Summary
Plaque rupture underlies most myocardial infarctions. Plaques vulnerable to rupture have thin fibrous caps, an excess of macrophages over vascular smooth muscle cells, large lipid cores, and depletion of collagen and other matrix proteins form the cap and lipid core. Production of matrix metalloproteinases from macrophages is prominent in human plaques, and studies in genetically modified mice imply a causative role for metalloproteinases in plaque vulnerability. Recent in-vitro studies on human monocyte-derived macrophages and on foam-cell macrophages generated in vivo suggest the existence of several macrophage phenotypes with distinct patterns of metalloproteinase expression. These phenotypes could play differing roles in cap, core and aneurysm formation.
Keywords: Atherosclerosis, atherothrombosis, extracellular matrix, inflammation, matrix metalloproteinases
Vulnerable plaque
There are more than 200,000 cases of myocardial infarction (MI) in the UK alone each year, and almost half this number of patients dies (http//:www.heartsats.org). MI results from coronary thrombosis at the site of an atherosclerotic plaque caused either by detachment of a large patch of surface endothelial cells or, more often, from rupture of the plaque cap. The characteristic features of ruptured plaques are: a thin fibrous cap; a higher ratio of macrophages to vascular smooth muscle cells (VSMCs) in the cap; less collagen, the main strength-giving component, in the cap; and a large, lipid-rich, collagen-poor necrotic core (1). The implication is that production of extracellular proteases from inflammatory cells degrades collagen and leads to mechanical weakness of the cap, particularly in the shoulder regions, which suffer high strain when the core is large and flexible (2). Intact plaques with the features of ruptured plaques listed above are believed vulnerable to subsequent rupture (vulnerable plaques), and patients with such plaques are thought vulnerable to MI (vulnerable patients). If so, pharmacological agents that reverse the features of vulnerable plaque will prevent MIs. So far this concept appears to hold true at least for statin drugs, which reduce the incidence of MI in clinical trials (3) and have biological effects such as inhibiting macrophage activation and protease production. Statins also reduce systemic and local plaque inflammation (4), the size of the lipid-rich necrotic core measured by magnetic resonance imaging (5), and the frequency of high-strain regions of the plaque cap measured by palpography in man (6). The present hope is to identify additional, maybe even more effective treatments to reverse plaque vulnerability and thereby prevent MIs.
Role of matrix metalloproteinases (MMPs) in plaque vulnerability
Loss of collagen and other extracellular matrix (ECM) components occurs in the highly-inflamed regions of plaque cap thereby reducing tensile strength; it also occurs in the lipid core, which promotes transfer of hydrodynamic forces during the cardiac cycle to the high-strain, shoulder regions of the plaque. For this reason much work has concerned the production of extracellular proteases, which occurs from all plaque-resident cells. However, amongst these, foam cell macrophages seem to be the predominant source. Serine proteinases, cysteinyl cathepsins, and MMPs are all present in plaques, and act in concert. However, MMPs have attracted most attention because they directly degrade ECM components and are efficient at neutral pH. The MMPs are a family of 23 genetically-related proteins that share several common properties including: a Zn2+-containing catalytic site; production as pro-forms that require proteolytic activation either inside or outside the cell; and inhibition by one or more of the four tissue inhibitors of metalloproteinases (TIMPs) (7). Most MMPs are secreted as pro-proteins and then become activated proteolytically by other MMPs, serine or cysteine proteinases (7); chemical activation by reactive oxygen species and nitric oxide is also possible (8). Alternatively the membrane-type MMPs and a few other MMPs are activated by furins in the endosome; the MT-MMPs remain attached to the outer plasma membrane surface. MMPs degrade collagens, elastins, the core proteins of proteoglycans, and ECM glycoproteins such as fibronectin and laminin. However, only MMPs-1, -2, -8, -13 and -14 actively degrade intact fibrillar collagens and may therefore have a special role in weakening plaques. Destruction of elastin, especially by MMPs-9 and -12, appears to have a role in outward remodelling and aneurysm formation (9). MMPs also cleave many cell surface molecules and extracellular regulators (7). They therefore influence not only matrix remodelling but the migration, proliferation and death of vascular cells (10). Death of macrophages leads to lipid core formation. Death of vascular smooth muscle cells (VSMCs) removes the main source of collagen synthesis which prevents vascular repair. Since VSMC are so important for plaque stability (11), MMPs and other extracellular proteases can have plaque stabilising effects by promoting migration and proliferation of VSMCs. For example, we and others showed using low-molecular-weight inhibitors and adenovirus-mediated TIMP gene transfer that MMPs are needed for smooth muscle cell migration and intima formation. Other studies used knockouts of MMP-2, MMP-9 and MMP-14 to directly implicate these MMPs in intima formation in mice (10).
