Abstract
During skeletal muscle excitation-contraction (EC) coupling, membrane depolarizations activate the sarcolemmal voltage-gated L-type Ca2+ channel (CaV1.1). CaV1.1 in turn triggers opening of the sarcoplasmic Ca2+ release channel (RyR1) via interchannel protein–protein interaction to release Ca2+ for myofibril contraction. Simultaneously to this EC coupling process, a small and slowly activating Ca2+ inward current through CaV1.1 is found in mammalian skeletal myotubes. The role of this Ca2+ influx, which is not immediately required for EC coupling, is still enigmatic. Interestingly, whole-cell patch clamp experiments on freshly dissociated skeletal muscle myotubes from zebrafish larvae revealed the lack of such Ca2+ currents. We identified two distinct isoforms of the pore-forming CaV1.1α1S subunit in zebrafish that are differentially expressed in superficial slow and deep fast musculature. Both do not conduct Ca2+ but merely act as voltage sensors to trigger opening of two likewise tissue-specific isoforms of RyR1. We further show that non-Ca2+ conductivity of both CaV1.1α1S isoforms is a common trait of all higher teleosts. This non-Ca2+ conductivity of CaV1.1 positions teleosts at the most-derived position of an evolutionary trajectory. Though EC coupling in early chordate muscles is activated by the influx of extracellular Ca2+, it evolved toward CaV1.1-RyR1 protein–protein interaction with a relatively small and slow influx of external Ca2+ in tetrapods. Finally, the CaV1.1 Ca2+ influx was completely eliminated in higher teleost fishes.
Keywords: calcium conductivity, evolution, ion channels, slow and fast muscle, zebrafish
Excitation-contraction (EC) coupling is understood as the mechanism linking membrane depolarization to the contraction of a muscle cell. Depolarization-induced activation of the sarcolemmal voltage-gated Ca2+ channel (CaV) triggers opening of the sarcoplasmic Ca2+ release channel (ryanodine receptor, RyR) to release from SR stores Ca2+ that is needed for myofibril contraction. Though in cardiac muscle the homologous isoform RyR2 is activated by the Ca2+ influx through CaV1.2, in skeletal muscle, conformational changes of CaV1.1 directly activate RyR1 via protein–protein interaction (1, 2). However, a simultaneously occurring Ca2+ current through CaV1.1 is observed in mammalian skeletal myotubes. In contrast to the large and rapidly activating currents that are needed for efficient RyR2 activation in cardiac myocytes (3, 4), the skeletal muscle CaV1.1 Ca2+ current is relatively small and slowly activating (5). Phylogenetically interesting is that EC coupling in skeletal muscle of very early chordates, such as amphioxus or tunicates, is activated by Ca2+ influx, and in lamprey muscles, maximum-force generation is dependent on Ca2+ currents, although direct CaV1.1-RyR coupling is already established (6). However, in cultured mammalian skeletal muscle cells the absence of extracellular Ca2+ or pharmacological blocking of CaV1.1 does not immediately affect intracellular Ca2+ release and contractions (7, 8). Despite the outcome of these short-term in vitro experiments, it remains unclear if the CaV1.1 Ca2+ influx into the intact muscle plays an essential role on the long-term.
Here we show that in skeletal muscle of teleost fishes the voltage-gated Ca2+ channel CaV1.1 is unable to conduct Ca2+ and, thus, turned from an ion channel to a pure voltage sensor to trigger RyR1 opening. Therefore, our findings suggest that on the level of the whole organism, Ca2+ influx via CaV1.1 is not required in teleost fishes. Furthermore, we discuss that this CaV1.1 nonconductivity stands at the topmost position of an evolutionary trajectory from Ca2+ influx-dependent, cardiac muscle-like EC coupling in skeletal muscles of early chordates to Ca2+ influx-independent EC coupling with small Ca2+ currents in mammals, and finally to the complete elimination of CaV1.1 Ca2+ influx in teleost fishes.
Results
Non–Ca2+-Conducting CaV1.1 Channels Perform EC Coupling in Zebrafish Skeletal Muscle.
Whole-cell patch clamp analysis of myotubes isolated from wild-type zebrafish larvae revealed the lack of voltage-dependent inward Ca2+ currents at physiologically relevant potentials between −40 and +70 mV (Fig. 1A). Also, replacement of the charge carrier Ca2+ by Ba2+ did not evoke inward L-type currents (Fig. 1D). However, correct voltage-dependent activation of the CaV1.1 channels was indicated by robust CaV1.1-specific gating currents (Fig. 1B) (9, 10) that reached the levels described for the Ca2+-conducting CaV1.1 in mouse myotubes (11). Gating currents result from depolarization-induced displacements of charges in the channel's voltage sensing elements, resulting in a conformational change of the channel complex (12). This steric effect is not only inducing channel opening but is also responsible for the direct coupling of CaV1.1 with the Ca2+ release channel RyR1 in the SR (2). To test for functional CaV1.1-RyR1 interaction, depolarization-induced intracellular Ca2+ release from the SR through RyR1 was recorded using the intracellular Ca2+ indicator dye Fluo-4. Robust Ca2+ transients indicated intact EC coupling in these myotubes (Fig. 1C). Thus, zebrafish CaV1.1 is able to undergo the voltage-dependent conformational change required for channel activation and to trigger EC coupling, but completely lost its function to conduct calcium ions.
