Abstract
Studying seemingly simple metathesis reactions between ZnCl2 and tBuMgCl has, surprisingly, revealed a much more complex chemistry involving mixed magnesium-zinc compounds that could be regarded as Mg-Zn hybrids. Thus, the reaction of equimolar amounts of ZnCl2 and tBuMgCl reveals the formation of the unprecedented mixed Mg-Zn complex [(THF)4Mg(μ-Cl)2Zn(tBu)(Cl)] (1), as a result of the co-complexation of the two anticipated exchange products of the metathesis. This magnesium zincate adopts a contacted ion-pair structure, closely related to Knochel’s pioneering “Turbo” Grignard reagents. Furthermore, a second coproduct identified in this reaction is the solvent-separated mixed magnesium-zinc chloride complex [{Mg(THF)6}2+{Zn2Cl6}2-] (3) that critically diminishes the amount of ZnCl2 available for the intended metathesis reaction to take place. In another surprising result, when the reaction is carried out by using an excess of 3 M equivalents of the Grignard reagent (closer to the catalytic conditions employed by synthetic chemists), solvent-separated magnesium trialkyl zincate [{Mg2Cl3(THF)6}+{Zn(tBu)3}-] (4) is obtained that can be viewed as a model for the active species involved in the increasingly important organic transformations of Grignard reagents catalysed by ZnCl2. Furthermore, preliminary reactivity studies reveal that complex 4 can be used as an effective new reagent for direct Zn-I exchange reactions that allow the preparation and structural identification of the magnesium tris(aryl) zincate [{Mg2Cl3(THF)6}+{Zn(p-Tol)3}-] (5) that represents the first example of complete 3-fold activation of a zincate in a Zn-I exchange reaction which, in turn, can efficiently be used as a precursor for Negishi cross-coupling reactions.
Organozinc compounds (together with organolithium and organomagnesium compounds) are amongst the most commonly used and vitally important reagents in synthesis, playing a key role in many fundamental organic transformations. Their main advantage is their unrivalled compatibility with a rich variety of organic functionalities that stems from the greater stability and more covalent character of Zn-C bonds (in comparison with Li-C or Mg-C bonds) (1). Together with the oxidative insertion of Zn metal into C-X bonds, metathesis reactions of ZnCl2 with more polar, more reactive organometallic reagents (typically organolithiums, RLi, or Grignard reagents, RMgX) are one of the most important synthetic tools to prepare organozinc reagents (2).
Related to this stoichiometric metathesis, a flurry of recent reports have established that the use of catalytic amounts of environmentally benign ZnCl2 with Grignard reagents greatly enhances the reactivity and selectivity of the latter in numerous cornerstone methodologies in organic synthesis such as alkylations (3, 4), nucleophilic substitutions (5), or cross-coupling applications (6). Mixed magnesium-zinc species R3ZnMgCl, resulting from the “in situ” metathesis reaction of the relevant Grignard reagent with the catalyst ZnCl2, seem to be implicated in these important transformations. However, their presence (as transient intermediates or defined compounds) is only assumed and there is no tangible spectroscopic or structural proof of their role in these vital, new synthetic methodologies. In fact, the number of organometallic compounds combining magnesium and zinc within the same molecule is scarce (7, 8), contrasting with the numerous reports on the synthesis of mixed-metal compounds (ates) containing an alkali-metal with either magnesium (magnesiates), or zinc (zincates), that show enhanced reactivity beyond the confines of the monometallic reagents from which they are derived (9–11).
In situ metathetical approaches are by far the most common vehicles of choice to prepare either these mixed magnesium-zinc putative intermediates or neutral organozinc reagents. Herein, we shed light on this fundamental synthetic tool, by isolating and characterising (structurally and spectroscopically) mixed-metal magnesium-zinc species (Mg-Zn hybrids) resulting from the reaction of ZnCl2 with tBuMgCl, which reveal that metathesis reactions and the constitutions of the products formed can be decidedly more complex than their simple reaction equations would suggest. Furthermore, we disclose their application to efficiently prepare tris(aryl) magnesium zincates via direct metal-halogen exchange reactions, one of the most important and commonly used methodologies in synthesis (12).
Results and Discussion
Stoichiometric Metathesis.
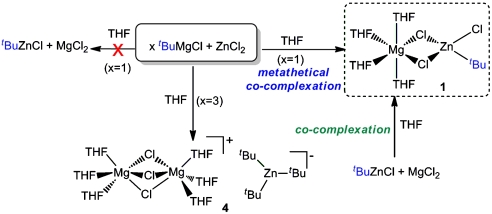
Firstly following the standard protocol used in the synthetic arena we reacted equimolar amounts of ZnCl2 dissolved in THF with a commercial solution of the Grignard reagent tBuMgCl in THF, from which a white solid was obtained almost instantaneously. Initially, we logically but mistakenly attributed this to the formation of MgCl2 (in fact it appears to be largely the hybrid magnesium-zinc chloride [{Mg(THF)6}2+{Zn2Cl6}2-] (3), vide infra) that was removed under filtration. Addition of hexane to the remaining solution allowed the crystallisation of a second product, namely the unexpected mixed magnesium-zinc mixed alkyl-chloride complex [(THF)4Mg(μ-Cl)2Zn(tBu)(Cl)] (1), as revealed by multinuclear NMR (1H and 13C) spectroscopy and X-ray crystallography, instead of the originally anticipated homometallic zinc compound tBuZnCl (Scheme 1). The isolated yield of 1 was a modest 10%.
Scheme 1.
