Abstract
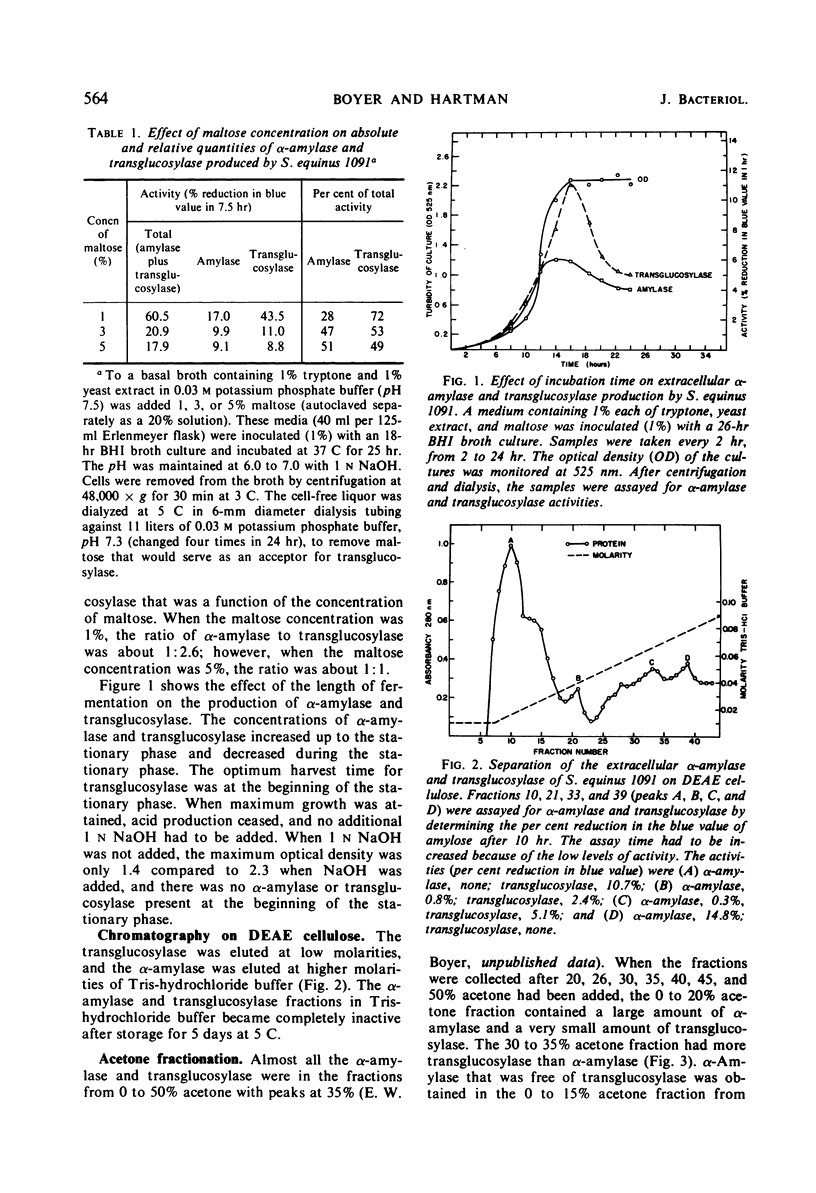
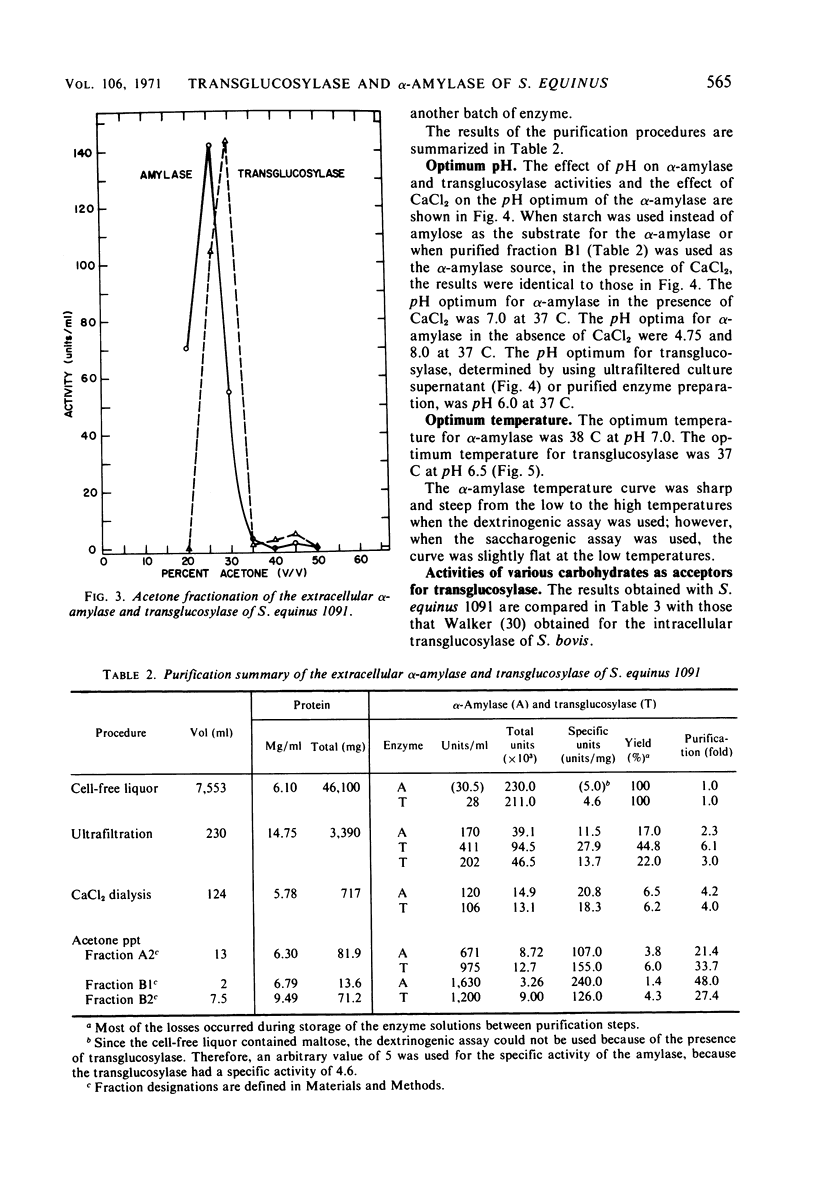
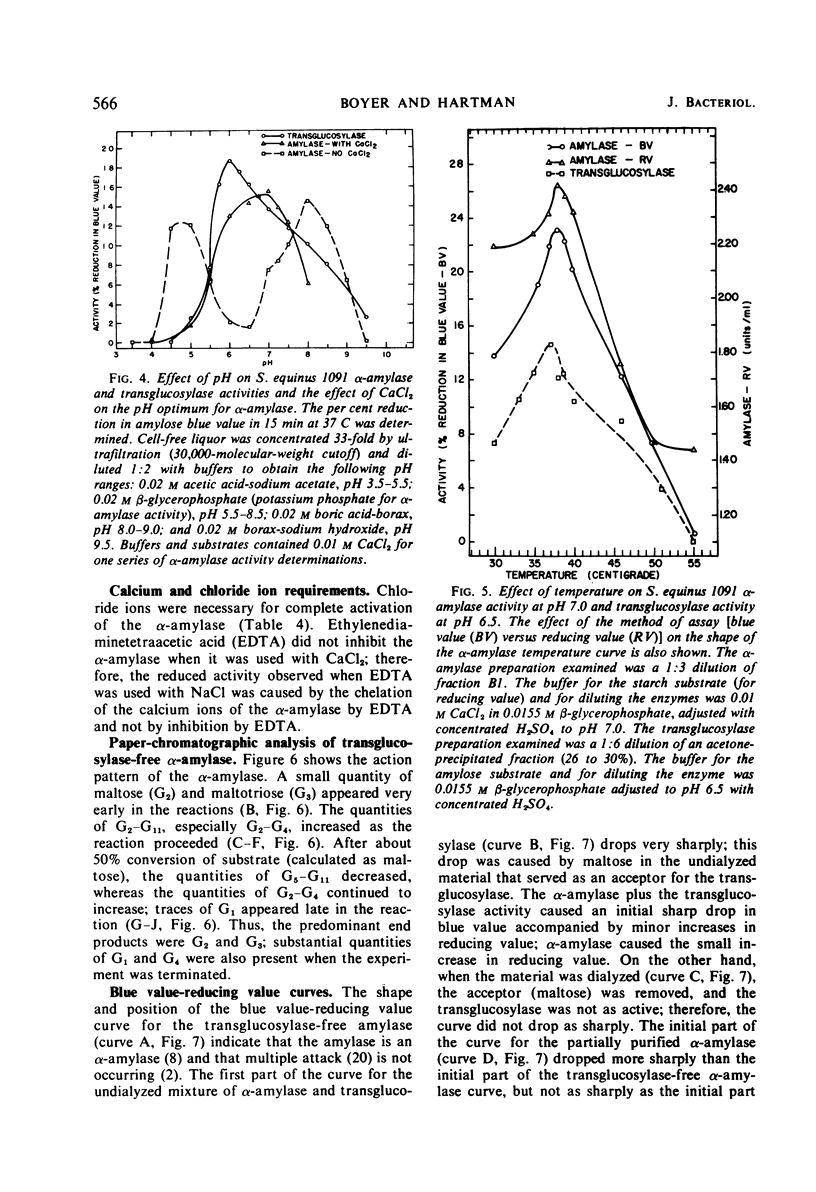
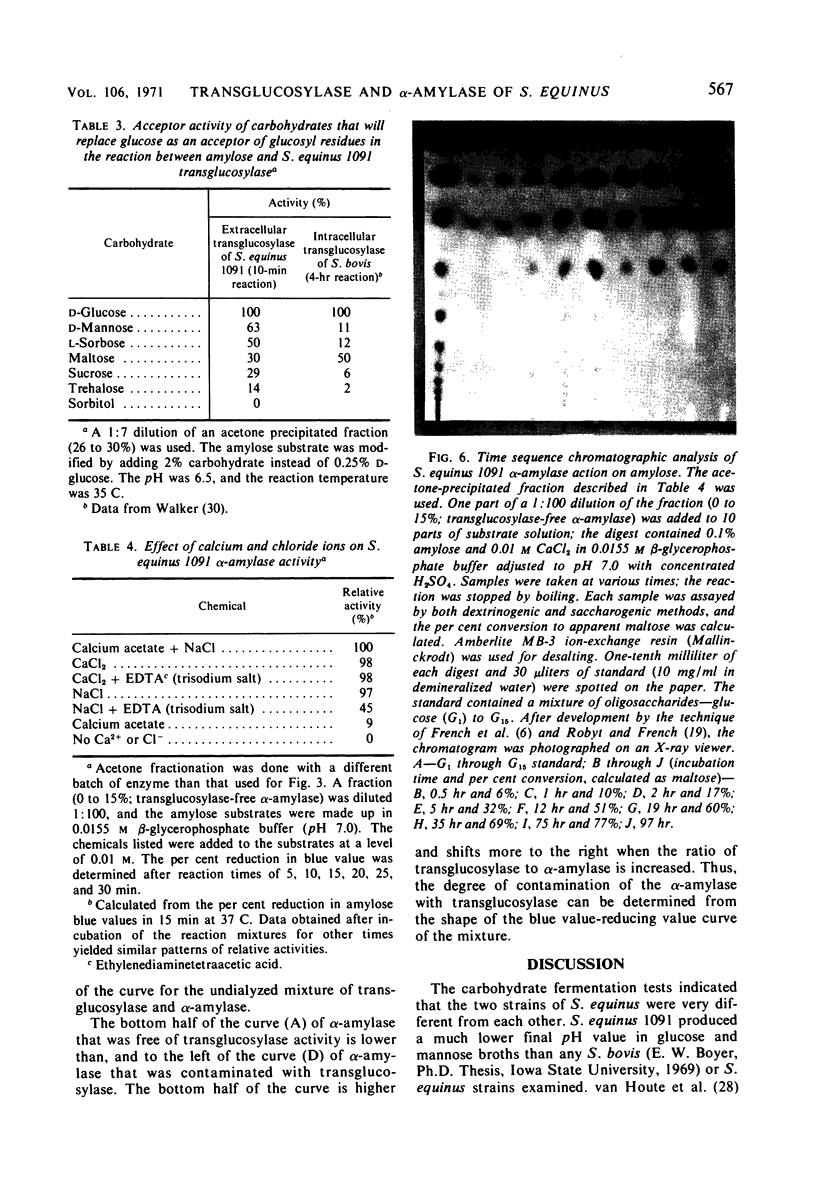
Culture filtrates of Streptococcus equinus 1091 contained α-amylase and transglucosylase. The effects of calcium carbonate, age of inoculum, concentration of maltose, and duration of the fermentation on α-amylase and transglucosylase production were determined. The extracellular α-amylase was purified 48-fold and was free of transglucosylase activity. The α-amylase (amylose substrate) required Cl− for maximum activity; ethylenediaminetetraacetic acid (EDTA) partially inhibited activity, but CaCl2 prevented EDTA inhibition. The temperature optimum was 38 C at pH 7.0, and the pH optimum was 7.0 at 37 C in the presence of CaCl2. Predominant final products of amylose hydrolysis, in order of decreasing prevalence, were maltose, maltotriose, maltotetraose, and glucose. The α-amylase showed no evidence of multiple attack. The extracellular transglucosylase was purified 27-fold, but a small amount of α-amylase remained. Transglucosylase activity (amylose substrate) was not increased in the presence of CaCl2. The temperature