Abstract
3,3′-Diindolylmethane (DIM) is a natural compound found in cruciferous vegetables that has antiproliferative and estrogenic activity. However, it is not clear whether the estrogenic effects are mediated through estrogen receptor (ER)α, ERβ, or both ER subtypes. We investigated whether DIM has ER subtype selectivity on gene transcription. DIM stimulated ERβ but not ERα activation of an estrogen response element upstream of the luciferase reporter gene. DIM also selectively activated multiple endogenous genes through ERβ. DIM did not bind to ERβ, indicating that it activates genes by a ligand-independent mechanism. DIM causes ERβ to bind regulatory elements and recruit the steroid receptor coactivator (SRC)-2 coactivator, which leads to the activation of ER target genes. Silencing of SRC-2 inhibited the activation of ER target genes, demonstrating that SRC-2 is required for transcriptional activation by DIM. Our results demonstrate that DIM is a new class of ERβ-selective compounds, because it does not bind to ERβ, but instead it selectively recruits ERβ and coactivators to target genes.
3,3’-Diindolylmethane represents a new class of ERβ-selective compounds, because they do not bind to ERβ, but selectively recruits ERβ and coactivators to target genes.
Estrogen signaling pathways are mediated by two estrogen receptors (ER), ERα and ERβ (1,2). Whereas the exact physiological roles of the two ER subtypes are unknown, it is clear that ERα and ERβ have different biological roles. The genes regulated by estradiol (E2) and selective estrogen receptor modulators with ERα are different from those regulated by ERβ (3). ERα and ERβ knockout mice exhibit different phenotypes (4). ERβ knockout mice have fewer corpora lutea, likely accounting for their observed subfertility (5). There is a widespread increase in the proliferation marker Ki-67 in luminal mammary epithelial cells of ERβ knockout mice, suggesting that ERβ is important for terminal differentiation of mammary epithelial cells (6). Prostate and myelogenous hyperplasia have been observed in ERβ knockout mice (7,8). These mice also show a loss of anxiety (9) and spatial learning (10) and develop depression-like behavior (11). These observations support a role for ERβ in behavior, mood, and affective disorders.
Brassica vegetables are rich in indole-3-carbinol (I3C), which upon exposure to gastric acid undergoes self-condensation to form 3,3′-diindolylmethane (DIM) (12). In cultured human breast cancer cells, radiolabeled I3C is converted to DIM, which accumulates in the nucleus of the cells (13). DIM exhibits both antiandrogenic and antiviral activities and is currently being tested in clinical trials for prostate cancer and cervical hyperplasia (14). DIM is currently among the most popular adjunct therapies for recurrent respiratory papillomatosis because of its effectiveness and low level of toxicity (14,15). Treatment of postmenopausal women with DIM led to an increase in the urinary estrogen metabolite 2-hydroxyestrone (16), which is associated with reduced breast cancer risk (17). Our studies have revealed that DIM is a potent inhibitor of breast (18,19) and prostate (20) cancer cell proliferation and an activator of estrogenic transcriptional pathways (21). However, the receptor specificity of the DIM estrogenic response remains to be investigated. Our objective was to determine whether DIM regulates gene transcription through ERα or ERβ. Here we show that DIM stimulated ERβ- but not ERα-mediated gene activation in U2OS, HeLa, and MDA-MB-231 cells, and even though DIM did not bind to ERβ, DIM activated ERβ-responsive genes in U2OS cells by recruiting steroid receptor coactivator 2 (SRC-2) and that silencing SRC-2 blocks this action. Our study demonstrated that DIM is a ligand-independent, selective activator of ERβ.
Materials and Methods
Cell culture
The U2OS, HeLa, and MDA-MB-231 cells were cultured and maintained as previously described (3,22). Briefly, cells were maintained in phenol red-free medium containing 5% stripped fetal bovine serum (Gemini Bio-Products, West Sacramento, CA; catalog no. 100-119), 2 mm glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. The U2OS cells expressing a tetracycline-inducible ERα or ERβ cDNA were prepared and maintained as previously described (3). The U2OS-ERβ cells stably transfected with a lentivirus expressing a scrambled or SRC-2 short hairpin RNA (shRNA) were prepared and maintained as previously described (23).