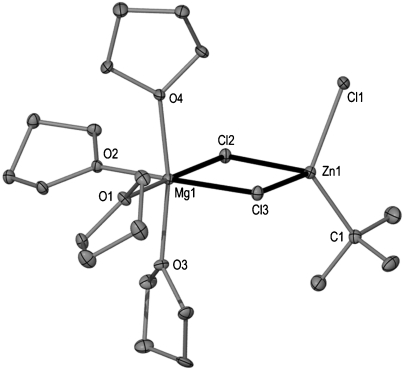
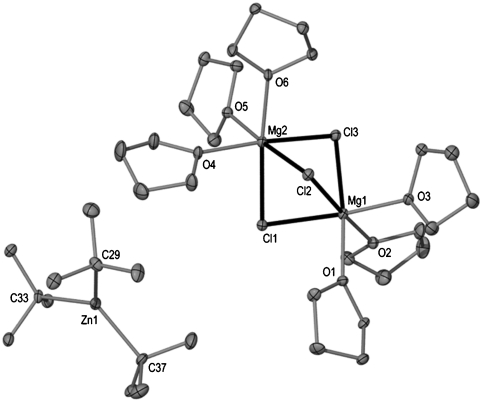
The molecular structure of 1 established by X-ray crystallographic studies (Fig. 1) confirms its heterobimetallic constitution, where both metals magnesium and zinc are intimately contacted through the presence of two chlorine bridges giving rise to a planar [Mg(μ-Cl)2Zn] four-membered ring. The zinc center completes its distorted tetrahedral coordination sphere by bonding to a terminal chlorine and a terminal tert-butyl group that was originally bound to magnesium. Magnesium achieves its larger distorted octahedral geometry by also bonding to four molecules of THF as well as to the Cl bridges. Tetrahedral Zn forms a strong sigma bond [Zn1-C1 2.000(3) Å] with the quaternary carbon of the formally anionic tert-butyl ligand that is marginally elongated to that found in discrete, linear  [1.977(4) Å] (13). Compound 1 exhibits a unique contacted ion-pair organometallic structure combining Mg with Zn and it can be envisaged as a “molecular salt” structure where the ionic MgCl2 fragment is trapped within a molecular framework before it can aggregate to build a lattice.* Thus, the two expected products of the metathesis reaction, tBuZnCl and MgCl2 have formed an unprecedented type of magnesium-zincate [Mg2+(ZnR3R′)2-] that can be regarded as a hybrid Mg-Zn Grignard reagent (14). The mixed-metal nature of 1 allows its comparison with Knochel’s Turbo Grignard reagents that combine a conventional Grignard reagent RMgX with LiCl (15–17), from which a myriad of successful regioselective functionalisations of a wide range of substituted aromatic and heterocyclic molecules have been reported. Furthermore, recent reports by the same group show that the addition of ZnCl2 to these bimetallic reagents to generate trimetallic systems can enhance their reactivity even further (18–20). In addition, Mulvey et al. have recently unveiled the structure of the related Turbo Hauser base [(THF)2Li(μ-Cl)2Mg(TMP)(THF)] (2) (Fig. 2) that exhibits a structural motif related to that of 1 where both metals are connected by two chlorine bridges with the remaining anionic ligand, in this case the amide TMP, is bonded to magnesium. Here, magnesium displays a tetrahedral geometry bonding to an additional molecule of THF; whereas lithium completes its coordination sphere with two molecules of THF (21). In 1, the greater electronegativity of zinc causes a reversal of the role played by magnesium that now acts as lithium (the cationic center) in 2, coordinating exclusively to the donor solvent (in addition to the two chlorine bridges) and transferring the anionic tBu ligand to stronger carbophile zinc.
[1.977(4) Å] (13). Compound 1 exhibits a unique contacted ion-pair organometallic structure combining Mg with Zn and it can be envisaged as a “molecular salt” structure where the ionic MgCl2 fragment is trapped within a molecular framework before it can aggregate to build a lattice.* Thus, the two expected products of the metathesis reaction, tBuZnCl and MgCl2 have formed an unprecedented type of magnesium-zincate [Mg2+(ZnR3R′)2-] that can be regarded as a hybrid Mg-Zn Grignard reagent (14). The mixed-metal nature of 1 allows its comparison with Knochel’s Turbo Grignard reagents that combine a conventional Grignard reagent RMgX with LiCl (15–17), from which a myriad of successful regioselective functionalisations of a wide range of substituted aromatic and heterocyclic molecules have been reported. Furthermore, recent reports by the same group show that the addition of ZnCl2 to these bimetallic reagents to generate trimetallic systems can enhance their reactivity even further (18–20). In addition, Mulvey et al. have recently unveiled the structure of the related Turbo Hauser base [(THF)2Li(μ-Cl)2Mg(TMP)(THF)] (2) (Fig. 2) that exhibits a structural motif related to that of 1 where both metals are connected by two chlorine bridges with the remaining anionic ligand, in this case the amide TMP, is bonded to magnesium. Here, magnesium displays a tetrahedral geometry bonding to an additional molecule of THF; whereas lithium completes its coordination sphere with two molecules of THF (21). In 1, the greater electronegativity of zinc causes a reversal of the role played by magnesium that now acts as lithium (the cationic center) in 2, coordinating exclusively to the donor solvent (in addition to the two chlorine bridges) and transferring the anionic tBu ligand to stronger carbophile zinc.
Fig. 1.
Molecular structure of 1 with hydrogen atoms omitted for clarity.
Fig. 2.
Comparison of the structures of Turbo Hauser Base [(THF)2Li(η-Cl)2Mg(TMP)(THF)] (2) and magnesium zinc hybrid 1.
Turning to the structure of 1 in solution, it is well known that solution constitutions of Grignard reagents are extremely complicated and direct comparison between solid structures and solution studies can often be difficult. Imamoto and Yamaguchi have elegantly proved by using coldspray ionization mass spectrometry (CSI-MS) that the dominant species of a complicated mixture in THF solution for RMgCl formulations is binuclear RMg2Cl3(THF)x (R = Me, tBu, Ph, Bz; x = 4–6) (22) that can be envisaged as a homometallic analog of 1, an adduct between MgCl2 and RMgCl solvated by THF molecules. We found that at room temperature the 1H NMR spectrum of 1 in d8-THF solution showed a sharp singlet at 0.99 ppm for the tBu group and two multiplets at 3.63 and 1.77 ppm for free protic THF (replaced by the large excess of d8-THF molecules), whose integration showed a tBu∶THF 1∶4 ratio identical to that found in the crystal structure. In addition, the 13C NMR spectrum of 1 displayed two resonances at 21.9 and 33.6 ppm for the quaternary carbon and the methyl groups of the tBu ligand, respectively. These 13C chemicals shifts are similar to those found for the related tBuZnCl species in the same deuterated solvent (21.0, 33.2 ppm). However, they differ significantly to those found in related organomagnesium compounds [ , 15.8, 36.0 ppm; tBuMgCl, 15.6, 35.8 ppm (the 13C{1H) NMR spectrum of tBuMgCl in deuterated THF is highly complex showing in addition three minor species that also contain tBu groups that is consistent with the complicated structure that Grignard reagents in general can exhibit in solution )] (22) suggesting that mixed-metal species 1 retains most of its “zinc character.” In addition, no changes in the 1H spectrum of 1 were observed when low temperature NMR studies were carried out at -40 °C, suggesting that, even at subambient temperatures, 1 retains its bimetallic constitution.
, 15.8, 36.0 ppm; tBuMgCl, 15.6, 35.8 ppm (the 13C{1H) NMR spectrum of tBuMgCl in deuterated THF is highly complex showing in addition three minor species that also contain tBu groups that is consistent with the complicated structure that Grignard reagents in general can exhibit in solution )] (22) suggesting that mixed-metal species 1 retains most of its “zinc character.” In addition, no changes in the 1H spectrum of 1 were observed when low temperature NMR studies were carried out at -40 °C, suggesting that, even at subambient temperatures, 1 retains its bimetallic constitution.
