. New Phytol 182: 421–428 [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Ma JF, Meharg AA, McGrath SP. (2009) Arsenic uptake and metabolism in plants. New Phytol 181: 777–794 [DOI] [PubMed] [Google Scholar]
- Zhao FJ, McGrath SP. (2009) Biofortification and phytoremediation. Curr Opin Plant Biol 12: 373–380 [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Wang JR, Barker JHA, Schat H, Bleeker PM, McGrath SP. (2003) The role of phytochelatins in arsenic tolerance in the hyperaccumulator Pteris vittata. New Phytol 159: 403–410 [DOI] [PubMed] [Google Scholar]
- Zhu YG, Sun GX, Lei M, Teng M, Liu YX, Chen NC, Wang LH, Carey AM, Deacon C, Raab A, et al. (2008) High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environ Sci Technol 42: 5008–5013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.