Abstract
The primary function of IL-7 is to promote maturation and survival of T cells. Through microarray expression analysis, we previously observed that human blood eosinophils express mRNA for IL-7Rα (CD127) and its common gamma chain (γc, CD132). The purpose of this study was to determine if eosinophils have functional IL-7 receptors and to assess the potential contribution of IL-7 to eosinophilic airway inflammation by evaluating its presence in bronchoalveolar lavage (BAL) fluid of subjects with atopic asthma before and after segmental bronchoprovocation with allergen. Immunoblot analysis revealed that CD127 is present in highly purified human blood eosinophils. Furthermore, eosinophils responded to IL-7 with phosphorylation of STAT5, upregulation of the activation marker CD69, and prolonged survival. Neutralization of GM-CSF, but not IL-5, significantly blunted these functional responses, suggesting that IL-7 mediates its effects by promoting eosinophil release of autologous GM-CSF. Notably, the suppressive effect of anti-GM-CSF on STAT5 phosphorylation occurred within 10 min of eosinophil exposure to IL-7. Thus, IL-7 likely activates eosinophil release of preformed, rather than newly synthesized GM-CSF. The biological relevance of IL-7 to eosinophilia in vivo was implicated in a study of airway allergen challenge in allergic asthmatics. IL-7 concentrations in BAL fluid increased significantly 48 h after segmental allergen challenge and were highly correlated with BAL eosinophils (r=0.7, p<0.001). In conclusion, the airway response to allergen is associated with the generation of IL-7, which may contribute to airway inflammation by promoting enhanced eosinophil activation and survival. Activation of eosinophils is a novel function for IL-7.
Keywords: eosinophils, allergy, lung
INTRODUCTION
IL-7 is required for T cell development in the thymus. In fact, a human mutation in the alpha chain of the IL-7 receptor (IL-7Rα/CD127) results in a severe combined immunodeficiency (SCID) phenotype characterized by a lack of T cells, with no discernable effect on NK cells or B cells (1,2). After thymic development, IL-7 is important for the homeostasis of naive T cell populations and for the generation and survival of memory CD4+ T cells (3). Because of its potent effect on survival and expansion of T cell populations, IL-7 is being developed as a therapeutic agent for T cell reconstitution (4–6) and neutralization of IL-7 has been suggested as a potential therapeutic for diseases such as rheumatoid arthritis, inflammatory bowel disease, atherosclerosis, multiple sclerosis and solid epithelial tumors (7–11). IL-7 is produced primarily by epithelial cells and is found predominantly in the thymus, bone marrow, and intestine (12).
The receptor for IL-7 is composed of a 60–90 kDa cytokine-specific α chain (IL-7Rα/CD127) and a 72 kDa common γ chain (γc/CD132) that is the primary signaling component for the IL-7 as well as IL-2, IL-4, IL-9, IL-15, and IL-21 receptors in several cell systems (12). Dimerization of IL-7Rα/γc activates their associated kinases Jak1 and Jak3, respectively, with activation of STAT5 being a primary downstream signaling event (12).
In addition to T cells, there are scattered reports of CD127 expression and/or cellular responsiveness to IL-7 by other cell types including human peripheral monocytes (13), endothelial cells (14), fibroblasts (15), and eosinophil progenitors in the bone marrow (16). Through a microarray analysis of eosinophil gene expression, we previously observed that human blood eosinophils express mRNA for CD127 and CD132 (17). However, the expression of functional IL-7Rα on mature circulating eosinophil has not been reported.
Eosinophils are associated with allergic diseases including asthma and are elevated in the lung after allergen exposure. Eosinophils are thought to contribute to airway obstruction, hyperresponsiveness, and tissue remodeling that are characteristic of asthma (18). Furthermore, there is growing evidence that human (19–21) as well as murine (22) eosinophils are important regulators of T cell recruitment and function.
The purpose of the current study was two-fold. First, using in vitro studies, we aimed to determine if highly purified human blood eosinophils express functional IL-7 receptors and to characterize the response of eosinophils to IL-7. Second, we sought to determine whether IL-7 is present in the airway of atopic subjects with asthma, whether it is enhanced by airway allergen challenge, and whether levels of IL-7 are associated with airway eosinophilia. Our overarching hypothesis was that IL-7 contributes to allergic airway inflammation associated with asthma through activation of eosinophils.
Materials and Methods
Human subjects
For the ex vivo analysis of IL-7 receptor function on human eosinophils, peripheral blood was obtained from normal or allergic donors. Subjects for the bronchoscopy study were allergic (skin prick test positive), with mild asthma (mean ± SEM for FEV1 was 97 ± 2 % of predicted and for methacholine PC20 was 5.7 ± 1.9 mg/ml) and included 9 males and 9 females between the ages of 19 and 32. Subjects were non-smokers, did not have a respiratory infection or asthma exacerbations within 30 days of study, and had not received long-acting β-agonists within 2 days, antihistamines or leukotriene antagonists within 7 days, or corticosteroids within 30 days of study enrollment. The University of Wisconsin-Madison Health Sciences Human Subjects Committee approved the study, and informed consent was obtained from all subjects.
Cell purification
Eosinophils were purified from heparinized peripheral blood as previously described (19). Briefly, the granulocyte fraction was obtained after centrifugation of HBSS-diluted blood over Percoll (1.090 g/ml), RBCs were lysed, and neutrophils, T cells, and monocytes were depleted, respectively, by anti-CD16, anti-CD3, and anti-CD14 immunomagnetic beads (AutoMac system, Miltenyi Biotec.). The resulting eosinophils were >99% pure and >97% viable. T cells, used as a positive control for immunoblotting, were purified from peripheral blood mononuclear cells by a negative selection kit for CD4+ T cells (Miltenyi Biotec).
Survival analysis
Purified blood eosinophils (1×106/ml) were cultured in 96-well flat-bottom tissue culture plates in 200 µl of RPMI-1640 (Cambrex) containing 1% fetal calf serum and 1% penicillin-streptomycin (Invitrogen). Multiple lots of recombinant human IL-7 at varying concentrations were tested from different manufactures including R&D Systems (Cat. No. 207-IL/CF), BD Biosciences (Cat. No. 554608), and Abcam (Cat. No. ab9629-10). GM-CSF (100 pg/ml, BD Biosciences) was used as a positive control. Neutralizing anti-GM-CSF Ab (Clone BVD2-23B6, Biosource International) and anti-IL-5 Ab (clone 14611.3, R&D Systems) were used at a concentration of 20 µg/ml. Each experimental condition was tested in duplicate or triplicate. Eosinophil viability was determined at 0, 48, and 72 h by trypan blue exclusion. At 0 h, the contents of three individual wells were counted. To obtain an accurate determination of cell survival, a consistent method for collecting and counting cells was established. Cell suspensions were pipette-mixed 25 times with a 100 µl pipetteman. A 1:1 dilution of cells and trypan blue (0.4 %) was prepared, pipette-mixed twelve times and 10 µl was added to a hemacytometer. Numbers of viable and dead cells were determined in eight × 1 mm2 areas of the counting chamber. Survival was determined by dividing the number of live cells at each time point by the number of live cells at 0 h and multiplying by 100. In PBMC “add back” experiments, eosinophil viability was determined by exclusion of propidium iodide (3 µg/ml) using flow cytometric analysis.
Flow cytometric analysis
For cell surface analysis of CD69, 1 × 105 eosinophils were stained using PE-conjugated anti-CD69 (clone TP1.55.3, Immunotech-Beckman Coulter) as previously described (23). Propidium iodide (3 µg/ml) was added to the stained cellular suspensions before analysis to allow electronic omission of any dead cells.
Detection of intracellular phospho-STAT5 by flow cytometric analysis was performed as previous described (19). In brief, purified blood eosinophils were incubated with 50 nM of IL-7 for 20 min, fixed with 2 % paraformaldehyde, permeabilized and stained with PE-conjugated anti-phospho-STAT 5 (Tyr -694, clone 47, BD Biosciences) or mouse IgG isotype control (BD Biosciences). Cells were analyzed with a BD FACSCaliber™ or FACScanII™ using 488nm wavelength laser excitation. Eosinophil populations were gated as previously described (23) and 5,000–10,000 photoelectric events were recorded. Data analyses were preformed with CellQuest version 3.3 software (BD Biosciences).