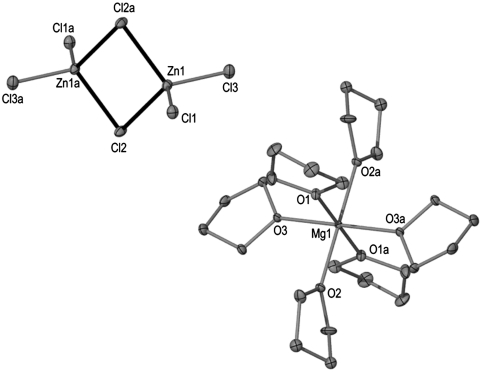
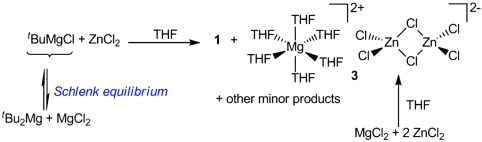
Because using this metathetical approach compound 1 is obtained only in poor yield, we next turned our attention to investigate in detail the composition of the white solid formed when ZnCl2 was reacted with tBuMgCl. This solid proved to be insoluble at room temperature in common organic solvents such as ether, THF, or toluene. Atomic absorption analysis of this solid revealed that it contains both magnesium and zinc. Furthermore, when some of this solid was dissolved in boiling THF, cooling the resulting solution afforded colorless crystals of the trinuclear monomagnesium-dizinc complex [{Mg(THF)6}2+{Zn2Cl6}2-] (3) as determined by X-ray crystallography. Complex 3 exhibits a solvent-separated structure (Fig. 3) where the cation is formed by an octahedral magnesium surrounded by six molecules of THF whereas the anionic moiety consists of two zinc atoms bonded to two chlorine bridges and two terminal ones in a distorted tetrahedral geometry. Several examples of other mixed-metal compounds containing either one of these two ions, separately but never together, have been reported (23–26). However, surprisingly a search of the Cambridge Crystallographic Database revealed that the 1∶2, Mg∶Zn stoichiometry found in compound 3 is extremely rare across the whole of mixed magnesium-zinc chemistry, with only three such compounds structurally characterized to date (27–29).
Fig. 3.
Anionic and cationic moieties of 3 with hydrogen atoms omitted for clarity.
The formation of 3 can be rationalised when the classical Schlenk equilibrium of Grignard reagents is taken into consideration (Scheme 2). Thus, tBuMgCl in THF solution will be in equilibrium with  and MgCl2, and on the addition of the ZnCl2 solution, the formation of the ionic mixed-metal species 3 must be greatly favored, probably due to its low solubility in THF. Adding support to this hypothesis, we managed to prepare 3 rationally by the reaction of MgCl2 with 2 M equivalents of ZnCl2 (Scheme 2). [Note that when compound 3 was prepared by using this co-complexation approach, the results for the Mg and Zn atomic absorption (Mg 3.87%, Zn 15.95%) were more satisfactory (theoretical compositions: Mg 3.03%, Zn 16.34%) than those found for the crude solid obtained by reaction of tBuMgCl and ZnCl2 (Mg 5.27%, Zn 13.14%), which suggest that in the latter method other magnesium species could also be involved]. The formation of 3 will not only have an effect on the Schlenk equilibrium, which will be driven towards the formation of more MgCl2, but also towards the amount of ZnCl2 readily available for the metathesis reaction to take place. Thus, strongly supporting this possibility, we found that the yield for the formation of the metathetical intermediate 1 can be greatly enhanced (almost quantitatively) when the co-complexation reaction of tBuZnCl with MgCl2 is carried out (Scheme 1).
and MgCl2, and on the addition of the ZnCl2 solution, the formation of the ionic mixed-metal species 3 must be greatly favored, probably due to its low solubility in THF. Adding support to this hypothesis, we managed to prepare 3 rationally by the reaction of MgCl2 with 2 M equivalents of ZnCl2 (Scheme 2). [Note that when compound 3 was prepared by using this co-complexation approach, the results for the Mg and Zn atomic absorption (Mg 3.87%, Zn 15.95%) were more satisfactory (theoretical compositions: Mg 3.03%, Zn 16.34%) than those found for the crude solid obtained by reaction of tBuMgCl and ZnCl2 (Mg 5.27%, Zn 13.14%), which suggest that in the latter method other magnesium species could also be involved]. The formation of 3 will not only have an effect on the Schlenk equilibrium, which will be driven towards the formation of more MgCl2, but also towards the amount of ZnCl2 readily available for the metathesis reaction to take place. Thus, strongly supporting this possibility, we found that the yield for the formation of the metathetical intermediate 1 can be greatly enhanced (almost quantitatively) when the co-complexation reaction of tBuZnCl with MgCl2 is carried out (Scheme 1).
Scheme 2.
Catalytic Conditions.
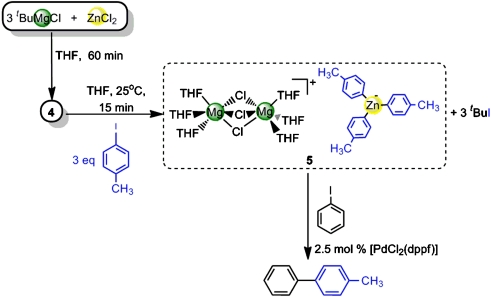
We next turned our attention to the study of the metathetical reaction of ZnCl2 with 3 M equivalents of tBuMgCl to prepare a magnesium trialkyl zincate and to be closer to the conditions used in catalytic applications (3–6). Compounds of this type have previously been suggested in the literature to be the active species in many zinc-catalysed organic reactions of Grignard reagents, however, very little is known about the true nature of these species. Thus, for example, Ishihara has recently developed a synthetic methodology using RMgCl in the presence of catalytic amounts of ZnCl2 that allows the alkylation of a wide range of ketones and aldimines minimizing the formation of undesired reduction or aldol side products (4). Experimentally, we found that on the addition of this excess of tBuMgCl to a solution of ZnCl2 in THF, a colorless solution was obtained which, on cooling, deposited crystals of the mixed-metal magnesium-rich zincate complex [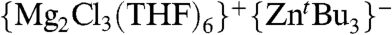 ] (4). Remarkably, no formation of insoluble product 3 is observed for this metathesis reaction that suggests that in the presence of an excess of Grignard reagent (here 3 M equivalents) the formation of mixed-metal species 4 must be greatly favored over the precipitation of 3.
] (4). Remarkably, no formation of insoluble product 3 is observed for this metathesis reaction that suggests that in the presence of an excess of Grignard reagent (here 3 M equivalents) the formation of mixed-metal species 4 must be greatly favored over the precipitation of 3.