Fig. 1.
Non–Ca2+-conducting Ca2+ channels in larval zebrafish skeletal myotubes. (A) Depolarizing test pulses to potentials unto +70 mV were unable to elicit Ca2+ currents at any potential (n = 14). (B) Robust voltage-dependent α1S gating is indicated by charge movements with a Qmax value of 10.45 ± 0.58 nC/μF and a half-maximal activation of −4.21 ± 1.36 mV (n = 20), assessed by integrating the “ON” component of α1S gating currents (Qon). (C) Depolarization-induced intracellular Ca2+ transients had a (ΔF/F0) max value of 4.44 ± 0.42 with half-maximal activation at −10.39 ± 0.75 mV (n = 17). (D) Also, 10 mM Ba2+ instead of 10 mM Ca2+ as a charge carrier did not awake any current at any potential (n = 6).
Two CaV1.1α1S Isoforms Are Differentially Expressed in Zebrafish.
The central α1S subunit forms the pore, the Ca2+ selectivity filter, and the voltage sensor of the skeletal muscle Ca2+ channel complex (12, 13), and thus primarily determines the channel’s conductivity properties. Therefore, we performed a BLAST search within the ENSEMBL zebrafish genomic assembly Zv7 with the coding sequence of the rabbit α1S subunit (rb-α1S) as a template (12). Surprisingly, two distinct genes coding for CaV1.1α1S subunits were identified in the zebrafish genome and were located on chromosomes 8 and 22. Both genes code for intact α1S subunits that contain all molecular characteristics, such as the interaction domains with the accessory β1a subunit (14) and with RyR1 (15). One gene, identified on chromosome 22, consists of 43 exons and codes for an α1S protein of 1,777 amino acids, hereafter named zf-α1S-a, with 66% sequence identity to rb-α1S. The second gene, identified on chromosome 8, is composed of 45 exons that code for the 1,847 amino acids of zf-α1S-b with 64% sequence identity to rb-α1S. The two zf-α1S isoforms share an amino acid homology of 73%. In a phylogenetic tree based on parsimony analysis of DNA sequences (Fig. 2A), both zf-α1S subunits cluster with rb-α1S, clearly separated from the clade comprising the cardiac α1C isoforms of rabbit and also zebrafish, consequently identifying them as α1S subunits. Ancestral α1 isoforms from the housefly Musca domestica (16) and the tunicate Halocynthia roretzi (17) were used as outgroups.
Fig. 2.
Two distinct α1S subunit isoforms are found in the zebrafish skeletal musculature. (A) Singlemost parsimonious tree of aligned DNA sequences groups both zebrafish α1S subunit isoforms (zf-α1S-a and zf-α1S-b) jointly with α1S of the rabbit (rb-α1S) and clearly separated from the clade comprising cardiac α1C subunits of both species. Bootstrap scores of 100% (1,000 replications) for all branches support evolutionary divergence. (B) Quantitative RT-PCR reveals different expression patterns for zf-α1S-a and zf-α1S-b. Data represent mean ± SEM. In deep fast muscle we almost exclusively detect zf-α1S-b, whereas in superficial slow muscle, zf-α1S-a is the predominant isoform (***P < 0.001 by Student's paired t test). (C) Data obtained for zf-α1S (B) closely match with expression levels of RyR1. (D–F) Whereas control experiments with rb-α1S (D) restored Ca2+ inward currents (Top and Middle) in murine α1S-null myotubes with Imax of −2.42 ± 0.18 pA/pF (n = 25), both, zf-α1S-a (n = 14) and zf-α1S-b (n = 12) were unable to restore any Ca2+ current (E and F). Ca2+ transients (Bottom) obtained with zf-α1S-a had (ΔF/F0) max values of 0.84 ± 0.12 (n = 15), with a half-maximal activation at 9.91 ± 1.54 mV, and thus were smaller (P < 0.001) and shifted toward more positive potentials (P < 0.01) when compared with zf-α1S-b [(ΔF/F0) max = 1.76 ± 0.2, with a half-maximal activation at 4.13 ± 1.4 mV, n = 17], which was comparable (P > 0.6) to rb-α1S [(ΔF/F0) max = 1.95 ± 0.24, with a half-maximal activation at 3.52 ± 1 mV, n = 30].
