Abstract
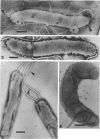
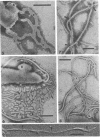
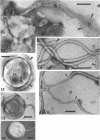
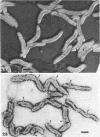
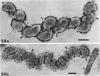
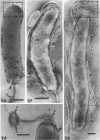
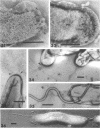
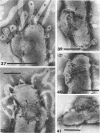
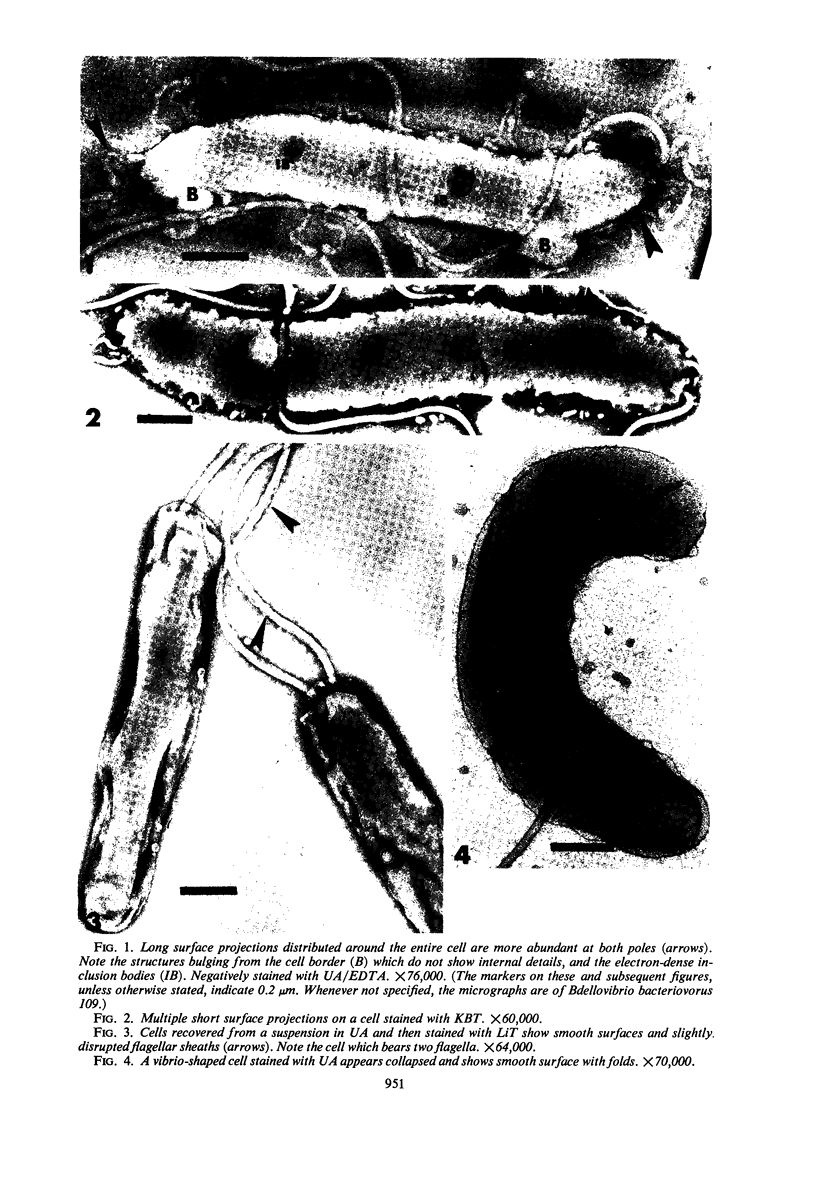
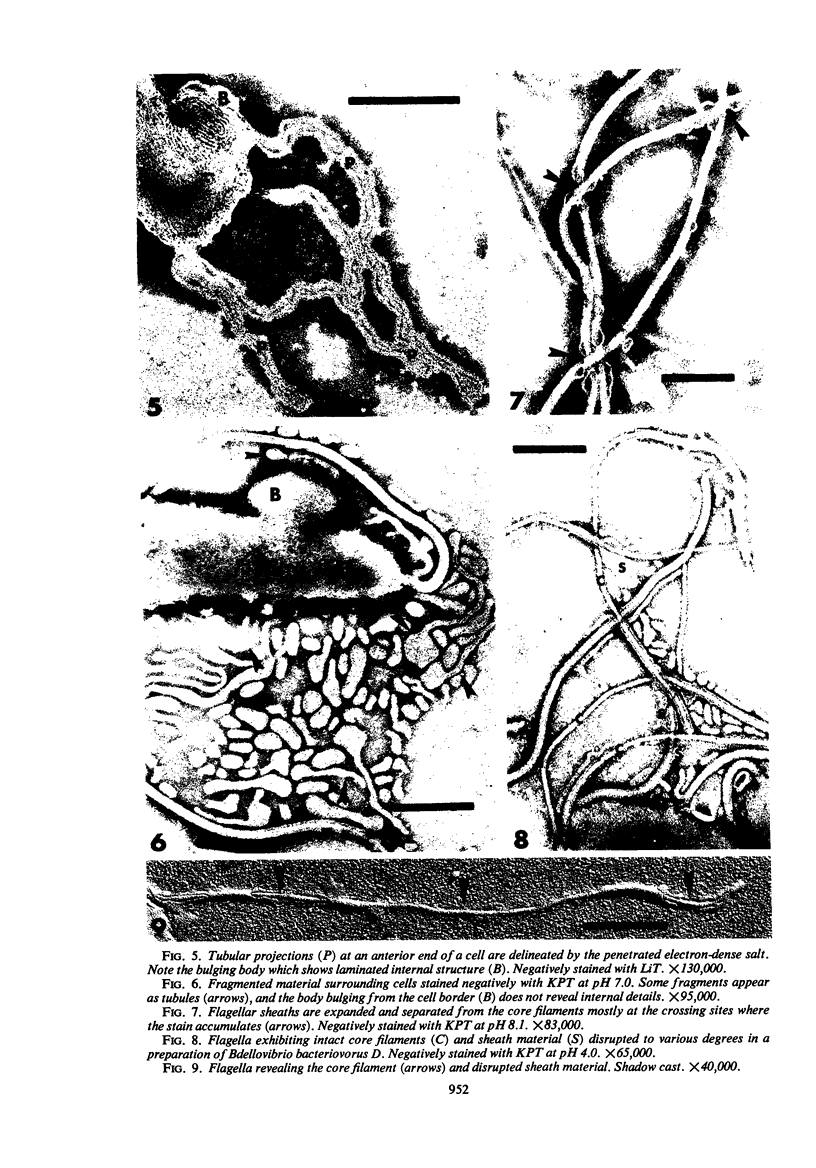
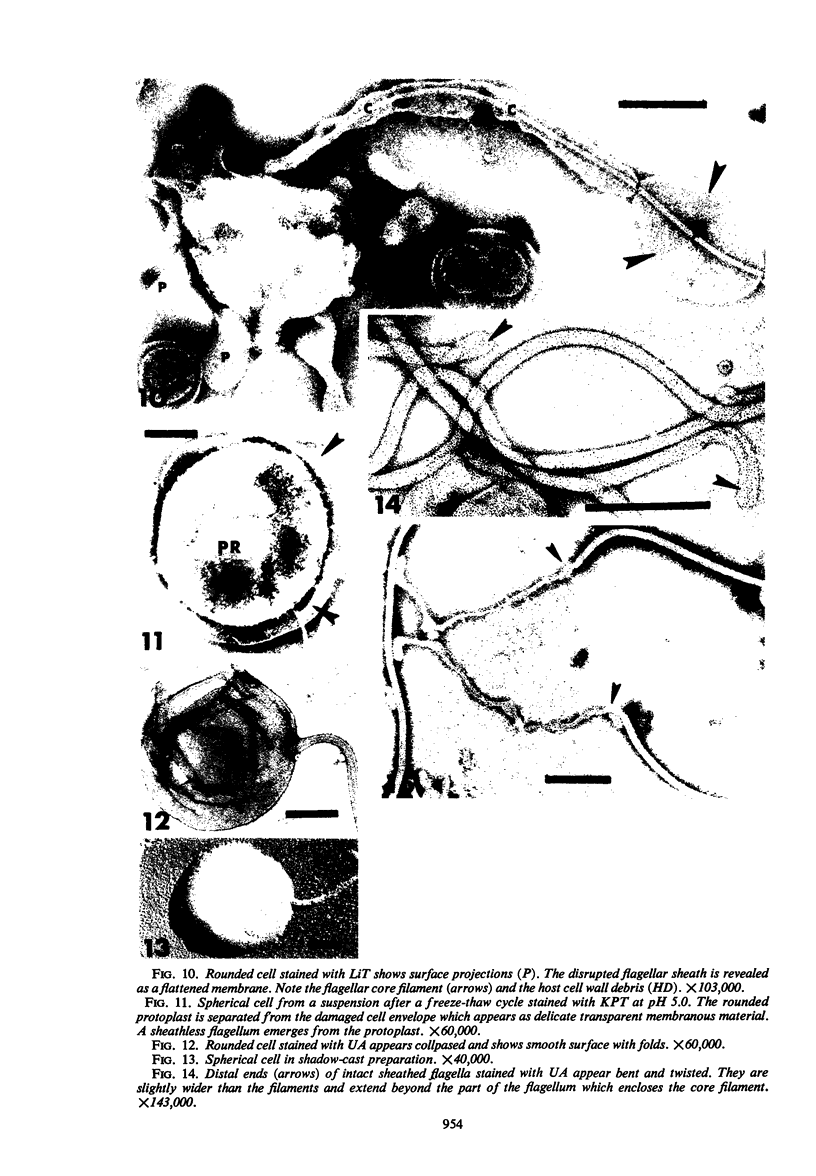
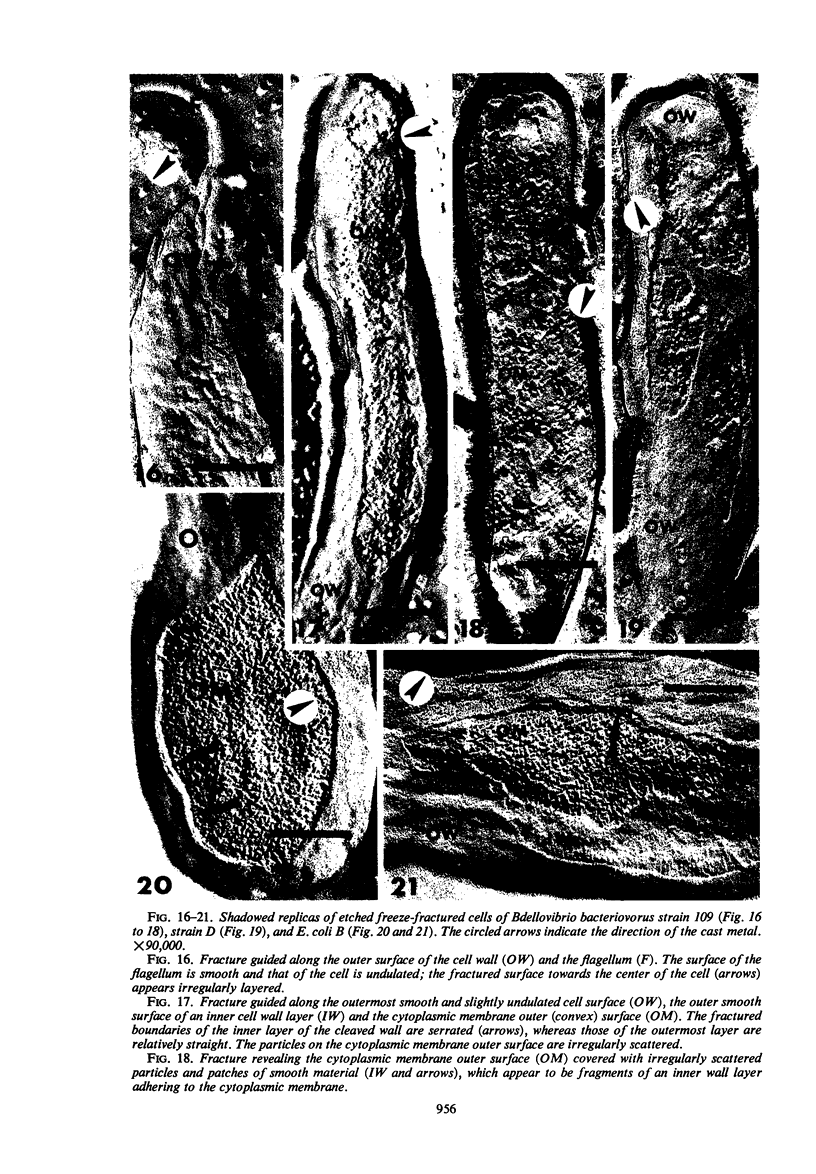
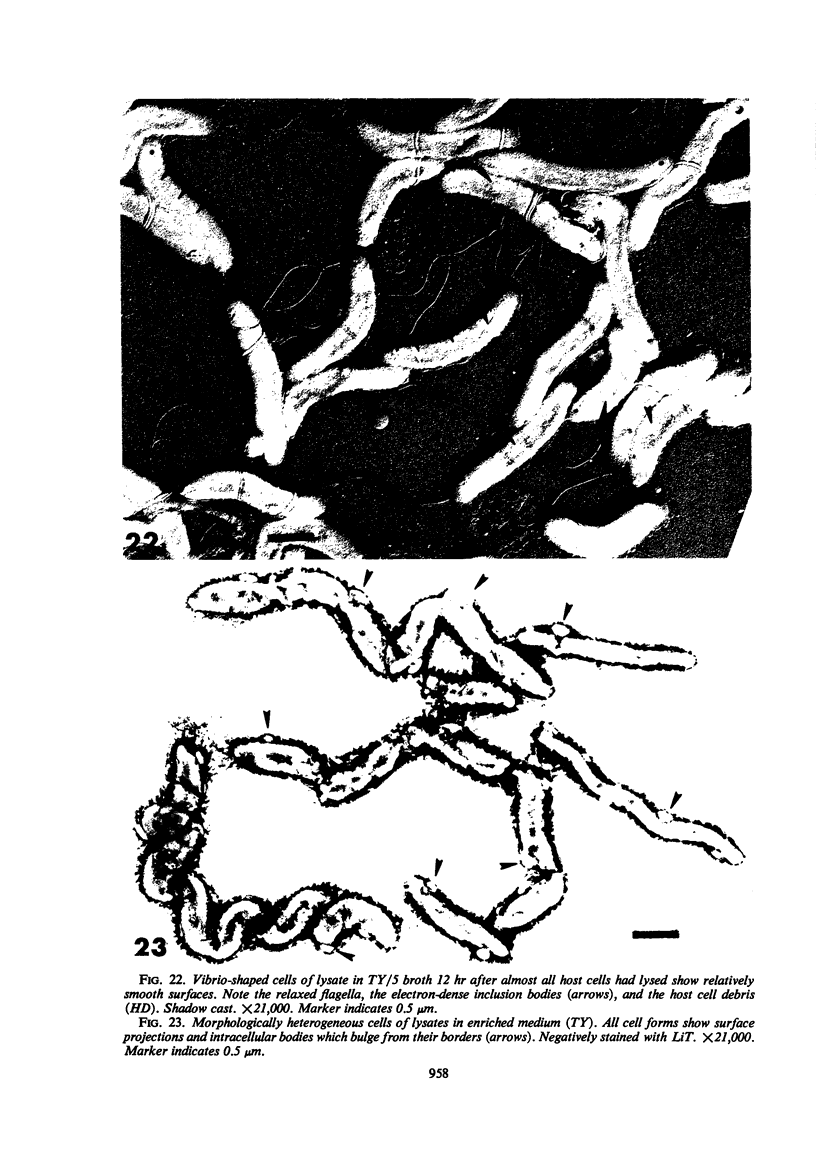
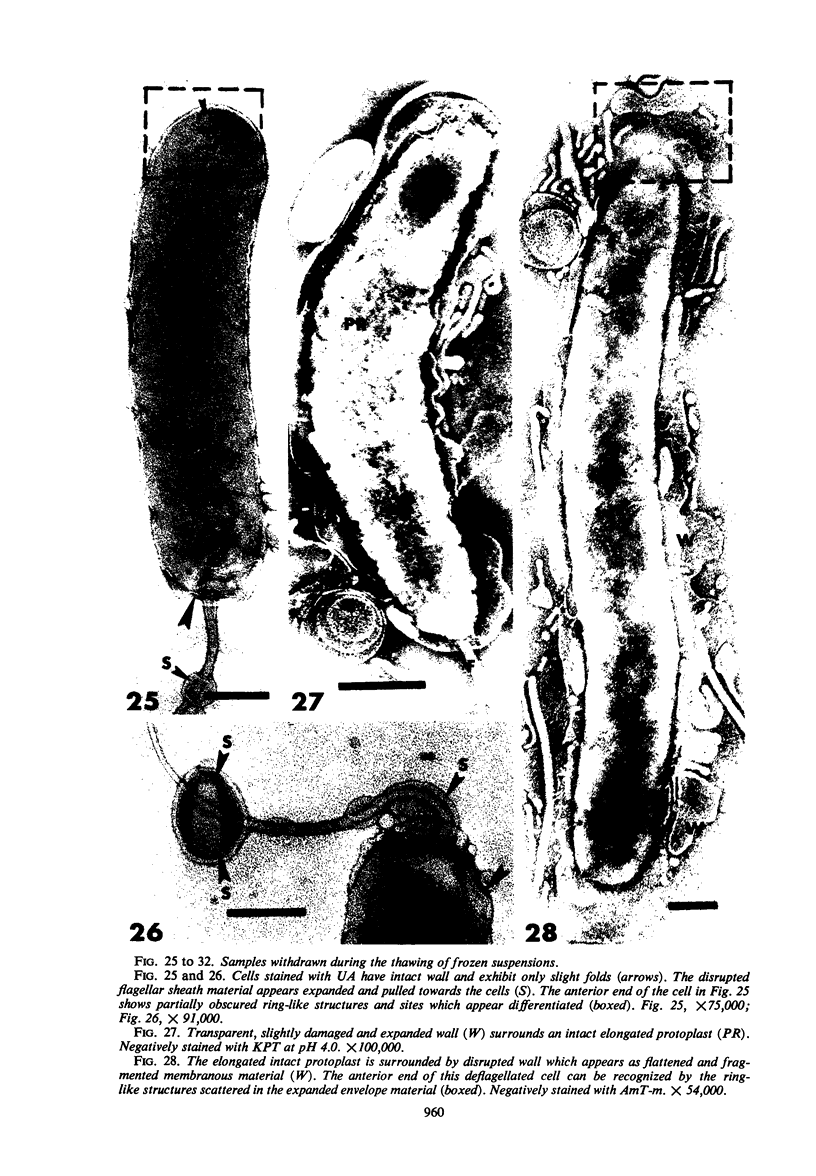
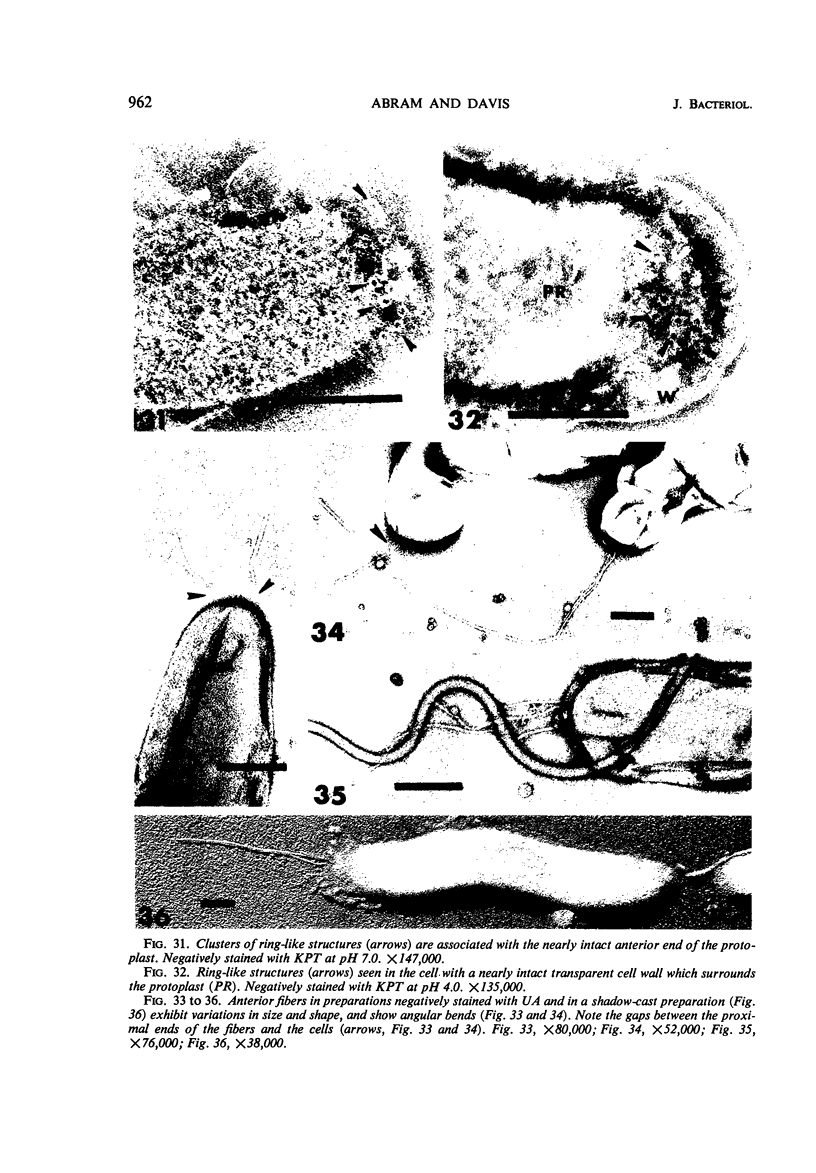
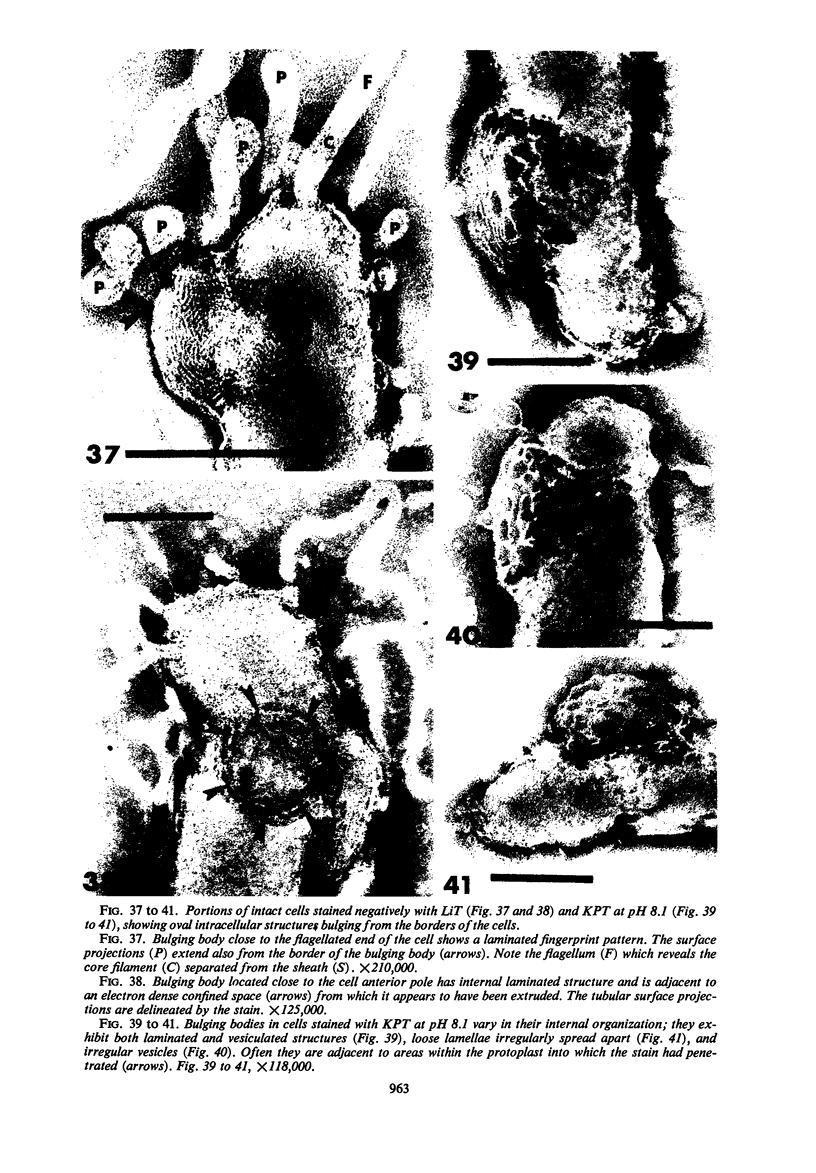
The structure of five parasitic strains of Bdellovibrio bacteriovorus was studied by electron microscope after negative staining and in shadow-case and etched freeze-fractured preparations. Special attention was paid to the cell wall and the flagellar sheath which is continuous with the wall or part of it. These structural components reveal distinct features which are induced by certain staining substances; they are exceedingly