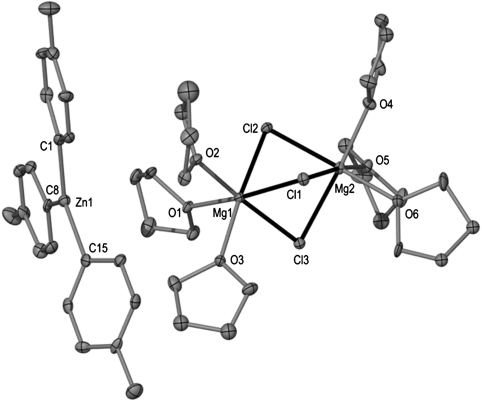
X-ray crystallographic studies established the solvent-separated ion-pair structure of 4, with a cationic moiety consisting of two distorted octahedral magnesium atoms sharing three chlorines and with three molecules of the solvent THF completing the coordination sphere of each magnesium (Fig. 4). The anionic part of the complex comprises a trigonal planar zinc center bonded to three tert-butyl groups. The binuclear cation {Mg2Cl3(THF)6}+ has been previously found to be part of the constitution of certain Grignard reagents in the solid state (22); whereas the structure of homoleptic  anion has been previously reported in the context of alkali-metal zincates (30–32). As previously seen for the formation of 1, the tert-butyl ligands have been transferred from Mg to the more electronegative Zn, and the byproduct of this metathesis reaction, MgCl2, has co-complexed with the relevant metathesis product (tBuZnCl for 1,
anion has been previously reported in the context of alkali-metal zincates (30–32). As previously seen for the formation of 1, the tert-butyl ligands have been transferred from Mg to the more electronegative Zn, and the byproduct of this metathesis reaction, MgCl2, has co-complexed with the relevant metathesis product (tBuZnCl for 1, 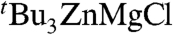 for 4). Despite this similarity, entirely different structural motifs are found and, whereas in 1 both metals are linked by two chlorine bridges, in 4 there is no possible metal-ligand-metal’ communication between Mg and Zn. Because structure and composition control reactivity (33), it can be anticipated that 1 and 4 may well exhibit distinct reactivities. Closely related to this idea, Oshima (5) has recently reported a study of nucleophilic substitutions of chlorosilanes with organomagnesium reagents in the presence of ZnCl2, finding that when the ratio RMgX∶ZnCl2 is 3∶1 (as in 4) the reaction occurs smoothly, affording comparable yields to those found when the reaction is carried out in the presence of catalytic amounts of ZnCl2; however, when the ratio between these reagents is changed to 2∶1 or 1∶1 (as in 1) no reaction is observed. Thus, Oshima’s reactivity studies, together with the fact that 4 is the product resulting from the metathesis reaction between 3 M equivalents of tBuMgCl and ZnCl2, suggests that mixed-metal solvent-separated ion pair structures such as that displayed by 4 could be the active or the coactive species in these catalytic transformations. (However, it must be noted that best results in these reactions are obtained when aryl magnesium reagents are employed, whereas with nBuMgBr only traces of the desired silane are obtained) (5).
for 4). Despite this similarity, entirely different structural motifs are found and, whereas in 1 both metals are linked by two chlorine bridges, in 4 there is no possible metal-ligand-metal’ communication between Mg and Zn. Because structure and composition control reactivity (33), it can be anticipated that 1 and 4 may well exhibit distinct reactivities. Closely related to this idea, Oshima (5) has recently reported a study of nucleophilic substitutions of chlorosilanes with organomagnesium reagents in the presence of ZnCl2, finding that when the ratio RMgX∶ZnCl2 is 3∶1 (as in 4) the reaction occurs smoothly, affording comparable yields to those found when the reaction is carried out in the presence of catalytic amounts of ZnCl2; however, when the ratio between these reagents is changed to 2∶1 or 1∶1 (as in 1) no reaction is observed. Thus, Oshima’s reactivity studies, together with the fact that 4 is the product resulting from the metathesis reaction between 3 M equivalents of tBuMgCl and ZnCl2, suggests that mixed-metal solvent-separated ion pair structures such as that displayed by 4 could be the active or the coactive species in these catalytic transformations. (However, it must be noted that best results in these reactions are obtained when aryl magnesium reagents are employed, whereas with nBuMgBr only traces of the desired silane are obtained) (5).
Fig. 4.
Anionic and cationic moieties of 4 with hydrogen atoms omitted for clarity.
We next studied the structure of 4 in solution using 1H and 13C NMR experiments which showed that in THF solution 4 is in dynamic equilibrium with its two monometallic components, 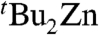 and tBuMgCl. Thus, when isolated crystals of 4 were dissolved in deuterated THF, four distinct resonances are observed in the tert-butyl region of the 1H NMR spectrum: a dominant singlet at 0.91 ppm that can be attributed to the main anionic
and tBuMgCl. Thus, when isolated crystals of 4 were dissolved in deuterated THF, four distinct resonances are observed in the tert-butyl region of the 1H NMR spectrum: a dominant singlet at 0.91 ppm that can be attributed to the main anionic  species but also three smaller resonances at 0.97, 0.88, and 0.86 ppm. that can be assigned to minor products
species but also three smaller resonances at 0.97, 0.88, and 0.86 ppm. that can be assigned to minor products 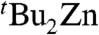 and tBuMgCl (two resonances), resp. Controlled addition experiments of
and tBuMgCl (two resonances), resp. Controlled addition experiments of 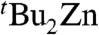 and tBuMgCl to solutions of 4 in deuterated THF (SI Text) confirmed that these minor species are in equilibrium with the mixed-metal compound 4. This equilibrium seems to be strikingly sensitive to the concentration of 4 in these THF-solutions, because under more dilute conditions disproportionation of 4 into its monometallic components is more favored. Based on the relative integration in the 1H NMR spectrum. the ratio
and tBuMgCl to solutions of 4 in deuterated THF (SI Text) confirmed that these minor species are in equilibrium with the mixed-metal compound 4. This equilibrium seems to be strikingly sensitive to the concentration of 4 in these THF-solutions, because under more dilute conditions disproportionation of 4 into its monometallic components is more favored. Based on the relative integration in the 1H NMR spectrum. the ratio 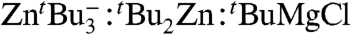 alters significantly from approximately 8∶1∶1 to 1∶1.3∶1.3 as the solution is made more dilute (SI Text). This solution scenario is probably the closest to that found under catalytic conditions. However, the position of this equilibrium will depend profoundly on what component reacts first with the organic substrate and on the starting concentration of the excess Grignard reagent present in the reaction mixture (34).
alters significantly from approximately 8∶1∶1 to 1∶1.3∶1.3 as the solution is made more dilute (SI Text). This solution scenario is probably the closest to that found under catalytic conditions. However, the position of this equilibrium will depend profoundly on what component reacts first with the organic substrate and on the starting concentration of the excess Grignard reagent present in the reaction mixture (34).
Applications in Zn-I Exchange Reactions.