The question now arises, why—in contrast to e.g., mammals—two α1S subunit isoforms are present in zebrafish, and whether they might follow specific expression patterns. Recently, two isoforms of the skeletal muscle RyR1 were identified in zebrafish (18), with RyR1a being expressed in superficial slow muscle (also called red muscle), which is aerobic and used for persistent swimming, and RyR1b as the predominant isoform in deep fast muscle (white muscle), the glycolytic musculature used for burst activity. To test if RyR1a and RyR1b might be explicit molecular interaction partners for zf-α1S-a and zf-α1S-b, respectively, we performed quantitative RT-PCR on each of these muscle layers, isolated from adult zebrafish. In deep fast muscle, zf-α1S-b was by far the predominant isoform, whereas in superficial slow muscle the expression level of zf-α1S-a was significantly higher compared with zf-α1S-b (Fig. 2B). The considerable signal obtained for zf-α1S-b in superficial slow muscle most likely results from a preparation artifact, because this superficial slow muscle layer is very thin in the relatively small zebrafish and thus hard to separate cleanly. Therefore, we performed the same experiments on the musculature of another species of the same superorder (Ostarioclupeomorpha), the sardine (Sardina pilchardus), in which the tissue separation is much clearer due to a robust superficial slow muscle layer of this very active swimmer. Here, a perfect separation of the two α1S and RyR1 isoforms in both muscle types was revealed (Fig. S1). As shown in Fig. 2 B and C, the expression patterns of zf-α1S-a and zf-α1S-b perfectly match with those of the two RyR1 isoforms, RyR1a and RyR1b.
Both zf-α1S Isoforms Do Not Conduct Ca2+.
To test the two distinct isoforms for their ability to conduct Ca2+, both zf-α1S cDNAs were generated by RT-PCR from zebrafish skeletal muscle and cloned into expression vector pGFP37, allowing N-terminal GFP tagging (7). Constructs were heterologously expressed in murine α1S-null myotubes (cell line GLT) (19) and patch-clamp analyzed. In stark contrast to rb-α1S controls (Fig. 2D), Ca2+ inward currents could not be detected either through zf-α1S-a (Fig. 2E) or zf-α1S-b (Fig. 2F). However, like the rb-α1S controls, both zf-α1S isoforms restored EC coupling with robust intracellular Ca2+ transients (Fig. 2 D–F). Notably, the Ca2+ transients recorded with zf-α1S-a were smaller and shifted toward more positive potentials compared with those obtained with zf-α1S-b (Table 1).
Table 1.
Intracellular Ca2+ transients recorded in expression experiments in GLT myotubes
| Construct | (ΔF/F0)max | 1/2V (ΔF/F0)max (mV) | n |
| rb-α1S | 1.73 ± 0.13 | 2.28 ± 0.75 | 67 |
| zf-α1S-a | 0.84 ± 0.12 * | 9.91 ± 1.54 ** | 15 |
| zf-α1S-b | 1.76 ± 0.2 * | 4.13 ± 1.4 ** | 17 |
| rb-α1S ME/TQ | 1.93 ± 0.22 | 1.15 ± 1.4 | 20 |
| rb-α1S N617D | 1.46 ± 0.23 | 1.92 ± 1.71 | 12 |
| rb-α1S D296K | 1.75 ± 0.3 | 1.38 ± 1.64 | 15 |
| RmRR | 1.96 ± 0.37 | 2.15 ± 2.3 | 5 |
Robust intracellular Ca2+ transients indicate intact EC coupling for all constructs *P < 0.001; **P < 0.01.
Non–Ca2+-Conducting CaV1.1 Channels Exist Also in Other Teleost Fishes.
The question arises whether non–Ca2+-conducting CaV1.1 channels are a common attribute of teleost fishes or are an exclusive trait of zebrafish or its order Cypriniformes. Therefore, we patch-clamp analyzed larval myotubes from a phylogenetically closely related fish, the pike characin (Ctenolucius hujeta, Characiformes) (Fig. 3A), and from ricefish medaka (Oryzias latipes, Beloniformes) (Fig. 3B), which is much higher in the phylogenetic scale. Results from both teleost species matched those from zebrafish. Again, no Ca2+ inward currents, but pronounced intra-cellular Ca2+ transients, were recorded.
Fig. 3.
Non–Ca2+-conducting Ca2+ channels are found also in other teleost orders. (A and B) In skeletal myotubes from the pike characin Ctenolucius hujeta (A) and the ricefish medaka Oryzias latipes (B), no Ca2+ inward currents could be recorded upon test pulses unto +70 mV (Upper), despite the presence of pronounced intracellular Ca2+ transients with (ΔF/F0) max values of 6.05 ± 0.37 (n = 10) for the pike characin and 3.7 ± 0.54 (n = 6) for medaka, respectively (Lower).
Charged Pore Residues Are Correlated with Ca2+ Conductance in Both α1S Isoforms.