susceptible to disruption by physical treatments, and in old cells often appear impaired. In freeze-fractured cells the wall shows characteristic fracturing tendencies not known in other microorganisms. These structural properties and features are distinct to Bdellovibrio wall and flagellar sheath, the structural integrity of which is a fundamental requirement for the infectivity and survival of this organism. The anterior end of Bdellovibrio is differentiated: 6 to 12 ring-like structures (9 to 12 nm, outer diameter) are built into its wall and several fibers (7 to 10 nm wide, up to 1.5 μm long) emerge from it. Intracellular structures, which are revealed as compact oval bodies bulging from the cell border and have internal laminated organization, are characteristic of Bdellovibrio after negative staining with certain compounds. These findings on the structure of parasitic Bdellovibrio substantiate previous observations indicating the uniqueness of this organism and add criteria for the identification of this genus.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D. ELECTRON MICROSCOPE OBSERVATIONS ON INTACT CELLS, PROTOPLASTS, AND THE CYTOPLASMIC MEMBRANE OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Mar;89:855–873. doi: 10.1128/jb.89.3.855-873.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANGHAM A. D., HORNE R. W. NEGATIVE STAINING OF PHOSPHOLIPIDS AND THEIR STRUCTURAL MODIFICATION BY SURFACE-ACTIVE AGENTS AS OBSERVED IN THE ELECTRON MICROSCOPE. J Mol Biol. 1964 May;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Structure of Escherichia coli after freeze-etching. J Bacteriol. 1970 Jan;101(1):304–313. doi: 10.1128/jb.101.1.304-313.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen myelin. Exp Cell Res. 1967 Mar;45(3):703–707. doi: 10.1016/0014-4827(67)90175-9. [DOI] [PubMed] [Google Scholar]
- Burger A., Drews G., Ladwig R. Wirtskreis und Infektionscyclus eines neu isolierten Bdellovibrio bacteriovorus-Stammes. Arch Mikrobiol. 1968 May 8;61(3):261–279. [PubMed] [Google Scholar]