Immunoblotting
For analysis of STAT 5 phosphorylation, purified eosinophils were stimulated for 10 and 20 min in the presence or absence IL-7 (50 nM) and cell lysates were prepared as previously described (24). In some experiments, eosinophils were pretreated with neutralizing Ab to GM-CSF (Biosource International) or isotype control (BD Pharmingen). Eosinophils (at the concentration of 25 × 106/ml) were sonicated in lysis buffer (20 mM Tris, pH 7.4, 137 mM NaCl, 1mM EDTA, 1 mM sodium ortho-vanadate, 20 mM β-glycerophosphate, 10 mM NaF, 1% Nonidet P-40, 0.25% deoxycholate, and 0.1% SDS) and insoluble material was removed by centrifugation at 15,800 × g/10 min/4°C. The soluble fraction was assayed for total protein and immunoblotted as previously described (24). Briefly, samples were resolved by SDS-PAGE, proteins were transferred to a PVDF membrane and probed with antibody specific for tyrosine-phosphorylated STAT5 (anti-pSTAT5A/B (Y694/Y699) Ab, Cat. No. 9351, Cell Signaling Technologies). Detection of immunoreactive bands was performed using Super Signal West Dura or Femto chemiluminescent substrate (Pierce). Equal protein loading was assured by immunoblotting the same cell lysates with antibody to total ERK1/2 (Upstate, Cat. No. 06-182).
For detection of the IL-7Rα subunit, CD127, by immunoblotting, lysates of purified eosinophils from 7 blood donors or T cells from a single donor were prepared in lysis buffer (20 mM Tris, pH 7.4, 137 mM NaCl, 1mM EDTA, 0.1% Triton-X-100) and the insoluble cellular fractions removed by centrifugation. Total protein was determined and immunoblotting was performed following electrophoresis under reducing conditions. Identically loaded gels were immunoblotted under the same conditions with either 1 µg/ml primary Ab specific for CD127 (R&D systems, MAB306) or an isotype control IgG1 Ab. Following immunoblotting, signal strength was quantified by densitometry analysis using NIH Image software.
Segmental bronchoprovocation with allergen (SBP-Ag) and bronchoalveolar lavage (BAL)
SBP-Ag and BAL were preformed as previously described (25,26). Allergens used in SBP were cat antigen (Fel d1, Bayer Allergy Products), ragweed (GS Ragweed mix, Greer Labs) and house dust mite (HDM, Dermatophagoides farinae, Miles Allergy Products) based on each subject’s skin test reactivity and correlative history. The allergen dose for SBP was based on the subject’s physiological response to a previous inhaled allergen challenge. At least 4 weeks prior to bronchoscopy, a graded inhaled antigen challenge was performed to determine the amount of antigen required to achieve a 20% fall in FEV1 (AgPD20). Bronchoscopy, segmental bronchoprovocation, and BAL were performed in two different bronchopulmonary segments as described (26). A total dose of 30% of the subject’s AgPD20 was administered incrementally to insure subject’s safety: 10% of the AgPD20 in the first segment and, when this dose was well tolerated, 20% in the second segment. Forty-eight hours later, a second bronchoscopy was performed by instilling a total of 160 ml of sterile 0.9% NaCl warmed to 37°C in each segment. BAL fluid recovered from the two segments was pooled for analysis. Cells were recovered by centrifugation and total cell count and differential performed. BAL fluid was stored at −80 °C and concentrated to 10 × before analysis by ELISA.
ELISA
A sensitive indirect ELISA was used as previously described (27). Reagents included monoclonal anti-human IL-7 (clone BVD10-40F6, BD Biosciences Pharmingen) or GM-CSF (Clone 6804, R&D Systems) Ab for capture, biotinylated monoclonal human anti-IL-7 (clone BVD10-11C10, BD Biosciences Pharmingen) or anti-GM-CSF (Clone 3209, R&D Systems) Ab for detection, and recombinant human IL-7 (R&D Systems) and GM-CSF (BD Biosciences Pharmingen) for preparation of standard curves. OD readings were obtained at a wavelength of 450nm in a Thermo Labsystem Multiskan Ascent microplate reader and data were analyzed using Ascent Software version 2.6. The sensitivity for each cytokine assay was <3 pg/ml.
Statistical analysis
Statistical analysis was performed using SigmaStat software (Jandel Scientific Software). A paired t-test was used to compare different cell culture treatments and distinguish 48 h SBP-Ag from baseline data. Correlations were made using the Spearman rank order correlation test. A p value of <0.05 was considered significant.
RESULTS
Evidence of IL-7 receptor on eosinophils
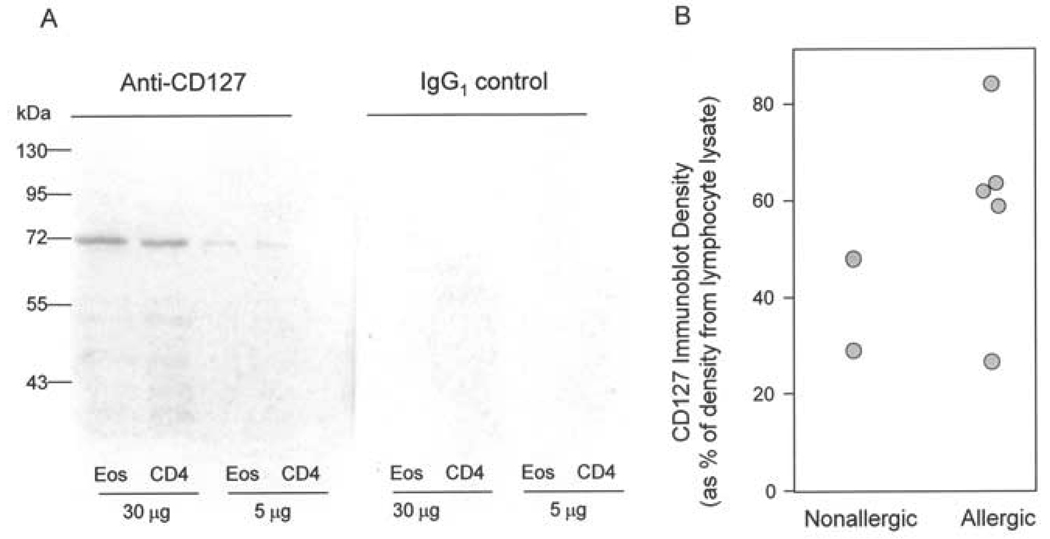
The IL-7 receptor is composed of an α chain (CD127) and a γ chain (CD132) that is common to IL-2R family cytokines. Eosinophil expression of CD132 has previously been reported (28,29). To determine whether eosinophils express CD127, immunoblot analysis of highly purified blood eosinophils was performed in parallel with purified CD4+ T cells. A protein of approximately 70–80 kDa was detected using a monoclonal Ab specific for CD127 (Figure 1A). CD127 was expressed on eosinophils from normal and allergic subjects. Densitometric analysis of the band detected in eosinophils from 7 separate blood donors demonstrated that the level of CD127 is between 26–84% of the expression in an equal mass of a standard lymphocyte preparation (Figure 1B). Because the non-eosinophil leukocyte contamination in our eosinophil preparations was <2% and the lysates from the eosinophil and T cell preparations yielded comparable protein mass per million cells (80 µg of protein per million cells for the T cell preparation vs 35–90 µg protein per million cells for the 7 eosinophil lysates), the immunoblot signal in the eosinophil lanes is not attributable to T cell contamination of the eosinophil preparations. It should also be noted that the immunoblot density of CD127 in the eosinophil preparations (Figure 1B) does not co-relate with the proportion of contaminating leukocytes.
Figure 1.
