Abstract
Human herpesvirus 8 (HHV-8) infection is present in 22.9% of Tobago men. However, seroprevalence and modes of transmission of HHV-8 among Tobago women are not known. HHV-8 seropositivity rates in Tobago women were examined and compared rates to Tobago men of similar ages. To assess possible modes of transmission, sexual behavior among Tobago women was examined to determine its association with HHV-8 seropositivity.
A cross-sectional study was conducted in 213 Tobago women, ages 18-65 years, who participated in the Tobago Cervical and Oral Cancer Screening Study. HHV-8 seropositivity was determined by a monoclonal immunofluorescence assay. Age-specific rates were compared to those previously observed in men. Logistic regression analyses were performed to determine the association between HHV-8 seropositivity and sexual behavior among the women.
HHV-8 seroprevalence among Tobago women was 14.1% (95% C.I., 10 - 19%), with no significant differences with men of similar age (p-value = .741). Age ≤ 17 years at first sexual intercourse was found to have a minimal significant association with HHV-8 seropositivity (O.R. = 2.51, 95% C.I. = 1.09-5.78) in women.
HHV-8 age-specific rates were similar between genders. Sexual activity may not be a major contributor to HHV-8 infection among Tobago women.
Keywords: Human herpesvirus 8, sexual transmission, Caribbean
Introduction
Human herpesvirus 8 (HHV-8), a member of the Family Herpesviridae and subfamily Gammaherpesvirinae, is the causal agent of Kaposi's sarcoma (KS) and primary effusion lymphoma and has been associated with multicentric Castleman's disease[Cesarman et al., 1995; Chang et al., 1994; Dupin et al., 1999; Soulier et al., 1995]. HHV-8 seroprevalence varies geographically and is considered endemic in Mediterranean countries where seropositivity rates are as high as 31% in Southern Italy[Serraino et al., 2006], and in Sub-Saharan Africa, where seropositivity reaches 87% in Botswana Africa[Engels et al., 2000]. KS is relatively common in these areas: incidence is as high as 8.8 and 30 per 100,000 population per year in Italy and Sub-Saharan Africa, respectively[Engels et al., 2000; Ferlay et al., 2004; Serraino et al., 2006; Vitale et al., 2001]. In the United States and parts of Northern Europe where KS is not prevalent, low to moderate HHV-8 seropositivity rates (3% to 23%) have been reported[Ablashi et al., 1999; Hoffman et al., 2004; Hudnall et al., 2003; Moore and Chang, 2001; Pellett et al., 2003].
HHV-8 infection is known to be a sexually transmitted disease (STD); however, the presence of HHV-8 infection among pre-pubertal children suggests that HHV-8 can also be transmitted non-sexually. Studies have suggested non-sexual transmission (i.e., saliva exchange) as the primary mode of HHV-8 transmission in hyper-endemic KS areas like Sub-Saharan Africa[Moore and Chang, 2001; Pica and Volpi, 2007; Schulz et al., 2002]. However, in non-KS endemic areas like the U.S. and Northern Europe, sexual transmission is believed to be the predominant mode of HHV-8 transmission among high-risk populations such as men having sex with men[Engels et al., 2007; Martro et al., 2007; Pica and Volpi, 2007; Smith et al., 1999] .
In the Caribbean islands, six studies[Ablashi et al., 1999; Engels et al., 1999; Fernandez et al., 2002; Hoffman et al., 2004; Lennette et al., 1996; Manns et al., 1998] have examined HHV-8 infection; however, only two studies[Engels et al., 1999; Fernandez et al., 2002] examined possible modes of HHV-8 transmission. To have a better understanding of HHV-8 infection in the Caribbean island of Tobago, we used rates from a previously conducted cross-sectional study among 215 healthy Tobago women, 18-65 years of age. In this population, we examined HHV-8 seropositivity rates, and investigated possible modes of transmission by analyzing available information on sexual behavior.
Methods
Study population
The Tobago Cervical and Oral Cancer Screening Study is a cross-sectional study conducted among healthy women to examine the prevalence of oral and cervical human papillomavirus (HPV) infections in Tobago[Ragin et al., 2007]. Between July and August 2004, study participants were recruited by posters, flyers, television, radio public announcements, presentations in churches, seminars with health care workers at the Tobago hospital, and word of mouth. Women who had a terminal illness, did not sign an informed consent, or were not the ages 18-65 years were excluded from participation in the study. Two-hundred sixteen women were approached, and 215 of them agreed to participate in the screening. Blood, cervical, and oral epithelial cells were collected at the study visit. Of the 215 women, blood samples were available for all except 2 study participants; as a result, 213 women were included in the present study. Demographic and health information, family medical history, and sexual lifestyle behaviors were collected through the Tobago Cervical and Head and Neck Cancer Health Assessment which included the standardized questionnaire by the University of Pittsburgh Head and Neck Cancer Program as well as elements from the Centers for Disease Control and Prevention's Sexual Lifestyle Questionnaire[Ragin et al., 2007]. The sexual lifestyle part of the assessment was self-administered in a private room, and then enclosed in a sealed envelope by the participant. At the end of the study, all of the participants’ assessments were mailed to the University of Pittsburgh for data entry and data analysis.