- Burnham J. C., Hashimoto T., Conti S. F. Electron microscopic observations on the penetration of Bdellovibrio bacteriovorus into gram-negative bacterial hosts. J Bacteriol. 1968 Oct;96(4):1366–1381. doi: 10.1128/jb.96.4.1366-1381.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- Diedrich D. L., Denny C. F., Hashimoto T., Conti S. F. Facultatively parasitic strain of Bdellovibrio bacteriovorus. J Bacteriol. 1970 Mar;101(3):989–996. doi: 10.1128/jb.101.3.989-996.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil A., Branton D. Changes in the plasma membrane of Escherichia coli during magnesium starvation. J Bacteriol. 1969 Jun;98(3):1320–1327. doi: 10.1128/jb.98.3.1320-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Lucy J. A. Electron microscopy of lipids: effects of pH and fixatives on the appearance of a macromolecular assembly of lipid micelles in negatively stained preparations. J Microsc. 1969;89(1):1–18. doi: 10.1111/j.1365-2818.1969.tb00645.x. [DOI] [PubMed] [Google Scholar]
- Guelin A., Lépine P., Lamblin D., Sisman J. Isolement d'un parasite bactérien actif sur les germes grampositifs à partir d'échantillons d'eau polluée. C R Acad Sci Hebd Seances Acad Sci D. 1968 Jun 24;266(26):2508–2509. [PubMed] [Google Scholar]