Evidence of IL-7 receptor expression on human eosinophils. A, Representative immunoblot analysis for CD127 in cell lysates from highly purified (>99%) eosinophils (Eos) or CD4+ T lymphocytes (CD4) immunoblotted with either a monoclonal antibody specific to CD127 (left panel) or an isotype control IgG1 (right panel). B, CD127 expression was analyzed by immunoblotting of 30 µg of cell lysate protein from 7 separate eosinophil donors and quantified by densitometry. For each donor, the immunoblot density is summarized as the % of CD127 signal from 30 µg of the positive control T lymphocyte cell lysate run on the same gel and immunoblot.
IL-7 promotes the ex vivo survival of eosinophils through autocrine induction of GM-CSF
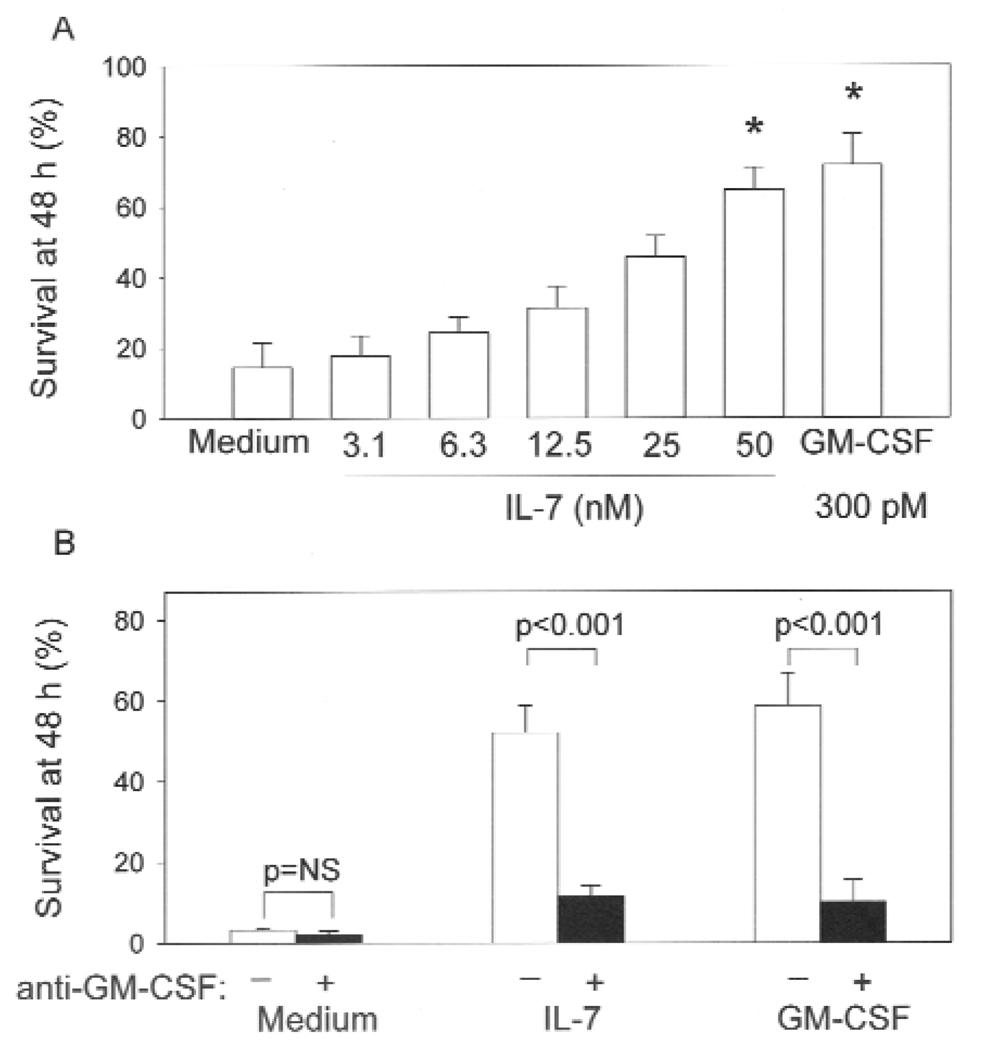
One of the primary functions of IL-7 is to induce anti-apoptotic signals in T cells (12). Similarly, IL-7 promoted the survival of eosinophils in a dose-dependent fashion (Figure 2). At the highest concentration of IL-7 tested, greater than 60 % of the initial number of eosinophils were alive at 48 h compared to < 20 % of cells incubated in medium alone (Figure 2, A). The level of survival induced by IL-7 was nearly equivalent to that induced by GM-CSF, which is a potent anti-apoptotic factor for eosinophils (30). GM-CSF can be produced in an autocrine fashion by eosinophils stimulated with factors such as cellular fibronectin (31), hyaluronan (32), and IL-15 (33). Thus, we sought to determine if IL-7 also induces autocrine production of GM-CSF. Detectable levels of GM-CSF by ELISA were not observed in culture supernatants from IL-7-stimulated eosinophils (data not shown); however, neutralization of GM-CSF had a potent inhibitory effect on IL-7-induced eosinophil survival (Figure 2 B), suggesting that the effect of IL-7 is through autocrine production of GM-CSF. In “add back” experiments where PBMCs (3% of the total cell concentration) were cultured with highly purified (>99.9%) eosinophils, there was no enhancement of IL-7-induced eosinophil survival (data not shown). Thus, the effect of IL-7 on eosinophil survival does not appear to be due to indirect production of GM-CSF by contaminating mononuclear cells.
Figure 2.
Effect of IL-7 on survival of human eosinophils. A, Eosinophil survival in the presence of increasing concentrations of IL-7 or 300 pM GM-CSF. The number of viable eosinophils at 48 h was determined by trypan blue exclusion and expressed as a percentage of the number of cells at 0 h (*p<0.05, n=7). B, Effect of GM-CSF neutralization on IL-7-induced eosinophil survival (n=3). Eosinophils were preincubated for 1 h in the presence (black bars) or absence (white bars) of anti-GM-CSF (20 µg/ml) and then cultured for 48 h with medium, IL-7 (50 nM), or GM-CSF (300 pM).