HHV-8 seropositivity rates of Tobago men, ages 40-65 years, were used as a comparison. These men represented the control group in a case-control study on the association between HHV-8 seropositivity and prostate cancer conducted in Tobago between 1997 and 2000[Hoffman et al., 2004]. Control male participants were drawn from a population-based prostate cancer screening study[Bunker et al., 2002], and had normal digital rectal examination (DRE) and serum prostate-specific antigen (PSA) values < 4.0 ng/mL[Bunker et al., 2002]. An additional 97 men, ages 40-65 years, from the Tobago prostate cancer screening study (n = 3201) were added to the existing controls (n = 62), giving a total of 159 men between the ages 40-65 years; their seropositivity rates were compared to those observed in a subset of the Tobago women (n = 122) belonging to the same age range (40-65 years)[Hoffman et al., 2004].
Demographic information for men was collected from the Tobago Prostate Cancer Screening Survey questionnaire. Sexual lifestyle behaviors were not collected during this survey. All blood samples were tested for antibodies against HHV-8 lytic antigens at the University of Pittsburgh.
All study participants signed an informed consent that was approved by the University of Pittsburgh Biomedical Institutional Review Board (IRB) and the IRB of the Division of Health and Social Services, Tobago House of Assembly.
Laboratory methods
A modified HHV-8 monoclonal antibody-enhanced immunofluorescence assay (mIFA) that assessed lytic antigens using the BCBL-1 cell line, as described elsewhere[Jenkins et al., 2002], was used to test blood specimens (plasma from Tobago women & serum from Tobago men) for HHV-8 seropositivity at the University of Pittsburgh. A HHV-8 seropositive result was reported for specimens that gave fluorescence at the dilution cut-off value of 1:100. For each mIFA run, known HHV-8 positive and negative sera were included. All blood specimens were tested in duplicates per lab run on 2 different days. A 10% random sample of blood specimens were tested twice in a blinded fashion per assay run. Agreement between duplicates was substantial [Landis and Koch, 1977] (Kappa intra-batch = 0.78, 95% confidence interval [C.I.], 0.38 – 1.00, based on n = 33 sample pairs; Kappa inter-batch = 0.61, 95% C.I., .46 - .77, based on n = 202 sample pairs with non-missing mIFA test results from the first two laboratory runs). HHV-8 antibody titers were also determined by mIFA on serially diluted serum samples (1:100 to 1:51,200). All blood specimens that were analyzed by mIFA were assessed microscopically by the same reader.
Data Analysis
The overall frequency and age-specific frequency distribution were used to measure the seroprevalence of HHV-8 infection among the Tobago women. Pearson's Chi-square test was used to examine whether there were any differences in HHV-8 seropositivity rates among these age groups (18-29, 30-39, 40-49, 50-59, 60-65 years). HHV-8 antibody titer of women who tested HHV-8 seropositive was examined by age groups. Fisher's exact test was used to determine whether there was a difference in antibody titer (low, < 800 versus high, ≥ 800) between younger (ages 18-39 years) and older (ages 40-65 years) women.
The Mantel-Haenszel Chi-square test was used to determine if there was a difference in the overall seroprevalence of HHV-8 infection between Tobago women and men of comparable age, after the Breslow-Day test for homogeneity was conducted. The Pearson's Chi-square test or Fisher's exact test (if appropriate) was used to examine whether there were any differences in age-specific HHV-8 seropositivity rates for each age group (40-49, 50-59, 60-65 years) between genders.
Logistic regression analyses were performed to assess the independent contribution of each study variable (age, marital status, history of cancer, oral and cervical HPV detection, and sexual lifestyle behavior variables) to HHV-8 sero-status, and to analyze the interaction of age and the study variables on HHV-8 sero-status.
Nine sexual behaviors plus results from HPV oral and cervical screening listed in Table I were scored (0 or1) based on a prior hypothesis of their likelihood of having a positive association with HHV-8 seropositivity. A score of 0 was given to the reference group (no risky behavior and/or no presence of HPV DNA); a score of 1 was given to the exposed group that had the risky behavior and/or presence of HPV DNA (Table I). The studied variable “Number of partners in the past 12 months” was collapsed to none or one partner (score = 0) in comparison to two or more partners (score = 1). Women who did not answer all 9 sexual behavior questions and had no HPV oral or cervical results were excluded from this analysis. To alleviate converging problems in the logistic regression model, sexual behavior scores were divided in the following categories: category 1 (scores 1-4), category 2 (score 5), category 3 (score 6), category 4 (score 7), and category 5 (scores 8-11).
Table I.
