Abstract
Background:
A comprehensive survey-based COPD severity score has usefulness for epidemiologic and health outcomes research. We previously developed and validated the survey-based COPD Severity Score without using lung function or other physiologic measurements. In this study, we aimed to further validate the severity score in a different COPD cohort and using a combination of patient-reported and objective physiologic measurements.
Methods:
Using data from the Function, Living, Outcomes, and Work cohort study of COPD, we evaluated the concurrent and predictive validity of the COPD Severity Score among 1,202 subjects. The survey instrument is a 35-point score based on symptoms, medication and oxygen use, and prior hospitalization or intubation for COPD. Subjects were systemically assessed using structured telephone survey, spirometry, and 6-min walk testing.
Results:
We found evidence to support concurrent validity of the score. Higher COPD Severity Score values were associated with poorer FEV1 (r = −0.38), FEV1% predicted (r = −0.40), Body mass, Obstruction, Dyspnea, Exercise Index (r = 0.57), and distance walked in 6 min (r = −0.43) (P < .0001 in all cases). Greater COPD severity was also related to poorer generic physical health status (r = −0.49) and disease-specific health-related quality of life (r = 0.57) (P < .0001). The score also demonstrated predictive validity. It was also associated with a greater prospective risk of acute exacerbation of COPD defined as ED visits (hazard ratio [HR], 1.31; 95% CI, 1.24-1.39), hospitalizations (HR, 1.59; 95% CI, 1.44-1.75), and either measure of hospital-based care for COPD (HR, 1.34; 95% CI, 1.26-1.41) (P < .0001 in all cases).
Conclusion:
The COPD Severity Score is a valid survey-based measure of disease-specific severity, both in terms of concurrent and predictive validity. The score is a psychometrically sound instrument for use in epidemiologic and outcomes research in COPD.
Measurement of disease severity is important for epidemiologic and outcomes research on patients with COPD. COPD severity measurement is important for accurately characterizing a study sample, for statistically controlling for disease severity, and for use as a study outcome measure. Epidemiologic studies of COPD often focus on large populations that reside in a broad geographic region (eg, 48 contiguous states of the United States).1 Consequently, disease severity measures that require lung function assessment, such as the Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging or the Body Mass, Obstruction, Dyspnea, Exercise (BODE) Index, may not always be feasible due to logistic constraints.2-4 A survey-based measure of COPD severity would be valuable in these situations, in addition to serving as a complementary severity tool even when physiologic measures can be obtained.
We previously developed and validated the comprehensive disease-specific COPD Severity Score for use in epidemiologic and health outcomes research.5,6 It was subsequently translated into Spanish and validated in a primary care setting in Spain.7 A major advantage of this score is that it does not require lung function or other physical measurements, facilitating its use in large-scale epidemiologic studies of COPD. Nationwide studies of COPD, for instance, may not include spirometry or other physiologic measurements because of cost or other logistic constraints. Our survey-based COPD Severity Score, which does not require such physical measurements, has value as a COPD-specific health outcome measure or to adjust for disease-specific severity.
In our original validation study, we only had lung function measurement for a subset of subjects. Using data from the Function, Living, Outcomes, and Work Study of COPD, we now independently validate the COPD Severity Score in a different large cohort of COPD in which lung function was measured on all subjects and longitudinal follow-up is available. In this different cohort study, we demonstrate that the COPD Severity Score is a simple, valid, and useful disease-specific severity measure that can be used in epidemiologic and health outcomes studies.
Materials and Methods
The Function, Living, Outcomes, and Work Study of COPD is an ongoing prospective cohort study of adult members of an integrated health care delivery system with a physician’s diagnosis of COPD. Recruitment methods have been previously reported in detail.8-10 We recruited a cohort of 1,202 Kaiser Permanente Medical Care Program members who were recently treated for COPD using a validated algorithm based both on health care use and pharmacy dispensing for COPD.11
At baseline assessment, we conducted structured telephone interviews that ascertained COPD severity, clinical history, and sociodemographic characteristics.8-10 Research clinic visits included spirometry and other physical assessments. The study was approved both by the University of California San Francisco Committee on Human Research and the Kaiser Foundation Research Institute’s institutional review board and all participants provided written informed consent.