IL-7 promotes morphological changes in eosinophil and upregulates CD69 through autocrine induction of GM-CSF
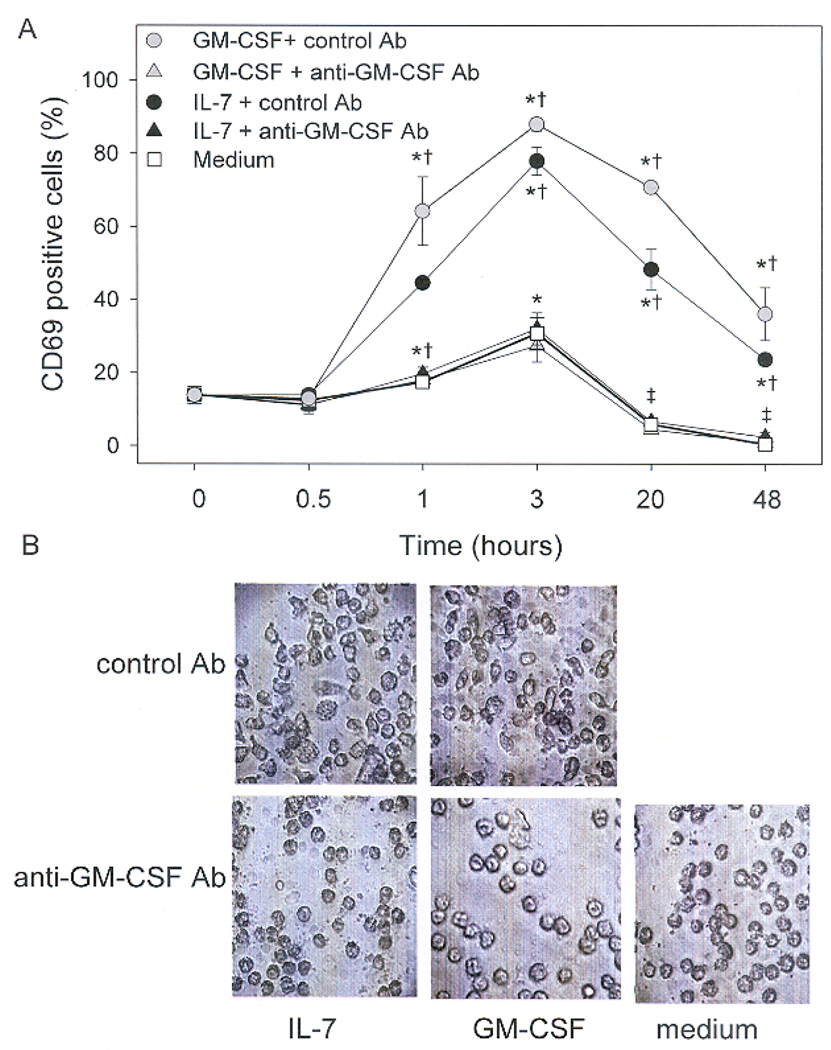
CD69 is a marker of cell activation that is found on the cell surface within hours of eosinophil activation by a variety of stimuli including GM-CSF (34). CD69 was upregulated by IL-7 within 1 h, peaked at 3 h, began to decrease within 20 h, but remained above background levels for at least 48 h (Figure 3A). The kinetics and magnitude of IL-7-induced upregulation of CD69 was similar to that induced by GM-CSF. Neutralization of GM-CSF completely abrogated IL-7 induced expression of CD69 at all time points (Figure 3A). As additional evidence of their activation by IL-7, eosinophils were shown to undergo morphological changes that have been associated with cellular activation (35). Cells cultured in IL-7 or GM-CSF became flat and more amoeboid (Figure 3 B). In the presence of neutralizing Ab to GM-CSF, eosinophils maintained the spherical characteristics of cells cultured in medium (Figure 3B). These data further support the notion that IL-7 induces autocrine release of GM-CSF by eosinophils. As with eosinophil viability, a role for contaminating monocytes was ruled out by demonstrating that addition of PBMCs (to 3% of the total cell population) to highly purified (>99.9%) eosinophils had no effect on IL-7-induced expression of CD69 by eosinophils (data not shown).
Figure 3.
Induction and kinetics of CD69 expression on eosinophils cultured with IL-7. Eosinophils were preincubated for 1 h with anti-GM-CSF (20 µg/ml, triangles) or an isotype control antibody (circles) and then cultured with medium (white squares), IL-7 (50 nM, black) or GM-CSF (300 pM, gray). Expression of CD69 was determined by flow cytometric analysis of eosinophils at 0, 0.5, 1, 3, 20, and 48 h. (n = 4, *p<0.05 compared to 0 h, †p< 0.05 compared to treatment plus anti-GM-CSF). B, Effect GM-CSF neutralization on eosinophil morphology 48 h after culture with IL-7. Eosinophils were treated with IL-7 (50nM), GM-CSF (300 pM), or medium in the presence of control antibody, or neutralizing antibody to GM-CSF (20 µg/ml).
IL-7 induces activation of STAT5 through autocrine induction of GM-CSF
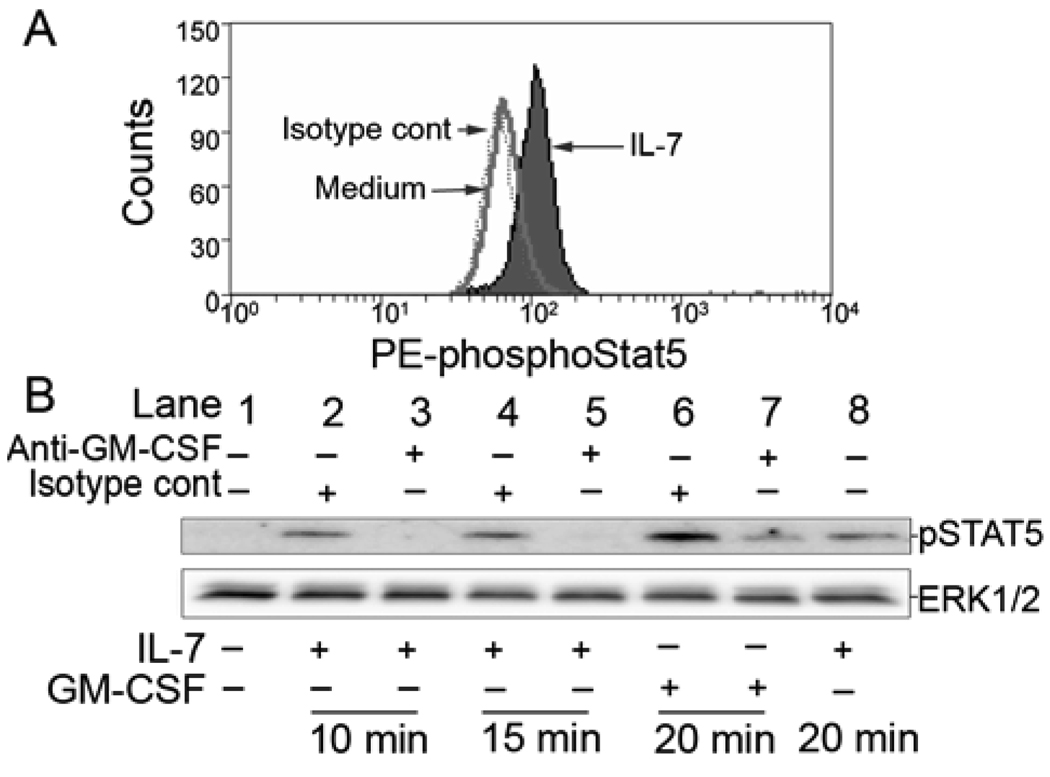
In T cells, IL-7 is a potent activator of STAT5 (36). Likewise, in purified human eosinophils, IL-7 induced phosphorylation of STAT5 as determined by intracellular flow cytometric analysis (Figure 4A). Addition of PBMCs (to 3% of the total cell concentration) to highly purified (>99.9%) eosinophils had no effect on levels of IL-7-induced phosphoSTAT5 in eosinophils (data not shown).
Figure 4.
Effect of IL-7 on eosinophil STAT5 phosphorylation. A, Intracellular flow cytometric analysis for detection of phosphotyrosine STAT5 in eosinophils cultured for 15 min in medium (dotted line) or IL-7 (50 nM, filled histogram). Isotype control is represented by solid line. Data are representative of eosinophils from 3 subjects. B. Eosinophils were preincubated with (upper labels) medium (lanes 1 and 8), isotype control antibody (20 µg/ml; lanes 2, 4, and 6), or a GM-CSF neutralizing antibody (20 µg/ml; lanes 3, 5, and 7) before stimulation with (bottom labels) medium (lane 1), 50 nM IL-7 (lanes 2–5 and 8), or 300 pM GM-CSF (lanes 6 and 7) for the indicated times. To ensure equal protein loading between samples, 60 µg of total protein was loaded and the immunoblots were reprobed for total ERK1/2 as a loading control (lower illustration). These data are representative of experiments from 3 eosinophil donors.
Using immunoblotting, an increase in phospho-STAT5 was detected within 10 min and persisted for at least 20 min (Figure 4B). Furthermore, an upward shift in the mobility of the STAT5 band was seen, consistent with the observed phosphorylation (not shown). STAT5 is also known to be activated by GM-CSF (24). Antibody neutralization of GM-CSF diminished both GM-CSF- and IL-7-induced phosphorylation of STAT5 (Figure 4B). Together the rapid kinetics of STAT5 phosphorylation stimulated by IL-7 and its neutralization by anti-GM-CSF, suggest that IL-7-stimulates autocrine production of GM-CSF from pre-formed intracellular stores. These data, coupled with data showing increased cellular activation and potentiation of eosinophil survival, suggest that both signaling processes and physiological endpoints in response to IL-7 occur as a result of GM-CSF released in an autocrine manner.