Over the recent years gene transfer, transgenic and knockout studies of MMPs and TIMPs have been conducted in mouse and rabbit models of atherosclerosis (see Table 1). In these studies, criteria of plaque vulnerability borrowed from human pathology included: increased plaque size; fewer VSMC; more macrophages; and decreased collagen content. Additional markers of instability included disruption of elastic fibres in the media at the base of plaques, intraplaque haemorrhage and the occurrence of buried fibrous caps, which may be the signature of silent plaque ruptures, as in man (12).
Table 1. Effects of MMP interventions in atherosclerotic plaque development and stability.
Results of overexpression (++) overexpression of activated enzymes (++a) and knockout (null) included increases (↑), decreases (↓) or no change (=) in the specified parameters. Missing or uncertain data is indicated as (?). Abbreviations: Ao = aorta, arch = aortic arch, BCA = brachiocephalic artery, root = aortic root.
| Number | Site | Size | SMC | Macrophages | ECM integrity |
Overall stability |
References |
|---|---|---|---|---|---|---|---|
| MMP-1 ++ | Root/arch | ↓ | = | = | ↓ | = | (18) |
| MMP-2 null | Root/arch | ↓ | ↓ | = | = | ↓ | (35) |
| MMP-3 null | Ao, BCA | ↑, ↑ | ?, ↓ | ↓, = | ↑, ? | ↑, ↓ | (21), (22) |
| MMP-7 null | BCA | = | ↑ | = | ? | = | (22) |
| MMP-9 null | Ao, BCA | ↓, ↑ | ?, ↓ | ↓, ↑ | ↑, = | ↑, ↓ | (23), (22) |
| MMP-9 ++ | Arch, BCA | =, = | =, = | =, = | =, = | =, ↓ | (19), (20) |
| MMP-9 ++a | Arch, BCA | = | = | ↓ | (19) | ||
| MMP-12 null | Ao, BCA | =, ↓ | =, ↑ | =, ↓ | =, ? | ↑, ↑ | (23), (22) |
| MMP-12 ++a | Ao | ↑ | ↑ | ↑ | ? | ? | (36) |
| MMP-13 null | Root | = | = | = | ↑ | ↑ | (37) |
| MMP-14 null | Root | = | = | = | ↑ | ↑ | (38) |
| TIMP-1 null | Root, Aorta | =, ↓ | =, ? | =, ↓ | ↓, ↓ | ↓, ↓ | (13), (14) |
| TIMP-1 ++ | Root, BCA | ↓, = | ?, = | ↓, = | ↑, ↑ | ↑, = | (16), (15) |
| TIMP-2 ++ | BCA | ↓ | ↑ | ↓ | ↑ | ↑ | (15) |
TIMP-1 knockout caused disruption of medial elastic layers in two studies (13, 14) (Table 1), which was associated in one study with increased macrophage content (13, 14). Conversely, systemic adenovirus-mediated gene transfer of TIMP-1 or TIMP-2 improved plaque stability (15, 16). Moreover, prolonged overexpression of TIMP-2, but not TIMP-1, prevented progression of established brachiocephalic artery plaques (15). The beneficial effects of TIMP-2 were associated with decreased macrophage invasion and apoptosis, suggesting that at least one MT-MMP promotes plaque instability, since TIMP-2 but not TIMP-1 inhibits several MT-MMPs. Interestingly, TIMPs appear to be more effective than low molecular weight MMP inhibitors in these models (17) for reasons that we do not yet understand. Perhaps systemically expressed TIMPs have preferential access to the vasculature.