We previously developed and validated the disease-specific COPD Severity Score for use in epidemiologic and outcomes research.5,6 Based on survey responses, the COPD severity score is composed of five overall aspects of COPD severity: respiratory symptoms, systemic corticosteroid use, other COPD medication use, previous hospitalization and intubation for respiratory disease, and home oxygen use. Each item was weighted (and assigned points) based on clinical aspects of the disease and its expected contribution to overall COPD severity. The weighting system was confirmed by factor analysis. Possible total scores range from 0 to 35, with higher scores reflecting more severe COPD.
In the current study, we reevaluated the concurrent validity of the COPD Severity Score by assessing its association with three aspects of health status defined a priori: pulmonary function and other physical measurements, physical health-related quality of life (HRQL), and functional limitations. We hypothesized that greater COPD severity would correlate with decrements in these three dimensions of health status.
To measure pulmonary function, we conducted spirometry according to American Thoracic Society Guidelines.12,13 Spirometry was performed using the EasyOne Frontline spirometer (ndd Medical Technologies; Chelmsford, MA), which is known for its reliability, accuracy, and durability.14,15 Percent predicted values were calculated using the race-ethnicity specific predictive equations derived from National Health And Nutrition Examination Survey III.16
Submaximal exercise performance was measured using the 6-min walk test, which has been widely used in studies of COPD.17,18 We used a standardized flat, straight course of 30 m in accordance with American Thoracic Society Guidelines.8,9,19 We also used the validated BODE Index, which is a multimodal measure of disease severity in COPD that combines respiratory symptoms and physiologic data.2 The BODE index is based on the body-mass index (B), the degree of airflow obstruction (O) measured by FEV1, grade of dyspnea (D) assessed by the modified Medical Research Council Dyspnea Scale, and exercise capacity (E) measured by the 6-min walk test. The BODE index predicts death and other poor outcomes in COPD.2,20,21
Physical HRQL was measured with the Short Form (SF)-12 Physical Component Summary score. The SF-12 is derived from the Medical Outcomes Study SF-36 instrument, which is the most widely used measure of generic health status. The Physical Component Summary score reflects an underlying physical dimension of physical HRQL and higher scores reflect more favorable health states.22 To measure disease-specific HRQL, we used the Airways Questionnaire 20 revised.23,24 This validated instrument has excellent psychometric properties for assessing HRQL in persons with airway disease, including COPD. Higher scores correspond to poorer HRQL.23,25
Self-reported functional limitation was measured using a previously validated approach used by Sternfeld and colleagues.26 The scale is composed of 10 questions that assess the degree of difficulty in multiple domains of basic physical functioning, such as pushing, stooping, kneeling, getting up from a standing position, lifting lighter or heavier objects, standing, sitting, standing from a seated position, walking up stairs, and walking in the neighborhood. Subjects who indicate “a lot of difficulty” with one or more functions or not doing a function because they were unable or they were told by a doctor not to do so are defined as having a self-reported functional limitation.26
Two additional questions were derived from the SF-36 scale to measure functional limitation.27 These items ask whether the respondent’s health limits him or her a lot, a little, or not at all in moderate activities (eg, moving a table, pushing a vacuum cleaner, bowling, or playing golf) or climbing several flights of stairs. Subjects who indicated “a lot” of limitation were defined as having functional limitation on each item.
To evaluate predictive validity, we studied the relation between the COPD Severity Score and the prospective risk of acute exacerbations of COPD. Although there is no universal definition of COPD exacerbations, ED visit or hospitalization for COPD are often used for research purposes.28,29 Therefore, we used Kaiser computerized databases to identify ED visits and hospitalization for COPD as proxy measure of acute COPD exacerbation. COPD-related hospitalization was defined as those with a principal International Classification of Diseases, 9th ed. discharge diagnosis code for COPD (491.xx, 492.xx, or 496.xx). COPD-related ED visits were identified as those with an International Classification of Diseases, 9th ed. code for COPD. A composite outcome for hospital-based care was defined as either an ED visit or hospitalization for COPD. The median duration of follow-up was 2.1 years (25th-75th interquartile range, 1.7-2.6 years). During the follow-up period, there were 76 hospitalizations and 244 ED visits for COPD.