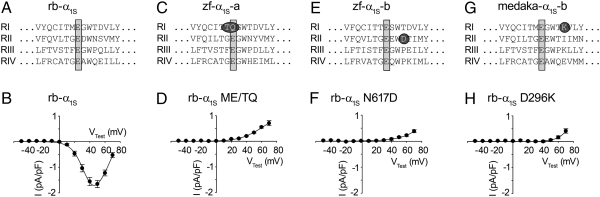
Surprisingly, zf-α1S / rb-α1S sequence alignments showed that at position 303 of zf-α1S-a, glutamate is consistently exchanged by glutamine when compared with rb-α1S (Fig. 4 A and C). In a properly folded conducting Ca2+ channel, the glutamate at this position is the first of four glutamates located in the pore-forming loops (p-loops) of the four homologous repeats (Fig. 4A), thus forming the selectivity filter for Ca2+ ions (20). Consequently, Gln303 in zf-α1S-a makes the formation of an intact Ca2+ selectivity filter doubtful. We tested the consequences of the hypothesized loss of an intact selectivity filter by exchanging the corresponding Glu292 together with the preceding Met291 to glutamine and threonine in the conducting rb-α1S, according to zf-α1S-a sequence. Construct rb-α1S ME/TQ was unable to recover L-type Ca2+ currents in heterologous expression experiments (Fig. 4D). In contrast to the prominent exchange at position 303 of zf-α1S-a, zf-α1S-b shows a perfect conservation of the essential Ca2+ selectivity filter glutamates (Glu312/Glu633/Glu1034/Glu1348) (Fig. 4E). Instead, in homologous repeat II of zf-α1S-b an additional negative charge (aspartate) in close proximity of the selectivity filter glutamate was identified, and this was able to completely block Ca2+ conductance when introduced into rb-α1S in point mutant rb-α1S N617D (Fig. 4F). Interestingly, this asparagine to aspartate mutation was also found in α1S-b of the pike characin, but not in α1S-b of the more distantly related medaka (Fig. 4G). Furthermore, a chimera of rb-α1S, containing the repeat II S5/p-loop/S6 region of medaka α1S-b, continued to show Ca2+ inward currents (Fig. S2). Unlike the addition of a negative charge (aspartate) in the repeat II p-loop of zf-α1S-b, in medaka-α1S-b, a conversion of a negative to a positive charge (aspartate to lysine compared with rb-α1S) was found in the repeat I p-loop, four residues C-terminal to the selectivity filter glutamate (Fig. 4G). Again, the corresponding point mutant rb-α1S D296K was unable to conduct Ca2+ inward currents (Fig. 4H). Intact EC coupling of all chimeras and point mutants mentioned previously was indicated by robust intracellular Ca2+ transients with (ΔF/F0) max values >1.4 (Table 1).
Fig. 4.
Amino acids incompatible with Ca2+ conductance. (A and B) In murine α1S-null myotubes, expression of the wild-type α1S from rabbit (rb-α1S), with a typical amino acid arrangement of a conducting channel in the pore region of homologous repeats I–IV (RI-RIV), could restore Ca2+ inward currents of 1.79 ± 0.16 pA/pF (n = 37). (C) In zf-α1S-a, a threonine and glutamine at position −1 and 0 relative to the selectivity filter (selectivity filter glutamates are indicated by gray bars) of homologous repeat I (RI) were identified and equally found in α1S-a of pike characin and medaka. (D) Corresponding replacement of residues Met291 and Glu292 to threonine and glutamine in rb-α1S (rb-α1S ME/TQ) completely abolished Ca2+ inward currents (n = 9). Similar results were obtained with (F) rb-α1S N617D (n = 8) in which Asn617 of rb-α1S was exchanged to aspartate as found in α1S-b of zebrafish (E) and pike characin, as well as with (H) rb-α1S D296K (n = 15) in which Asp296 was exchanged to lysine as found in α1S-b of medaka (G).
Non–Ca2+-Conducting CaV1.1 Channels Are a Common Trait of Higher Teleosts.
We tested for the presence of one of three marker amino acids incompatible with CaV1.1 Ca2+ conductance, in a large selection of teleosts using sets of degenerated α1S-specific primers (Table S1) that amplify short fragments from genomic DNA or cDNA coding for the p-loop regions of homologous repeats I and II of α1S-a and α1S-b. Sequence analysis of these fragments from 32 species of 17 different orders of teleost fishes (Figs. S3 and S4) revealed the presence of two α1S isoforms in all teleosts from the ancestral branch Osteoglossomorpha (Notopterus notopterus) to the most recently branching Beloniformes (Oryzias latipes; Fig. 5). The marker amino acids threonine and glutamine in the selectivity filter of zf-α1S-a were identified in α1S-a of all tested higher-teleost fishes starting with the clade comprising the Cypriniformes. Interestingly, sequence analysis of α1S-b from numerous teleosts revealed either the lysine at position +4 relative to the selectivity filter in repeat I (Fig. S3) or the aspartate at position +3 relative to the selectivity filter in repeat II (Fig. S4), strictly correlating with their position on the evolutionary tree (Fig. 5). Consequently, higher teleosts exclusively express non–Ca2+-conducting CaV1.1 channels. However, none of the three marker amino acid positions were found in the ancestrally branching superorders Osteoglossomorpha and Elopomorpha, and thus it is feasible that basal teleosts, like nonteleost fishes and tetrapods, still have Ca2+-conducting CaV1.1 channels.
