Abstract
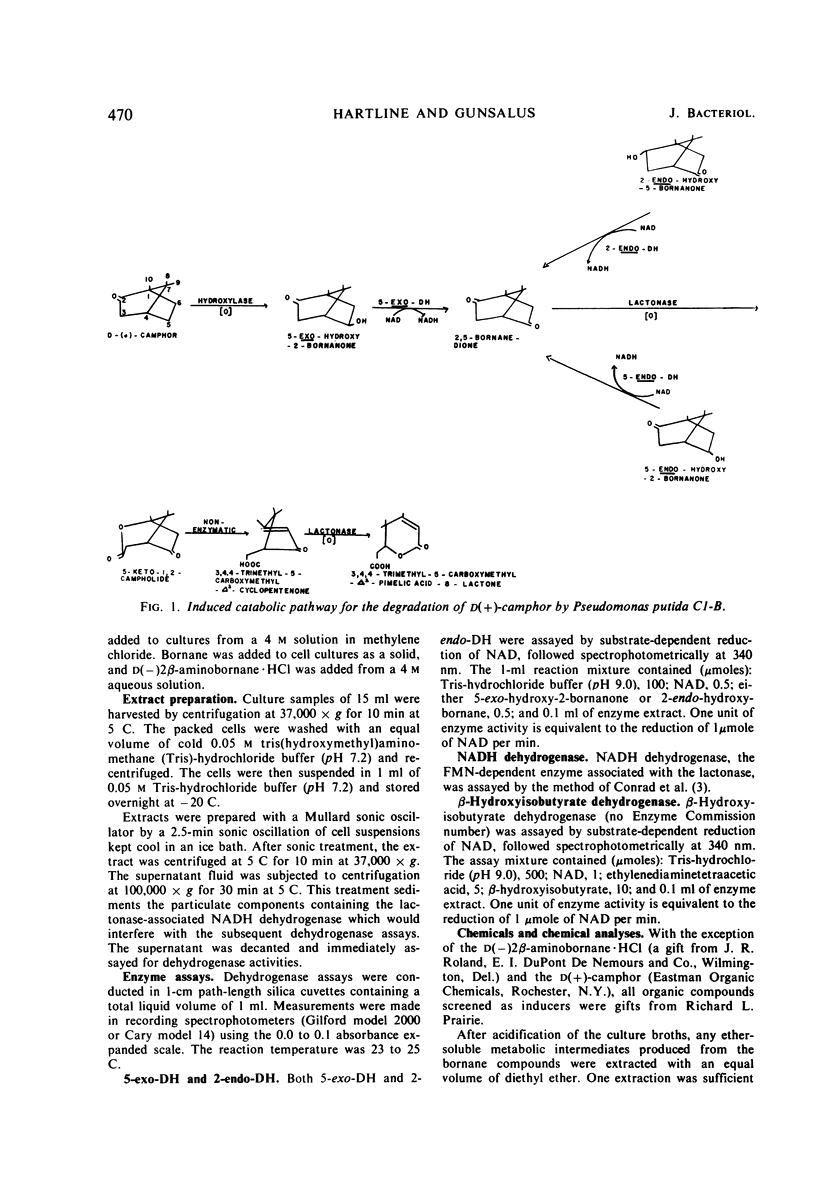
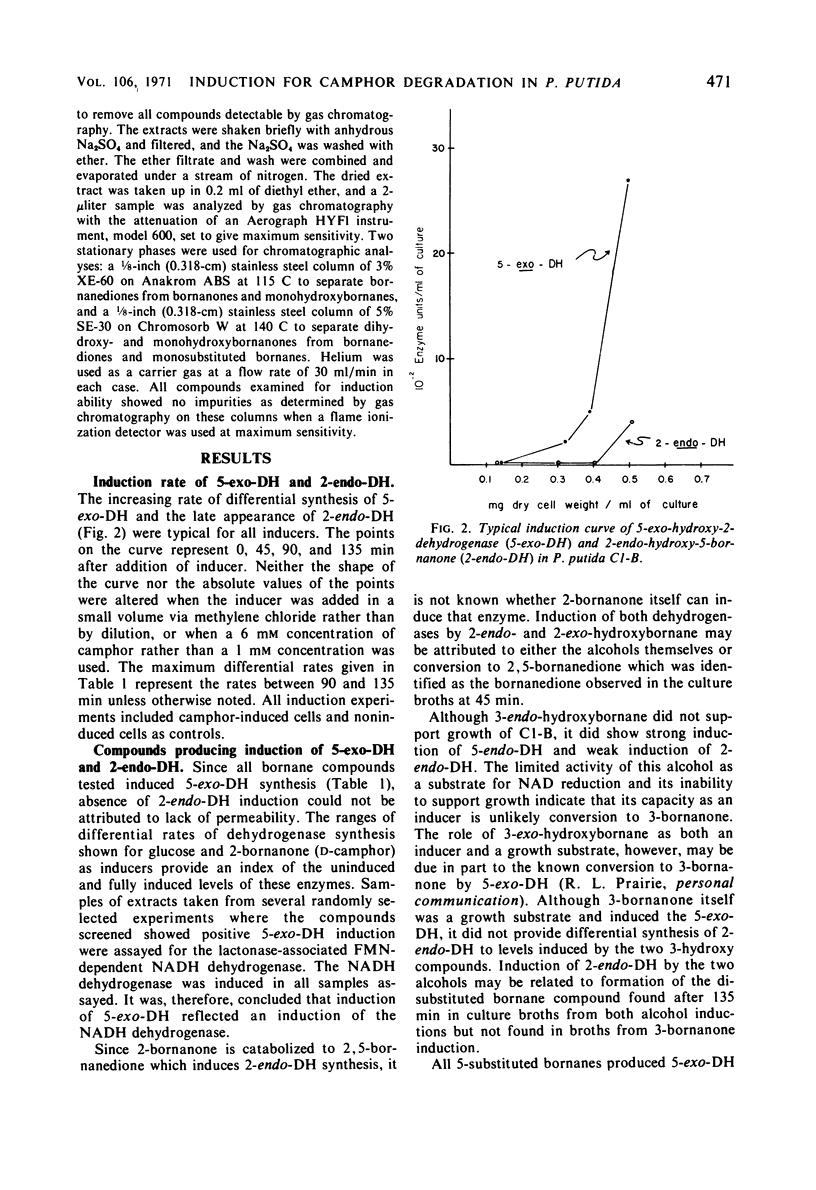
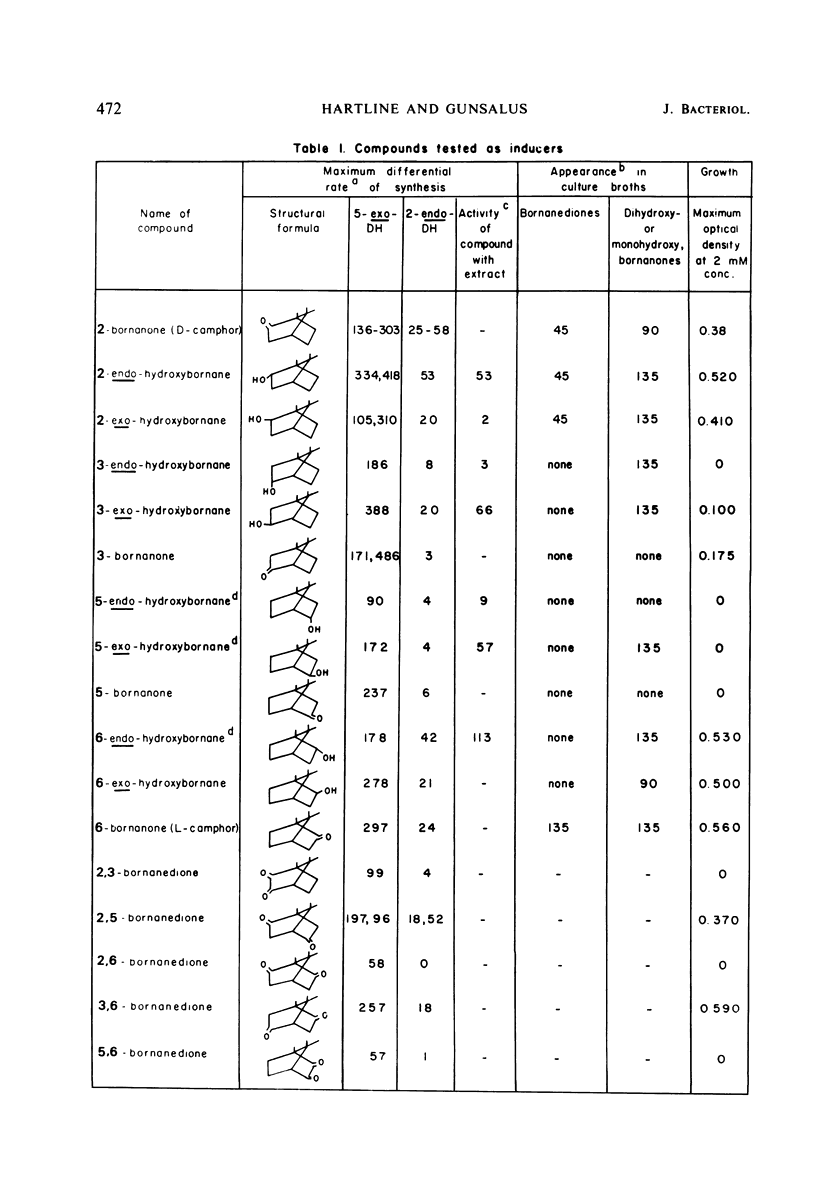
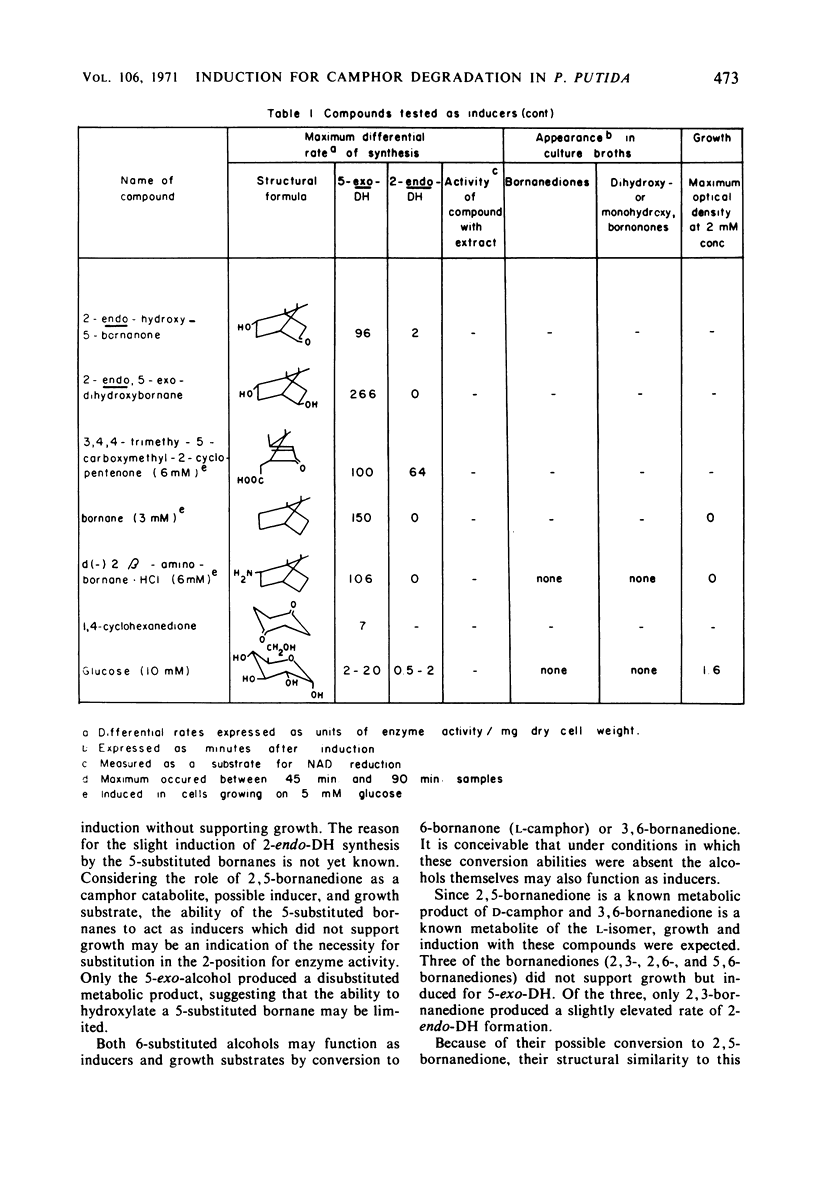
The ability of bornane and substituted bornanes to induce the early enzymes for d(+)-camphor degradation and control of these enzymes by catabolite repression were studied in a strain of a Pseudomonas putida. Bornane and 20 substituted bornane compounds showed induction. Of these 21 compounds, bornane and 8 of the substituted bornanes provided induction without supporting growth. Oxygen, but not nitrogen, enhanced the inductive potency of the unsubstituted bornane ring. All bornanedione isomers caused induction, and those with substituents on each of the three consecutive carbon atoms, including the methyl group at