Statistical analysis was conducted using SAS 9.1 (SAS, Inc.; Cary, NC). We evaluated concurrent validity by examining the Pearson correlation between the COPD Severity Score and physical measurements (eg, pulmonary function) and HRQL. To control for age, sex, race, and educational attainment, the Pearson partial correlation was calculated. Logistic regression analysis was used to study the association between COPD Severity Score and the risk of functional limitations. We used Cox proportional hazards regression to elucidate the impact of the COPD Severity Score on the prospective risk of ED visits, hospitalizations, and hospital-based care for COPD (composite endpoint of ED visits and hospitalizations). In a secondary analysis, we re-estimated the Cox regression after controlling for FEV1 and BODE scores as additional physiology-based measures.
In both Cox and logistic regression analyses, we also controlled for age, sex, race, smoking history, and educational attainment. The risk estimates (ie, odds ratio [OR] or hazard ratio [HR]) were expressed per 0.5 SD of COPD Severity Score (ie, 3.04 points), an approximation of the minimal clinically important difference.30
As a sensitivity analysis, we repeated the analysis in less severe and more severe disease strata defined by the GOLD criteria. To evaluate a more severe spectrum of COPD, we examined those with GOLD stage II or greater defined as an FEV1/FVC ratio < 0.70 and FEV1 < 80% predicted. This stratum is consistent with the focus of the Burden of Obstructive Lung Disease stratregy.31 The less severe stratum was defined as less than GOLD stage II.
Results
Concurrent Validity of the COPD Severity Score
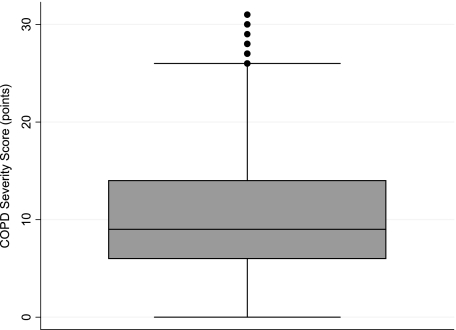
Baseline characteristics of the cohort are shown in Table 1 and the COPD Severity Score distribution is shown in Figure 1. Higher COPD Severity Score values were associated with poorer FEV1 (r = −0.38), FEV1% (r = −0.40), BODE Index (r = 0.57), and distance walked in 6 min (r = −0.43) (P < .0001 in all cases) (Table 2). The score also correlated with poorer generic health status (r = −0.49) and worse disease-specific HRQL (r = 0.57) (Table 3). In all cases, the correlations were similar in the subset of cohort members with COPD classified as GOLD stage II or greater. In the less severe stratum, the correlations were less strong for lung function measures (but still statistically significant). Correlations adjusted for age, sex, race, and educational attainment were highly similar to the unadjusted correlations.
Table 1.
—Baseline Characteristics of Function, Living, Outcomes, and Work Study Cohort of COPD
| Characteristic | Entire Cohort (N = 1,202) | GOLD Stage < II (n = 460) | GOLD Stage ≥ II (n = 742) |
| Age, y | 58 (6) | 56 (6.6) | 59 (6) |
| Male sex | 511 (43%) | 165 (36%) | 346 (47%) |
| White, non-Hispanic race/ethnicity | 810 (67%) | 280 (61%) | 530 (71%) |
| FEV1, L | 1.79 (0.78) | 2.37 (0.68) | 1.44 (0.60) |
| FEV1, % predicted | 62% (23%) | 83% (16%) | 49% (17%) |
| BODE Index, points | 2.9 (2.4) | 1.8 (1.9) | 3.6 (2.4) |
| COPD Severity Scorea | 10.3 (6.1) | 8.4 (5.4) | 11.4 (6.2) |
Values given are mean (SD) or No. (%). BODE 5 Body Mass, Obstruction, Dyspnea, Exercise; GOLD 5 Global Initiative for Chronic Obstructive Lung Disease.