Potential Risk Factors for Human Herpesvirus 8 (HHV-8) Infection in Tobago Women, ages 18-65 years
| Variables (Reference/ Exposed Groups) | Number in Reference/Exposed Groups | Odds ratio* | 95% Confidence Interval |
|---|---|---|---|
| Marital Status (married/widow vs. single/other) | 118/92 | .74 | .31-1.76 |
| History of Cancer (no/yes) | 198/8 | 2.17 | .40 – 11.94 |
| Age at 1st sexual intercourse (age ≥18 vs. age ≤ 17) | 106/87 | 2.51 | 1.09 – 5.78 |
| Number of lifetime partners (one partner vs. 2 or more partners) | 29/164 | .81 | .28 – 2.33 |
| Ever diagnosed with a STD (no vs. yes) | 175/21 | 1.84 | .61 – 5.50 |
| Sexual intercourse in the past 12 months (no vs. yes) | 25/170 | 1.65 | .42 – 6.42 |
| Number of partners in the past 12 months (none vs. one partner; none vs. two or more partners) | 17/153 | .76 | .19 – 3.13 |
| 17/43 | .79 | .17 – 3.76 | |
| Condom used during vaginal sex (sometimes/frequently vs. no) | 104/82 | .86 | .37 – 2.00 |
| Ever had oral sex (no vs. yes) | 59/128 | .63 | .26 – 1.55 |
| Condom used during oral sex (sometimes/always vs. no) | 16/111 | 1.18 | .25 – 5.73 |
| Partner performed oral sex (no vs. occasionally/frequently) | 33/153 | .61 | .22 – 1.69 |
| HPV detected in oral cavity (no vs. yes) | 197/15 | 1.58 | .42 – 5.97 |
| HPV detected in cervix (no vs. yes) | 137/75 | .92 | .4 – 2.11 |
Note.
= age-adjusted, HPV= Human papillomavirus, STD= sexually transmitted disease, vs. = versus.
All data analyses were conducted with SPSS version 12.0; an alpha level of 0.05, two-sided, was set for all the analyses.
Results
Study population characteristics
A total of 213 Tobago women, ages 18-65 years, was included in the study. The median age was 41.0 years (25-75% percentile, 35-48 years). Of these women, 83.1% identified themselves as African-Caribbean, 7% as mixed race, and 1.9% as East-Indian; 8% of the women did not report their race. The majority of women reported that they were married at the time of interview (51.6%).
The HHV-8 sero-status of 159 Tobago men, ages 40-65 years, were compared to that of the subset of Tobago women of comparable age (n = 122). The median age of the male study population was 59.0 years (25-75% percentile, 53-63 years). The median age of the female subset study population was 47.0 years (25-75% percentile, 45-53 years). The majority of the female subset and of the men reported that they were married at the time of the interview (62.3% and 71.7%, respectively).
HHV-8 seroprevalence
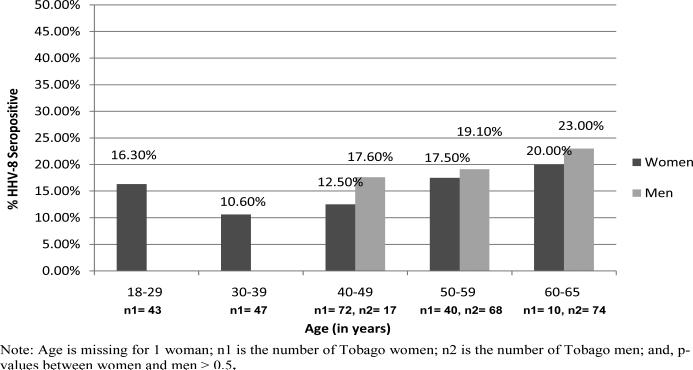
An overall seroprevalence of 14.1% (95% C.I., 10 - 19%) for antibodies against HHV-8 lytic antigens was found among Tobago women. HHV-8 seroprevalence point estimates varied across age groups, with the lowest prevalence observed in the age group 30-39 years, and the highest prevalence observed in the age group 60-65 years (Figure 1). However, there were no significant differences in HHV-8 age-specific seropositivity rates among the age groups (18-29, 30-39, 40-49, 50-59, 60-65 years) (p-value = .835).
Figure 1.
HHV-8 Seropositivity Rates According to Age in Tobago Women, ages 18-65 years, and Tobago Men, ages 40-65 years
HHV-8 antibody titer distribution
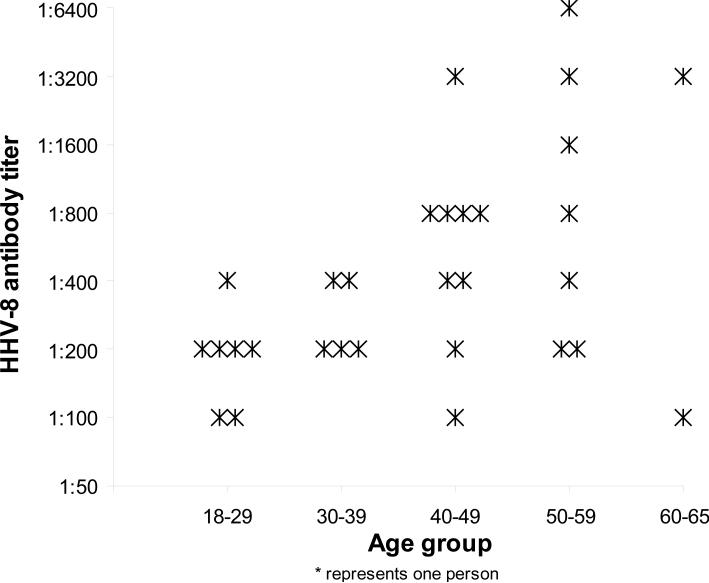
There were 30 HHV-8 seropositive women; their IgG antibody titer against HHV-8 lytic antigens ranged from 1:100 to 1:6400 (Figure 2). The median antibody titer was 400 (25-75% percentile, 200-800) among the seropositive women. Overall, women, ages 40-65 years, were more likely to have an antibody titer of ≥ 800 (8.2%, n = 10/122) than women, ages 18-39 years (0%, n = 0/90) (p-value = .006).