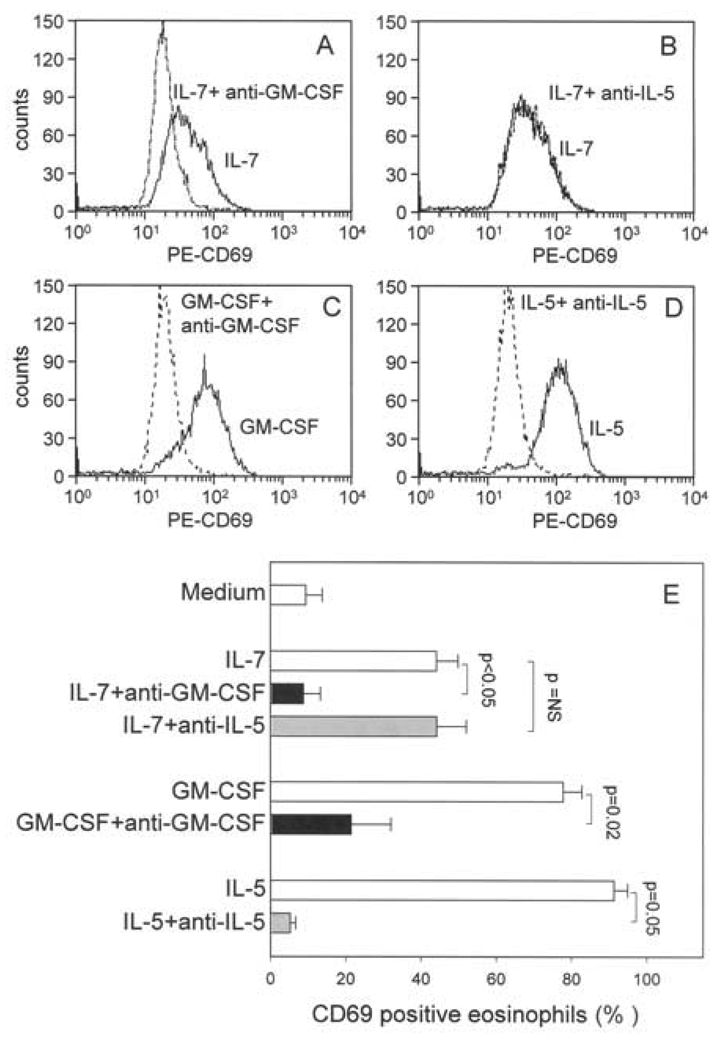
Neutralization of IL-5 does not block IL-7-mediated activation of eosinophils
IL-5 has previously been shown to mediate IL-7-induced eosinophil colony formation from human bone marrow cells (16). To determine if autocrine release of IL-5 also contributes to IL-7-mediated activation of mature circulating eosinophils, cells were pretreated with neutralizing Ab to IL-5 prior to addition of IL-7. In contrast to neutralization of GM-CSF (Figure 5A and E), blocking IL-5 had no effect on IL-7-induced expression of CD69 (Figure 5B and E). The neutralizing Ab efficacy was confirmed in Figure 5C, D and E. Furthermore, based on analysis of PI exclusion/uptake by flow cytometric analysis, anti-IL-5 had no effect on IL-7-enhanced eosinophil viability after 48 h of culture. For example, in one experiment, the viability of eosinophils cultured for 48 h with IL-7 was reduced from 78% to 41% in the presence of neutralizing Ab to GM-CSF, but remained at 78% in the presence of anti-IL-5. The efficacy of the neutralizing Ab was confirmed by the observation that IL-5-induced viability was decreased from 83% to 22% in the presence of anti-IL-5. An identical pattern was observed for eosinophils from a different donor. Thus, in contrast to the effects of IL-7 on differentiation of eosinophil progenitors (16), the effect of IL-7 on survival and expression of CD69 on mature circulating eosinophils appears to be through autocrine induction of GM-CSF and not IL-5.
Figure 5.
Effect of neutralization of GM-CSF or IL-5 on IL-7-induced eosinophils expression of CD69. A-D. Representative histogram with solid lines depicting stimulant and hatched lines representing stimulant plus neutralizing Ab. Purified eosinophils were stimulated (3 h) with IL-7 in the presence of neutralizing Ab to GM-CSF (A) or IL-5 (B). The efficacy of the Abs was confirmed by blocking CD69 expression induced by GM-CSF (C) or IL-5 (D) using the respective neutralizing Ab. E. Summary data of three experiments from different blood donors.
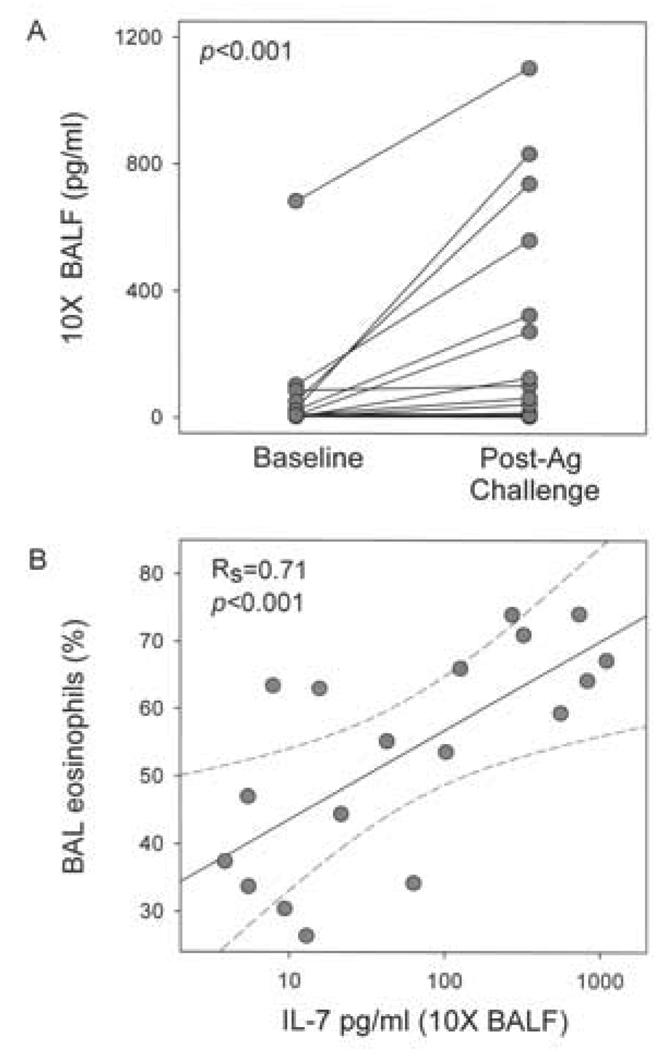
Concentrations of IL-7 increase in BAL fluid following airway allergen challenge and correlate with BAL eosinophil
To explore the potential in vivo relevance of IL-7 to allergen-induced eosinophilic airway inflammation, IL-7 levels were determined in BAL fluid obtained before and 48 h after segmental allergen challenge in allergic asthma subjects. The total number of BAL cells increased from a mean ± SEM of 36 ± 4 million at baseline to 403 ± 72 million (p<0.001) 48 h after SBP-Ag. The percentage of eosinophils increased from 2 ± 1 % to 53 ± 4 %. At baseline, IL-7 was detectable (>3 pg/ml) in 10 × concentrated BAL fluid from 12 of 18 subjects. Concentrations increased from a mean ± SEM of 58 ± 37 pg/ml at baseline to 236 ± 80 pg/ml 48 h after SBP-Ag (Figure 6A). Notably, after Ag challenge, there was a statistically significant (rs=0.71, p<0.001) correlation between IL-7 levels and the percentage of BAL eosinophils (Figure 6B).
Figure 6.
IL-7 concentrations in BAL fluid before and 48 h after SBP-Ag. A, Concentrations of IL-7 were determined by ELISA analysis of 10 × concentrated BAL fluid obtained immediately before (baseline) and 48 h after SBP-Ag (n=18) B, Correlation between levels of IL-7 and percentage of eosinophils in BAL fluid 48 h after SBP-Ag (Rs=0.71, p<0.001).