- LUCY J. A., GLAUERT A. M. STRUCTURE AND ASSEMBLY OF MACROMOLECULAR LIPID COMPLEXES COMPOSED OF GLOBULAR MICELLES. J Mol Biol. 1964 May;8:727–748. doi: 10.1016/s0022-2836(64)80121-2. [DOI] [PubMed] [Google Scholar]
- Lépine P., Guélin A., Sisman J., Lamblin D. Etude au microscope électronique de la lyse de Salmonella par Bdellovibrio bacteriovorus. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jun 19;264(25):2957–2960. [PubMed] [Google Scholar]
- MOOR H. DIE GEFRIER-FIXATION LEBENDER ZELLEN UND IHRE ANWENDUNG IN DER ELEKTRONENMIKROSKOPIE. Z Zellforsch Mikrosk Anat. 1964 Apr 28;62:546–580. [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Nanninga N. Ultrastructure of the cell envelope of Escherichia coli B after freeze-etching. J Bacteriol. 1970 Jan;101(1):297–303. doi: 10.1128/jb.101.1.297-303.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsen C. C. Fine structure of the mesosome and nucleoid in frozen-etched Bacillus subtilis. Arch Mikrobiol. 1968;61(1):40–47. doi: 10.1007/BF00704290. [DOI] [PubMed] [Google Scholar]