Figure 2.
The Frequency of HHV-8 Antibody Titer in HHV-8 Seropositive Tobago Women by Age Groups (N=30)
Comparison of HHV-8 seropositivity rates between Tobago women and men
Across age groups 40-65 years, HHV-8 seropositivity rates were 14.8% (95% C.I., 9.0 - 22.3%) in women and 20.8% (95% C.I., 14.7 - 27.9%) in men. Adjusting for age, the test of homogeneity (p-value = .946) and differences in HHV-8 seropositivity between men and women were not statistically significant (p-value = .741) (Figure 1). However, in each age group, Tobago women had a lower HHV-8 seropositivity rate than their male counterparts (Figure 1).
Risk factors for HHV-8 infection
HHV-8 seropositivity was analyzed in relation to sexual lifestyle behavior in women (Table I). HHV-8 seropositivity was not statistically associated with any sexual behavior variables, except for “age at first sexual intercourse”. Tobago women who reported to have their first sexual intercourse at ≤ 17 years of age were 2.51-fold more likely (95% C.I., 1.09-5.78) to be HHV-8 seropositive than women who reported to have their first sexual intercourse at ≥ 18 years of age. In addition, marital status, history of cancer, and HPV DNA detected in oral and cervical cavity were not associated with HHV-8 sero-status.
No significant interactions were observed between age and any studied variable
Out of the 9 sexual behavior variables plus 2 HPV screening variables listed in Table I, the combined sexual behavior scores (including HPV screening results) ranged from 1 to 9 with a mean score of 6.3 (95% C.I., 6-6.5) among 115 women. In the logistic regression model, the number of women in the combined sexual behavior score categories were the following: 15 in category 1 (scores 1-4), 15 in category 2 (score 5), 30 in category 3 (score 6), 35 in category 4 (score 7), and 20 in category 5 (scores 8-11).There was no statistically significant association between HHV-8 seropositivity and the combined sexual behavior scores after adjusting for age (p-value = .282). There was no statistically significant association with HHV-8 seropositivity when the combined sexual behavior scores were divided into two categories: low score of < 6 (6.7% seropositive, n = 2/30) and high score of > = 6 (17.6% seropositive, n = 15/85) (p-value = .231).
Discussion
This study indicates that HHV-8 infection is present among Tobago women at a frequency similar to that previously reported in men[Engels et al., 2007; Hoffman et al., 2004; Plancoulaine et al., 2004; Serraino et al., 2006]. The seropositivity rate observed among these women is comparable to rates found among women in the Mediterranean area[Cattani et al., 2003; Masini et al., 2000; Tanzi et al., 2005]. As for KS, this incidence has been reported to range from 0.2 to 2.8 per 100,000 population per year among women in the Mediterranean region[Davidovici et al., 2001; Tanzi et al., 2005; Vitale et al., 2001]. In Tobago, no cases of KS have been reported for women and men during the period of 1994-1997[Dr. Elizabeth Quamina Cancer Registry, 2008; Ferlay et al., 2004]. The lack of KS in Tobago despite the moderate rates of HHV-8 infection may be due to the immune status of the population, genetic make-up that prevents disease development/progression to clinical stage, possible environmental factors (for example, diet), under-reporting of KS to health officials, or a small population size.
A gradual increase in HHV-8 seropositivity was observed among Tobago women, from ages 30-39 years to ages 60-65 years. Women ages 18-29 years had a higher HHV-8 seropositivity in comparison to women ages 30-49 years; this higher seropositivity among younger women may be due to a cohort effect. Women ≥ 40 years of age had a higher prevalence of antibody titer ≥ 1:800 than women ages ≤ 39 years. The reason for the higher antibody titers among older women may be due to cumulative exposure to HHV-8. As for the male controls in Hoffman et al. study, the overall median antibody titer among these seropositive men (n = 15/159), ages 40-65 years, was lower (median = 400) in comparison to women (n = 18/122) of similar ages (median = 800)[Hoffman et al., 2004]. The reason for the lower median antibody titers among men in comparison to women is not known.
As for gender and HHV-8 seropositivity with age, the overall HHV-8 seropositivity rate as well as the rates across age groups (40-49, 50-59, 60-65 years) were higher in Tobago men in comparison to Tobago women; however, these seropositivity rates did not significantly differ. This lack of significant gender difference in seropositivity rates is consistent with studies conducted in Sub-Saharan Africa[Plancoulaine et al., 2004], Italy[Serraino et al., 2006], and the United States[Engels et al., 2007; Hudnall et al., 2003].
Few studies have examined HHV-8 infection in Caribbean populations. Studies conducted in Dominican Republic[Lennette et al., 1996], Cuba[Fernandez et al., 2002], and Haiti[Lennette et al., 1996] found moderate HHV-8 seropositivity rates for antibodies against HHV-8 lytic antigens, 13%, 16.9%, and 29%, respectively. These seropositivity rates are similar to rates found in Tobago. Also, no gender difference in HHV-8 seropositivity rate found in the Cuba study[Fernandez et al., 2002] is consistent to the present study's finding; the study conducted in Dominican Republic and Haiti did not report gender seropositivity rates[Lennette et al., 1996].
