Abstract
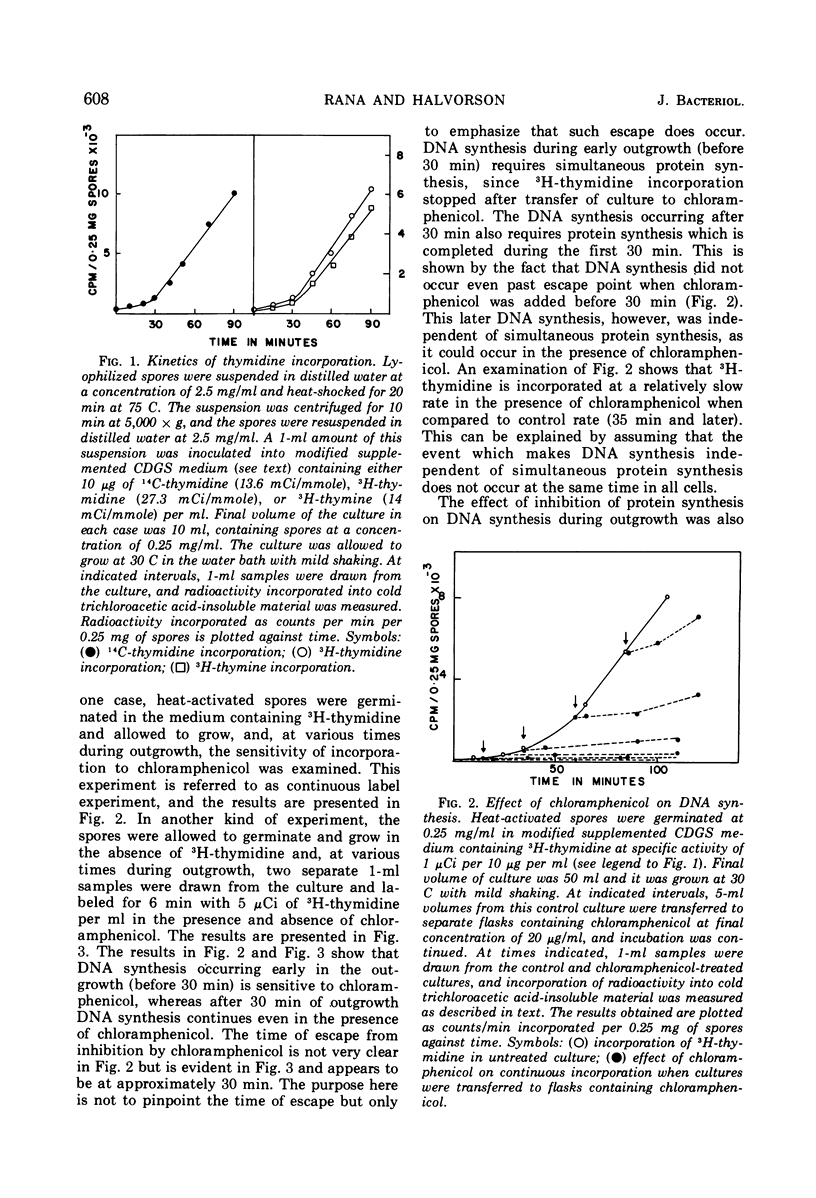
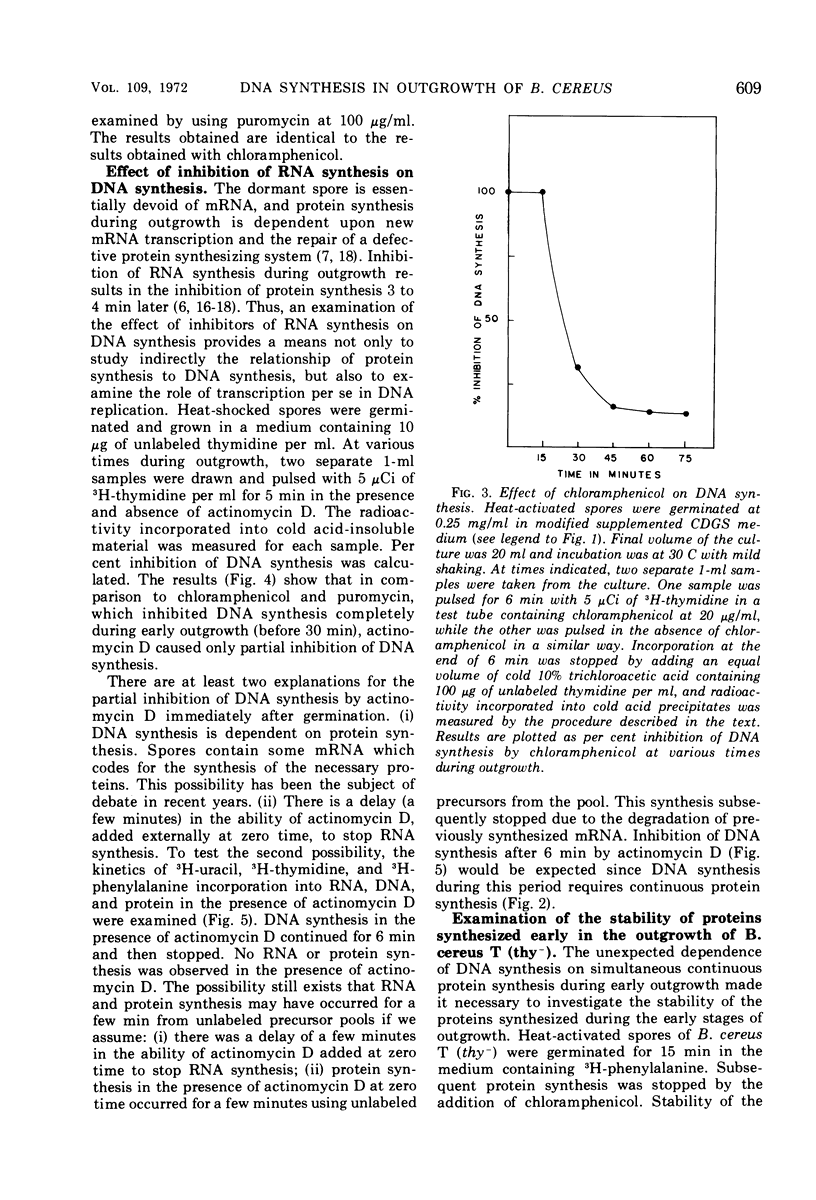
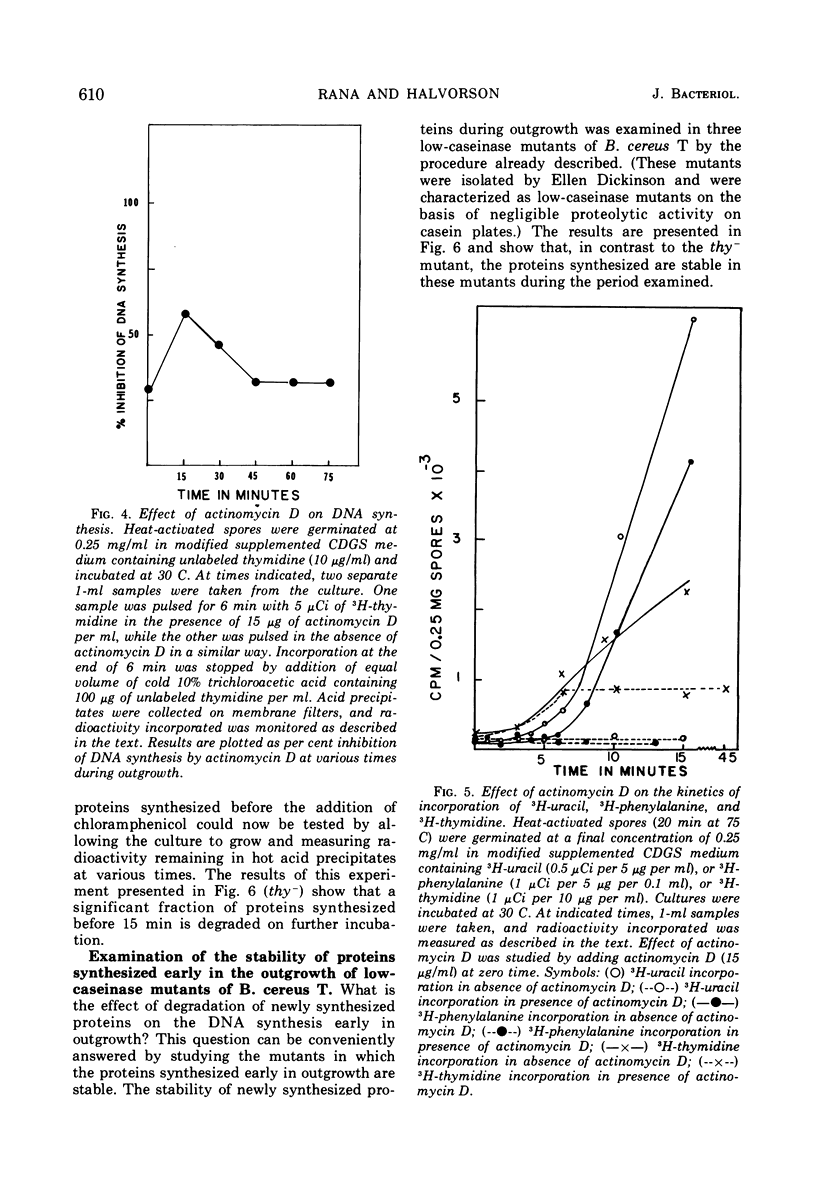
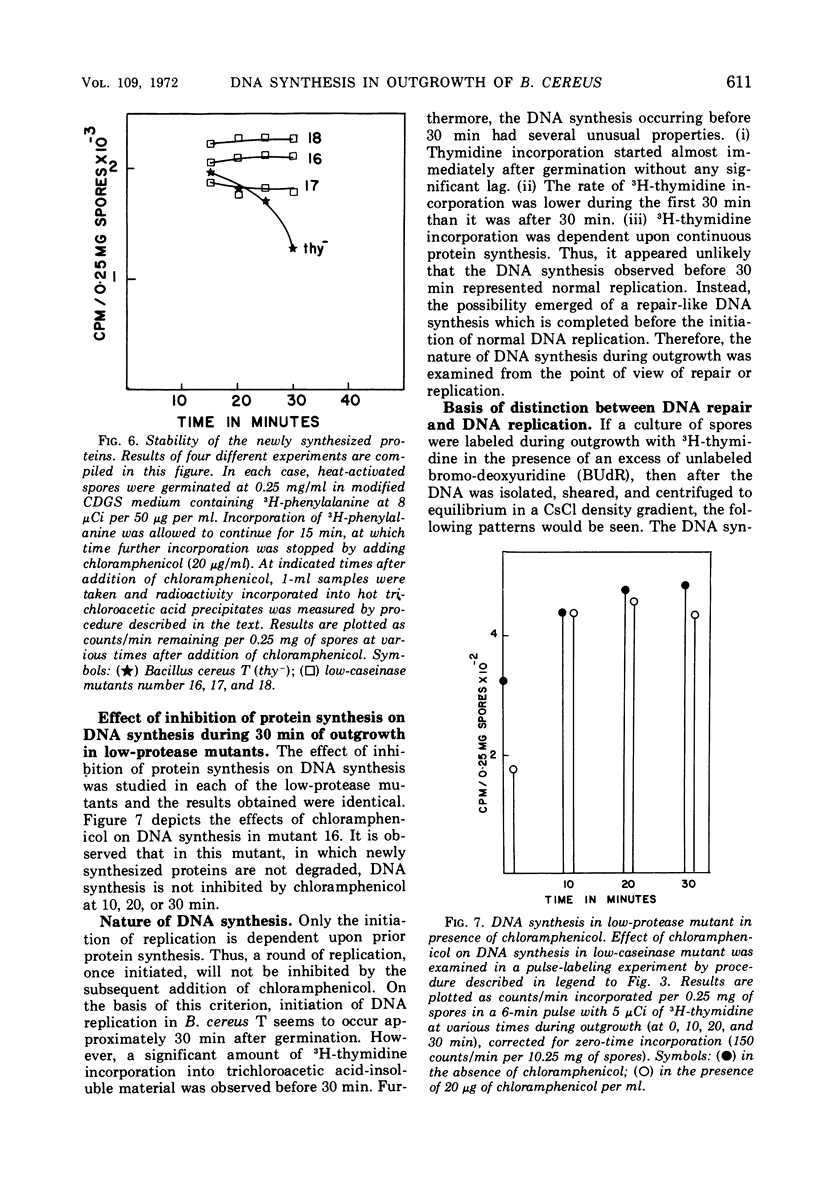
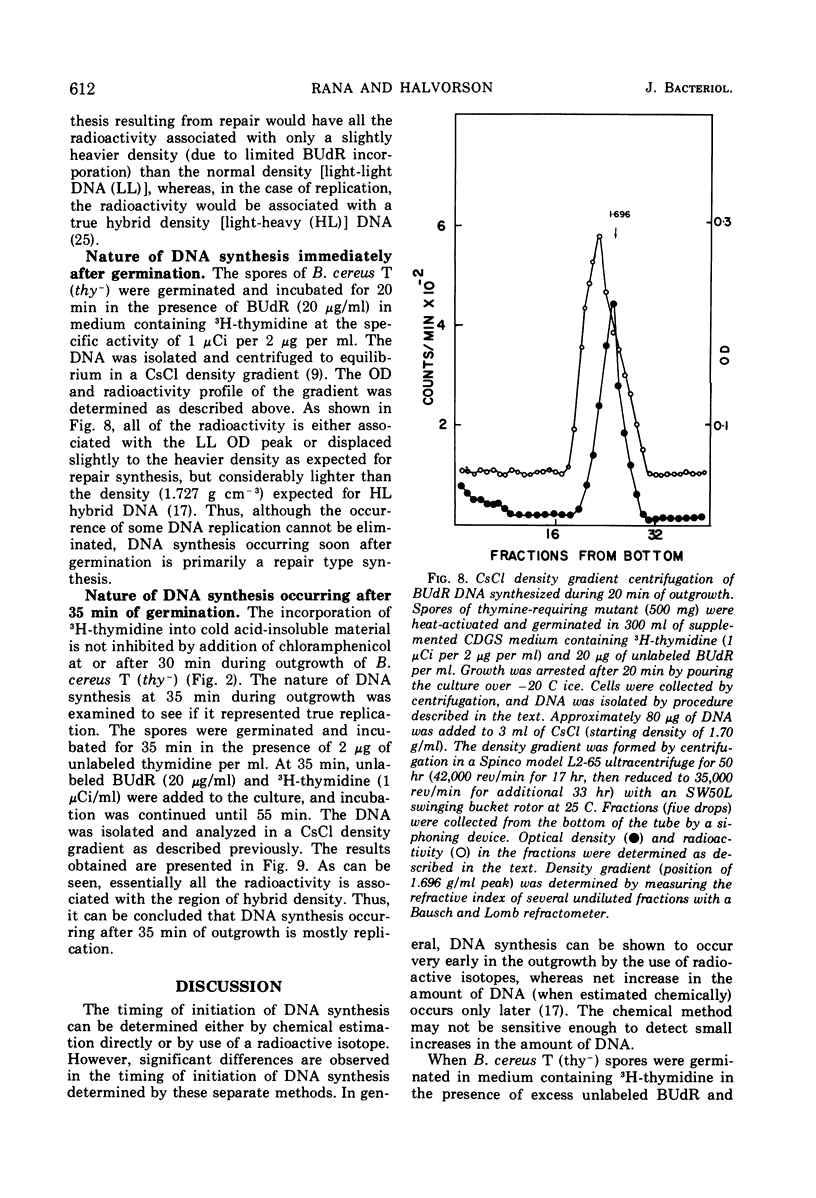
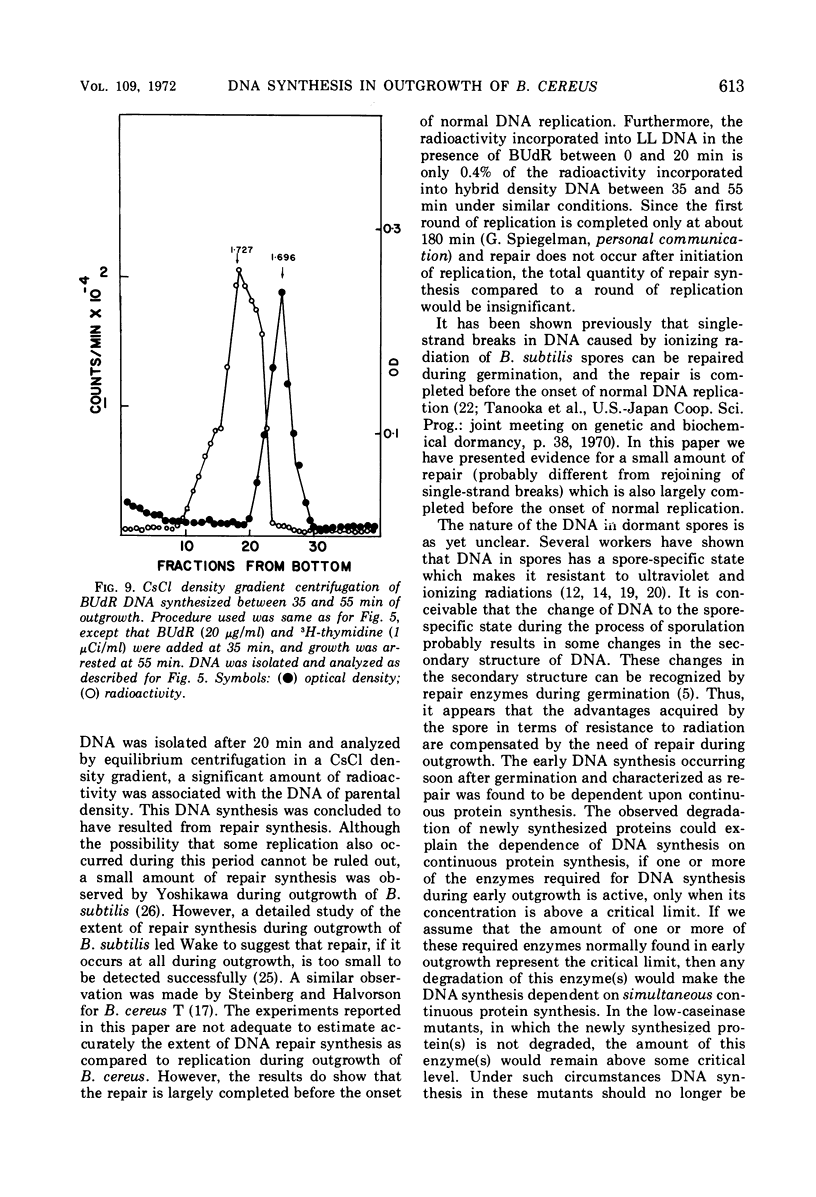
Deoxyribonucleic acid (DNA) synthesis during early outgrowth of spores of Bacillus cereus T (thy−) has been examined. 14C-thymidine incorporated begins 2 to 5 min after germination and continues at a slow rate up to 30 min, after which the rate of 14C-thymidine incorporation