Transfection assay
U2OS, HeLa, or MDA-MB-231 cells were collected, transferred to a cuvette, and then electroporated with a gene pulser (Bio-Rad Laboratories, Hercules, CA) as previously described using 3 μg ERE-tk-Luc and 1 μg ERα or ERβ expression vectors (24,25). Experiments were done in triplicate, and the mean ± se was calculated and statistical analysis was performed using the Prism curve-fitting program (GraphPad Software, version 3.03). Results are representative of data collected from at least three experiments.
ER binding assay
U2OS-ERα stable cells grown in 12-well dishes were treated for 18 h with or without 1 μg/ml doxycycline. After the treatment, cells were incubated at 37 C for 2 h with 5 nm [3H]E2 (specific activity 110 Ci/mmol; PerkinElmer Life Science, Boston, MA) in the absence and presence of E2 or DIM, as stated in the figure legends. After washing four times with 1 ml ice-cold 0.1% BSA in PBS, the cells were lysed with absolute ethanol and bound [3H]E2 measured by scintillation counting. Total binding of [3H]E2 was expressed as counts per minute per 100,000 cells. The amount of radioactivity in the cells not treated with doxycycline was considered nonspecific binding. Experiments were done in triplicate, and the mean ± se was calculated and statistical analysis was performed using the Prism curve-fitting program (GraphPad Software, version 3.03). Results are representative of data collected from at least three experiments.
Real-time RT-PCR
Total RNA was isolated using Aurum Total RNA Mini Kit (Bio-Rad) according to the manufacturer’s protocol. RT was performed with 1 μg total RNA using the iScript cDNA synthesis kit (Bio-Rad). Real-time PCR was performed using SYBR Green Supermix with an iCycler thermal cycler (Bio-Rad). The data were collected and analyzed using the comparative Ct (threshold cycle) method using β-actin as the reference gene as previously described (26). Experiments were done in triplicate, and the mean ± se was calculated and statistical analysis was performed using the Prism curve-fitting program (GraphPad Software, version 3.03). Results are representative of data collected from at least three experiments.
ChIP
Cells were treated as indicated in the figure legends and then were cross-linked, collected, and lysed as previously described (3,27). Immunoprecipitations were performed overnight at 4 C with anti-ERα (catalog no. sc-543X; Santa Cruz Biotechnology, Santa Cruz, CA), anti-ERβ (6A12 catalog no. GTX70178, 14C8 catalog no. GTX70174, and 7B10 catalog no. GTX70182; GeneTex, San Antonio, TX), or anti-SRC-2 (GRIP1; catalog no. ab9261; Abcam, Cambridge, MA). PCR was done with primers as described previously (22,28). Experiments were done in triplicate, and the mean ± se was calculated and statistical analysis was performed using the Prism curve-fitting program (GraphPad Software, version 3.03). Results are representative of data collected from at least three experiments.
Results
DIM selectively activates reporter genes through ERβ
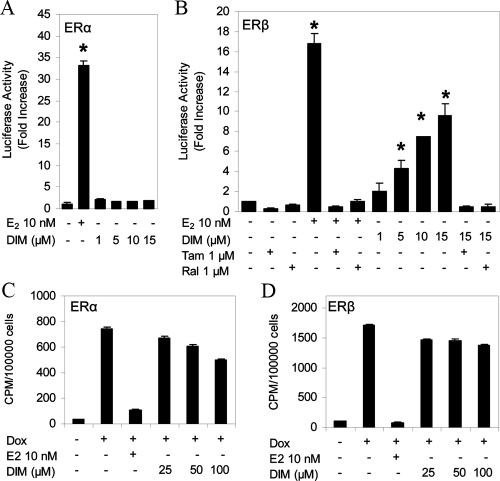
We initially examined the effects of DIM with ERα and ERβ using standard luciferase (Luc) reporter assays. U2OS osteosarcoma cells were cotransfected with a classical estrogen response element (ERE) upstream of a minimal thymidine kinase (tk) promoter (ERE-tk-Luc) and expression vectors for human ERα or ERβ. DIM did not activate ERE-tk-Luc in cells transfected with ERα (Fig. 1A) but produced a concentration-dependent activation with ERβ (Fig. 1B). Treatment with 15 μm DIM resulted in a 10-fold induction of Luc activity with ERβ, compared with a 17-fold activation observed with E2. Treatment with higher concentrations of DIM did not result in an increase in activation (data not shown). The selective estrogen receptor modulators raloxifene and tamoxifen blocked the activation by DIM (Fig. 1B), confirming that the effect of DIM is mediated through ERβ.