optimum was 37 C at pH 6.5, and the pH optimum was 6.0 at 37 C. Carbohydrates that served as acceptors for the transglucosylase to degrade amylose were, in order of decreasing acceptor efficiency: d-glucose, d-mannose, l-sorbose, maltose, sucrose, and trehalose. The extracellular transglucosylase of S. equinus 1091 synthesized higher maltodextrins in the medium when the cells were grown in the presence of maltose.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdullah M., French D., Robyt J. F. Multiple attack by alpha-amylases. Arch Biochem Biophys. 1966 Jun;114(3):595–598. doi: 10.1016/0003-9861(66)90385-7. [DOI] [PubMed] [Google Scholar]
- DEIBEL R. H. THE GROUP D STREPTOCOCCI. Bacteriol Rev. 1964 Sep;28:330–366. doi: 10.1128/br.28.3.330-366.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNICAN L. K., SEELEY H. W. Starch hydrolysis by Strepto-coccus equinus. J Bacteriol. 1962 Feb;83:264–269. doi: 10.1128/jb.83.2.264-269.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French D., Mancusi J. L., Abdullah M., Brammer G. L. Separation of starch oligosaccharides by high temperature paper chromatography. J Chromatogr. 1965 Aug;19(2):445–447. doi: 10.1016/s0021-9673(01)99480-4. [DOI] [PubMed] [Google Scholar]