the bridgehead carbon, showed induction without supporting growth. Although it was not possible to obtain experimental data for a case of absolute gratuitous induction by compounds not supporting growth, indirect evidence in support of gratuitous induction is presented. It is proposed that the ability of P. putida to tolerate the unusually high degree of possible gratuitous induction observed for camphor catabolism may be related to the infrequent occurrence of bicyclic ring structures in nature. Survival of an organism with a broad specificity for gratuitous induction is discussed. Glucose and succinate, but not glutamate, produced catabolite repression of the early camphor-degrading enzymes. Pathway enzymes differ in their degree of sensitivity to succinate-provoked catabolite repression. The ability of a compound to produce catabolite repression is not, however, directly related to the duration of the lag period (diauxic lag) between growth on camphor and growth on the repressing compound.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONRAD H. E., DUBUS R., NAMTVEDT M. J., GUNSALUS I. C. MIXED FUNCTION OXIDATION. II. SEPARATION AND PROPERTIES OF THE ENZYMES CATALYZING CAMPHOR LACTONIZATION. J Biol Chem. 1965 Jan;240:495–503. [PubMed] [Google Scholar]
- Cushman D. W., Tsai R. L., Gunsalus I. C. The ferroprotein component of a methylene hydroxylase. Biochem Biophys Res Commun. 1967 Mar 9;26(5):577–583. doi: 10.1016/0006-291x(67)90104-0. [DOI] [PubMed] [Google Scholar]
- Gunsalus I. C., Bertland A. U., 2nd, Jacobson L. A. Enzyme induction and repression in anabolic and catabolic pathways. Arch Mikrobiol. 1967;59(1):113–122. doi: 10.1007/BF00406322. [DOI] [PubMed] [Google Scholar]
- Hedegaard J., Gunsalus I. C. Mixed function oxidation. IV. An induced methylene hydroxylase in camphor oxidation. J Biol Chem. 1965 Oct;240(10):4038–4043. [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Trudgill P. W., DuBus R., Gunsalus I. C. Mixed function oxidation. V. Flavin interaction with a reduced diphosphopyridine nucleotide dehydrogenase, one of the enzymes participating in camphor lactonization. J Biol Chem. 1966 Mar 10;241(5):1194–1205. [PubMed] [Google Scholar]
- Yu C. A., Gunsalus I. C. Monoxygenases. VII. Camphor ketolactonase I and the role of three protein components. J Biol Chem. 1969 Nov 25;244(22):6149–6152. [PubMed] [Google Scholar]