Mice do not actively express MMP-1 but mice transgenic for human MMP-1 in macrophages were found to have smaller plaques with less collagen (18) (Table 1). Over-expressing a native pro-form of MMP-9 in macrophages did not affect plaque stability in ApoE null mice (19). However, over-expressing a mutated form of MMP-9 that becomes fully auto-activated produced high levels of plaque instability (19). Adenovirus-mediated over-expression of MMP-9 produced instability of advanced plaques caused by collar implantation into ApoE null mice (20). These studies illustrate an important principle: both the nature of MMPs expressed and the level of activity are crucial in determining whether controlled remodelling or matrix destruction and hence instability occurs.
When MMP knockouts were studied, both protective and deleterious effects were documented (Table 1). MMP-2 knockout reduced VSMC accumulation in plaques, suggesting less plaque stability. MMP-3 knockout increased plaque size in two studies. Macrophage content and elastin breaks were decreased in one study (21), implying greater stability, but smooth muscle cell content was decreased and buried fibrous layers increased in the second, implying less stability (22). Since MMP-3 potentially increases migration of both macrophages and smooth muscle cells through the ECM, the net effect on plaque composition could vary depending on the experimental conditions. MMP-7 deletion had little effect except to increase VSMC content in one study (22). MMP-9 knockout reduced plaque size, macrophage content and elastin breaks in one study (23), but increased plaque size, macrophage content and buried fibrous layers in another (22), which led to opposite conclusions on plaque stability. These ambiguous findings (Table 1) might be explained by experimental variables such as the diet, timing or site of plaques chosen for study influencing the levels of active MMP achieved. MMP-12 knockout (22, 23) decreased both elastin breaks (23) and buried fibrous layers (22), while active MMP-12 over-expression in rabbits increased plaque size and inflammation. Hence there is a consensus that MMP-12 impairs plaque stability. Knockout of the collagenases, MMP-13 and -14, had little effect on macrophage or VSMC content but increased collagen in plaques, which implies greater stability. Taken together, the results are consistent with our thinking that some MMPs (particularly normal levels of MMP-2 and -9) promote plaque stability by promoting VSMC migration and cap formation. Others, including MMP-12, -13, and -14 and high levels of activated MMP-9 cause matrix destruction or increase plaque inflammation, which are associated with plaque rupture.
Macrophage phenotypes in atherosclerosis
Apart from scavenging cholesterol to form foam cells, plaque macrophages participate in an arterial immune-inflammatory reaction most likely initiated by oxidised phospholipids and cholesterol derivatives derived from low-density lipoprotein (LDL) (24). Enzymic and oxidative modification of LDL triggers both innate and acquired immunity (24). Among the lymphocytes, CD4+ T-helper (Th) cells predominate in plaques. Initially present in a Th0 ground state, Th cells can differentiate to Th1 cells, which secrete and respond to interferon (IFN-γ) or to Th2 cells, which secrete and respond to interleukin (IL)-4. Interestingly, IFN-γ blocks Th2 differentiation while IL-4 blocks Th1 differentiation so there is a tendency for the immune response to become polarised. Th1 cells activate macrophages to produce MHC antigens and a host of pro-inflammatory products, while Th2 cells tend to calm inflammation, but promote humoral immunity and allergy. Not surprisingly, therefore, IFN-γ promotes atherosclerosis formation (25). The role of IL-4 is more ambiguous; approximately equal numbers of studies show IL-4 increases or decreases atherosclerosis (25). Other cytokines, particularly IL-10 and transforming growth factor (TGF)-β have consistently anti-inflammatory roles. They are preferentially produced by T regulatory (Treg) lymphocytes (25). These and other T-cell subtypes maintain stable properties in culture and when removed and then re-injected into other animals and are therefore regarded as a lineage of differentiated cells.