Range 0-31 points (median 9 points) in overall cohort.
Figure 1.
Box plot of COPD Severity Score. The shaded area shows the 25th to 75th interquartile range of scores (the line through the shaded area is the median score). The upper and lower lines show the upper and lower adjacent values, respectively (eg, upper adjacent value is 75th percentile value + 1.5 × interquartile range).
Table 2.
—Correlation Between COPD Severity Score and Physical Measures: Pulmonary Function, BODE Index, and Distance Walked in 6 Min
| Entire Cohort (N = 1,202) |
GOLD Stage < II (n = 460) |
GOLD Stage ≥ II (n = 742) |
||||
| Characteristic | Unadjusted Correlation | Adjusted Correlationa | Unadjusted Correlation | Adjusted Correlationa | Unadjusted Correlation | Adjusted Correlationa |
| FEV1 | −0.38 | −0.41 | −0.19 | −0.21 | −0.37 | −0.39 |
| FEV1, % predicted | −0.40 | −0.41 | −0.22 | −0.22 | −0.39 | −0.39 |
| BODE Indexb | 0.57 | 0.56 | 0.47 | 0.44 | 0.57 | 0.55 |
| Distance walked in 6 minc | −0.43 | −0.43 | −0.36 | −0.34 | −0.46 | −0.47 |
All P < .0001. See Table 1 for expansion of abbreviations.
Partial correlation adjusted for age, sex, race, and educational attainment.
Higher BODE Index scores indicate poorer health status.
6-min walk test.
Table 3.
—Relation Between COPD Severity Score and Health-Related Quality of Life
| Entire Cohort (N = 1,202) |
GOLD Stage < II (n = 460) |
GOLD Stage ≥ II (n = 742) |
||||
| HRQL Measure | Unadjusted Correlation | Adjusted Correlationa | Unadjusted Correlation | Adjusted Correlationa | Unadjusted Correlation | Adjusted Correlationa |
| Generic physical health status (SF-12 Physical Component Summary Score) | −0.49 | −0.48 | −0.52 | −0.50 | −0.47 | −0.45 |
| Disease-specific HRQL (AQ-20R) | 0.57 | 0.57 | 0.59 | 0.57 | 0.58 | 0.56 |
All P< .0001. Higher scores on SF-12 indicate more favorable health status; higher scores on AQ-20R indicate poorer health status. AQ-20R = Airways Questionnaire 20 Revised; HRQL = health-related quality of life; SF = short form. See Table 1 for expansion of other abbreviations.
Partial correlation adjusted for age, sex, race, and educational attainment.
Higher COPD severity scores were associated with a greater risk of self-reported functional limitation (OR per 0.5 SD increment in severity score, 1.60; 95% CI, 1.48-1.73), limitation in moderate activities (OR, 1.64; 95% CI, 1.52-1.78), and limitation in stair climbing (OR, 1.68; 95% CI, 1.55-1.82) (Table 4). Findings were similar among subjects with lesser and greater disease severity defined by GOLD category.
Table 4.
—COPD Severity Score and the Risk of Functional Limitations
| Functional Limitation Measure | Entire Cohort (N = 1,202) | GOLD Stage < II (n = 460) | GOLD Stage ≥ II (n = 742) |
| Self-reported functional limitationa | 1.60 (1.48-1.73) | 1.56 (1.36-1.78) | 1.63 (1.48-1.80) |
| Limitation in moderate activitiesb | 1.64 (1.52-1.78) | 1.75 (1.50-2.03) | 1.61 (1.46-1.77) |
| Limitation in climbing several flights of stairs | 1.68 (1.55-1.82) | 1.73 (1.50-2.00) | 1.63 (1.47-1.80) |
Values expressed as odds ratio (95% CI). All P < .0001. Logistic regression analysis controlling for age, sex, race, and educational attainment. See Table 1 for expansion of abbreviations.