DISCUSSION
We have established that highly purified human blood eosinophils express IL-7Rα (CD127) and that IL-7 stimulation of eosinophils leads to phosphorylation of STAT5. Moreover, we demonstrated a novel function of IL-7 for enhancement of human eosinophil survival and upregulation of the activation marker CD69. An in vivo relevance for IL-7 was suggested by the observation that levels of IL-7 were highly correlated with eosinophils in BAL fluid of allergic asthma subjects 48 h after airway allergen challenge. These data suggest that IL-7 may play a role in allergen-induced eosinophilic airway inflammation that is associated with asthma.
The primary function of IL-7 is considered to be regulation of the development and survival of T cells; however, there have been sporadic reports that IL-7 can also affect nonlymphoid cells. For example, IL-7 has been shown to activate human monocyte tumoricidal activity and to induce their production of IL-6, IL-1, TNF-α and IL-8 (13,37). Although a direct effect of IL-7 on mature eosinophils has not been previously reported, IL-7 has been shown to stimulate eosinophil colony formation from bone marrow cells (16). There is also indirect in vivo evidence associating eosinophils and IL-7. Eosinophils have long been noted in the thymus (38,39), which is a principal location for IL-7 production. Although there is growing evidence that eosinophils can influence T cell function (19,20), the significance of eosinophils at this T cell site remains a mystery. Eosinophil infiltrates have been reported in murine tumors engineered to overexpress IL-7 (40,41) and in colonic mucosa of mice with selective over-expression of IL-7 in the colon (42). Conversely, eosinophils were lacking in a mouse model of colitis with targeted IL-7 deletion (43). Despite these scattered inferences to eosinophils and IL-7, to our knowledge, this is the first report to demonstrate the IL-7Rα chain on eosinophils and to show their activation by IL-7.
Evidence that IL-7 can activate eosinophils included induction of STAT5 phosphorylation, stimulation of morphological changes consistent with cellular activation/migration, upregulation of CD69 on the cell surface, and enhanced eosinophil survival. The effects of IL-7 were significantly reduced in the presence of blocking antibodies to GM-CSF, but not anti-IL-5 suggesting that IL-7 activation of these particular eosinophil functions is mediated via autocrine production of GM-CSF. Neutralization of GM-CSF reduced STAT5 phosphorylation within 10 min of eosinophil exposure to IL-7. The rapid kinetics of the anti-GM-CSF-mediated effect raises the possibility that IL-7 can induce autocrine release of preformed GM-CSF. Indeed, there are reports that GM-CSF is stored in crystalloid granules of eosinophils (44). The kinetics of inhibition of GM-CSF activity is reminiscent of eosinophil storage and rapid release of preformed RANTES (45) and IL-4 (46), which are reported to be stored in specialized small secretory vesicles and selectively released by a process known as piecemeal degranulation (47,48). Bandeira-Melo and colleagues showed that eotaxin-induced IL-4 release could not be measured by ELISA, but was instead detected by a unique gel-based dual antibody detection assay (49). Thus, the fact that we were not able to directly detect GM-CSF production by eosinophils is not unexpected. A number of reports have demonstrated that eosinophil survival induced by factors such as IL-15 (33), hyaluronic acid (32), or TNF-α plus fibronectin (32) can be reversed by neutralization of GM-CSF, yet demonstrable levels of GM-CSF could not be measure by ELISA. Furthermore, it has been shown that the continuous presence of minute amounts of GM-CSF are sufficient to inhibit eosinophil apoptosis (30).
We recognize that there are inherent limitations to our studies. First, nanomolar concentrations of IL-7 were necessary for activation of eosinophils. This observation is consistent with what has been reported for IL-7-induced release of GM-CSF by T cells (50). The reason why GM-CSF release by T cells and eosinophils requires relatively high concentrations of IL-7 is not known. There is evidence that human T cells express both high and low affinity IL-7 receptors (51). It is possible that IL-7-induced GM-CSF release occurs through activation of low affinity receptors, and thus requires higher concentrations of IL-7 to achieve effective cell signaling and functional cellular responses. The report that IL-7 can stimulate GM-CSF production by T cells (50) raises a second potential limitation. It is possible that the small numbers of T cells or other mononuclear cells present in our eosinophils preparations (generally <2%) contribute to the effects of IL-7 on eosinophils. This scenario is unlikely based on our observation that an eosinophil preparation of 99.9% purity responded to IL-7 and addition of 3% PBMC did not further enhance IL-7-induced eosinophil survival, expression of CD69 or levels of STAT5 phosphorylation. Finally, although we have clearly demonstrated the presence of CD127 mRNA expression (17) and the protein by Western blot analysis (Figure 1), the detectable level of the receptor by flow cytometric analysis is low and inconsistent. The fact IL-7Rα is detected by immunoblotting at levels between 26 and 84% of the expression in an equal mass of a standard lymphocyte preparation and that eosinophils respond to stimulation with IL-7 with respect to multiple biological readouts, is consistent with an IL-7 receptor conformation in eosinophils that limits binding or accessibility of the monoclonal antibodies used for flow cytometry. We suspect that in eosinophils, the receptors may be sequestered in multi-protein complexes, caveolae or in other cellular microdomains that mask the epitope detected by the monoclonal antibody used for flow cytometric analysis.
In concert with our in vitro studies demonstrating a direct effect of IL-7 on eosinophils, we have also shown that airway eosinophilia induced by allergen challenge of allergic asthma subjects is associated with increased BAL fluid concentrations of IL-7. The source of IL-7 in BAL fluid was not determined in this study. Epithelial cells are the principal source of IL-7 in tissues where IL-7 is predominant including the thymus, bone marrow, and intestine (12). While there is a paucity of studies on IL-7 in the airway, there is some evidence that BAL cells express IL-7. An early report documented IL-7 mRNA in BAL cells from lung transplant patients (52) and a more recent study demonstrated IL-7 staining of alveolar macrophages and epithelial cells in cytospin preparations of BAL cells from patients with concurrent infection with HIV-associated tuberculosis (53). Further studies of BAL cells and bronchial biopsies are required to determine the source of IL-7 in the airway of asthma subjects following allergen challenge. There is also a need to determine if these cells are directly activated by allergen or indirectly affected by factors produced by other IL-7-responsive cells such as T cells and monocytes. For example, IL-7 synthesis by epithelial cells can be upregulated by IFN-γ, IL-1, or TNF-α (54,55).
The presence of IL-7 adds to the redundancy of eosinophil survival factors in the airway after allergen exposure. In asthma, one could envision that IL-7 may provide a pathway for GM-CSF production and enhanced survival of eosinophils in the event of deficient production by prominent GM-CSF-producing cells such as airway epithelial cells and/or alveolar macrophages. Furthermore, the ability of IL-7 to promote eosinophil survival and induce the eosinophil survival factor GM-CSF may contribute to eosinophilia in nonatopic airway diseases that are not typically associated with increased levels of the eosinophil-specific cytokine IL-5.
In summary, we have established a novel function for IL-7 in the activation of eosinophils and potentiation of their survival, and have demonstrated that levels of IL-7 in BAL fluid are increased by airway allergen challenge and parallel the influx of eosinophils, thus supporting the notion that IL-7 contributes to airway inflammation by promoting eosinophil activation and survival.
Acknowledgments
The authors wish to thank our Research Coordinators, Mary Jo Jackson, R.N. and Erin Billmeyer, R.N. for patient recruitment, screening, and assistance with bronchoscopies; Keith Meyer, M.D. for assistance with bronchoscopies; Rose DeGrauw, B.S. for BAL processing, Hun Sun Chung, M.S. for assistance with immunoblotting, Anne Brooks, B.S. and Beth Schwantes, B.S. for eosinophil purification, and Julie Sedgwick, Ph.D. for overseeing the eosinophil purification process and for helpful scientific discussion.