Fig. 5.
Non–Ca2+-conducting CaV1.1 channels are a common trait of all higher teleost fishes. Sequencing of a phylogenetic wide range of teleost fishes revealed that two distinct α1S isoforms (α1S-a and α1S-b) are present in all teleosts. Marker amino acids indicating non-Ca2+ conductivity (boxed in black) were identified in α1S-a and α1S-b of all higher teleosts starting with the clade comprising the order Cypriniformes. The positions of the selectivity filter glutamates are indicated by gray bars. Phylogenetic tree is drawn after the Tree of Life web project (21).
Discussion
Ca2+ Influx–Independent EC Coupling Without Simultaneous CaV1.1 Ca2+-Conductance Sets Teleosts at a Most-Derived Position Concerning the Evolution of Chordate Skeletal Muscle EC Coupling.
Early chordates such as tunicates or cephalochordates (amphioxus) were shown to activate RyR via influx of extracellular Ca2+ (Ca2+ influx-dependent EC coupling; see Fig. 6) (22, 23) with rapidly activating Ca2+ currents (24), rather resembling the kinetics of cardiac CaV1.2 currents. At this phylogenetic stage, CaV channels are clustered opposite to RyR but are ultrastructurally arbitrarily arranged (25). This cardiac-like CaV arrangement, together with the lack of an intact α1S II-III loop critical domain (15) (Fig. S5), explains the inability for direct CaV-RyR coupling. The functional arrangement of CaV1.1 in groups of four (tetrads), a prerequisite for direct CaV1.1–RyR1 interaction, can first be observed in hagfish and lamprey (25). In these species, blocking of extracellular Ca2+ influx could no more fully obstruct EC coupling (24, 26, 27), even though CaV1.1 Ca2+ influx was found to still contribute to maximum force generation in EC coupling in lampreys (6). In tetrapods, from frog to human, the tetrads changed their arrangement inside the orthogonal arrays, and skeletal-muscle EC coupling became independent of Ca2+ influx (29), although a small and slowly activating CaV1.1 Ca2+ current can be observed, whose role is largely unknown. In higher-teleost fishes, the CaV1.1 Ca2+ current completely vanished, in part due to substantial sequence exchanges in or close to the selectivity filter of both CaV1.1α1S isoforms, and consequently, RyR1 activation is solely achieved by direct CaV1.1–RyR1 interaction. This clear evolutionary trajectory points to an evolutionary pressure on the reduction of CaV1.1 Ca2+ currents.
Fig. 6.
Non–Ca2+-conducting CaV1.1 channels position higher teleost fishes as most derived group concerning the evolution of skeletal muscle EC coupling. In Ca2+ influx-dependent EC coupling (22, 23) of early prochordates, such as tunicates or amphioxus, RyR opening is induced via rapidly activating Ca2+ currents (Ca2+ current) (24), and CaV channels are arbitrarily arranged in clusters (CaV clusters) opposite RyR (25). Later, hagfish and lampreys developed direct CaV1.1-RyR1 coupling (24, 26, 27) with CaV1.1 arranged in groups of four (tetrads) (25). In tetrapods, the tetrads changed their disposition within the array, and a slowly activating Ca2+ current is still observed, despite that EC coupling is independent of the Ca2+ influx. The Ca2+ inward current completely vanished in teleost fishes, and EC coupling is solely induced via CaV1.1-RyR1 protein–protein interaction. This is true for both distinct CaV1.1α1S isoforms (α1S-a and α1S-b) that are present in higher teleosts, concordant with the hypothesis of a whole-genome duplication (WGD) at the basis of the teleost linage (28). Selectivity filter glutamates are boxed in gray, marker amino acids indicating non-Ca2+ conductivity in black. Phylogenetic tree is drawn after the Tree of Life web project (21).
Organ Differentiation in Teleost Skeletal Muscle.
We identified two different non–Ca2+-conducting isoforms of the pore-forming CaV1.1α1S subunit in teleost fishes. CaV1.1 α1S-a and α1S-b are differentially expressed in superficial slow and deep fast musculature, respectively, which have repeatedly been shown to be very diverse musculature types regarding their transcriptome and physiology (30–32). We further showed that the expression pattern of the two different α1S subunits nicely matches the expression pattern of the two RyR1 isoforms of teleost skeletal muscle, suggesting the presence of two distinct musculature-type specific CaV1.1-RyR1 couplons. This two-couplon adaptation is an intriguing example of how the whole-genome duplication at the basis of the teleost linage (WGD; see Fig. 6) continues into isoform formation during phylogenetic organ differentiation. Future studies will investigate the putative physiological and biophysical differences of EC coupling in these two different skeletal musculatures.
