Abstract
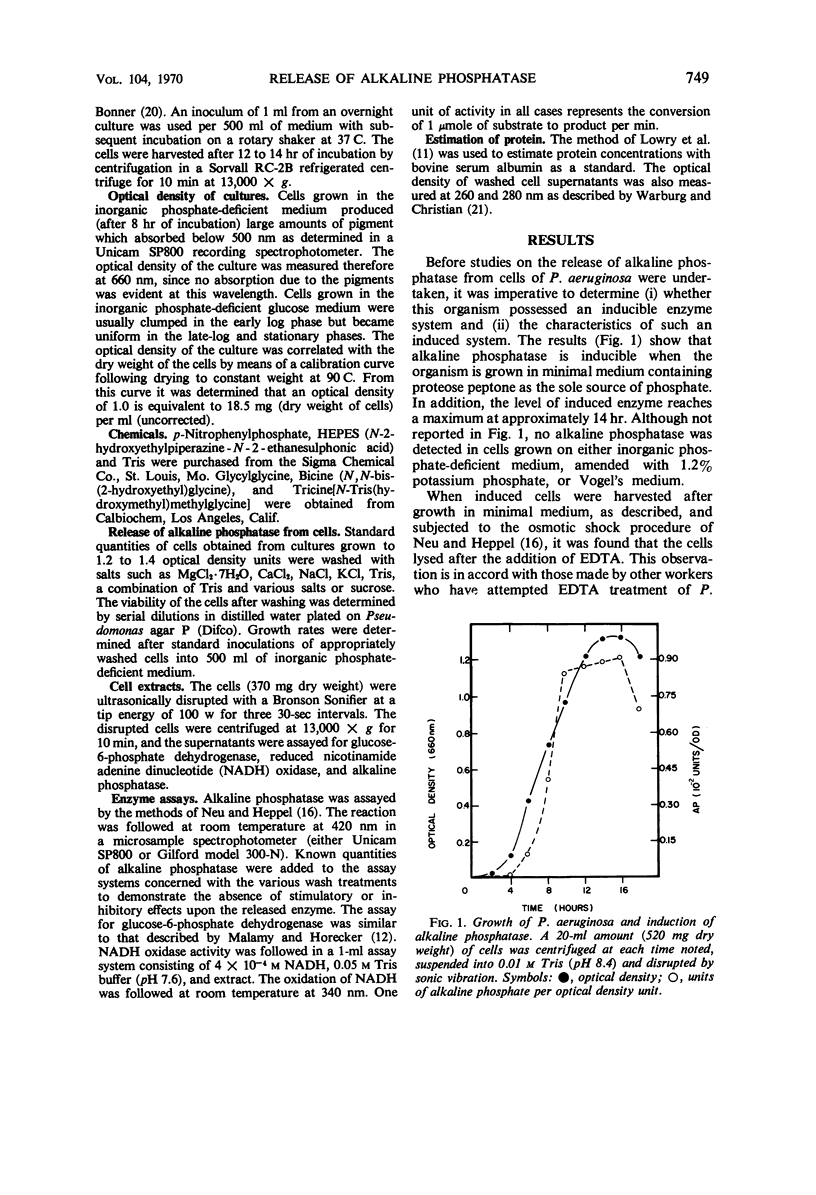
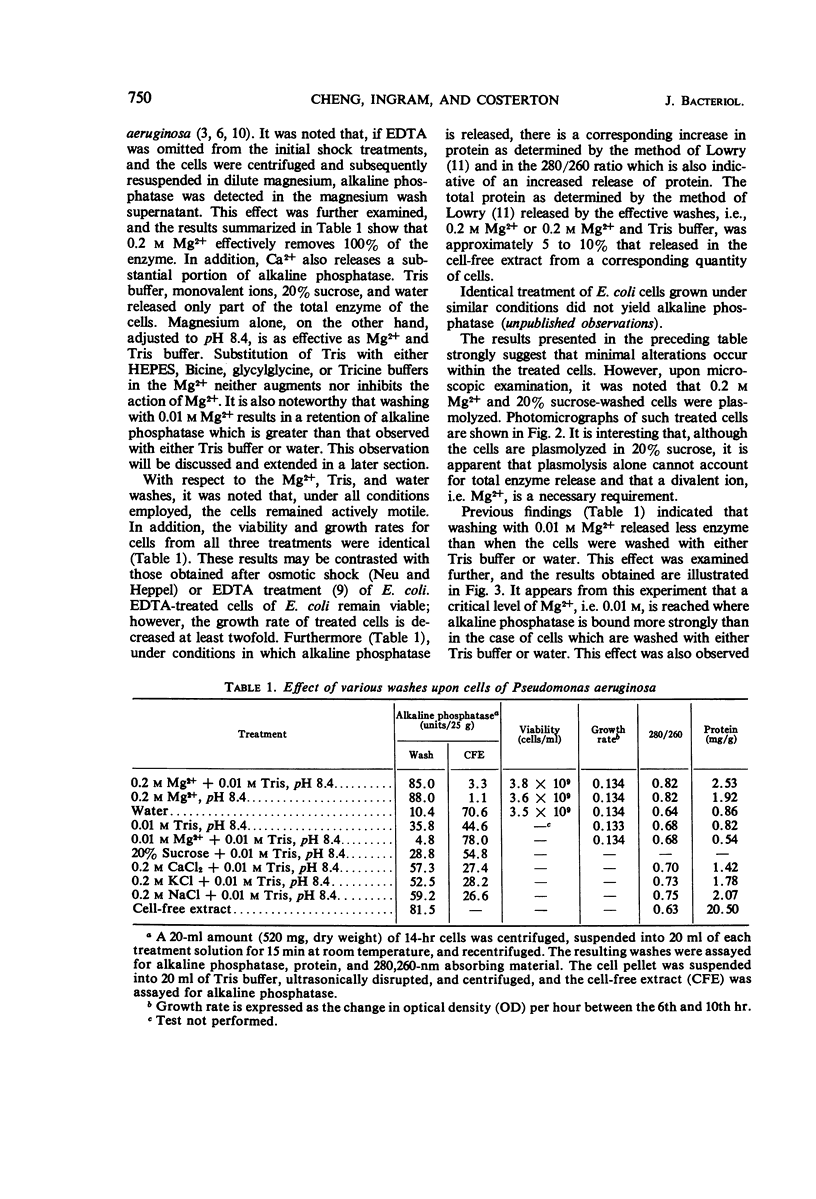
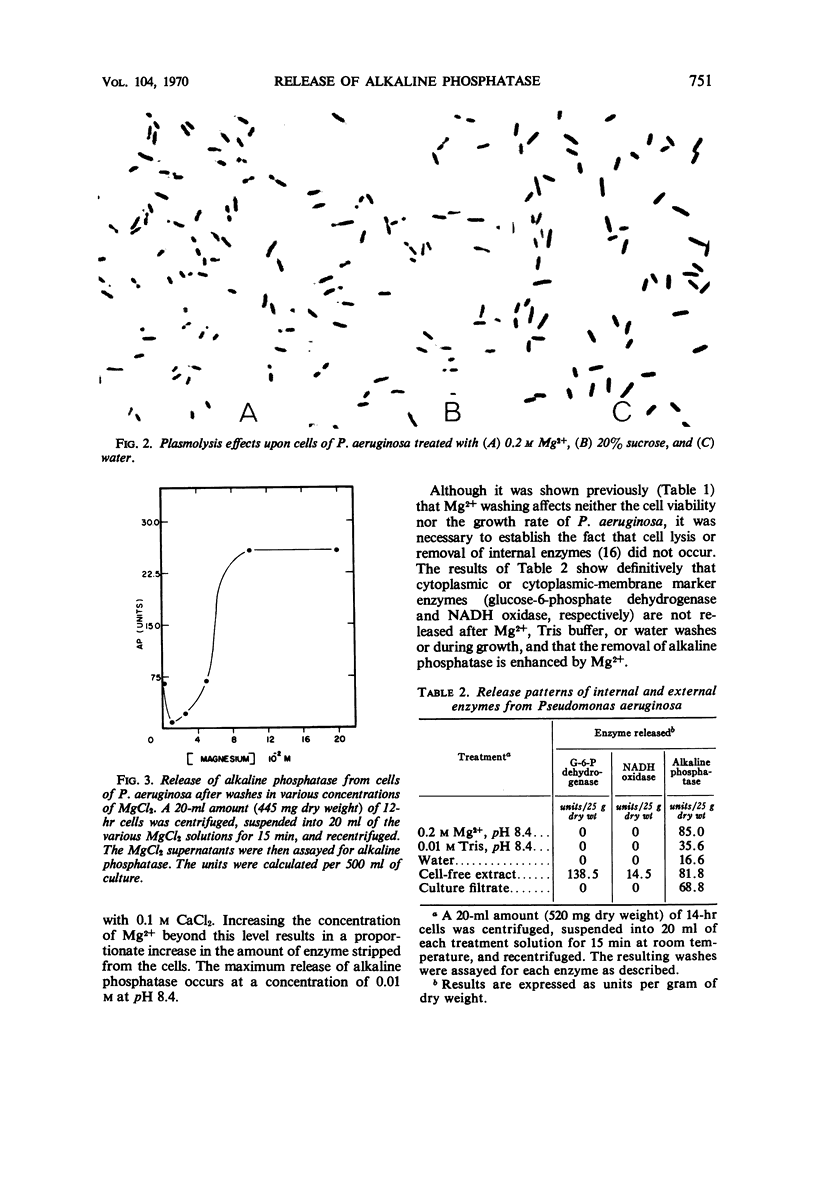
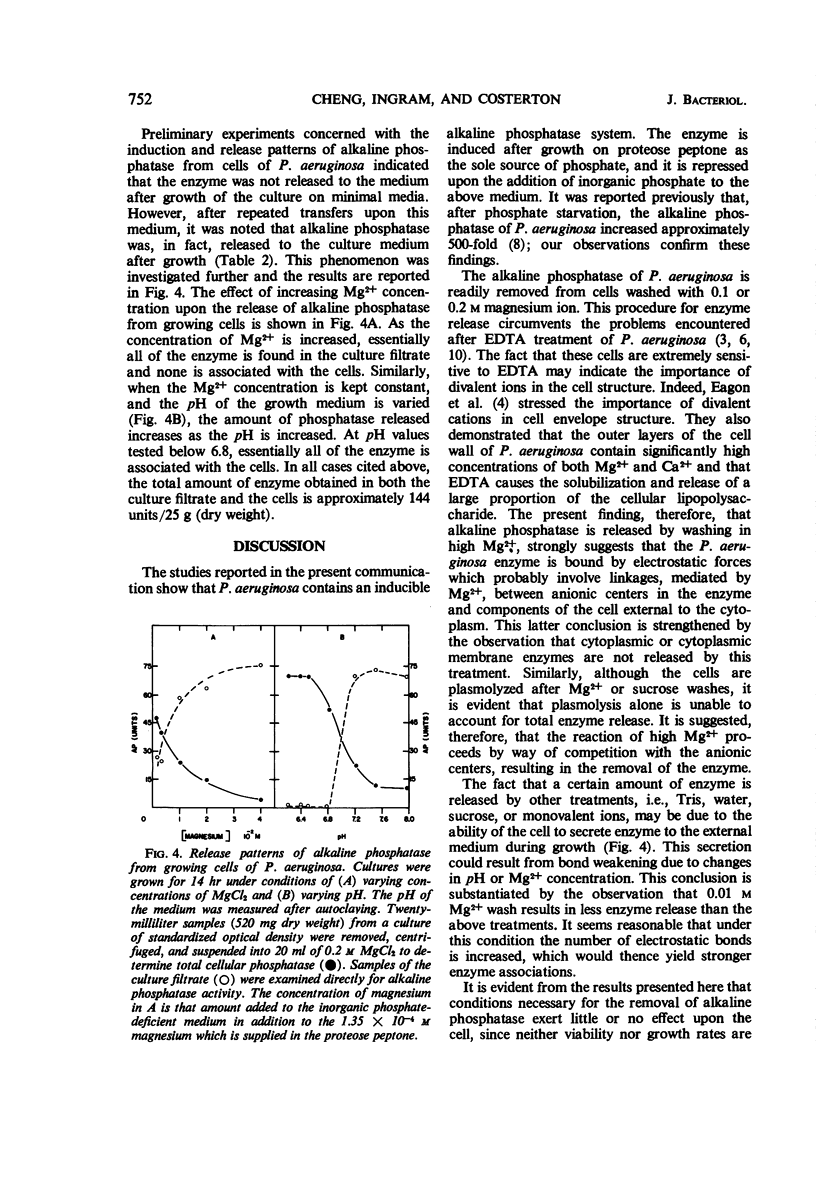
Pseudomonas aeruginosa ATCC 9027 contains an inducible alkaline phosphatase. The enzyme is readily removed from 14-hr cells by washes in 0.2 m MgCl2, pH 8.4. Similar washes in tris(hydroxymethyl)aminomethane buffer, 20% sucrose, monovalent ions, or water partially release enzyme from the cells. The release of alkaline phosphatase is correlated with an increased release of protein and retention of internal enzymes. The effect of 0.2 m MgCl2 washing upon the cells is minimal since both viability and growth rates remain unchanged as compared to water washing. Although cells are plasmolyzed in both 0.2 m MgCl2 and 20% sucrose, it is evident that plasmolysis alone is unable to account for total enzyme release and that a divalent metal, i.e. Mg2+, augments the release pattern. Growing cells in the presence of increasing concentrations of MgCl2 or at