Figure 1.
DIM selectively activates ERE transcription through ERβ. A single copy of the vitellogenin A2 ERE upstream of the minimal tk promoter (ERE-tk-Luc) was cotransfected into U2OS cells with expression vectors for human ERα (A) or ERβ (B). After transfection, the cells were treated with 10 nm E2 or increasing concentrations of DIM in the absence or presence of 1 μm tamoxifen (Tam) or raloxifene (Ral), and Luc activity was measured. U2OS cells stably transfected with a tetracycline-inducible human ERα (C) or ERβ (D) were incubated with 5 nm [3H]E2 in the absence or presence of 100 nm E2 or DIM as indicated. Cells were treated with doxycycline (Dox) to induce expression of ERα or ERβ. Total binding of [3H]E2 was determined by scintillation counting. Each data point is the average of triplicate determinations ± sem. *, Significant difference between treatment and control at a level of P ≤ 0.05.
Phytoestrogens found in soybeans, such as genistein, bind to ERβ with a 7- to 30-fold higher affinity, compared with ERα (29). These data suggest that DIM may act as an ERβ-selective agonist by binding to ERβ with a higher affinity than ERα. However, an ER binding assay found that DIM did not bind to ERα (Fig. 1C) or ERβ (Fig. 1D) at a concentration (15 μm) that leads to reporter activation in U2OS cells.
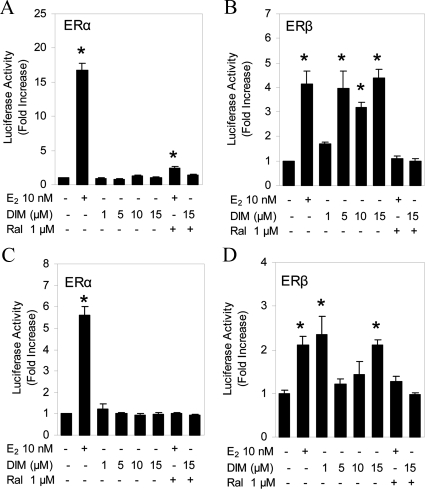
DIM did not induce ERE-tk-Luc activity with ERα in HeLa (Fig. 2A) or MDA-MB-231 (Fig. 2C) cells. Similar to U2OS cells, DIM activated ERE-tk-Luc with ERβ to the same magnitude as E2 in HeLa (Fig. 2B) and MDA-MB-231 (Fig. 2D) cells. The activation of the ERE by E2 and DIM was abolished by raloxifene in both cell types. Together, these studies demonstrate that DIM selectively activates ERβ in three different cell types.
Figure 2.
DIM is an ERβ-selective activator in HeLa and MDA-MB-231 cells. HeLa human cervical cells were cotransfected with ERE-tk-Luc and an expression vector for human ERα (A) or ERβ (B). MDA-MB-231 human breast cancer cells were cotransfected with ERE-tk-Luc and an expression vector for human ERα (C) or ERβ (D). The cells were treated for 18 h, and then Luc activity was measured. Each data point is the average of triplicate determinations ± sem. *, Significant difference between treatment and control at a level of P ≤ 0.05.