In the present study, HHV-8 seropositivity rates were higher than rates reported in other Caribbean populations. For example, in Jamaica, three studies reported low HHV-8 seropositivity rates of 0.68% among women attending gynecology clinics, 2.7% among blood donors (ages 18 to 63 years), and 3.6% among blood donors (ages 18 to 64 years); in these studies, a whole virus enzyme-linked immunoassay and/or immunofluorescence assay testing for antibodies against lytic and latent HHV-8 antigens were used [Ablashi et al., 1999; Engels et al., 1999; Manns et al., 1998]. In Trinidad, HHV-8 seropositivity rate of 1.3% among female and male blood donors (ages 18 to 64 years) was found by using a whole virus enzyme-linked immunoassay [Ablashi et al., 1999]. The low HHV-8 seropositivity rates found in Trinidad and Jamaica populations in comparison to the present study population may be due to differences in study populations or in the sensitivity of the serological assay used to examine HHV-8 infection. The serological assay, mIFA, used in the present study has been reported to have a sensitivity of 89.9% and specificity of 97.5% [Pellett et al., 2003]. Based on studies that used serological assays to examine patterns of change in HHV-8 antibody responses, antibody titers have shown to decrease over time in some individuals[Quinlivan et al., 2001; Zavitsanou et al., 2006]. Therefore, HHV-8 serological assays may not be sensitive enough to detect all HHV-8 exposed individuals. The decline in antibody titers suggests that HHV-8 infection may be underestimated in cross-sectional studies.
In this study, a significant association between HHV-8 sero-status and age at first sexual intercourse was found among women. A study conducted among female prostitutes and age-matched controls in Oviedo and Barcelona, Spain found a similar association between HHV-8 seropositive status and age at first sexual intercourse[de Sanjose et al., 2002]; but, this association was not confirmed by studies conducted among U.S. women [Cannon et al., 2001; Goedert et al., 2003]. A study conducted in Cuba which included women and men did not find an association between HHV-8 seropositivity and age at first sexual intercourse[Fernandez et al., 2002]. The minimal association between HHV-8 seropositivity and age at first sexual intercourse reported in the present study suggests that sexual activity may not be the predominate mode of HHV-8 transmission in Tobago.
Sexual transmission may not be the only mode of HHV-8 transmission in Tobago. In this study, several variables that assess sexual lifestyle behaviors were not associated with HHV-8 sero-status among the women. In addition, the detection of HPV DNA in oral or cervical cavity was not associated with HHV-8 seropositivity. The lack of associations between seropositivity and several sexual behavior variables is consistent with previous studies that examined similar sexual lifestyle behaviors and HHV-8 sero-status among women[Engels et al., 2007; Goedert et al., 2003].
Non-sexual transmission of HHV-8 may play a role in Tobago. Evidence of HHV-8 infection among pre-pubertal children in other populations suggests non-sexual routes of HHV-8 transmission, and point at saliva as the probable route of transmission[Cattani et al., 2003; Cunha et al., 2005; Pica and Volpi, 2007; Rezza et al., 2000]. Saliva is known to have the highest viral load and viral shedding in comparison to other sites such as peripheral blood mononuclear cells, urine, semen, and prostate[Cannon et al., 2003; Gandhi et al., 2004; Pauk et al., 2000; Taylor et al., 2004]. Due to the age of the present study population, ≥18 years of age, evidence of non-sexual transmission through oral transmission was not observed. However, to evaluate whether oral transmission through sexual behaviors was associated with HHV-8 sero-status, women who participated in oral sexual activities and/or had the presence of HPV DNA in their oral cavity were examined and found to have higher risk for HHV-8 infection than their counterparts. Tobago women were less likely to be seropositive if they ever had oral sex or if they had partners who performed oral sex on them; in contrast, they were more likely to be seropositive if they did not use condoms during oral sex or if HPV was detected in their oral cavity. However, none of these associations were statistically significant.
There are some limitations in the present study. One limitation is that for Tobago men, sexual lifestyle behavior data were not available. A recent study has shown sexual lifestyle behaviors such as duration of sexual activity in years, the number of lifetime sex partners, and co-infection with other STDs to be associated with HHV-8 seropositive status among heterosexual men[Engels et al., 2007]. Another limitation is the limited power to examine the association between HHV-8 sero-status and sexual lifestyle behaviors among women. Sexual lifestyle behavior variables were missing in some of the women's responses; however, this missing data was less than 14% for most variables.
The present study is the first to examine the relationship between several sexual lifestyles behaviors and HHV-8 infection in the Caribbean. A good estimation of HHV-8 seroprevalence among women, ages 18 to 65 years, based on a 95% confidence interval width of < 10% was calculated. This study had enough study participants to detect a doubling of HHV-8 seropositivity (from 15% to 30%) with 80% power to test at alpha level of 0.05, two-sided.
In conclusion, the present study provides evidence that HHV-8 infection is present in Tobago. However, sexual activity may not be a major contributor in acquiring HHV-8 infection. Non-sexual transmission of HHV-8 may also occur in Tobago; however, evidence of this transmission remains not known. Understanding possible modes of HHV-8 transmission will help design programs aimed at interrupting this viral infection in populations. Further studies, especially longitudinal studies, are needed to examine HHV-8 infection, transmission, and its relationship with possible associated malignancies in Tobago.