“Unable to do” or “severe limitation” in a battery of basic physical activities (see “Materials and Methods” section).
Health limits “a lot” in performing moderate activities, such as moving a table, pushing a vacuum cleaner, bowling, or playing golf.
Predictive Validity of the COPD Severity Score
Higher COPD Severity Scores were associated with a greater prospective risk of acute exacerbation of COPD requiring emergency health care use, including ED visits (HR, 1.31; 95% CI, 1.24-1.39), hospitalizations (HR, 1.59; 95% CI, 1.44-1.75), or either indicator of hospital-based care for COPD (HR, 1.34; 95% CI, 1.26-1.41) (Table 5). The risk estimates were highly similar among the less severe and more severe strata defined by GOLD grouping.
Table 5.
—COPD Severity Score and the Prospective Risk of Acute Exacerbations of COPD
| Emergency Health Care Use for COPD | Entire Cohort (N = 1,202) | GOLD Stage < II (n = 460) | GOLD Stage ≥ II (n = 742) |
| ED visit | 1.31 (1.24-1.39) | 1.33 (1.18-1.50) | 1.32 (1.26-1.41) |
| Hospitalization | 1.59 (1.44-1.75) | 1.42 (1.09-1.86)a | 1.60 (1.43-1.79) |
| Any hospital-based careb | 1.34 (1.26-1.41) | 1.30 (1.16-1.45) | 1.36 (1.27-1.45) |
Values expressed as hazard ratio (95% CI). P < .0001 unless otherwise indicated. Cox proportional hazards analysis controlling for age, sex, race, and educational attainment. Hazard ratio is per 0.5 SD increment in COPD Severity Score. See Table 1 for expansion of abbreviation.
P = .01.
ED visit or hospitalization for COPD.
In a secondary analysis, we reestimated the predictive validity of the COPD Severity Score after controlling for FEV1 and BODE Index as physiology-based severity indicators. Higher COPD Severity Score values remained associated with a greater longitudinal risk of ED visits (HR, 1.17; 95% CI, 1.09-1.25), hospitalizations (HR, 1.31; 95% CI, 1.16-1.48), and hospital-based care for COPD (HR, 1.18; 95% CI, 1.10-1.26) (P < .0001 in all cases).
Discussion
In our original report of the COPD Severity Score, we indicated that the validation in a separate and larger cohort would be advantageous.5 In this analysis, based on an entirely independent COPD cohort, we now have further demonstrated the validity of the COPD Severity Score. The score has excellent concurrent validity compared with diverse measures of lung function, exercise capacity, HRQL, and functional limitation. It also has predictive validity for acute exacerbations of COPD leading to hospital-based health care use. Therefore, the score is a psychometrically sound severity instrument and can be used in epidemiologic and outcomes research in COPD.
The COPD Severity Score can be used easily in survey-based research because it is simple to administer and does not require pulmonary function measurement or other physiologic assessments. It was originally validated in a population-based national cohort study of subjects with self-reported physician diagnosis of chronic bronchitis, emphysema, or COPD. The current study examined a very different cohort of COPD patients who were recruited from a large managed care organization and had both health-care use with diagnostic coding for COPD and pharmacy dispensing of COPD medications. Remarkably, the current analysis found the identical correlation between COPD Severity Score and FEV1 percent predicted as reported in the original study, which had available spirometry only in a subset of subjects (r = −0.40 in both cases). The strong evidence of validity in two very different COPD cohorts, which was demonstrated using a diverse set of measures, provides compelling evidence that the COPD Severity Score has substantive validity for research purposes.
The BODE Index has become a widely used measure of COPD severity because it has substantive concurrent and predictive validity.2,20,21 However, it requires measurement of lung function and distance walked in 6 min, which may not be logistically feasible in large-scale epidemiologic studies of geographically diverse populations. The survey-based COPD Severity Score, which has predictive validity even after controlling for FEV1 and BODE Index, provides a robust assessment of COPD severity without measurement of physiologic variables.