DIM selectively activates endogenous gene transcription through ERβ
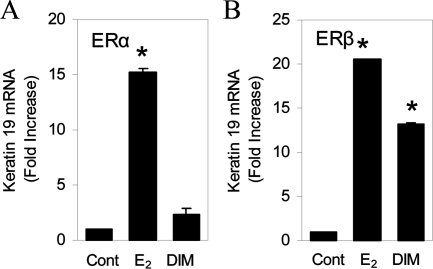
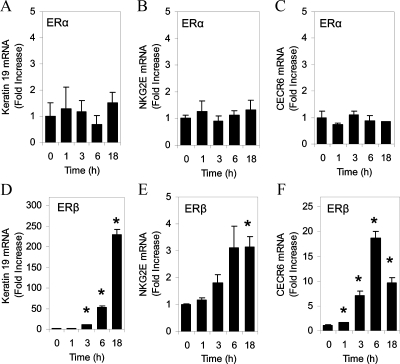
To determine whether the ER-subtype selectivity also is observed with endogenous genes, the effects of DIM were examined in U2OS cells stably transfected with a tetracycline-inducible ERα or ERβ using the keratin 19 gene, which is a known ER target gene (30). We have previously described the level of expression of ERβ in these cells (26). E2 activated the keratin 19 gene in both U2OS-ERα and U2OS-ERβ cells (Fig. 3, A and B), whereas DIM significantly increased keratin 19 mRNA levels only in the U2OS-ERβ cells (Fig. 3B). A study of the kinetics of the DIM-mediated activation of the keratin 19 gene revealed that treating U2OS-ERα cells for up to 18 h did not stimulate the K19, NKG2E, and CECR6 genes (Fig. 4, A–C), whereas treatment of U2OS-ERβ cells with DIM resulted in a time-dependent induction of keratin 19 expression, with a maximal induction of 230-fold at 18 h (Fig. 4D). DIM produced a time-dependent 3-fold induction of the NKG2E gene after 18 h treatment in U2OS-ERβ cells (Fig. 4E). DIM also activated the CECR6 gene in U2OS-ERβ cells (Fig. 4F). The induction of CECR6 gene peaked at 18-fold after 6 h DIM exposure and then decreased at 18 h.
Figure 3.
DIM selectively activates transcription of the endogenous keratin 19 gene. U2OS cells stably transfected with human ERα (A) or ERβ (B) were treated with 10 nm E2 or 15 μm DIM for 3 h. The level of mRNA was measured by real-time PCR. Each data point is the average of triplicate determinations ± sem. *, Significant difference between treatment and control at a level of P ≤ 0.05.
Figure 4.
DIM selectively activates transcription of the human keratin 19, NKG2E, and CECR6 genes. U2OS cells stably transfected with human ERα (A–C) or ERβ (D–F) were treated with 10 nm E2 or 15 μm DIM for 1, 2, 6, or 18 h. The level of mRNA was measured by real-time PCR. Each data point is the average of triplicate determinations ± sem. *, Significant difference between treatment and control at a level of P ≤ 0.05.
DIM causes the selective recruitment of ERβ and SRC-2 to target genes
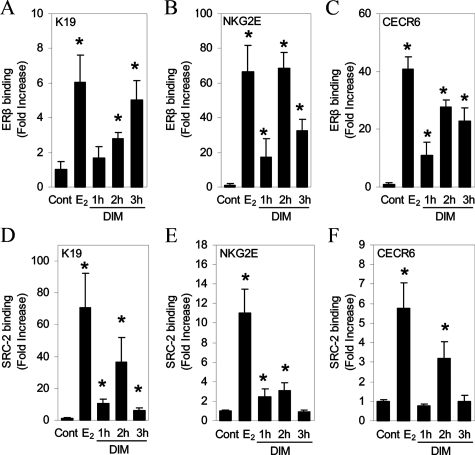
Our observations that DIM treatment selectively activates ERβ-responsive genes suggest that DIM might induce the recruitment of ERβ to these genes to regulate transcription. To investigate this possibility, we performed chromatin immunoprecipitation (ChIP) assays on the keratin 19 gene because E2 recruits ER to an ERE in U2OS-ERβ cells along with RNA polymerase II and coregulatory proteins (3,22,31). E2 treatment resulted in recruitment of ERβ to the keratin 19 gene. DIM treatment also resulted in recruitment of ERβ to the keratin 19 gene after 2 h treatment (Fig. 5A). ERβ recruitment to the keratin 19 gene was maintained for 3 h treatment. ChIP analysis of the NKG2E gene was also performed because ER is recruited to this gene in U2OS-ERβ cells (28). DIM treatment for 2 h resulted in maximal recruitment of ERβ to the NKG2E promoter in U2OS-ERβ cells to the same level as E2 (Fig. 5B). We also analyzed the CECR6 gene for recruitment of ERβ in U2OS-ERβ cells. Treatment of U2OS-ERβ cells with DIM resulted in recruitment of ERβ to the CECR6 gene at 1 h (Fig. 5C) with a maximal recruitment at 2 h that was maintained for 3 h.