Responses to T-cells are not mediated directly but rather through their influence on macrophages. Macrophages are derived from blood monocytes, which were recently shown to exist predominantly in two different lineages identifiable by different expression patterns of cell-surface markers including Ly6C (function presently unknown) and P-selectin in mice (26), and CD16 and P-selectin in humans (27). Ly6Chigh monocytes appear more inflammatory, accumulate in the circulation in atherosclerosis-prone mice, and are recruited preferentially into inflammatory foci, including atherosclerotic plaques (27). Adherence of monocytes to the endothelium or ECM up-regulates secretion of a wide range of inflammatory mediators, and also MMPs, which presumably help monocytes migrate into sites of inflammation. Prostaglandin production appears to be an important trigger for these events (28). Monocytes subsequently differentiate to macrophages under the influence of growth factors, particularly M-CSF (see Fig. 1). Most inflammatory macrophages, including foam cell macrophages (FCM), probably derive from the Ly6Chigh monocyte population, although this remains a hypothesis (26). During maturation, macrophages become ‘primed’ to respond to pro-inflammatory cytokines, acutely through transcription factor pathways that include most prominently nuclear factor (NF)-κB. They can also become loaded with modified LDL to become FCM, which, in plaques, contain active, nuclear localised NF-κB (29). Other cytokines including IFN-γ and IL-4 activate distinct signalling cascades, and appear to bring about additional changes in cell phenotype. M1 cells generated in response to IFN-γ have the ‘classic’ pro-inflammatory nature, while so-called M2a cells produced in response to IL-4 have an ‘alternative’ activation state (Fig. 1) (30). FCM carrying M1 or M2 markers have recently been identified in mouse atherosclerotic plaques (31). Other M2 types include M2b resident macrophages (e.g. Kupfer cells), that may derive selectively from Ly6Clow monocytes (26), and M2c macrophages that have responded to anti-inflammatory cytokines such as IL-10 (30). Whether these macrophages subtypes represent a lineage of differentiated cells or simply different phenotypes that can interconvert is currently much less well-defined than for T-cells and monocytes.
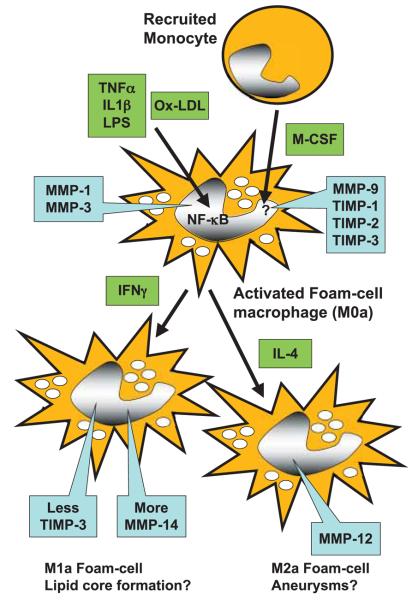
Figure 1. A working model for regulation of MMP expression by different macrophages phenotypes.
Recruited monocytes up-regulate MMP-9 and TIMPs1–3 during differentiation into macrophages in response to M-CSF. MMP-1 and -3 are up-regulated by inflammatory mediators or oxidised-LDL through NF-κB. Further differentiation to M1 cells in response to IFN-γ up-regulates MMP-14 and down-regulates TIMP-3. Differentiation to M2 cells up-regulates MMP-12.
