Abstract
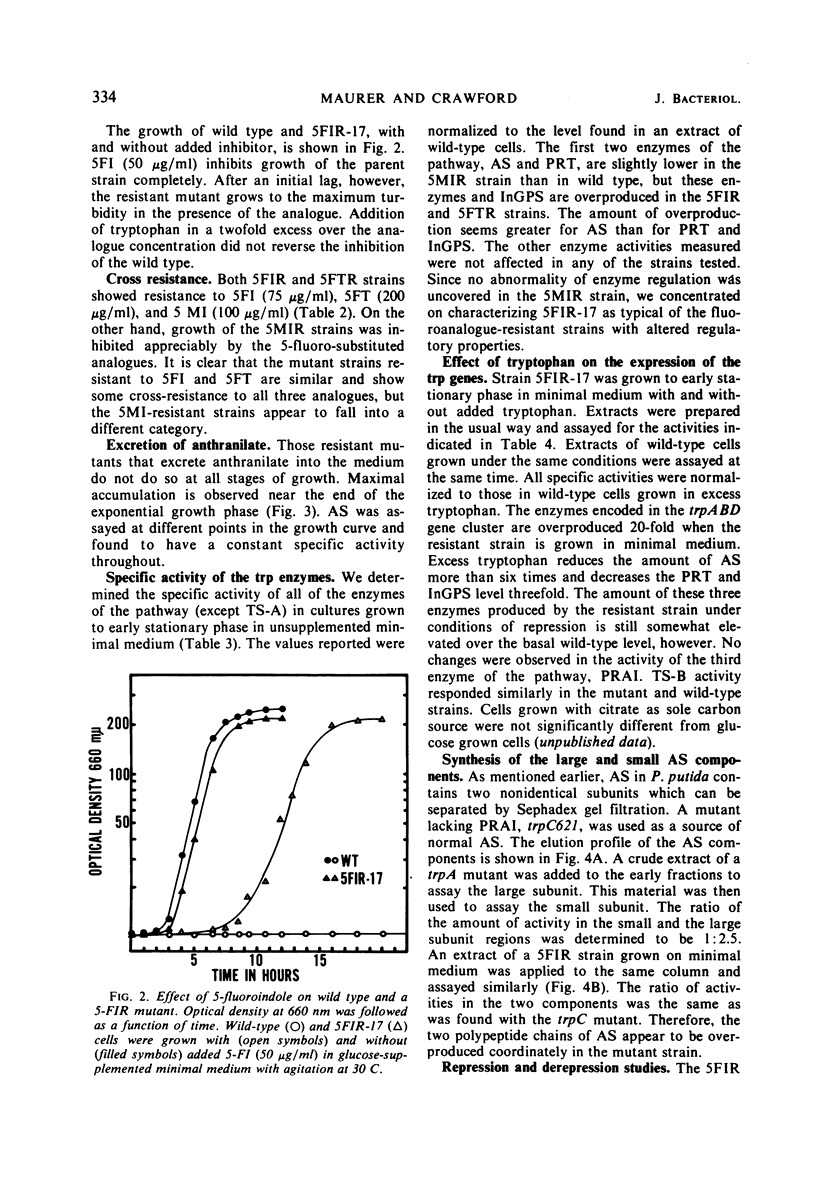
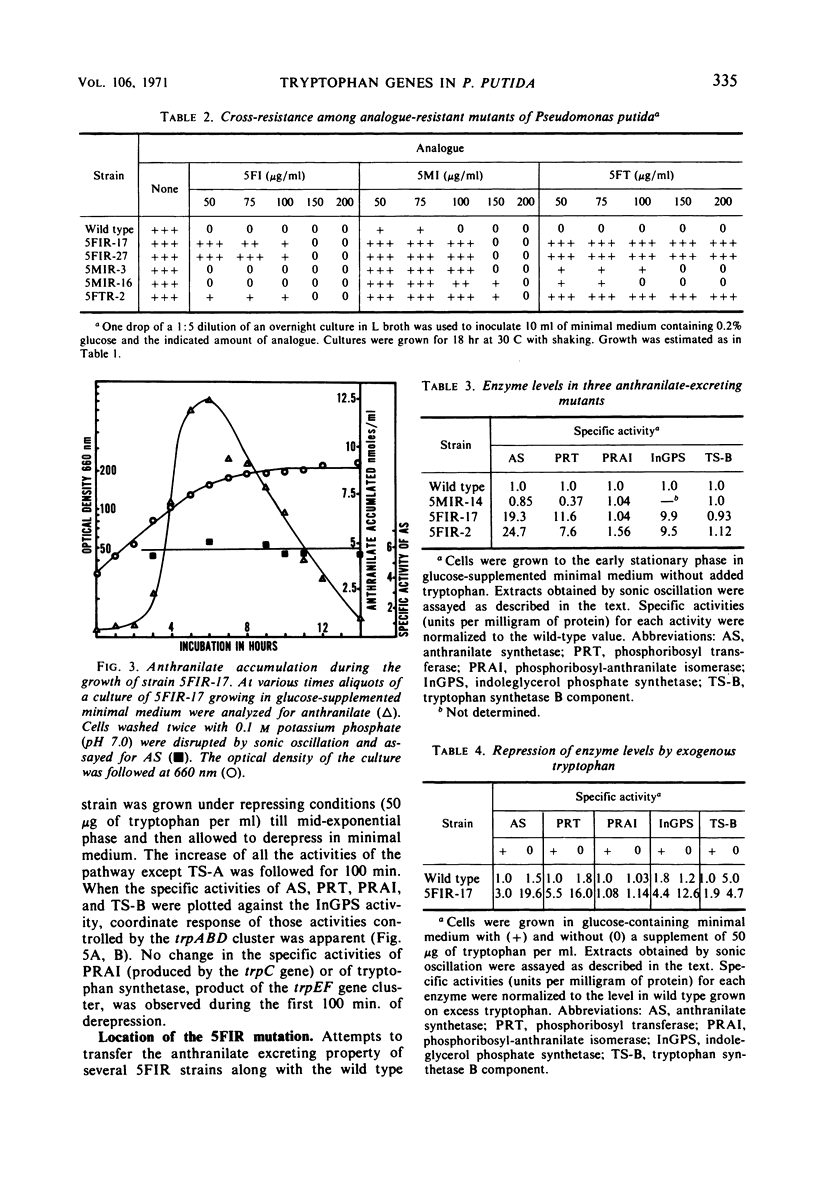
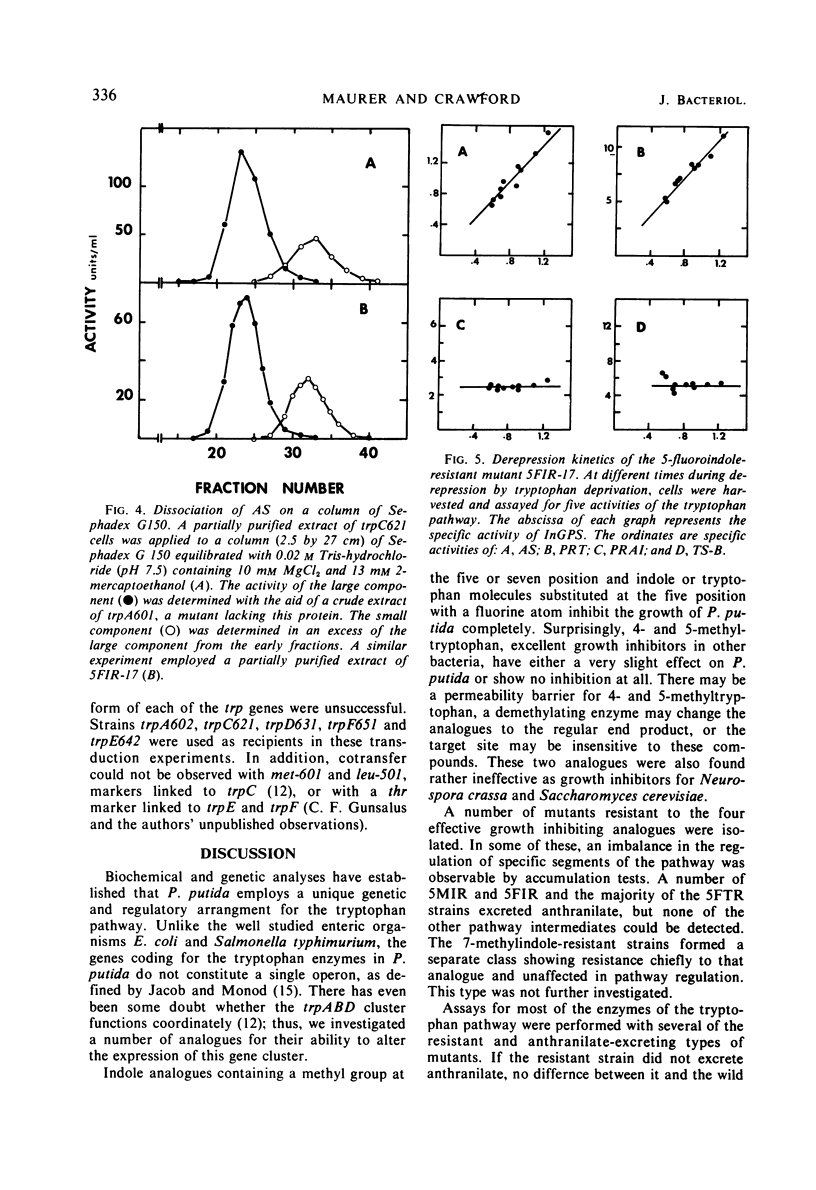
Three indole analogues, 5-methylindole, 5-fluoroindole, and 7-methylindole, and the tryptophan analogue 5-fluorotryptophan were found to inhibit the growth of wild-type Pseudomonas putida. Mutants resistant to these analogues were obtained. Some of the 5-fluoroindole- and 5-fluorotryptophan-resistant strains exhibit an abnormality in the regulation of certain trp genes. These strains excrete anthranilate when grown in minimal medium in the presence or absence of the inhibitor. In these strains, the trpA, B, and D gene products, the first, second, and fourth enzymes of the tryptophan pathway, are produced in 20-fold excess over the normal wild-type level. The other enzymes of the pathway are unaffected. Exogenous tryptophan is still able to repress the expression of the trpABD cluster somewhat. Similarity between the 5-fluoroindole- and 5-fluorotryptophan-resistant strains suggests that the former compound becomes effective through conversion to the latter. Repression and derepression experiments with two anthranilate-excreting, 5-fluoroindole-resistant strains showed coordinate variation of the affected enzymes. The