Figure 5.
DIM recruits ERβ and SRC-2 to the keratin 19, NKG2E, and CECR6 genes. U2OS cells stably transfected with human ERβ were treated with 10 nm E2 or 15 μm DIM for 1, 2, or 3 h, and then ChIP was performed using antibodies to ERβ (A–C) or SRC-2 (D–F). Immunoprecipitated chromatin fragments were PCR amplified and quantitated by real-time PCR. Each data point is the average of triplicate determinations ± sem. *, Significant difference between treatment and control at a level of P ≤ 0.05.
Because the SRC-2 coactivator is recruited to ERβ target genes in U2OS cells after E2 treatment (28), we next sought to determine whether DIM also recruits SRC-2 to target genes. DIM induced recruitment of SRC-2 to the keratin 19 gene in U2OS-ERβ cells (Fig. 5D) at 1 h. A maximal 36-fold induction of SRC-2 recruitment occurred after 2 h DIM treatment, which was maintained after 3 h. SRC-2 was recruited to the NKG2E gene at 1 h treatment (Fig. 5E) and was maximally recruited at 2 h after treatment. DIM treatment led to a 3-fold induction of SRC-2 binding to the CECR6 gene after 2 h treatment (Fig. 5F). DIM did not recruit SRC-1 and SRC-3 coactivators to the K19, NKG2E, or CECR6 genes (data not shown). The kinetics of DIM-mediated recruitment of ERβ and SRC-2 to target gene promoters are consistent with previously reported results (3,32). These results demonstrate that DIM causes the selective recruitment of ERβ and the SRC-2 coactivator to target genes.
SRC-2 is necessary for the activation of ERβ target genes by DIM
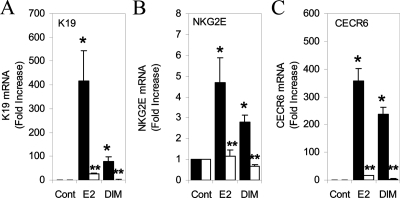
To further explore the functional role of SRC-2 in the transcriptional regulation of ERβ-responsive genes, we used RNA interference (RNAi) to silence SRC-2. The effectiveness of the silencing of SRC-2 in these stably transfected cells has been previously described (23). The effect of DIM on expression of the keratin 19, NKG2E, and CECR6 genes was examined in U2OS-ERβ cells stably expressing a scrambled or SRC-2 shRNA. DIM treatment resulted in an 80-fold expression of keratin 19 in scrambled shRNA-expressing cells. Silencing of SRC-2 reduced keratin 19 expression by DIM to 1.5-fold (Fig. 6A). NKG2E expression by DIM was reduced from 2.5-fold in scrambled cells to control levels in SRC-2 shRNA cells (Fig. 6B). Expression of the CECR6 gene was reduced from 160-fold to control levels after SRC-2 silencing (Fig. 6C). In all three cases, E2-mediated target gene expression was significantly reduced after SRC-2 gene silencing, consistent with previous data showing SRC-2 is recruited to these genes by E2 (28). These results demonstrate that silencing of SRC-2 results in a reduction of DIM-mediated activation of ERβ target genes.
Figure 6.
SRC-2 mediates transcription of the keratin 19, NKG2E, and CECR6 genes by DIM. U2OS cells stably transfected with human ERβ and a scrambled (black bars) or SRC-2 (white bars) shRNA were treated with 10 nm E2 or 15 μm DIM for 18 h. The level of keratin 19 (A), NKG2E (B), and CECR6 (C) mRNA was measured by real-time PCR. Each data point is the average of triplicate determinations ± sem. *, Significant difference compared with control at a level of P ≤ 0.05; **, Significant difference compared with U2OS cells stably transfected with a scrambled shRNA.
Discussion
DIM is the major in vivo product from indole-3-carbinol, which is found in cruciferous vegetables, such as broccoli, cauliflower, cabbage, and Brussels sprouts. DIM has been shown to have weak ligand-independent estrogenic activity (21) using reporter genes in MCF-7 cells. Here we have examined whether DIM has selective ER subtype activity.