increased pH values results in an almost total secretion of the enzyme to the culture filtrate. The findings suggest that P. aeruginosa alkaline phosphatase is linked to the exocytoplasmic region through divalent metal ion, presumably Mg2+, bridges.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockman R. W., Heppel L. A. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry. 1968 Jul;7(7):2554–2562. doi: 10.1021/bi00847a016. [DOI] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Production of L-asparaginase II by Escherichia coli. J Bacteriol. 1968 Dec;96(6):2043–2048. doi: 10.1128/jb.96.6.2043-2048.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGON R. G., CARSON K. J. LYSIS OF CELL WALLS AND INTACT CELLS OF PSEUDOMONAS AERUGINOSA BY ETHYLENEDIAMINE TETRAACETIC ACID AND BY LYSOZYME. Can J Microbiol. 1965 Apr;11:193–201. doi: 10.1139/m65-025. [DOI] [PubMed] [Google Scholar]
- Eagon R. G., Simmons G. P., Carson K. J. Evidence for the presence of ash and fivalent metals in the cell wall of Pseudomonas aeruginosa. Can J Microbiol. 1965 Dec;11(6):1041–1042. doi: 10.1139/m65-144. [DOI] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Hou C. I., Gronlund A. F., Campbell J. J. Influence of phosphate starvation on cultures of Pseudomonas aeruginosa. J Bacteriol. 1966 Oct;92(4):851–855. doi: 10.1128/jb.92.4.851-855.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- MALLETTE M. F., COWAN C. I., CAMPBELL J. J. GROWTH AND SURVIVAL OF ESCHERICHIA COLI IN MEDIUM LIMITED IN PHOSPHATE. J Bacteriol. 1964 Apr;87:779–785. doi: 10.1128/jb.87.4.779-785.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D. Selection of sucrose-dependent Escherichia coli to obtain envelope mutants and fragile cultures. Science. 1966 Aug 19;153(3738):892–894. doi: 10.1126/science.153.3738.892. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chou J. Release of surface enzymes in Enterobacteriaceae by osmotic shock. J Bacteriol. 1967 Dec;94(6):1934–1945. doi: 10.1128/jb.94.6.1934-1945.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Rogers D. Osmotic pools in Escherichia coli. Science. 1968 Feb 2;159(3814):531–532. doi: 10.1126/science.159.3814.531. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Olsen R. Expression and localization of Escherichia coli alkaline phosphatase synthesized in Salmonella typhimurium cytoplasm. J Bacteriol. 1968 Nov;96(5):1601–1605. doi: 10.1128/jb.96.5.1601-1605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Voelz H., Ortigoza R. O. Cytochemistry of phosphatases in Myxococcus xanthus. J Bacteriol. 1968 Oct;96(4):1357–1365. doi: 10.1128/jb.96.4.1357-1365.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B., Glaser L. An E. coli mutant with cryptic UDP-sugar hydrolase and altered metabolite regulation. Biochem Biophys Res Commun. 1968 Jun 10;31(5):671–677. doi: 10.1016/0006-291x(68)90614-1. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G. The sensitivity of pseudomonads to ethylenediaminetetra-acetic acid. J Gen Microbiol. 1967 Apr;47(1):67–76. doi: 10.1099/00221287-47-1-67. [DOI] [PubMed] [Google Scholar]