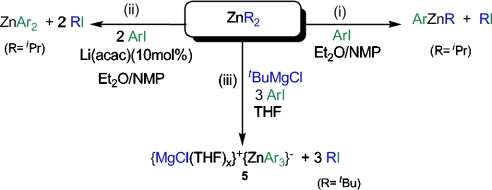
Metal-halogen exchange reactions constitute one of the most powerful synthetic tools to functionalise aromatic molecules (35). Zn-halogen exchange reactions of aromatic halides are particularly important because they allow the preparation of arylzinc compounds that are key precursors in Negishi cross-coupling processes (36–38). Whereas more polar (and therefore more reactive) organometallics such as organolithium or organomagnesuium reagents are well established metal-halogen exchange reagents, less polar dialkylzinc compounds are more reluctant to react with aryl iodides and the presence of additives or catalytic conditions is required for the Zn-I exchange reaction to occur at a reasonable rate. Thus, Knochel has elegantly shown that 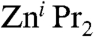 fails to react with aryl iodides when THF is used as a solvent; however, when a mixture of Et2O/NMP (NMP = N-methylpyrrolidinone) is employed, the desired aryl(alkyl)zinc product ArZni Pr can be obtained in quantitative yields (Scheme 3, i). The addition of catalytic amounts of inorganic salts such as Li(acac) (Scheme 3, ii) activates the second i Pr group of the organozinc reagent, allowing the preparation of highly functionalised diaryl zinc derivatives via direct Zn-I exchange (39). Furthermore, results from the same research group have revealed that the presence of magnesium salts on the reaction of alkyl iodides (more reactive than aryl derivatives) with
fails to react with aryl iodides when THF is used as a solvent; however, when a mixture of Et2O/NMP (NMP = N-methylpyrrolidinone) is employed, the desired aryl(alkyl)zinc product ArZni Pr can be obtained in quantitative yields (Scheme 3, i). The addition of catalytic amounts of inorganic salts such as Li(acac) (Scheme 3, ii) activates the second i Pr group of the organozinc reagent, allowing the preparation of highly functionalised diaryl zinc derivatives via direct Zn-I exchange (39). Furthermore, results from the same research group have revealed that the presence of magnesium salts on the reaction of alkyl iodides (more reactive than aryl derivatives) with 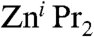 accelerated the Zn-I exchange process by a factor of 200 times compared to the reaction with neat
accelerated the Zn-I exchange process by a factor of 200 times compared to the reaction with neat 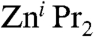 (40). Encouraged by these results, we decided to investigate the reactivity of trialkyl magnesium zincate 4 towards 4-iodo-toluene to establish if the three tert-butyl groups can be activated to undergo Zn-I exchange allowing the formation of a homoleptic tris(aryl) magnesium zincate {ZnAr3}- and three equivalents of tBuI (Scheme 3, iii).
(40). Encouraged by these results, we decided to investigate the reactivity of trialkyl magnesium zincate 4 towards 4-iodo-toluene to establish if the three tert-butyl groups can be activated to undergo Zn-I exchange allowing the formation of a homoleptic tris(aryl) magnesium zincate {ZnAr3}- and three equivalents of tBuI (Scheme 3, iii).
Scheme 3.
Thus, 3 M equivalents of 4-iodotoluene were added to a solution of 4 (prepared in situ as described above) in THF, affording a pale yellow solution that was allowed to stir at room temperature for 15 min before being placed in the freezer (at -30 °C) which after 24 h deposited colorless crystals of [{Mg2Cl3(THF)6}+{Zn(p-Tol)3}-] (5) (p-Tol = 4-Me-C6H4) in a 60% yield, as determined by 1H and 13C NMR spectroscopy and X-ray crystallography. To elaborate, the 1H NMR spectrum of 5 in deuterated THF displayed two doublets at 7.67 and 6.87 ppm and a singlet at 2.21 ppm that can be assigned to the aromatic and the methyl groups of the para-tolyl ligand, resp. In addition, two multiplets were observed at 3.61 and 1.77 corresponding to free THF molecules, whose integration showed a THF∶Ar 2∶1 ratio. The most notable feature of the 13C{1H} NMR spectrum is the presence of a signal at 162.8 ppm for the ipso carbon, which is now directly bonded to zinc, that is remarkably more downfield than in the aryl iodide (91.1 ppm).
The molecular structure of 5 determined by X-ray crystallographic studies provided confirmation that the Zn-I exchange reaction has successfully occurred, via the activation of the three tert-butyl groups of 4 to form the new tris(aryl) Mg-Zn hybrid 5 (vide infra). In close analogy to its precursor 4, 5 displays a solvent-separated ion-pair structure, containing the same binuclear cation {Mg2Cl3(THF)6}+, whereas its anionic counterpart is constituted by a trigonal planar zinc center σ-bonded to three para-tolyl groups (Fig. 5).
Fig. 5.
Anionic and cationic moieties of 5 with hydrogen atoms omitted for clarity.
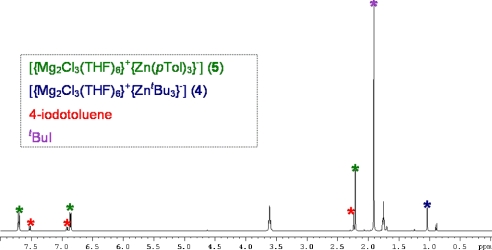
To shed more light on the reaction of 4 with 4-iodotoluene and to distinguish between the possibility that 5 is made either by metal-halogen exchange or by an alternative pathway (such as a disproportionation process of a plausible {ZnAr2(tBu)} intermediate species), we next carried out the reaction by using deuterated THF as the solvent, monitoring the reaction directly by 1H NMR spectroscopy (Fig. 6). These studies revealed that, after 30 min, 83% of compound 4 has been converted into tris(aryl)zincate 5, establishing unequivocally that the latter is the result of a direct Zn-I exchange between hybrid 4 and 3 M equivalents of 4-iodotoluene. The formation of coproduct tBuI could be detected in the 1H NMR spectrum (singlet at 1.91 ppm), which also showed that this coproduct reacts very slowly with 5 (after 4 h at room temperature, traces of a different arylzinc species and 4-tert-butyl-toluene can be observed, SI Text). These preliminary studies prove that direct Zn-I exchange under mild conditions (room temperature, short reaction times) can be accomplished when magnesium trialkylzincate 4 is employed. Furthermore, these reactions occur with a high atom economy (41) because all three tert-butyl groups on 4 take part in the Zn-I exchange process. Within the context of lithium tri- and tetrazincates, Uchiyama, and Sakamoto (42–44) have reported their application in Zn-halogen exchange reactions, applying this methodology to a wide range of organic substrates. However, to the best of our knowledge, these reactions require the use of equimolar amounts of the aryl halide and the lithium zincate and, therefore, only one of the alkyl ligands of the mixed-metal reagent is consumed in the initial reaction, requiring then in the quenching step, the presence of an excess of the relevant electrophile because the remaining alkyl groups on the zincate can also be transferred.