MMP production in macrophages and foam cells
Regulation of MMP production from macrophages has been widely studied in vitro (28). As summarised in Table 2, positive mediators include pro-inflammatory cytokines, tumour necrosis factor (TNF)-α and IL-1β, ligands for Toll-like receptors (TLRs) and engagement of the CD40/CD40L complex. Interestingly, however, the pro-inflammatory Th1 cytokine, IFN-γ, inhibits expression of most MMPs. The Th2 cytokine, IL-4, also inhibits secretion of most MMPs but increases expression of MMP-12. Anti-inflammatory cytokines, IL-10 and TGF-β inhibit MMP expression. Effects on TIMP expression are generally less pronounced (Table 2).
Table 2. MMP regulation in macrophages.
Effects of agonist and antagonists were measured on MMP mRNA and/or protein seretion form mature primary macrophages. For references consult the review (28). Abbreviations: CD40L, CD40 ligand; ConA, concanavalin A; IFN, interferon; IL, interleukin; IκB, inhibitor or nuclear factor κB; LDL, low-density lipoptein; LPS, lipopolysachharide; MMP, metalloproteinase; Ox, oxidised; PMA, phorbol myristate acetate; TNF, tumour necrosis factor.
| MMP | Agonist | Antagonist |
|---|---|---|
| MMP-1 | CD40L, cholesterol- feeding, LPS, S. aureus |
Cerivastatin, IFN-γ, IκBα, IL-4, IL-10, simvasatin |
| MMP-2 | LPS, T-cell medium or membranes |
Cerivastatin, simvasatin |
| MMP-3 | CD40L, cholesterol- feeding, LPS |
Cerivastatin, IFN-γ, IκBα, simvasatin |
| MMP-8 | CD40L, IL-1β, LPS, TNFα | |
| MMP-9 | IL-1β, LPS, ox-LDL, S. aureus, T-lymphocyte medium or membranes, TNFα |
Cerivastatin, fluvastatin, IFN-γ, IL-4, IL-10 |
| MMP-11 | CD40L | |
| MMP-12 | CD40L, cholesterol- feeding, IL-4, LPS |
IFN-γ |
| MMP-13 | LPS | IFN-γ |
| MMP-14 | LPS, TNFα | IFN-γ |
| MMP-16 | Ox-LDL, TNFα | |
| TIMP-1 | IL-6, IL-10, LPS, Ox-LDL, S. aureus |
IL-4, IL-8, IFNγ, Ox-LDL |
| TIMP-2 | None | |
| TIMP-3 | None | IFN-γ |
We study foam cells formed in vivo and relate this to MMP regulation and functions including migration, invasion, proliferation and apoptosis measured ex vivo (32-34). Foam cells are obtained from polyurethane sponges implanted under the dorsal skin of rabbits. The foreign body reaction causes infiltration of macrophages, all of which are positive for the lineage marker RAM-11 (32). In rabbits fed normal chow all cells are non-foamy macrophages (NFM), while in animals fed a cholesterol-enriched diet all are foam-cell macrophages (FCM). This approach yields pure, functionally-intact foam cells in much larger quantities than obtainable by collagenase digestion from plaques. Unlike NFM, FCM show constitutively active NF-κB, confirming that they are in an activated state (32). Interestingly, these FCM constitutively express several MMPs, including MMP-1, -3 -9 -12 and -14 as well as TIMPs-1, -2 and -3 (32-34). The expression of MMPs-1 and -3 alone is inhibited by blocking activation of NF-κB. Based on the in-vitro studies (Table 2) and our ex-vivo experiments we infer that recently recruited macrophages exist in a differentiated state that we designate M0 (Fig. 1). These macrophages can be activated through NF-κB to an M0a state that secretes MMP-1 and MMP-3. In many inflammatory conditions this probably happens through mediators such as TNF-α and IL-1β; especially in infections activation of TLRs may be superadded; during foam cell formation, however, NF-κB becomes constitutively activated and MMPs-1 and -3 are therefore permanently turned on. As a result, FCM expressing MMPs-1 and -3 are present in early fatty streaks and during subsequent fibrous cap formation. Our study showing less SMC accumulation in MMP-3 null mice (22) suggests that MMP-3 plays an active part in cap formation, but more direct evidence is needed.