Abbreviations used in this paper
- BAL
bronchoalveolar lavage
- Rs
correlation coefficients determined by Spearman’s test
- SBP-Ag
segmental bronchoprovocation with allergen.
Footnotes
This work was supported in part by an institutional Specialized Center of Research grant (NIH HL56396), a Program Project grant (NIH HL088594), and the University of Wisconsin General Clinical Research Center grant (NIH M01RR03186).
Disclosures
The authors have no financial conflict of interest.
Reference List
- 1.Giliani S, Mori L, de Saint BG, Le Deist F, Rodriguez-Perez C, Forino C, Mazzolari E, Dupuis S, Elhasid R, Kessel A, Galambrun C, Gil J, Fischer A, Etzioni A, Notarangelo LD. Interleukin-7 receptor alpha (IL-7Ralpha) deficiency: cellular and molecular bases. Analysis of clinical, immunological, and molecular features in 16 novel patients. Immunol. Rev. 2005;203:110–126. doi: 10.1111/j.0105-2896.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 2.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol. Rev. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 3.Guimond M, Fry TJ, Mackall CL. Cytokine signals in T-cell homeostasis. J Immunother. 2005;28:289–294. doi: 10.1097/01.cji.0000165356.03924.e7. [DOI] [PubMed] [Google Scholar]
- 4.Snyder KM, Mackall CL, Fry TJ. IL-7 in allogeneic transplant: clinical promise and potential pitfalls. Leuk. Lymphoma. 2006;47:1222–1228. doi: 10.1080/10428190600555876. [DOI] [PubMed] [Google Scholar]
- 5.Nunnari G, Pomerantz RJ. IL-7 as a potential therapy for HIV-1-infected individuals. Expert. Opin. Biol. Ther. 2005;5:1421–1426. doi: 10.1517/14712598.5.11.1421. [DOI] [PubMed] [Google Scholar]
- 6.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damas JK, Waehre T, Yndestad A, Otterdal K, Hognestad A, Solum NO, Gullestad L, Froland SS, Aukrust P. Interleukin-7-mediated inflammation in unstable angina: possible role of chemokines and platelets. Circulation. 2003;107:2670–2676. doi: 10.1161/01.CIR.0000070542.18001.87. [DOI] [PubMed] [Google Scholar]
- 8.Al Rawi MA, Mansel RE, Jiang WG. Interleukin-7 (IL-7) and IL-7 receptor (IL-7R) signalling complex in human solid tumours. Histol. Histopathol. 2003;18:911–923. doi: 10.14670/HH-18.911. [DOI] [PubMed] [Google Scholar]
- 9.Totsuka T, Kanai T, Nemoto Y, Makita S, Okamoto R, Tsuchiya K, Watanabe M. IL-7 Is essential for the development and the persistence of chronic colitis. J. Immunol. 2007;178:4737–4748. doi: 10.4049/jimmunol.178.8.4737. [DOI] [PubMed] [Google Scholar]
- 10.Svejgaard A. The immunogenetics of multiple sclerosis. Immunogenetics. 2008;60:275–286. doi: 10.1007/s00251-008-0295-1. [DOI] [PubMed] [Google Scholar]
- 11.Churchman SM, Ponchel F. Interleukin-7 in rheumatoid arthritis. Rheumatology. (Oxford) 2008;47:753–759. doi: 10.1093/rheumatology/ken053. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Alderson MR, Tough TW, Ziegler SF, Grabstein KH. Interleukin 7 induces cytokine secretion and tumoricidal activity by human peripheral blood monocytes. J. Exp. Med. 1991;173:923–930. doi: 10.1084/jem.173.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dus D, Krawczenko A, Zalecki P, Paprocka M, Wiedlocha A, Goupille C, Kieda C. IL-7 receptor is present on human microvascular endothelial cells. Immunol. Lett. 2003;86:163–168. doi: 10.1016/s0165-2478(03)00018-x. [DOI] [PubMed] [Google Scholar]
- 15.Huang M, Sharma S, Zhu LX, Keane MP, Luo J, Zhang L, Burdick MD, Lin YQ, Dohadwala M, Gardner B, Batra RK, Strieter RM, Dubinett SM. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J. Clin. Invest. 2002;109:931–937. doi: 10.1172/JCI14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vellenga E, Esselink MT, Straaten J, Stulp BK, De Wolf JT, Brons R, Giannotti J, Smit JW, Halie MR. The supportive effects of IL-7 on eosinophil progenitors from human bone marrow cells can be blocked by anti-IL-5. J. Immunol. 1992;149:2992–2995. [PubMed] [Google Scholar]
- 17.Bates ME, Liu LY, Esnault S, Stout BA, Fonkem E, Kung V, Sedgwick JB, Kelly EA, Bates DM, Malter JS, Busse WW, Bertics PJ. Expression of IL-5- and GM-CSF-responsive genes in blood and airway eosinophils. Am J Respir Cell Mol. Biol. 2004;30:736–743. doi: 10.1165/rcmb.2003-0234OC. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen EA, Ochkur SI, Lee NA, Lee JJ. Eosinophils and asthma. Curr. Allergy Asthma Rep. 2007;7:18–26. doi: 10.1007/s11882-007-0026-y. [DOI] [PubMed] [Google Scholar]
- 19.Liu LY, Bates ME, Jarjour NN, Busse WW, Bertics PJ, Kelly EA. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J. Immunol. 2007;179:4840–4848. doi: 10.4049/jimmunol.179.7.4840. [DOI] [PubMed] [Google Scholar]
- 20.Liu LY, Mathur SK, Sedgwick JB, Jarjour NN, Busse WW, Kelly EAB. Human airway and peripheral blood eosinophils enhance Th1 and Th2 cytokine secretion. Allergy. 2006;61:589–598. doi: 10.1111/j.1398-9995.2006.01060.x. [DOI] [PubMed] [Google Scholar]
- 21.Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, Ghahary A, Moqbel R. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J. Allergy Clin. Immunol. 2007;119:1313–1320. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Liu LY, Jarjour NN, Busse WW, Kelly EA. Chemokine receptor expression on human eosinophils from peripheral blood and bronchoalveolar lavage fluid following segmental antigen challenge. J. Allergy Clin. Immunol. 2003;112:556–562. doi: 10.1016/s0091-6749(03)01798-6. [DOI] [PubMed] [Google Scholar]
- 24.Stout BA, Bates ME, Liu LY, Farrington NN, Bertics PJ. IL-5 and granulocyte-macrophage colony-stimulating factor activate STAT3 and STAT5 and promote Pim-1 and cyclin D3 protein expression in human eosinophils. J Immunol. 2004;173:6409–6417. doi: 10.4049/jimmunol.173.10.6409. [DOI] [PubMed] [Google Scholar]
- 25.Calhoun WJ, Jarjour NN, Gleich GJ, Stevens CA, Busse WW. Increased airway inflammation with segmental versus aerosol antigen challenge. Am. Rev. Respir. Dis. 1993;147:1465–1471. doi: 10.1164/ajrccm/147.6_Pt_1.1465. [DOI] [PubMed] [Google Scholar]
- 26.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EAB. Decreased expression of membrane IL-5R∀ on human eosinophils: I. Loss of membrane IL-5alpha on eosinophils and increased soluble IL-5R alpha in the airway after antigen challenge. J. Immunol. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 27.Kelly EA, Rodriguez RR, Busse WW, Jarjour NN. The effect of segmental bronchoprovocation with allergen on airway lymphocyte function. Am. J. Respir. Crit. Care Med. 1997;156:1421–1428. doi: 10.1164/ajrccm.156.5.9703054. [DOI] [PubMed] [Google Scholar]
- 28.Conesa A, Tassinari P, Aldrey O, Taylor P, Bianco NE, De Sanctis JB. Interleukin-2 induces peroxide production by primed normodense eosinophils of patients with asthma. Allergy Asthma Proc. 2003;24:27–33. [PubMed] [Google Scholar]