Ca2+ Influx in Mammalian Skeletal Muscle—A Tolerated Remnant or a Physiological Necessity?
Apparently, all aspects of teleost fish skeletal muscle work appropriately without Ca2+ influx through CaV1.1—a fact that also sheds light on the question about the role of the CaV1.1 Ca2+ influx into mammalian skeletal muscle. Together with the findings that pharmacological blocking of CaV1.1 or elimination of extracellular Ca2+ does not inhibit EC coupling (7, 8, 29), it is manifested that the Ca2+ influx in tetrapods is largely dispensable and could be seen as a tolerated remnant of the ancestral Ca2+influx-dependent skeletal muscle EC coupling. However, Ca2+ influx in mammalian skeletal muscle seems to be variably regulated, e.g., by the accessory CaV1.1 subunits α2δ-1 (33) and γ (34) or by retrograde current amplification, induced by RyR1-CaV1.1 interaction (35). Ca2+ current density is also massively up-regulated in developing skeletal muscle by expression of an alternatively spliced α1S subunit (36). A possible role of this regulated Ca2+ influx might be in Ca2+ homeostasis by, e.g., SR store filling. However, experimental attempts to evaluate the importance of this Ca2+ influx for muscle function in vivo have not yet been realizable due to the lack of an appropriate system. The presence of non–Ca2+-conducting Ca2+ channels in teleost fishes now provides evidence that on the organism level, skeletal muscle is completely functional without any CaV1.1 Ca2+ influx even on the long term. However, it is still feasible that principal differences in the physiology of Ca2+ handling in mammalian and teleost skeletal muscle might exist, and that in mammalian muscle the Ca2+ influx is indeed indispensable. To address this question, a knock-in mouse model carrying a nonconducting CaV1.1 and a transgenic zebrafish strain that expresses a conducting CaV1.1 in skeletal muscle will be valuable tools in further studies.
Taken together, this report might serve as the starting point for a diverse range of further studies in the fields of skeletal muscle physiology, biophysics of ion channel pores, and evolutionary biology of skeletal muscle function.
Materials and Methods
Cell Culture.
Myotubes of zebrafish, pike characin, and medaka were isolated and cultured exactly as described in detail (9). The murine α1S-null line GLT was cultured and transfected as described (19, 37).
Biophysical Analysis.
Myotubes were analyzed for L-type Ca2+ currents and gating currents by using the whole-cell patch-clamp technique in combination with intracellular Ca2+ recordings by loading the cells with the Ca2+-sensitive dye Fluo-4 as described for GLT (38) and zebrafish myotubes (39). Charge carrier was 10 mM Ca2+, or in one control experiment, 10 mM Ba2+ (Fig. 1D).
Quantitative RT-PCR.
RNA samples of deep fast and superficial slow muscle tissue from adult zebrafish or from sardine were isolated using the RNeasy Kit (Qiagen) and reverse-transcribed using random primers and M-MLV reverse transcriptase (Promega). All qPCR assays were designed with at least one primer spanning an exon–exon boundary. Primer sequences are found in Table S2. To generate absolute standard curves for all targets DNA fragments of 400–500 bp containing the qPCR primer annealing regions were cloned into the pGEM-T Easy vector (Promega) and sequence fidelity checked (Eurofins MWG Operon). PCR fragments generated from the pGEM constructs were quantified using the Quant-iT PicoGreen dsDNA Quantitation Kit (Invitrogen) and used at concentrations of 1 × 102 – 1 × 105 molecules/PCR as templates for the site-specific standard curves. All qPCRs were performed on a Stratagene MX3000P using MESA GREEN qPCR Mastermix Plus for SYBR Assay (Eurogentec). Absolute copy numbers of samples were calculated using MxPro 4.0 software (Stratagene). All expression values are given as copy number of target gene divided by the copy number of the housekeeping gene succinate dehydrogenase complex, subunit A (SDHA) (40).
Molecular Cloning.
zf-α1S-a and zf-α1S-b cDNA fragments were PCR generated from reverse-transcribed RNA isolated from zebrafish skeletal muscle. Generation of zf-α1S constructs and of point mutations into rb-α1S cDNA, all contained in expression plasmid pGFP37 (7), is described in SI Materials and Methods.
Genomic and cDNA Sequencing.
Tissue samples of various species were collected from alive (tail fin clip) or freshly killed fish. gDNA was isolated immediately or after transportation in 100% alcohol. RNA was isolated and reverse transcribed as described previously for quantitative RT-PCR. α1S-specific but species-unspecific degenerate primers were used to PCR-generate fragments coding for the α1S subunit pore regions of homologous repeats I and II carrying marker amino acids for nonconductivity (Table S1).