Important study strengths include the large size of the COPD cohort, broad spectrum of disease severity, systematic collection of physiologic and functional status data, prospective ascertainment of health care use, and subject diversity in terms of age, gender, race-ethnicity, and socioeconomic status. Further, recruitment from a large health plan helps to ensure generalizability to patients who are being treated for COPD in clinical practice.
Our study is also subject to limitations. Although the inclusion criteria required health care use for COPD, misclassification of COPD could have occurred. Our COPD definition required concomitant treatment with COPD medications to increase its specificity. In addition, all patients had a physician diagnosis of COPD and reported having the condition. Confirmation of study results among persons with GOLD stage II or greater strongly indicates that our findings were not driven by systematic diagnostic misclassification. This reinforces our previous validation of the subject selection algorithm based on medical record review.11 Nonetheless, we acknowledge the possibility of diagnostic heterogeneity within our cohort and cannot completely exclude the possibility that this could affect some of the findings, most likely by biasing toward the null. Aside from geographic considerations, the generalizability of these findings may be limited in application to populations without access to health care. Moreover, the COPD Severity Score has not been systematically assessed in other linguistic or cultural contexts.
In addition, we performed multiple statistical tests, which raises the possibility of false-positive results (ie, type 1 error). Because we specified our analyses a priori, however, we did not explicitly adjust for multiple comparisons.32 Also, the very small P values make false-positive results highly unlikely.
In summary, the COPD Severity Score is a simple, valid, and robust survey-based instrument that can be applied in epidemiologic studies to adjust for disease severity or analyze health outcomes. It correlates with, but does not require the measurement of, pulmonary function that can be logistically impossible or prohibitively expensive to measure in large-scale epidemiologic studies conducted over broad geographic regions.
Supplementary Material
Acknowledgments
Author contributions: Dr Eisner: contributed to designing the study, designing and performing the analysis, and writing the manuscript.
Dr Omachi: contributed to the analysis and writing the manuscript.
Dr Katz: contributed to writing the manuscript.
Dr Yelin: contributed to interpreting the data and writing the manuscript.
Dr Iribarren: contributed to designing the study and writing the manuscript.
Dr Blanc: contributed to designing the study, analyzing the data, and writing the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- BODE Index
Body Mass, Obstruction, Dyspnea, Exercise Index
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- HR
hazard ratio
- HRQL
health-related quality of life
- OR
odds ratio
- SF
short form
Footnotes
Funding/Support: This study was funded by the National Heart, Lung, and Blood Institute [Grant R01 HL077618], National Institutes of Health and Flight Attendants Medical Research Institute, UCSF Bland Lane Center of Excellence in Secondhand Smoke. Dr Eisner was also supported by the National Heart, Lung, and Blood Institute [K24 HL 097245].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Trupin L, Earnest G, San Pedro M, et al. The occupational burden of chronic obstructive pulmonary disease. Eur Respir J. 2003;22(3):462–469. doi: 10.1183/09031936.03.00094203. [DOI] [PubMed] [Google Scholar]
- 2.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 3.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 4.Fabbri LM, Hurd SS GOLD Scientific Committee. Global strategy for the diagnosis, management and prevention of COPD: 2003 update. Eur Respir J. 2003;22(1):1–2. doi: 10.1183/09031936.03.00063703. [DOI] [PubMed] [Google Scholar]
- 5.Eisner MD, Trupin L, Katz PP, et al. Development and validation of a survey-based COPD severity score. Chest. 2005;127(6):1890–1897. doi: 10.1378/chest.127.6.1890. [DOI] [PubMed] [Google Scholar]