We have demonstrated that DIM selectively triggers ERβ-mediated transcriptional pathways rather than ERα-mediated pathways. DIM activated ERE-tk-Luc and the keratin 19, NKG2E, and CECR6 genes in U2OS-ERβ cells but not in U2OS-ERα cells. Whereas the magnitude of regulation was equivalent to that of E2 for some endpoints, DIM was less potent. Nevertheless, the concentration of DIM needed for ERβ activation is within the range of levels achievable through dietary intake (33). These results indicate that DIM can trigger ERβ-mediated transcriptional pathways at physiological levels. We demonstrated that DIM does not bind to ERα or ERβ, indicating that the effects of DIM are mediated by unliganded ERβ. ChIP studies showed that DIM stimulates recruitment of ERβ to the keratin 19, NKG2E, and CECR6 genes. The recruitment of ERβ was associated with the recruitment of the SRC-2 to the keratin 19, NKG2E, and CECR6 genes. The kinetics of SRC-2 recruitment by DIM was consistent with previously observed SRC-2 recruitment after E2 treatment (28). Silencing of SRC-2 using RNAi inhibited the DIM-induced activation of the keratin 19, NKG2E, and CECR6 genes. These findings demonstrate that DIM activates the unliganded form of ERβ, which is capable of binding to ER regulatory elements leading to recruitment of SRC-2 and transcription of target genes.
The activation of ERβ by DIM may be linked to the DIM-mediated activation of kinases (21,34), including MAPK, which is known to phosphorylate ER (35). Other potential targets of DIM are the coactivators. DIM treatment led to recruitment of SRC-2 to ER target genes. Silencing of SRC-2 using RNAi prevented transcription of ER target genes by DIM, revealing SRC-2 as a key target in the DIM effect. Interestingly, SRC-2 is phosphorylated by p38 MAPK, which is activated by DIM (36). Tiling arrays and ChIP sequencing methods have found that some ER regulatory sites require transcription factors such as FoxA1 and HSF-2 for transcriptional activation (25,37). Even though DIM did not bind to ERβ, ChIP studies revealed that ERβ bound to ERE present in the keratin 19, NKG2E, and CECR6 genes, resulting in recruitment of SRC-2 and subsequent transcription of these genes. DIM treatment did not activate the keratin 19, NKG2E, or CECR6 genes in the presence of ERα. Future studies are required to determine whether DIM activates ERβ, coactivators, or transcription factors by posttranslational modifications, resulting in ERβ-mediated transcription.
Most of the attention has been directed at developing ERβ agonists that bind selectively to ERβ. Several ERβ-selective compounds, including ERB-041 (38) and diarylpropionitrile (39) have been identified by their selective binding to ERβ. We previously showed that MF101 (22) and liquiritigenin (32) bind similarly to ERα and ERβ, but they activate only ERβ transcriptional pathways. Here we show that DIM represents a third class of ERβ-selective compounds that activate transcription in a ligand-independent fashion.
Footnotes
This work was supported by Grants CA69056 and CA102360 from the National Institutes of Health.
Disclosure Summary: O.I.V., E.F.S., G.L.F., and L.F.B. have nothing to declare. D.C.L. has received financial support for research from Bionovo, Inc., a biotechnology company with commercial interests in estrogen receptors.
First Published Online February 16, 2010
Abbreviations: ChIP, Chromatin immunoprecipitation; DIM, 3,3′-diindolylmethane; E2, estradiol; ER, estrogen receptor; ERE, estrogen response element; I3C, indole-3-carbinol; Luc, luciferase; RNAi, RNA interference; shRNA, short hairpin RNA; SRC-2, steroid receptor coactivator 2; tk, thymidine kinase.