Supplementary Material
Acknowledgments
We thank Sandra Schleret for technical assistance; Tobias Grabner, Dominik Exner, and Martin Eichelberger for support in collecting fish samples; Sabine Geiger-Schredelseker for expert help with qPCR; and Dr. Christian Sturmbauer for expert help with the parsimony analysis and for comments on the manuscript. This work was supported by FWF Austrian Science Fund Grants FWF-16098-B04 and FWF-DK-W1101-B12 (to M.G.) and of Medizinische Forschungsförderung Innsbruck Grant MFI-6180 (to J.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: Sequences for zf-α1S-a and zf-α1S-b have been deposited in the GenBank database (accession nos. FJ769223 and AY495698).
This article contains supporting information online at www.pnas.org/cgi/content/full/0912153107/DCSupplemental.
References
- 1.Schneider MF, Chandler WK. Voltage dependent charge movement of skeletal muscle: A possible step in excitation-contraction coupling. Nature. 1973;242:244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- 2.Rios E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987;325:717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- 3.Tanabe T, Mikami A, Numa S, Beam KG. Cardiac-type excitation-contraction coupling in dysgenic skeletal muscle injected with cardiac dihydropyridine receptor cDNA. Nature. 1990;344:451–453. doi: 10.1038/344451a0. [DOI] [PubMed] [Google Scholar]
- 4.García J, Tanabe T, Beam KG. Relationship of calcium transients to calcium currents and charge movements in myotubes expressing skeletal and cardiac dihydropyridine receptors. J Gen Physiol. 1994;103:125–147. doi: 10.1085/jgp.103.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donaldson PL, Beam KG. Calcium currents in a fast-twitch skeletal muscle of the rat. J Gen Physiol. 1983;82:449–468. doi: 10.1085/jgp.82.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasledov GA, Katina IE, Zhitnikova IuV. Characteristics of functioning of electromechanical coupling in striated muscles of higher and lower vertebrates (Translated from Russian) Biofizika. 2002;47:716–727. [PubMed] [Google Scholar]
- 7.Grabner M, Dirksen RT, Beam KG. Tagging with green fluorescent protein reveals a distinct subcellular distribution of L-type and non-L-type Ca2+ channels expressed in dysgenic myotubes. Proc Natl Acad Sci USA. 1998;95:1903–1908. doi: 10.1073/pnas.95.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkens CM, Kasielke N, Flucher BE, Beam KG, Grabner M. Excitation-contraction coupling is unaffected by drastic alteration of the sequence surrounding residues L720-L764 of the α1S II-III loop. Proc Natl Acad Sci USA. 2001;98:5892–5897. doi: 10.1073/pnas.101618098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schredelseker J, et al. The β1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc Natl Acad Sci USA. 2005;102:17219–17224. doi: 10.1073/pnas.0508710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ono F, Higashijima S, Shcherbatko A, Fetcho JR, Brehm P. Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. J Neurosci. 2001;21:5439–5448. doi: 10.1523/JNEUROSCI.21-15-05439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams BA, Tanabe T, Mikami A, Numa S, Beam KG. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990;346:569–572. doi: 10.1038/346569a0. [DOI] [PubMed] [Google Scholar]
- 12.Tanabe T, et al. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- 13.Arikkath J, Campbell KP. Auxiliary subunits: Essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 14.De Waard M, Witcher DR, Pragnell M, Liu H, Campbell KP. Properties of the α 1-β anchoring site in voltage-dependent Ca2+ channels. J Biol Chem. 1995;270:12056–12064. doi: 10.1074/jbc.270.20.12056. [DOI] [PubMed] [Google Scholar]
- 15.Kugler G, Weiss RG, Flucher BE, Grabner M. Structural requirements of the dihydropyridine receptor α1S II-III loop for skeletal-type excitation-contraction coupling. J Biol Chem. 2004;279:4721–4728. doi: 10.1074/jbc.M307538200. [DOI] [PubMed] [Google Scholar]
- 16.Grabner M, et al. Insect calcium channels. Molecular cloning of an α1-subunit from housefly (Musca domestica) muscle. FEBS Lett. 1994;339:189–194. doi: 10.1016/0014-5793(94)80413-3. [DOI] [PubMed] [Google Scholar]
- 17.Okagaki R, et al. The maternal transcript for truncated voltage-dependent Ca2+ channels in the ascidian embryo: A potential suppressive role in Ca2+ channel expression. Dev Biol. 2001;230:258–277. doi: 10.1006/dbio.2000.0119. [DOI] [PubMed] [Google Scholar]
- 18.Hirata H, et al. Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease. Development. 2007;134:2771–2781. doi: 10.1242/dev.004531. [DOI] [PubMed] [Google Scholar]