- Greenwood C. T., Milne E. A. Starch degrading and synthesizing enzymes: a discussion of their properties and action pattern. Adv Carbohydr Chem Biochem. 1968;23:281–366. doi: 10.1016/s0096-5332(08)60171-x. [DOI] [PubMed] [Google Scholar]
- HOBSON P. N., MACPHERSON M. Amylases of Clostridium butyricum and a Streptococcus isolated from the rumen of the sheep. Biochem J. 1952 Dec;52(4):671–679. doi: 10.1042/bj0520671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVVY G. A., MARSH C. A. Preparation and properties of beta-glucuronidase. Adv Carbohydr Chem. 1959;14:381–428. doi: 10.1016/s0096-5332(08)60227-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Narayanan A. S., Shanmugasundaram E. R. Studies on amylase of Fusarium vasinfectum. Arch Biochem Biophys. 1967 Feb;118(2):317–322. doi: 10.1016/0003-9861(67)90355-4. [DOI] [PubMed] [Google Scholar]
- Niven C. F., Smiley K. L., Sherman J. M. The Hydrolysis of Arginine by Streptococci. J Bacteriol. 1942 Jun;43(6):651–660. doi: 10.1128/jb.43.6.651-660.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plapp B. V., Cole R. D. Purification and characterization of bovine liver beta-glucuronidase. Arch Biochem Biophys. 1966 Sep 26;116(1):193–206. doi: 10.1016/0003-9861(66)90027-0. [DOI] [PubMed] [Google Scholar]