- Remsen C. C., Watson S. W., Waterbury J. B., Trüper H. G. Fine structure of Ectothiorhodospira mobilis Pelsh. J Bacteriol. 1968 Jun;95(6):2374–2392. doi: 10.1128/jb.95.6.2374-2392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Horne R. W. Reassociation of purified lipopolysaccharide and phospholipid of the bacterial cell envelope: electron microscopic and monolayer studies. J Bacteriol. 1967 May;93(5):1705–1721. doi: 10.1128/jb.93.5.1705-1721.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOLP H., STARR M. P. BDELLOVIBRIO BACTERIOVORUS GEN. ET SP. N., A PREDATORY, ECTOPARASITIC, AND BACTERIOLYTIC MICROORGANISM. Antonie Van Leeuwenhoek. 1963;29:217–248. doi: 10.1007/BF02046064. [DOI] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Factors affecting the intracellular parasitic growth of Bdellovibrio bacteriovorus developing within Escherichia coli. J Bacteriol. 1969 Feb;97(2):912–923. doi: 10.1128/jb.97.2.912-923.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Isolation and characterization of host-independent Bdellovibrios. J Bacteriol. 1969 Nov;100(2):769–785. doi: 10.1128/jb.100.2.769-785.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P., Mandel M. Deoxyribonucleic acid characterization of Bdellovibrios. J Bacteriol. 1969 Nov;100(2):786–790. doi: 10.1128/jb.100.2.786-790.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Structure of the flagellum of Bdellovibrio bacteriovorus. J Bacteriol. 1968 May;95(5):1952–1955. doi: 10.1128/jb.95.5.1952-1955.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo M. Morphological and physiological aspects of the interaction of Bdellovibrio with host bacteria. Curr Top Microbiol Immunol. 1969;50:174–204. doi: 10.1007/978-3-642-46169-9_6. [DOI] [PubMed] [Google Scholar]
- Simpson F. J., Robinson J. Some energy-producing systems in Bdellovibrio bacteriovorus, strain 6-5-S. Can J Biochem. 1968 Aug;46(8):865–873. doi: 10.1139/o68-129. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Baigent N. L. Parasitic interaction of Bdellovibrio bacteriovorus with other bacteria. J Bacteriol. 1966 May;91(5):2006–2017. doi: 10.1128/jb.91.5.2006-2017.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp H. Bdellovibrio bacteriovorus--ein räuberischer Bakterienparisit. Naturwissenschaften. 1968 Feb;55(2):57–63. doi: 10.1007/BF00599479. [DOI] [PubMed] [Google Scholar]
- Varon M., Shil M. Interacton of Bdellovibrio bacteriovorus and host bacteria. I. Kinetic studies of attachment and invasion of Escherichia coli B by Bdellovibrio bacteriovorus. J Bacteriol. 1968 Mar;95(3):744–753. doi: 10.1128/jb.95.3.744-753.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon M., Shilo M. Interaction of Bdellovibrio bacteriovorus and host bacteria. II. Intracellular growth and development of Bdellovibrio bacteriovorus in liquid cultures. J Bacteriol. 1969 Jul;99(1):136–141. doi: 10.1128/jb.99.1.136-141.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. The isolation of protoplasts from Bacillus megaterium by controlled treatment with lysozyme. J Bacteriol. 1953 Dec;66(6):688–695. doi: 10.1128/jb.66.6.688-695.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R. S., Koo V. M. Penetration of red cell membranes by some membrane-associated particles. Proc Soc Exp Biol Med. 1968 Jun;128(2):353–357. doi: 10.3181/00379727-128-33013. [DOI] [PubMed] [Google Scholar]