- 19.Powell JA, Petherbridge L, Flucher BE. Formation of triads without the dihydropyridine receptor α subunits in cell lines from dysgenic skeletal muscle. J Cell Biol. 1996;134:375–387. doi: 10.1083/jcb.134.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Ellinor PT, Sather WA, Zhang JF, Tsien RW. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 1993;366:158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- 21.Maddison DR, Schulz KS. Tree of Life Web Project. 2009 Available at http://tolweb.org/ [Google Scholar]
- 22.Nakajo K, Chen L, Okamura Y. Cross-coupling between voltage-dependent Ca2+ channels and ryanodine receptors in developing ascidian muscle blastomeres. J Physiol. 1999;515:695–710. doi: 10.1111/j.1469-7793.1999.695ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagiwara S, Henkart MP, Kidokoro Y. Excitation-contraction coupling in amphioxus muscle cells. J Physiol. 1971;219:233–251. doi: 10.1113/jphysiol.1971.sp009659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers CM, Brown ER. Differential sensitivity to calciseptine of L-type Ca2+ currents in a ‘lower’ vertebrate (Scyliorhinus canicula), a protochordate (Branchiostoma lanceolatum) and an invertebrate (Alloteuthis subulata) Exp Physiol. 2001;86:689–694. doi: 10.1111/j.1469-445x.2001.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 25.Di Biase V, Franzini-Armstrong C. Evolution of skeletal type e-c coupling: A novel means of controlling calcium delivery. J Cell Biol. 2005;171:695–704. doi: 10.1083/jcb.200503077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue I, Tsutsui I, Bone Q, Brown ER. Evolution of skeletal muscle excitation-contraction coupling and the appearance of dihydropyridine-sensitive intramembrane charge movement. Proc R Soc Lond B Biol Sci. 1994;255:181–187. [Google Scholar]
- 27.Inoue I, Tsutsui I, Bone Q. Excitation-contraction coupling in skeletal and caudal heart muscle of the hagfish Eptatretus burgeri Girard. J Exp Biol. 2002;205:3535–3541. doi: 10.1242/jeb.205.22.3535. [DOI] [PubMed] [Google Scholar]
- 28.Jaillon O, et al. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature. 2004;431:946–957. doi: 10.1038/nature03025. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N’-tetracetic acid. Biochim Biophys Acta. 1972;267:605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- 30.Bryson-Richardson RJ, et al. Myosin heavy chain expression in zebrafish and slow muscle composition. Dev Dyn. 2005;233:1018–1022. doi: 10.1002/dvdy.20380. [DOI] [PubMed] [Google Scholar]
- 31.Østbye TK, et al. The two myostatin genes of Atlantic salmon (Salmo salar) are expressed in a variety of tissues. Eur J Biochem. 2001;268:5249–5257. doi: 10.1046/j.0014-2956.2001.02456.x. [DOI] [PubMed] [Google Scholar]
- 32.Buss RR, Drapeau P. Physiological properties of zebrafish embryonic red and white muscle fibers during early development. J Neurophysiol. 2000;84:1545–1557. doi: 10.1152/jn.2000.84.3.1545. [DOI] [PubMed] [Google Scholar]
- 33.Obermair GJ, et al. The Ca2+ channel α2δ-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of α1S or excitation-contraction coupling. J Biol Chem. 2005;280:2229–2237. doi: 10.1074/jbc.M411501200. [DOI] [PubMed] [Google Scholar]
- 34.Andronache Z, et al. The auxiliary subunit γ1 of the skeletal muscle L-type Ca2+ channel is an endogenous Ca2+ antagonist. Proc Natl Acad Sci USA. 2007;104:17885–17890. doi: 10.1073/pnas.0704340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakai J, et al. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380:72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- 36.Tuluc P, et al. A CaV1.1 Ca2+ channel splice variant with high conductance and voltage-sensitivity alters EC coupling in developing skeletal muscle. Biophys J. 2009;96:35–44. doi: 10.1016/j.bpj.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuhuber B, et al. Association of calcium channel α1S and β1a subunits is required for the targeting of β1a but not of α1S into skeletal muscle triads. Proc Natl Acad Sci USA. 1998;95:5015–5020. doi: 10.1073/pnas.95.9.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss RG, et al. Functional analysis of the R1086H malignant hyperthermia mutation in the DHPR reveals an unexpected influence of the III-IV loop on skeletal muscle EC coupling. Am J Physiol Cell Physiol. 2004;287:C1094–C1102. doi: 10.1152/ajpcell.00173.2004. [DOI] [PubMed] [Google Scholar]
- 39.Schredelseker J, Dayal A, Schwerte T, Franzini-Armstrong C, Grabner M. Proper restoration of excitation-contraction coupling in the dihydropyridine receptor β1-null zebrafish relaxed is an exclusive function of the β1a subunit. J Biol Chem. 2009;284:1242–1251. doi: 10.1074/jbc.M807767200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang R, Dodd A, Lai D, McNabb WC, Love DR. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin (Shanghai) 2007;39:384–390. doi: 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.