Fig. 6.
1H NMR spectrum of [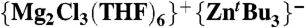 ] (4) in deuterated thf solution on the addition of 3 M equivalents of 4-iodotoluene after 30 min at room temperature.
] (4) in deuterated thf solution on the addition of 3 M equivalents of 4-iodotoluene after 30 min at room temperature.
Because Mg-Zn hybrid 5 possesses three covalent Zn-C (aryl) bonds within an anionic-activated “ate” structure, it could potentially be an excellent precursor in Negishi cross-coupling reactions (36–38). Therefore, to evaluate the synthetic utility of 5, we carried out test reactions of solutions of 5 (prepared in situ) with 3 M equivalents of iodobenzene in the presence of 2.5 mol% [PdCl2(dppf)] [dppf = 1,1′-bis(diphenylphosphino)ferrocene] (Scheme 4). These reactions proved extremely promising, affording 4-methylbiphenyl 6 in a 65% yield (this yield could be improved to 93% when isolated crystals of 5 were employed) (Scheme 4) showing the excellent potential this mixed Mg-Zn synthetic methodology has for the preparation of unsymmetrical biaryls and providing a proof of concept that tris(aryl)zincates are effective precursors for Pd-catalysed Negishi cross-coupling reactions.
Scheme 4.
Concluding Remarks.
A systematic study of the “simple” metathesis reaction of ZnCl2 with variable amounts of the Grignard reagent tBuMgCl has been carried out that offers a closer insight into the multicomponent species involved in this fundamental synthetic tool. When only 1 M equivalent of the Grignard reagent is employed, a hybrid Grignard [(THF)4Mg(μ-Cl)2Zn(tBu)(Cl)] (1) is obtained as a result of the co-complexation of the two exchange products of the metathesis reaction. Furthermore, a second coproduct of this reaction is solvent-separated [{Mg(THF)6}2+{Zn2Cl6}2-] (3) that diminishes the amount of ZnCl2 available to react with tBuMgCl. When three equivalents of the Grignard reagent are employed the magnesium trialkylzincate [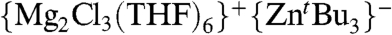 ] (4) is obtained and the formation of side product 3 is significantly inhibited. Solvent-separated ion pair complex 4 can be envisaged as a model for the active species in the organic transformations of Grignard reagents by using catalytic amounts of ZnCl2. Collectively, results highlight that metathesis reactions and the constitution of the organometallic species formed can be a bewilderingly complicated subject, involving the formation of several distinct organometallic products that poses the intriguing question: “Do all, some, or any of the reactivities previously attributed to homometallic reagents, prepared through this commonly used route, actually belong to one or more mixed-metal Mg-Zn species?”
] (4) is obtained and the formation of side product 3 is significantly inhibited. Solvent-separated ion pair complex 4 can be envisaged as a model for the active species in the organic transformations of Grignard reagents by using catalytic amounts of ZnCl2. Collectively, results highlight that metathesis reactions and the constitution of the organometallic species formed can be a bewilderingly complicated subject, involving the formation of several distinct organometallic products that poses the intriguing question: “Do all, some, or any of the reactivities previously attributed to homometallic reagents, prepared through this commonly used route, actually belong to one or more mixed-metal Mg-Zn species?”
Building connections between organic and inorganic chemistry, we investigated the application of magnesium trialkylzincate 4 in Zn-I exchange reactions. These studies revealed that the three tert-butyl groups in 4 are active towards reaction with 4-iodotoluene, allowing the preparation of tris(aryl) zincate 5 whose structure has been determined by X-ray crystallography. Furthermore, it was established that this compound can be effectively used as a precursor in Negishi cross-coupling reactions. Further investigations on the application of this mixed magnesium-zinc approach to metal-halogen exchange reactions to other substituted aromatic substrates are currently underway in our laboratory.
Materials and Methods
For more details on the general conditions employed, crystal data, full experimental details and the NMR spectral data see SI Text.
Synthesis of [(THF)4Mg(μ-Cl)2Zn(tBu)(Cl)] (1).
A solution of tert-butylmagnesium chloride (2 mL of a 1 M solution in THF, 2 mmol) in THF (10 mL) was cooled to 0 °C, and zinc chloride (2 mL of a 1 M solution in diethyl ether, 2 mmol) was added dropwise, resulting in the formation of a white precipitate. After stirring for 1 h at 0 °C, the solid was removed by filtration. The filtrate was concentrated in vacuo (to approx. 3 mL), and then hexane (2 mL) was added. The resulting colorless solution was then transferred to the freezer (-30 °C) and after 48 h a crop of colorless crystals of 1 were isolated (average isolated yield = 0.11 g, 10%). The isolated yield of 1 could be improved to ∼95% by reacting tBuZnCl (2 mmol) with MgCl2 (2 mmol) in THF.
Synthesis of [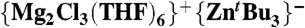 ] (4).
] (4).
A solution of tert-butylmagnesium chloride (6 mL of a 1 M solution in THF, 6 mmol) in THF (10 mL) was cooled to 0 °C, and zinc chloride (2 mL of a 1 M solution in diethyl ether, 2 mmol) was added dropwise. The resulting colorless solution was stirred for 1 h at 0 °C, concentrated in vacuo (to approx. 3 mL), and then transferred to the freezer (-30 °C). After 24 h, a crop of colorless crystals of [(THF)4MgCl2] were isolated (0.54 g, 1.4 mmol). To the filtrate was added hexane (2 mL) and the resulting solution returned to the freezer for 48 h. A batch of colorless crystals of 4 were isolated (average crystalline yield = 0.34 g, 19%). Compound 4 could also be prepared using an alternative co-complexation route by reacting tBuMgCl with  .
.
Synthesis of [{Mg2Cl3(THF)6}+{Zn(p-Tol)3}-] (5).
Compound 4 was generated in situ by adding 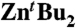 (0.36 g, 2 mmol in 10 mL THF) to a solution of tert-butylmagnesium chloride (1 M solution in THF, 2 mL, 2 mmol) in THF (10 mL). 4-iodotoluene (1.308 g, 6 mmol) was then introduced and the resulting yellow solution stirred for 30 min at room temperature. The volatiles (including tBuI) were then removed in vacuo, then THF (20 mL) was added and the solution stirred at room temperature for 1 h. The solution was concentrated in vacuo, and then transferred to the freezer for 48 h. A batch of colourless crystals of 5 were isolated (average crystalline yield = 0.994 g, 60%). Compound 5 can also be obtained when compound 4 is prepared by using the metathetical approach (3 M equivalents of tBuMgCl and ZnCl2), however, the resulting crystalline samples of 5 proved to be contaminated by co-crystallisation of [(THF)4MgCl2].