- 6.Omachi TA, Yelin EH, Katz PP, et al. The COPD Severity Score: a dynamic prediction tool for health care utilization. COPD. 2008;5(6):339–346. doi: 10.1080/15412550802522700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miravitlles M, Llor C, de Castellar R, Izquierdo I, Baró E, Donado E. Validation of the COPD severity score for use in primary care: the NEREA study. Eur Respir J. 2009;33(3):519–527. doi: 10.1183/09031936.00087208. [DOI] [PubMed] [Google Scholar]
- 8.Eisner MD, Blanc PD, Yelin EH, et al. COPD as a systemic disease: impact on physical functional limitations. Am J Med. 2008;121(9):789–796. doi: 10.1016/j.amjmed.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisner MD, Iribarren C, Yelin EH, et al. Pulmonary function and the risk of functional limitation in chronic obstructive pulmonary disease. Am J Epidemiol. 2008;167(9):1090–1101. doi: 10.1093/aje/kwn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanc PD, Iribarren C, Trupin L, et al. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax. 2009;64(1):6–12. doi: 10.1136/thx.2008.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidney S, Sorel M, Quesenberry CP, Jr, et al. COPD and incident cardiovascular disease hospitalizations and mortality. Kaiser Permanente Medical Care Program. Chest. 2005;128(4):2068–2075. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 12.Statement of the American Thoracic Society Standardization of spirometry—1987 update. Am Rev Respir Dis. 1987;136(5):1285–1298. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society Standardization of spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 14.Walters JA, Wood-Baker R, Walls J, Johns DP. Stability of the EasyOne ultrasonic spirometer for use in general practice. Respirology. 2006;11(3):306–310. doi: 10.1111/j.1440-1843.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Padilla R, Vázquez-García JC, Márquez MN, et al. Latin American COPD Prevalence Study (PLATINO) Team The long-term stability of portable spirometers used in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care. 2006;51(10):1167–1171. [PubMed] [Google Scholar]
- 16.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- 18.Sciurba F, Criner GJ, Lee SM, et al. National Emphysema Treatment Trial Research Group. Six-minute walk distance in chronic obstructive pulmonary disease: reproducibility and effect of walking course layout and length. Am J Respir Crit Care Med. 2003;167(11):1522–1527. doi: 10.1164/rccm.200203-166OC. [DOI] [PubMed] [Google Scholar]
- 19.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 20.Martinez FJ, Han MK, Andrei AC, et al. National Emphysema Treatment Trial Research Group. Longitudinal change in the BODE index predicts mortality in severe emphysema. Am J Respir Crit Care Med. 2008;178(5):491–499. doi: 10.1164/rccm.200709-1383OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong KC, Lu SJ, Soh CS. Does the multidimensional grading system (BODE) correspond to differences in health status of patients with COPD? Int J Chron Obstruct Pulmon Dis. 2006;1(1):91–96. doi: 10.2147/copd.2006.1.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Hajiro T, Nishimura K, Jones PW, et al. A novel, short, and simple questionnaire to measure health-related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(6):1874–1878. doi: 10.1164/ajrccm.159.6.9807097. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Eisner MD, Katz PP, Yelin EH, Blanc PD. Measuring disease-specific quality of life in obstructive airway disease: validation of a modified version of the airways questionnaire 20. Chest. 2006;129(6):1644–1652. doi: 10.1378/chest.129.6.1644. [DOI] [PubMed] [Google Scholar]
- 25.Alemayehu B, Aubert RE, Feifer RA, et al. Comparative analysis of two quality-of-life instruments for patients with chronic obstructive pulmonary disease. Value Health. 2002;5(5):437–442. doi: 10.1046/J.1524-4733.2002.55151.x. [DOI] [PubMed] [Google Scholar]
- 26.Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156(2):110–121. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 28.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan VS, Ramsey SD, Make BJ, Martinez FJ. Physiologic variables and functional status independently predict COPD hospitalizations and emergency department visits in patients with severe COPD. COPD. 2007;4(1):29–39. doi: 10.1080/15412550601169430. [DOI] [PubMed] [Google Scholar]
- 30.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 31.Buist AS, McBurnie MA, Vollmer WM, et al. BOLD Collaborative Research Group International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 32.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.