Expression of MMP-12 is up-regulated in response to IL-4 and down-regulated by IFN-γ, suggesting a link to the M2 pheno-type (9). On the other hand, we found that arginase-I, fibronectin and thrombospondin, which are markers for M2 macrophages, are down-regulated in rabbit FCM (34), which suggests that FCM preparations also contain at least some cells with M1 character. This is consistent with upregulation of scavenger receptor LOX-1 in rabbit foam cells (34) and several additional markers in mouse plaque FCM (31). Interestingly FCM showing either arginase-I down-regulation or MMP-12 up-regulation were selectively located in the deep layers of advanced rabbit and human plaques (34). All this evidence fits with the hypothesis that M0 macrophages subsequently differentiate towards a mixture of M1 and M2 phenotypes as plaques progress (Fig. 1).
More recently we found that TIMP-3 is down-regulated in FCM compared to NFM (33). Furthermore, a distinct TIMP-3 negative subpopulation was identified, which was highly invasive through matrigel, proliferative and prone to undergo apoptosis. All these parameters were normalised by addition of exogenous TIMP-2 or TIMP-3 but not TIMP-1, implicating the activity of an MT-MMP. Consistent with this, we found that the TIMP-3 negative subpopulation selectively over-expresses MMP-14. Moreover inhibitory antibodies against MMP-14 mimic the effects of adding exogenous TIMP-3 (33). This FCM sub-population may have M1-like properties, since IFN-γ down-regulates TIMP-3 in macrophages (unpublished observations). TIMP-3 negative and MMP-14 positive macrophages were again localised in the deep layers of advanced rabbit and human plaques, intermingled with TIMP-3 positive and MMP-14 negative FCM (33).
Based on these observations we have formulated the working hypothesis shown in Figure 1. This differs from most previous schemes in two important respects: 1) we add a differentiated M0 phenotype as a precursor of both M1 and M2 and 2) we consider that each phenotype (not only M2) can be acted on by pro-inflammatory mediators leading to activated states (MXa). Foam cell formation seems to switch cells to a prolonged activated state that is preserved during culture ex vivo (32). What remains of critical importance is the final outcome of the inflammatory response. Most commonly (e.g. after an acute infection) the provoking agent is eliminated and tissue integrity is restored, albeit perhaps with scar formation. Less commonly (e.g. persistent infections or foreign bodies, autoimmune reactions) a state of chronic granuloma formation ensues. This consists of a complex of leucocytes in all stages of activation together with connective tissue cells and capillaries. We suggest that stable atherosclerotic plaques are in effect chronic granulomas of the artery wall. Relatively rarely, a third endpoint is reached – ulceration. Ulceration of the arterial intima is followed by thrombosis, which can provoke MI leading to death. We hypothesise that activated macrophage subpopulations make distinct contributions to plaque instability. For example, previous work has suggested a selective role for M2 macrophages in aneurysm formation, in part through the so-called matrix metalloelastase, MMP-12 (9). Our data showing that TIMP-3-negative, MMP-14-positive foam cells are more invasive and destructive of matrigel, localise to the deep areas of plaques, and are more likely to undergo apoptosis, suggests a potential role for this subpopulation in lipid core formation (33). These proposals have, however, not yet been tested formally in any experimental model. M1- and M2-like phenotypes have been observed in mouse plaques (31), but whether they can derive from a common M0 precursor is still unclear. Whether M1 and M2 are inter-convertible is also unknown, but this should be amenable to testing. Since the mediators of T-cell and macrophage phenotypes closely converge, it will be a challenge to devise strategies to selectively delete M1 and M2 macrophages. However, genomics, in particular, may help to identify drugs targets to ameliorate adverse macrophage phenotypes. The hope is that these would prevent MIs without affecting the normal ability of macrophages to fight infections and mediate tissue repair.