(0.36 g, 2 mmol in 10 mL THF) to a solution of tert-butylmagnesium chloride (1 M solution in THF, 2 mL, 2 mmol) in THF (10 mL). 4-iodotoluene (1.308 g, 6 mmol) was then introduced and the resulting yellow solution stirred for 30 min at room temperature. The volatiles (including tBuI) were then removed in vacuo, then THF (20 mL) was added and the solution stirred at room temperature for 1 h. The solution was concentrated in vacuo, and then transferred to the freezer for 48 h. A batch of colourless crystals of 5 were isolated (average crystalline yield = 0.994 g, 60%). Compound 5 can also be obtained when compound 4 is prepared by using the metathetical approach (3 M equivalents of tBuMgCl and ZnCl2), however, the resulting crystalline samples of 5 proved to be contaminated by co-crystallisation of [(THF)4MgCl2].
Supplementary Material
ACKNOWLEDGEMENTS.
We thank Prof. R. E. Mulvey for many helpful discussions and Dr. P. Allan for her assistance with atomic absorption analysis. We thank the Royal Society (University Research Fellowship to E. H.), the Faculty of Science, University of Strathclyde (starter grant to E. H.), the 7th European Community Framework Programme (Marie Curie Intra European Fellowship to P.G.A.), and the EPSRC (DTA award to M.D.M.) for their generous sponsorship of this research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913307107/DCSupplemental.
Data deposition: The atomic coordinates have been deposited in the Cambridge Structural Database (CCDC).
*Note that this structure was first presented at the RSC Conference on Main Group Chemistry, Manchester, September 11, 2009 by J. Z. Chua, P. Garcia-Alvarez, M. D. McCall, and E. Hevia.
References
- 1.Rappoport Z, Marek I, editors. The chemistry of organozinc compounds. Chichester, UK: Wiley; 2006. (Patai Series). [Google Scholar]
- 2.Knochel P, Jones P. In: Organozinc Reagents: A Practical Approach. Harwood LM, Moody CJ, editors. Oxford, UK: Oxford Univ Press; 1999. [Google Scholar]
- 3.Jansen JFGA, Feringa BL. Zinc(II) catalysed conjugate addition of Grignard reagents to α,β-unsaturated ketones. Chem Commun. 1989:741–742. [Google Scholar]
- 4.Hatano M, Suzuki S, Ishihara K. Highly efficient alkylation to ketones and aldimines with Grignard reagents catalyzed by zinc(II) chloride. J Am Chem Soc. 2006;128:9998–9999. doi: 10.1021/ja0628405. [DOI] [PubMed] [Google Scholar]
- 5.Murakami K, Yorimitsu H, Oshima K. Zinc-catalyzed nucleophilic substitution reaction of chlorosilanes with organomagnesium reagents. J Org Chem. 2009;74:1415–1417. doi: 10.1021/jo802433t. [DOI] [PubMed] [Google Scholar]
- 6.Stude C, Breit B. Zinc-catalyzed enantiospecific sp3-sp3 cross-coupling of α-hydroxy ester triflates with Grignard reagents. Angew Chem Int Ed. 2009;47:5451–5455. doi: 10.1002/anie.200800733. [DOI] [PubMed] [Google Scholar]
- 7.Rijnberg E, et al. Synthesis and structual characterization of the homoleptic magnesium bis zinc ate complex Mg(THF)6[Zn(CH2Ph)3]2. J Organomet Chem. 1997;541:181–185. [Google Scholar]
- 8.Krieger M, et al. Die kristallstrukturen der triarylzinkate [Mg2Br3(THF)6][ZnPh3] und [MgBr(THF)5][ZnMes3] Z Anorg Allg Chem. 1998;624:1387–1388. [Google Scholar]
- 9.Mulvey RE. Modern ate chemistry: Applications of synergic mixed alkali-metal—magnesium or -zinc reagents in synthesis and structure building. Organometallics. 2006;25:1060–1075. [Google Scholar]
- 10.Mulvey RE, Mongin F, Uchiyama M, Kondo Y. Deprotonative metalation using ate compounds: Synergy, synthesis, and structure building. Angew Chem Int Ed. 2007;46:3802–3824. doi: 10.1002/anie.200604369. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy AR, Klett J, Mulvey RE, Wright DS. Synergic sedation of sensitive anions: Alkali-mediated zincation of cyclic ethers and ethene. Science. 2009;326:706–708. doi: 10.1126/science.1178165. [DOI] [PubMed] [Google Scholar]
- 12.Clayden J. Organolithiums: Selectivity for Synthesis. Vol. 23. Oxford, UK: Pergamon, Elsevier Science; 2002. pp. 111–148. (Tetrahedron Organic Chemistry Series). [Google Scholar]
-
13.Lewiński J, et al. From discrete linear
 molecules to 1D coordination polymers and 2D fabrics. J Am Chem Soc. 2007;129:3096–3098. doi: 10.1021/ja067229k. [DOI] [PubMed] [Google Scholar]
molecules to 1D coordination polymers and 2D fabrics. J Am Chem Soc. 2007;129:3096–3098. doi: 10.1021/ja067229k. [DOI] [PubMed] [Google Scholar] - 14.Jin L, et al. Revelation of the difference between arylzinc reagents prepared from aryl Grignard and aryllithium reagents respectively: Kinetic and structural features. J Am Chem Soc. 2009;131:16656–16657. doi: 10.1021/ja908198d. During the submission of this paper and within the context of oxidative homocoupling of ArZnX reagents, a structure similar to 1 albeit highly disordered over two sites, with a Ph substituent in place of a tBu group, has been reported in an early view article. [DOI] [PubMed] [Google Scholar]
- 15.Krasovskiy A, Knochel P. A LiCl-mediated Br/Mg exchange reaction for the preparation of functionalized aryl- and heteroarylmagnesium compounds from organic bromides. Angew Chem Int Ed. 2004;43:3333–3336. doi: 10.1002/anie.200454084. [DOI] [PubMed] [Google Scholar]
- 16.Krasovskiy A, Krasovskaya V, Knochel P. Mixed Mg/Li amides of the type R2NMgCl·LiCl as highly efficient bases for the regioselective generation of functionalized aryl and heteroaryl magnesium compounds. Angew Chem Int Ed. 2006;45:2958–2961. doi: 10.1002/anie.200504024. [DOI] [PubMed] [Google Scholar]
- 17.Wunderlich SH, Knochel P. (tmp)2Zn·2MgCl2·2LiCl: A chemoselective base for the directed zincation of sensitive arenes and heteroarenes. Angew Chem Int Ed. 2007;46:7685–7688. doi: 10.1002/anie.200701984. [DOI] [PubMed] [Google Scholar]