locus conferring resistance and excretion is not linked by transduction to any of the trp genes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balbinder E., Callahan R., 3rd, McCann P. P., Cordaro J. C., Weber A. R., Smith A. M., Angelosanto F. Regulatory mutants of the tryptophan operon of Salmonella typhimurium. Genetics. 1970 Sep;66(1):31–53. doi: 10.1093/genetics/66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus I. C. Autonomous replication of a defective transducing phage in Pseudomonas putida. Virology. 1969 May;38(1):92–104. doi: 10.1016/0042-6822(69)90131-7. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOY C. H., GIBSON F. 1-(o-Carboxyphenylamino)-1-deoxyribulose. A compound formed by mutant strains of Aerobacter aerogenes and Escherichia coli blocked in the biosynthesis of tryptophan. Biochem J. 1959 Aug;72:586–597. doi: 10.1042/bj0720586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enatsu T., Crawford I. P. Enzymes of the tryptophan synthetic pathway in Pseudomonas putida. J Bacteriol. 1968 Jan;95(1):107–112. doi: 10.1128/jb.95.1.107-112.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARGIE B., HOLLOWAY B. W. ABSENCE OF CLUSTERING OF FUNCTIONALLY RELATED GENES IN PSEUDOMONAS AERUGINOSA. Genet Res. 1965 Jul;6:284–299. doi: 10.1017/s0016672300004158. [DOI] [PubMed] [Google Scholar]
- Gunsalus C., Gunsalus C. F., Chakrabarty A. M., Sikes S., Crawford I. P. Fine structure mapping of the tryptophan genes in Pseudomonas putida. Genetics. 1968 Nov;60(3):419–435. doi: 10.1093/genetics/60.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Tryptophanyl transfer RNA synthetase and expression of the tryptophan operon in the trpS mutants of Escherichia coli. Genetics. 1969 Mar;61(3):521–538. doi: 10.1093/genetics/61.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Queener S. F., Gunsalus I. C. Anthranilate synthase enzyme system and complementation in Pseudomonas species. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1225–1232. doi: 10.1073/pnas.67.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMERVILLE R. L., YANOFSKY C. STUDIES ON THE REGULATION OF TRYPTOPHAN BIOSYNTHESIS IN ESCHERICHIA COLI. J Mol Biol. 1965 Apr;11:747–759. doi: 10.1016/s0022-2836(65)80032-8. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wegman J., Crawford I. P. Tryptophan synthetic pathway and its regulation in Chromobacterium violaceum. J Bacteriol. 1968 Jun;95(6):2325–2335. doi: 10.1128/jb.95.6.2325-2335.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]