Acknowledgment
The authors would like to thank the staff in the Tobago Health Studies Office in Scarborough, Tobago, Trinidad & Tobago for their help and support in conducting this research study.
Sources of Financial Support: This study was supported in part by 2R25 CA057703 11A2 (Education Program in Cancer Research) to A.C.M., 1 P20 CA132385-01to E.T., and R13 CA130596A and 1KL2 RR024154-02 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research to C.C.R.
Footnotes
The authors do not have any information to disclosure that might pose any conflict of interest.
Part of the study's data was presented at the following meeting: Proceedings of the 98th Annual Meeting of the American Association for Cancer Research; April 2007; Los Angeles, CA. Abstract # 1746.
Conflict of Interest:
Alicia C. McDonald, “No conflict”
Camille C. Ragin, “No conflict”
Frank J. Jenkins, “No conflict”
Joel Weissfeld, “No conflict”
John Wilson, “No conflict”
Victor W. Wheeler, “No conflict”
Jaquie B. Wilson, “No conflict”
Clareann H. Bunker, “No conflict”
Emanuela Taioli, “No conflict”
References
- Ablashi D, Chatlynne L, Cooper H, Thomas D, Yadav M, Norhanom AW, Chandana AK, Churdboonchart V, Kulpradist SA, Patnaik M, Liegmann K, Masood R, Reitz M, Cleghorn F, Manns A, Levine PH, Rabkin C, Biggar R, Jensen F, Gill P, Jack N, Edwards J, Whitman J, Boshoff C. Seroprevalence of human herpesvirus-8 (HHV-8) in countries of Southeast Asia compared to the USA, the Caribbean and Africa. Br J Cancer. 1999;81(5):893–897. doi: 10.1038/sj.bjc.6690782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker CH, Patrick AL, Konety BR, Dhir R, Brufsky AM, Vivas CA, Becich MJ, Trump DL, Kuller LH. High prevalence of screening-detected prostate cancer among Afro-Caribbeans: the Tobago Prostate Cancer Survey. Cancer Epidemiol Biomarkers Prev. 2002;11(8):726–729. [PubMed] [Google Scholar]
- Cannon MJ, Dollard SC, Black JB, Edlin BR, Hannah C, Hogan SE, Patel MM, Jaffe HW, Offermann MK, Spira TJ, Pellett PE, Gunthel CJ. Risk factors for Kaposi's sarcoma in men seropositive for both human herpesvirus 8 and human immunodeficiency virus. Aids. 2003;17(2):215–222. doi: 10.1097/00002030-200301240-00012. [DOI] [PubMed] [Google Scholar]
- Cannon MJ, Dollard SC, Smith DK, Klein RS, Schuman P, Rich JD, Vlahov D, Pellett PE, Group HIVERS Blood-borne and sexual transmission of human herpesvirus 8 in women with or at risk for human immunodeficiency virus infection. N Engl J Med. 2001;344(9):637–643. doi: 10.1056/NEJM200103013440904. [DOI] [PubMed] [Google Scholar]
- Cattani P, Cerimele F, Porta D, Graffeo R, Ranno S, Marchetti S, Ricci R, Capodicasa N, Fuga L, Amico R, Cherchi G, Gazzilli M, Zanetti S, Fadda G. Age-specific seroprevalence of Human Herpesvirus 8 in Mediterranean regions. Clin Microbiol Infect. 2003;9(4):274–279. doi: 10.1046/j.1469-0691.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332(18):1186–1191. doi: 10.1056/NEJM199505043321802. [see comment] [DOI] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266(5192):1865–1869. doi: 10.1126/science.7997879. [see comment] [DOI] [PubMed] [Google Scholar]
- Cunha AMG, Caterino-de-Araujo A, Costa SCB, Santos-Fortuna E, Boa-Sorte NCA, Goncalves MS, Costa FF, Galvao-Castro B. Increasing seroprevalence of human herpesvirus 8 (HHV-8) with age confirms HHV-8 endemicity in Amazon Amerindians from Brazil. J Gen Virol. 2005;86(Pt 9):2433–2437. doi: 10.1099/vir.0.81087-0. [DOI] [PubMed] [Google Scholar]
- Davidovici B, Karakis I, Bourboulia D, Ariad S, Zong J, Benharroch D, Dupin N, Weiss R, Hayward G, Sarov B, Boshoff C. Seroepidemiology and molecular epidemiology of Kaposi's sarcoma-associated herpesvirus among Jewish population groups in Israel. J Natl Cancer Inst. 2001;93(3):194–202. doi: 10.1093/jnci/93.3.194. [DOI] [PubMed] [Google Scholar]
- de Sanjose S, Marshall V, Sola J, Palacio V, Almirall R, Goedert JJ, Bosch FX, Whitby D. Prevalence of Kaposi's sarcoma-associated herpesvirus infection in sex workers and women from the general population in Spain. Int J Cancer. 2002;98(1):155–158. doi: 10.1002/ijc.10190. [DOI] [PubMed] [Google Scholar]
- Dr. Elizabeth Quamina Cancer Registry TNCRoTT . North Central Regional Health Authority; Mount Hope, Trinidad W.I.: 2008. [Google Scholar]
- Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande JP, Weiss RA, Alitalo K, Boshoff C. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc Natl Acad Sci U S A. 1999;96(8):4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels EA, Atkinson JO, Graubard BI, McQuillan GM, Gamache C, Mbisa G, Cohn S, Whitby D, Goedert JJ. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J Infect Dis. 2007;196(2):199–207. doi: 10.1086/518791. [see comment] [DOI] [PubMed] [Google Scholar]
- Engels EA, Eastman H, Ablashi DV, Wilks RJ, Braham J, Manns A. Risk of transfusion-associated transmission of human herpesvirus 8. J Natl Cancer Inst. 1999;91(20):1773–1775. doi: 10.1093/jnci/91.20.1773. [DOI] [PubMed] [Google Scholar]
- Engels EA, Sinclair MD, Biggar RJ, Whitby D, Ebbesen P, Goedert JJ, Gastwirth JL. Latent class analysis of human herpesvirus 8 assay performance and infection prevalence in sub-saharan Africa and Malta. Int J Cancer. 2000;88(6):1003–1008. doi: 10.1002/1097-0215(20001215)88:6<1003::aid-ijc26>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Bray F, Pisani P, Parkin D. Globocan 2002: Cancer Incidence, Mortality, and Prevalence Worldwide IARC CancerBase. IARC Press; 2004. [Google Scholar]
- Fernandez L, Serraino D, Rezza G, Lence J, Ortiz RM, Cruz T, Vaccarella S, Sarmati L, Andreoni M, Franceschi S. Infection with human herpesvirus type 8 and human T-cell leukaemia virus type 1 among individuals participating in a case-control study in Havana City, Cuba. Br J Cancer. 2002;87(11):1253–1256. doi: 10.1038/sj.bjc.6600613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Koelle DM, Ameli N, Bacchetti P, Greenspan JS, Navazesh M, Anastos K, Greenblatt RM. Prevalence of human herpesvirus-8 salivary shedding in HIV increases with CD4 count. J Dent Res. 2004;83(8):639–643. doi: 10.1177/154405910408300811. [DOI] [PubMed] [Google Scholar]
- Goedert JJ, Charurat M, Blattner WA, Hershow RC, Pitt J, Diaz C, Mofenson LM, Green K, Minkoff H, Paul ME, Thomas DL, Whitby D, Women and Infants Transmission S Risk factors for Kaposi's sarcoma-associated herpesvirus infection among HIV-1-infected pregnant women in the USA. Aids. 2003;17(3):425–433. doi: 10.1097/00002030-200302140-00017. [DOI] [PubMed] [Google Scholar]
- Hoffman LJ, Bunker CH, Pellett PE, Trump DL, Patrick AL, Dollard SC, Keenan HA, Jenkins FJ. Elevated seroprevalence of human herpesvirus 8 among men with prostate cancer. J Infect Dis. 2004;189(1):15–20. doi: 10.1086/380568. [DOI] [PubMed] [Google Scholar]
- Hudnall SD, Chen T, Rady P, Tyring S, Allison P. Human herpesvirus 8 seroprevalence and viral load in healthy adult blood donors. Transfusion. 2003;43(1):85–90. doi: 10.1046/j.1537-2995.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- Jenkins FJ, Hoffman LJ, Liegey-Dougall A. Reactivation of and primary infection with human herpesvirus 8 among solid-organ transplant recipients. J Infect Dis. 2002;185(9):1238–1243. doi: 10.1086/340237. [DOI] [PubMed] [Google Scholar]
- Landis J, Koch G. The Measurement of Observer Agreement for Categorical Data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Lennette ET, Blackbourn DJ, Levy JA. Antibodies to human herpesvirus type 8 in the general population and in Kaposi's sarcoma patients. Lancet. 1996;348(9031):858–861. doi: 10.1016/S0140-6736(96)03240-0. [see comment] [DOI] [PubMed] [Google Scholar]
- Manns A, Strickler HD, Hanchard B, Manassaram DM, Waters D, Ablashi DV. Age- and sex-specific seroprevalence of human herpesvirus 8 in Jamaica. J Natl Cancer Inst. 1998;90(14):1102–1104. doi: 10.1093/jnci/90.14.1102. [comment] [DOI] [PubMed] [Google Scholar]
- Martro E, Esteve A, Schulz TF, Sheldon J, Gambus G, Munoz R, Whitby D, Casabona J, Euro-Shaks study g Risk factors for human Herpesvirus 8 infection and AIDS-associated Kaposi's sarcoma among men who have sex with men in a European multicentre study. Int J Cancer. 2007;120(5):1129–1135. doi: 10.1002/ijc.22281. [DOI] [PubMed] [Google Scholar]
- Masini C, Abeni DD, Cattaruzza MS, Capuano M, Pedicelli C, Cerimele F, Pasquini P, Cerimele D, Fadda G, Cattani P. Antibodies against human herpesvirus 8 in subjects with non-venereal dermatological conditions. Br J Dermatol. 2000;143(3):484–490. doi: 10.1111/j.1365-2133.2000.03699.x. [DOI] [PubMed] [Google Scholar]
- Moore PS, Chang Y. In: Kaposi's Sarcoma-Associated Herpesvirus 8 (HHV-8) Knipe D, Howley P, editors. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. pp. 2996–3018. [Google Scholar]