- 29.Simon HU, Plotz S, Simon D, Seitzer U, Braathen LR, Menz G, Straumann A, Dummer R, Levi-Schaffer F. Interleukin-2 primes eosinophil degranulation in hypereosinophilia and Wells' syndrome. Eur. J. Immunol. 2003;33:834–839. doi: 10.1002/eji.200323727. [DOI] [PubMed] [Google Scholar]
- 30.Esnault S, Malter JS. Minute quantities of granulocyte-macrophage colony-stimulating factor prolong eosinophil survival. J. Interferon Cytokine Res. 2001;21:117–124. doi: 10.1089/107999001750069980. [DOI] [PubMed] [Google Scholar]
- 31.Meerschaert J, Vrtis RF, Shikama Y, Sedgwick JB, Busse WW, Mosher DF. Engagement of alpha4beta7 integrins by monoclonal antibodies or ligands enhances survival of human eosinophils in vitro. J. Immunol. 1999;163:6217–6227. [PubMed] [Google Scholar]
- 32.Esnault S, Malter JS. Hyaluronic acid or TNF-alpha plus fibronectin triggers granulocyte macrophage-colony-stimulating factor mRNA stabilization in eosinophils yet engages differential intracellular pathways and mRNA binding proteins. J Immunol. 2003;171:6780–6787. doi: 10.4049/jimmunol.171.12.6780. [DOI] [PubMed] [Google Scholar]
- 33.Hoontrakoon R, Chu HW, Gardai SJ, Wenzel SE, McDonald P, Fadok VA, Henson PM, Bratton DL. Interleukin-15 inhibits spontaneous apoptosis in human eosinophils via autocrine production of granulocyte macrophage-colony stimulating factor and nuclear factor-kappaB activation. Am. J. Respir. Cell Mol. Biol. 2002;26:404–412. doi: 10.1165/ajrcmb.26.4.4517. [DOI] [PubMed] [Google Scholar]
- 34.Hartnell A, Robinson DS, Kay AB, Wardlaw AJ. CD69 is expressed by human eosinophils activated in vivo in asthma and in vitro by cytokines. Immunology. 1993;80:281–286. [PMC free article] [PubMed] [Google Scholar]
- 35.Gervais FG, Cruz RP, Chateauneuf A, Gale S, Sawyer N, Nantel F, Metters KM, O'Neill GP. Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J Allergy Clin Immunol. 2001;108:982–988. doi: 10.1067/mai.2001.119919. [DOI] [PubMed] [Google Scholar]
- 36.Lin JX, Leonard WJ. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 2000;19:2566–2576. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 37.Standiford TJ, Strieter RM, Allen RM, Burdick MD, Kunkel SL. IL-7 up-regulates the expression of IL-8 from resting and stimulated human blood monocytes. J. Immunol. 1992;149:2035–2039. [PubMed] [Google Scholar]
- 38.Throsby M, Herbelin A, Pleau JM, Dardenne M. CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J. Immunol. 2000;165:1965–1975. doi: 10.4049/jimmunol.165.4.1965. [DOI] [PubMed] [Google Scholar]
- 39.Lee I, Yu E, Good RA, Ikehara S. Presence of eosinophilic precursors in the human thymus: evidence for intra-thymic differentiation of cells in eosinophilic lineage. Pathol. Int. 1995;45:655–662. doi: 10.1111/j.1440-1827.1995.tb03518.x. [DOI] [PubMed] [Google Scholar]
- 40.Hock H, Dorsch M, Kunzendorf U, Qin Z, Diamantstein T, Blankenstein T. Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin 2, interleukin 4, interleukin 7, tumor necrosis factor, or interferon gamma. Proc. Natl. Acad. Sci. U. S. A. 1993;90:2774–2778. doi: 10.1073/pnas.90.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McBride WH, Thacker JD, Comora S, Economou JS, Kelley D, Hogge D, Dubinett SM, Dougherty GJ. Genetic modification of a murine fibrosarcoma to produce interleukin 7 stimulates host cell infiltration and tumor immunity. Cancer Res. 1992;52:3931–3937. [PubMed] [Google Scholar]
- 42.Watanabe M, Ueno Y, Yajima T, Okamoto S, Hayashi T, Yamazaki M, Iwao Y, Ishii H, Habu S, Uehira M, Nishimoto H, Ishikawa H, Hata J, Hibi T. Interleukin 7 transgenic mice develop chronic colitis with decreased interleukin 7 protein accumulation in the colonic mucosa. J. Exp. Med. 1998;187:389–402. doi: 10.1084/jem.187.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freeden-Jeffry U, Davidson N, Wiler R, Fort M, Burdach S, Murray R. IL-7 deficiency prevents development of a non-T cell non-B cell-mediated colitis. J. Immunol. 1998;161:5673–5680. [PubMed] [Google Scholar]
- 44.Levi-Schaffer F, Lacy P, Severs NJ, Newman TM, North J, Gomperts B, Kay AB, Moqbel R. Association of granulocyte-macrophage colony-stimulating factor with the crystalloid granules of human eosinophils. Am. Soc. Hematol. 1995;85:2579–2586. [PubMed] [Google Scholar]
- 45.Lacy P, Mahmudi-Azer S, Bablitz B, Hagen SC, Velazquez JR, Man SF, Moqbel R. Rapid mobilization of intracellularly stored RANTES in response to interferon-gamma in human eosinophils. Blood. 1999;94:23–32. [PubMed] [Google Scholar]
- 46.Melo RC, Spencer LA, Perez SA, Ghiran I, Dvorak AM, Weller PF. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6:1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moqbel R, Coughlin JJ. Differential secretion of cytokines. Sci. STKE. 2006;2006:e26. doi: 10.1126/stke.3382006pe26. [DOI] [PubMed] [Google Scholar]
- 48.Melo RC, Spencer LA, Dvorak AM, Weller PF. Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J. Leukoc. Biol. 2007 doi: 10.1189/jlb.0707503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bandeira-Melo C, Gillard G, Ghiran I, Weller PF. EliCell: a gel-phase dual antibody capture and detection assay to measure cytokine release from eosinophils. J. Immunol. Meth. 2000;244:105–115. doi: 10.1016/s0022-1759(00)00264-7. [DOI] [PubMed] [Google Scholar]
- 50.Aiello FB, Keller JR, Klarmann KD, Dranoff G, Mazzucchelli R, Durum SK. IL-7 induces myelopoiesis and erythropoiesis. J. Immunol. 2007;178:1553–1563. doi: 10.4049/jimmunol.178.3.1553. [DOI] [PubMed] [Google Scholar]
- 51.Kondo M, Takeshita T, Higuchi M, Nakamura M, Sudo T, Nishikawa S, Sugamura K. Functional participation of the IL-2 receptor gamma chain in IL-7 receptor complexes. Science. 1994;263:1453–1454. doi: 10.1126/science.8128231. [DOI] [PubMed] [Google Scholar]
- 52.Whitehead BF, Stoehr C, Wu CJ, Patterson G, Burchard EG, Theodore J, Clayberger C, Starnes VA. Cytokine gene expression in human lung transplant recipients. Transplantation. 1993;56:956–961. doi: 10.1097/00007890-199310000-00034. [DOI] [PubMed] [Google Scholar]
- 53.Kibiki GS, Myers LC, Kalambo CF, Hoang SB, Stoler MH, Stroup SE, Houpt ER. Bronchoalveolar neutrophils, interferon gamma-inducible protein 10 and interleukin-7 in AIDS-associated tuberculosis. Clin. Exp. Immunol. 2007 doi: 10.1111/j.1365-2249.2007.03330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood. 2000;96:1873–1878. [PubMed] [Google Scholar]
- 55.Oshima S, Nakamura T, Namiki S, Okada E, Tsuchiya K, Okamoto R, Yamazaki M, Yokota T, Aida M, Yamaguchi Y, Kanai T, Handa H, Watanabe M. Interferon regulatory factor 1 (IRF-1) and IRF-2 distinctively up-regulate gene expression and production of interleukin-7 in human intestinal epithelial cells. Mol. Cell Biol. 2004;24:6298–6310. doi: 10.1128/MCB.24.14.6298-6310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]