- ROBYT J., FRENCH D. Action pattern and specificity of an amylase from Bacillus subtilis. Arch Biochem Biophys. 1963 Mar;100:451–467. doi: 10.1016/0003-9861(63)90112-7. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., French D. Multiple attach hypothesis of alpha-amylase action: action of porcine pancreatic, human salivary, and Aspergillus oryzae alpha-amylases. Arch Biochem Biophys. 1967 Oct;122(1):8–16. doi: 10.1016/0003-9861(67)90118-x. [DOI] [PubMed] [Google Scholar]
- SEELEY H. W., DAIN J. A. Starch hydrolyzing streptococci. J Bacteriol. 1960 Feb;79:230–235. doi: 10.1128/jb.79.2.230-235.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH D. G., SHATTOCK P. M. The serological grouping of Streptococcus equinus. J Gen Microbiol. 1962 Dec;29:731–736. doi: 10.1099/00221287-29-4-731. [DOI] [PubMed] [Google Scholar]
- WALKER G. J. A TRANSGLUCOSYLASE OF STREPTOCOCCUS BOVIS. Biochem J. 1965 Feb;94:299–308. doi: 10.1042/bj0940299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER G. J. THE CELL-BOUND ALPHA-AMYLASES OF STREPTOCOCCUS BOVIS. Biochem J. 1965 Feb;94:289–298. doi: 10.1042/bj0940289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER G. J., WHELAN W. J. The mechanism of carbohydrase action. 4. The mechanism of D-enzyme action. Biochem J. 1957 Dec;67(4):548–551. doi: 10.1042/bj0670548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESMEYER H., COHN M. The characterization of the pathway of maltose utilization by Escherichia coli. II. General properties and mechanism of action of amylomaltase. Biochim Biophys Acta. 1960 Apr 22;39:427–439. doi: 10.1016/0006-3002(60)90195-5. [DOI] [PubMed] [Google Scholar]
- Walker G. J., Hope P. M. Degradation of starch granules by some amylolytic bacteria from the rumen of sheep. Biochem J. 1964 Feb;90(2):398–408. doi: 10.1042/bj0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J. Metabolism of the reserve polysaccharide of Streptococcus mitis: Properties of a transglucosylase. Biochem J. 1966 Dec;101(3):861–872. doi: 10.1042/bj1010861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houte J., Winkler K. C., Jansen H. M. Iodophilic polysaccharide synthesis, acid production and growth in oral streptococci. Arch Oral Biol. 1969 Jan;14(1):45–61. doi: 10.1016/0003-9969(69)90020-x. [DOI] [PubMed] [Google Scholar]