References
- Chambon P 2005 The nuclear receptor superfamily: a personal retrospect on the first two decades. Mol Endocrinol 19:1418–1428 [DOI] [PubMed] [Google Scholar]
- Koehler KF, Helguero LA, Haldosén LA, Warner M, Gustafsson JA 2005 Reflections on the discovery and significance of estrogen receptor β. Endocr Rev 26:465–478 [DOI] [PubMed] [Google Scholar]
- Kian Tee M, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC 2004 Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors α and β. Mol Biol Cell 15:1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Harrell JC, Korach KS 2005 Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol 67:285–308 [DOI] [PubMed] [Google Scholar]
- Harris HA 2007 Estrogen receptor-β: recent lessons from in vivo studies. Mol Endocrinol 21:1–13 [DOI] [PubMed] [Google Scholar]
- Förster C, Mäkela S, Wärri A, Kietz S, Becker D, Hultenby K, Warner M, Gustafsson JA 2002 Involvement of estrogen receptor β in terminal differentiation of mammary gland epithelium. Proc Natl Acad Sci USA 99:15578–15583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim GJ, Wang L, Andersson S, Nagy N, Kis LL, Zhang Q, Mäkelä S, Warner M, Gustafsson JA 2003 Disruption of the estrogen receptor β gene in mice causes myeloproliferative disease resembling chronic myeloid leukemia with lymphoid blast crisis. Proc Natl Acad Sci USA 100:6694–6699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson JA, Rissman EF 2005 Lack of functional estrogen receptor β influences anxiety behavior and serotonin content in female mice. Physiol Behav 84:157–163 [DOI] [PubMed] [Google Scholar]
- Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA 2002 Disruption of estrogen receptor β gene impairs spatial learning in female mice. Proc Natl Acad Sci USA 99:3996–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ 2005 17 β-Estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-β knockout (BERKO) mice. Psychopharmacology (Berl) 179:637–643 [DOI] [PubMed] [Google Scholar]
- Grose KR, Bjeldanes LF 1992 Oligomerization of indole-3-carbinol in aqueous acid. Chem Res Toxicol 5:188–193 [DOI] [PubMed] [Google Scholar]
- Staub RE, Feng C, Onisko B, Bailey GS, Firestone GL, Bjeldanes LF 2002 Fate of indole-3-carbinol in cultured human breast tumor cells. Chem Res Toxicol 15:101–109 [DOI] [PubMed] [Google Scholar]
- Auborn KJ 2002 Therapy for recurrent respiratory papillomatosis. Antivir Ther 7:1–9 [PubMed] [Google Scholar]
- Wiatrak BJ 2003 Overview of recurrent respiratory papillomatosis. Curr Opin Otolaryngol Head Neck Surg 11:433–441 [DOI] [PubMed] [Google Scholar]
- Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF 2004 Pilot study: effect of 3,3′-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer 50:161–167 [DOI] [PubMed] [Google Scholar]
- Kabat GC, O'Leary ES, Gammon MD, Sepkovic DW, Teitelbaum SL, Britton JA, Terry MB, Neugut AI, Bradlow HL 2006 Estrogen metabolism and breast cancer. Epidemiology 17:80–88 [DOI] [PubMed] [Google Scholar]
- Chang X, Tou JC, Hong C, Kim HA, Riby JE, Firestone GL, Bjeldanes LF 2005 3,3′-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis 26:771–778 [DOI] [PubMed] [Google Scholar]
- Hong C, Kim HA, Firestone GL, Bjeldanes LF 2002 3,3′-Diindolylmethane (DIM) induces a G1 cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of p21(WAF1/CIP1) expression. Carcinogenesis 23:1297–1305 [DOI] [PubMed] [Google Scholar]
- Vivar OI, Lin CL, Firestone GL, Bjeldanes LF 2009 3,3′-Diindolylmethane induces a G1 arrest in human prostate cancer cells irrespective of androgen receptor and p53 status. Biochem Pharmacol 78:469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong H, Riby JE, Firestone GL, Bjeldanes LF 2004 Potent ligand-independent estrogen receptor activation by 3,3′-diindolylmethane is mediated by cross talk between the protein kinase A and mitogen-activated protein kinase signaling pathways. Mol Endocrinol 18:291–302 [DOI] [PubMed] [Google Scholar]