- Pauk J, Huang ML, Brodie SJ, Wald A, Koelle DM, Schacker T, Celum C, Selke S, Corey L. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med. 2000;343(19):1369–1377. doi: 10.1056/NEJM200011093431904. [see comment] [DOI] [PubMed] [Google Scholar]
- Pellett PE, Wright DJ, Engels EA, Ablashi DV, Dollard SC, Forghani B, Glynn SA, Goedert JJ, Jenkins FJ, Lee TH, Neipel F, Todd DS, Whitby D, Nemo GJ, Busch MP, Retrovirus Epidemiology Donor S Multicenter comparison of serologic assays and estimation of human herpesvirus 8 seroprevalence among US blood donors. Transfusion. 2003;43(9):1260–1268. doi: 10.1046/j.1537-2995.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- Pica F, Volpi A. Transmission of human herpesvirus 8: an update. Curr Opin Infect Dis. 2007;20(2):152–156. doi: 10.1097/QCO.0b013e3280143919. [DOI] [PubMed] [Google Scholar]
- Plancoulaine S, Abel L, Tregouet D, Duprez R, van Beveren M, Tortevoye P, Froment A, Gessain A. Respective roles of serological status and blood specific antihuman herpesvirus 8 antibody levels in human herpesvirus 8 intrafamilial transmission in a highly endemic area. Cancer Res. 2004;64(23):8782–8787. doi: 10.1158/0008-5472.CAN-04-2000. [DOI] [PubMed] [Google Scholar]
- Quinlivan EB, Wang RX, Stewart PW, Kolmoltri C, Regamey N, Erb P, Vernazza PL, Swiss HIVCS Longitudinal sero-reactivity to human herpesvirus 8 (KSHV) in the Swiss HIV Cohort 4.7 years before KS. J Med Virol. 2001;64(2):157–166. doi: 10.1002/jmv.1031. [DOI] [PubMed] [Google Scholar]
- Ragin CCR, Wheeler VW, Wilson JB, Bunker CH, Gollin SM, Patrick AL, Taioli E. Distinct distribution of HPV types among cancer-free Afro-Caribbean women from Tobago. Biomarkers. 2007;12(5):510–522. doi: 10.1080/13547500701340384. [DOI] [PubMed] [Google Scholar]
- Rezza G, Tchangmena OB, Andreoni M, Bugarini R, Toma L, Bakary DK, Glikoutou M, Sarmati L, Monini P, Pezzotti P, Ensoli B. Prevalence and risk factors for human herpesvirus 8 infection in northern Cameroon. Sex Transm Dis. 2000;27(3):159–164. doi: 10.1097/00007435-200003000-00008. [DOI] [PubMed] [Google Scholar]
- Schulz TF, Sheldon J, Greensill J. Kaposi's sarcoma associated herpesvirus (KSHV) or human herpesvirus 8 (HHV8). Virus Res. 2002;82(12):115–126. doi: 10.1016/s0168-1702(01)00394-x. [DOI] [PubMed] [Google Scholar]
- Serraino D, Cerimele D, Piselli P, Aztori L, Farchi F, Carletti F, Navarra A, Masala MV, Rezza G. Infection with human herpesvirus type 8 and Kaposi's sarcoma in Sardinia. Infection. 2006;34(1):39–42. doi: 10.1007/s15010-006-5025-8. [DOI] [PubMed] [Google Scholar]
- Smith NA, Sabin CA, Gopal R, Bourboulia D, Labbet W, Boshoff C, Barlow D, Band B, Peters BS, de Ruiter A, Brown DW, Weiss RA, Best JM, Whitby D. Serologic evidence of human herpesvirus 8 transmission by homosexual but not heterosexual sex. J Infect Dis. 1999;180(3):600–606. doi: 10.1086/314926. [DOI] [PubMed] [Google Scholar]
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86(4):1276–1280. [see comment] [PubMed] [Google Scholar]
- Tanzi E, Zappa A, Caramaschi F, Amendola A, Lasagna D, Gatti L, Ascoli V, Rezza G, Zanetti AR. Human herpesvirus type 8 infection in an area of Northern Italy with high incidence of classical Kaposi's sarcoma. J Med Virol. 2005;76(4):571–575. doi: 10.1002/jmv.20400. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Chohan B, Lavreys L, Hassan W, Huang M-L, Corey L, Ashley Morrow R, Richardson BA, Mandaliya K, Ndinya-Achola J, Bwayo J, Kreiss J. Shedding of human herpesvirus 8 in oral and genital secretions from HIV-1-seropositive and - seronegative Kenyan women. J Infect Dis. 2004;190(3):484–488. doi: 10.1086/421466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale F, Briffa DV, Whitby D, Maida I, Grochowska A, Levin A, Romano N, Goedert JJ. Kaposi's sarcoma herpes virus and Kaposi's sarcoma in the elderly populations of 3 Mediterranean islands. Int J Cancer. 2001;91(4):588–591. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1089>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Zavitsanou A, Sypsa V, Petrodaskalaki M, Psichogiou M, Katsoulidou A, Boletis J, Hadjiconstantinou V, Karalis D, Kalapothaki V, Hatzakis A. Human herpesvirus 8 infection in hemodialysis patients. Am J Kidney Dis. 2006;47(1):167–170. doi: 10.1053/j.ajkd.2005.09.019. [DOI] [PubMed] [Google Scholar]