Acknowledgments
Financial support:
The authors' work is supported by the British Heart Foundation and the European Vascular Genomics Network.
References
- 1.Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 Supplement 1):C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 2.Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006;69:625–635. doi: 10.1016/j.cardiores.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 4.Crisby M, Nordin-Fredriksson G, Shah PK, et al. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: Implications for plaque stabilization. Circulation. 2001;103:926–933. doi: 10.1161/01.cir.103.7.926. [DOI] [PubMed] [Google Scholar]
- 5.Underhill HR, Yuan C, Zhao XQ, et al. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: a high-resolution magnetic resonance imaging trial. Am Heart J. 2008;155:584 e1–8. doi: 10.1016/j.ahj.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Van Mieghem CA, McFadden EP, de Feyter PJ, et al. Noninvasive detection of subclinical coronary atherosclerosis coupled with assessment of changes in plaque characteristics using novel invasive imaging modalities: the Integrated Biomarker and Imaging Study (IBIS) J Am Coll Cardiol. 2006;47:1134–1142. doi: 10.1016/j.jacc.2005.09.075. [DOI] [PubMed] [Google Scholar]
- 7.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Rajagopalan S, Meng XP, Ramasamy S, et al. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas. J Clin Invest. 2004;114:300–308. doi: 10.1172/JCI19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newby AC. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006;69:614–624. doi: 10.1016/j.cardiores.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Clarke MC, Figg N, Maguire JJ, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JL, Carson K, Williams HM, et al. Plaque rupture after short periods of fat-feeding in the apolipoprotein E knockout mouse: Model characterisation, and effects of pravastatin treatment. Circulation. 2005;111:1422–1430. doi: 10.1161/01.CIR.0000158435.98035.8D. [DOI] [PubMed] [Google Scholar]
- 13.Lemaitre V, Soloway PD, D'Armiento J. Increased medial degradation with pseudo-aneurysm formation in apolipoprotein E-knockout mice deficient in tissue inhibitor of metalloproteinases-1. Circulation. 2003;107:333–338. doi: 10.1161/01.cir.0000044915.37074.5c. [DOI] [PubMed] [Google Scholar]
- 14.Silence J, Collen D, Lijnen HR. Reduced athero-sclerotic plaque but enhanced aneurysm formation in mice with inactivation of the tissue inhibitor of metalloproteinase-1 (TIMP-1) gene. Circ Res. 2002;90:897–903. doi: 10.1161/01.res.0000016501.56641.83. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JL, Baker AH, Oka K, et al. Suppression of atherosclerotic plaque progression and instability by tissue inhibitor of metalloproteinase-2: Involvement of macrophage migration and apoptosis. Circulation. 2006;113:2435–2444. doi: 10.1161/CIRCULATIONAHA.106.613281. [DOI] [PubMed] [Google Scholar]
- 16.Rouis M, Adamy C, Duverger N, et al. Adenovirus-mediate overexpression of tissue inhibitor of metalloproteinase-1 reduces atherosclerotic lesions in apolipoprotein E deficient mice. Circulation. 1999;100:533–540. doi: 10.1161/01.cir.100.5.533. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JL, Fritsche-Danielson R, Behrendt M, et al. Effect of broad-spectrum matrix metalloproteinase inhibition on atherosclerotic plaque stability. Cardiovasc Res. 2006;71:586–595. doi: 10.1016/j.cardiores.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Lemaitre V, O'Byrne TK, Borczuk AC, et al. ApoE knockout mice expressing human matrix metalloproteinase-1 in macrophages have less advanced atherosclerosis. J Clin Invest. 2001;107:1227–1234. doi: 10.1172/JCI9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gough PJ, Gomez IG, Wille PT, et al. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Nooijer R, Verkleij CJN, von der Thusen JH, et al. Lesional overexpression of matrix metalloproteinase-9 promotes intraplaque hemorrhage in advanced lesions but not at earlier stages of atherogenesis. Arterioscler Thromb Vasc Biol. 2006;26:340–346. doi: 10.1161/01.ATV.0000197795.56960.64. [DOI] [PubMed] [Google Scholar]