- 18.Dong Z, et al. Direct zincation of functionalized aromatics and heterocycles by using a magnesium base in the presence of ZnCl2. Chem Eur J. 2009;15:457–468. doi: 10.1002/chem.200801558. [DOI] [PubMed] [Google Scholar]
- 19.Rohbogner CJ, Wunderlich SH, Closoki GC, Knochel P. New mixed Li/Mg and Li/Mg/Zn amides for the chemoselective metallation of arenes and heteroarenes. Eur J Org Chem. 2009:1781–1795. [Google Scholar]
- 20.Piller FM, et al. Preparation of polyfunctional arylmagnesium, arylzinc, and benzylic zinc reagents by using magnesium in the presence of LiCl. Chem Eur J. 2009;15:7192–7202. doi: 10.1002/chem.200900575. [DOI] [PubMed] [Google Scholar]
- 21.García-Alvarez P, et al. Unmasking representative structures of TMP-active hauser and Turbo-Hauser bases. Angew Chem Int Ed. 2008;47:8079–8081. doi: 10.1002/anie.200802618. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto S, Imamoto T, Yamaguchi K. Constitution of Grignard Reagent RMgCl in Tetrahydrofuran. Org. Lett. 2001;3:1793–1795. doi: 10.1021/ol010048x. [DOI] [PubMed] [Google Scholar]
- 23.Sun W-Y, Xie J, Huang C-K, Ueyama N. Novel tripodal chelating ligand for appending and encapsulating metal ions. Crystal structure of a parachute-like hydrogen bonded complex. Chem Commun. 2000:1429–1430. [Google Scholar]
- 24.Johansson FB, Bond AD, Mackenzie CJ. Functional tetrametallic linker modules for coordination polymers and metal—organic frameworks. Inorg Chem. 2007;46:2224–2236. doi: 10.1021/ic062131s. [DOI] [PubMed] [Google Scholar]
- 25.Bond AD, et al. The first observation of the [Cp3Mn]- anion; structures of hexagonal [(η2-Cp)3MnK·1.5thf] and ion-separated [(η2-Cp)3Mn]2[Mg(thf)6].2thf. Chem Commun. 2001:1956–1957. doi: 10.1039/b106366a. [DOI] [PubMed] [Google Scholar]
- 26.Fornies J, et al. The first structurally characterized homoleptic aryl-manganese(III) compound and the corresponding isoleptic and isoelectronic chromium(II) derivative. Organometallics. 2005;24:3266–3271. [Google Scholar]
- 27.Clegg W, Little IR, Straughan BP. Zinc carboxylate complexes: structural characterization of the mixed-metal linear trinuclear complexes MZn2(crot)6(base)2 (M = Mn, Co, Ni, Zn, Cd, Mg, Ca, Sr; crot- = crotonate(1-); base=quinoline, 6-methylquinoline) Inorg Chem. 1988;27:1916–1923. [Google Scholar]
- 28.Clegg W, Harbron DR, Straughan BP. Structures of the mixed-metal carboxylate base adducts [MgZn2(crotonate)6(4-vinylpyridine)2] and [MgCo2(crotonate)6(4-vinylpyridine)4] Acta Crystallogr C. 1991;47:267–270. [Google Scholar]
- 29.Ruben M, Walther D, Knake R, Goris H, Beckert R. Fixation of carbon dioxide by oxalic amidinato magnesium complexes: Structures and reactions of trimetallic magnesium carbamato and related complexes. Eur J Inorg Chem. 2000:1055–1064. [Google Scholar]
- 30.Boss SR, et al. Ligand and metal effects on the formation of main-group polyhedral clusters. Angew Chem Int Ed. 2003;42:5593–5596. doi: 10.1002/anie.200351921. [DOI] [PubMed] [Google Scholar]
- 31.Boss SR, et al. Encapsulation of hydride by molecular main group metal clusters: Manipulating the source and coordination sphere of the interstitial ion. Dalton Trans. 2006:5574–5582. doi: 10.1039/b612782g. [DOI] [PubMed] [Google Scholar]
- 32.Clegg W, et al. Structurally defined reactions of sodium TMP-zincate with nitrile compounds: Synthesis of a salt-like sodium sodiumdizincate and other unexpected ion-pair products. Angew Chem Int Ed. 2008;47:731–734. doi: 10.1002/anie.200704341. [DOI] [PubMed] [Google Scholar]
- 33.Mulvey RE. Avant-garde metalating agents: Structural basis of alkali-metal-mediated metalation. Acc Chem Res. 2009;42:743–755. doi: 10.1021/ar800254y. [DOI] [PubMed] [Google Scholar]
- 34.Hurley ER, He X, Brown SN, Henderson KW. Dramatic effect of aggregation on rates and thermodynamics of stereoisomerization of magnesium enolates. J Am Chem Soc. 2009;131:6056–6057. doi: 10.1021/ja809716j. [DOI] [PubMed] [Google Scholar]
- 35.Knochel P. Handbook of Functionalised Organometallics. Weinheim, Germany: Willey-VCH; 2005. pp. 270–273. [Google Scholar]
- 36.Negishi E. Palladium- or nickel-catalyzed cross coupling. A new selective method for carbon-carbon bond formation. Acc Chem Res. 1982;15:340–348. [Google Scholar]
- 37.Negishi E, Valente LF, Kobayashi M. Palladium-catalyzed cross-coupling reaction of homoallylic or homopropargylic organozincs with alkenyl halides as a new selective route to 1,5-dienes and 1,5-enynes. J Am Chem Soc. 1980;102:3298–3299. [Google Scholar]
- 38.Manolikakes G, Dong Z, Mayr H, Li J, Knochel P. Negishi cross-couplings compatible with unprotected amide functions. Chem Eur J. 2009;15:1324–1328. doi: 10.1002/chem.200802349. [DOI] [PubMed] [Google Scholar]
- 39.Kneisel FF, Dochnahl M, Knochel P. Nucleophilic catalysis of iodine-zinc exchange reaction: Preparation of highly functionalised diaryl zinc compounds. Angew Chem Int Ed. 2004;43:1017–1021. doi: 10.1002/anie.200353316. [DOI] [PubMed] [Google Scholar]
- 40.Micouin L, Knochel P. Iodine-zinc exchange reactions mediated by i- Pr 2Zn. A new preparation for secondary zinc reagents. Synlett. 1997:327–328. [Google Scholar]
- 41.Trost BM. The atom economy-a search for synthetic efficiency. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- 42.Kondo Y, Takazawa N, Yamazaki C, Sakamoto T. Halogen-zinc exchange reaction with lithium trimethylzincate. J Org Chem. 1994;59:4717–4718. [Google Scholar]
- 43.Kondo Y, Fujinami M, Uchiyama M, Sakamoto T. Lithium tri-tert-butylzincate as a chemoselective metallating reagent for functionalized organic halides. J Chem Soc Perkin Trans 1. 1997:799–800. [Google Scholar]
- 44.Furuyama T, et al. Development of highly chemoselective bulky zincate complex, tBu4ZnLi2: Design, structure, and practical applications in small-/macromolecular synthesis. Chem Eur J. 2008;14:10348–10356. doi: 10.1002/chem.200800536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.