- Cvoro A, Paruthiyil S, Jones JO, Tzagarakis-Foster C, Clegg NJ, Tatomer D, Medina RT, Tagliaferri M, Schaufele F, Scanlan TS, Diamond MI, Cohen I, Leitman DC 2007 Selective activation of estrogen receptor-β transcriptional pathways by an herbal extract. Endocrinology 148:538–547 [DOI] [PubMed] [Google Scholar]
- Cvoro A, Tatomer D, Tee MK, Zogovic T, Harris HA, Leitman DC 2008 Selective estrogen receptor-β agonists repress transcription of proinflammatory genes. J Immunol 180:630–636 [DOI] [PubMed] [Google Scholar]
- An J, Tzagarakis-Foster C, Scharschmidt TC, Lomri N, Leitman DC 2001 Estrogen receptor β-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J Biol Chem 276:17808–17814 [DOI] [PubMed] [Google Scholar]
- Levy N, Zhao X, Tang H, Jaffe RB, Speed TP, Leitman DC 2007 Multiple transcription factor elements collaborate with estrogen receptor α to activate an inducible estrogen response element in the NKG2E gene. Endocrinology 148:3449–3458 [DOI] [PubMed] [Google Scholar]
- Levy N, Paruthiyil S, Zhao X, Vivar OI, Saunier EF, Griffin C, Tagliaferri M, Cohen I, Speed TP, Leitman DC 8 September 2009 Unliganded estrogen receptor-β regulation of genes is inhibited by tamoxifen. Mol Cell Endocrinol 10.1016/j.mce.2009.08.030 [DOI] [PubMed] [Google Scholar]
- Burakov D, Crofts LA, Chang CP, Freedman LP 2002 Reciprocal recruitment of DRIP/mediator and p160 coactivator complexes in vivo by estrogen receptor. J Biol Chem 277:14359–14362 [DOI] [PubMed] [Google Scholar]
- Levy N, Tatomer D, Herber CB, Zhao X, Tang H, Sargeant T, Ball LJ, Summers J, Speed TP, Leitman DC 2008 Differential regulation of native estrogen receptor-regulatory elements by estradiol, tamoxifen, and raloxifene. Mol Endocrinol 22:287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S 1998 Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonists/antagonists. Mol Pharmacol 54:105–112 [DOI] [PubMed] [Google Scholar]
- Choi I, Gudas LJ, Katzenellenbogen BS 2000 Regulation of keratin 19 gene expression by estrogen in human breast cancer cells and identification of the estrogen responsive gene region. Mol Cell Endocrinol 164:225–237 [DOI] [PubMed] [Google Scholar]
- Cvoro A, Tzagarakis-Foster C, Tatomer D, Paruthiyil S, Fox MS, Leitman DC 2006 Distinct roles of unliganded and liganded estrogen receptors in transcriptional repression. Mol Cell 21:555–564 [DOI] [PubMed] [Google Scholar]
- Mersereau JE, Levy N, Staub RE, Baggett S, Zogovic T, Chow S, Ricke WA, Tagliaferri M, Cohen I, Bjeldanes LF, Leitman DC 2008 Liquiritigenin is a plant-derived highly selective estrogen receptor β agonist. Mol Cell Endocrinol 283:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HT, Schaldach CM, Firestone GL, Bjeldanes LF 2003 Plant-derived 3,3′-diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J Biol Chem 278:21136–21145 [DOI] [PubMed] [Google Scholar]
- Xue L, Firestone GL, Bjeldanes LF 2005 DIM stimulates IFNγ gene expression in human breast cancer cells via the specific activation of JNK and p38 pathways. Oncogene 24:2343–2353 [DOI] [PubMed] [Google Scholar]
- Zhao C, Dahlman-Wright K, Gustafsson JA 2008 Estrogen receptor β: an overview and update. Nucl Recept Signal 6:e003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigo DE, Basu A, Nierth-Simpson EN, Weldon CB, Dugan CM, Elliott S, Collins-Burow BM, Salvo VA, Zhu Y, Melnik LI, Lopez GN, Kushner PJ, Curiel TJ, Rowan BG, McLachlan JA, Burow ME 2006 p38 mitogen-activated protein kinase stimulates estrogen-mediated transcription and proliferation through the phosphorylation and potentiation of the p160 coactivator glucocorticoid receptor-interacting protein 1. Mol Endocrinol 20:971–983 [DOI] [PubMed] [Google Scholar]
- Krum SA, Miranda-Carboni GA, Lupien M, Eeckhoute J, Carroll JS, Brown M 2008 Unique ERα cistromes control cell type-specific gene regulation. Mol Endocrinol 22:2393–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, Frail DE, Henderson RA, Zhu Y, Keith Jr JC 2003 Evaluation of an estrogen receptor-β agonist in animal models of human disease. Endocrinology 144:4241–4249 [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA 2001 Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 44:4230–4251 [DOI] [PubMed] [Google Scholar]