- 21.Silence J, Lupu F, Collen D, et al. Persistence of atherosclerotic plaque but reduced aneurysm formation in mice with stromelysin-1 (MMP-3) gene inactivation. Arterioscler Thromb Vasc Biol. 2001;21:1440–1445. doi: 10.1161/hq0901.097004. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JL, George SJ, Newby AC, et al. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci USA. 2005;102:15575–15580. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luttun A, Lutgens E, Manderveld A, et al. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein E-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation. 2004;109:1408–1414. doi: 10.1161/01.CIR.0000121728.14930.DE. [DOI] [PubMed] [Google Scholar]
- 24.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 25.Tedgui A, Mallat Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 26.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 27.Libby P, Nahrendorf M, Pittet MJ, et al. Diversity of denizens of the atherosclerotic plaque: not all monocytes are created equal. Circulation. 2008;117:3168–3170. doi: 10.1161/CIRCULATIONAHA.108.783068. [DOI] [PubMed] [Google Scholar]
- 28.Newby AC. Metalloproteinase Expression in Monocytes and Macrophages and its Relationship to Atherosclerotic Plaque Instability. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.173898. in print. [DOI] [PubMed] [Google Scholar]
- 29.Brand K, Page S, Rogler G, et al. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 31.Bouhlel MA, Derudas B, Rigamonti E, et al. PPAR-gamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Chase A, Bond M, Crook MF, et al. Role of nuclear factor-κB activation in metalloproteinase-1, -3 and -9 secretion by human macrophages in vitro and rabbit foam cells produced in vivo. Arterioscler Thromb Vasc Biol. 2002;22:765–771. doi: 10.1161/01.atv.0000015078.09208.92. [DOI] [PubMed] [Google Scholar]
- 33.Johnson JL, Sala-Newby GB, Ismail Y, et al. Low tissue inhibitor of metalloproteinases 3 and high matrix metalloproteinase 14 levels defines a subpopulation of highly invasive foam-cell macrophages. Arterioscler Thromb Vasc Biol. 2008;28:1647–1653. doi: 10.1161/ATVBAHA.108.170548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas AC, Sala-Newby GB, Ismail Y, et al. Genomics of foam cells and nonfoamy macrophages from rabbits identifies arginase-I as a differential regulator of nitric oxide production. Arterioscler Thromb Vasc Biol. 2007;27:571–577. doi: 10.1161/01.ATV.0000256470.23842.94. [DOI] [PubMed] [Google Scholar]
- 35.Kuzuya M, Nakamura K, Sasaki T, et al. Effect of MMP-2 deficiency on atherosclerotic lesion formation in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:1120–1125. doi: 10.1161/01.ATV.0000218496.60097.e0. [DOI] [PubMed] [Google Scholar]
- 36.Liang J, Liu E, Yu Y, et al. Macrophage metalloelastase accelerates the progression of atherosclerosis in transgenic rabbits. Circulation. 2006;113:1993–2001. doi: 10.1161/CIRCULATIONAHA.105.596031. [DOI] [PubMed] [Google Scholar]
- 37.Deguchi J-O, Aikawa E, Libby P, et al. Matrix metalloproteinase-13/Collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation. 2005;112:2708–2715. doi: 10.1161/CIRCULATIONAHA.105.562041. [DOI] [PubMed] [Google Scholar]
- 38.Schneider F, Sukhova GK, Aikawa M, et al. Matrix-metalloproteinase-14 deficiency in bone-marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques. Circulation. 2008;117:931–939. doi: 10.1161/CIRCULATIONAHA.107.707448. [DOI] [PubMed] [Google Scholar]