increases considerably. Early DNA synthesis up to 30 min after germination is dependent upon simultaneous protein synthesis. The examination of the stability of proteins synthesized soon after germination shows that they are susceptible to intracellular degradation. The evidence provided here indicates that protein degradation is the cause of observed dependence of DNA synthesis on simultaneous protein synthesis. The DNA synthesis occurring soon after germination is primarily a repair type synthesis which is followed by the onset of normal replication approximately 30 min after germination.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. L., Sueoka N. Phase transitions in ribonucleic acid synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1968 Jan;59(1):153–160. doi: 10.1073/pnas.59.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALASSA G. [Ribonucleic acid from Bacillus subtilis spores]. Biochim Biophys Acta. 1963 Jul 30;72:497–500. doi: 10.1016/0006-3002(63)90272-5. [DOI] [PubMed] [Google Scholar]
- Balassa G., Contesse G. Synthèses macromoléculaires au cours de la germination des spores de B. subtilis. I. Cinétique. Ann Inst Pasteur (Paris) 1965 Nov;109(5):683–705. [PubMed] [Google Scholar]
- CHURCH B. D., HALVORSON H., HALVORSON H. O. Studies on spore germination: its independence from alanine racemase activity. J Bacteriol. 1954 Oct;68(4):393–399. doi: 10.1128/jb.68.4.393-399.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan J. E., Jr, Setlow R. B. Thymine Photoproducts but not Thymine Dimers Found in Ultraviolet-Irradiated Bacterial Spores. Science. 1965 Jul 16;149(3681):308–310. doi: 10.1126/science.149.3681.308. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Halvorson H. O. Evidence for a defective protein synthesizing system in dormant spores of Bacillus cereus. Arch Biochem Biophys. 1968 Mar 11;123(3):622–632. doi: 10.1016/0003-9861(68)90182-3. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A., Sueoka N. Sequential replication of the Bacillus subtilis chromosome. IV. Genetic mapping by density transfer experiment. J Mol Biol. 1967 Jul 28;27(2):349–368. doi: 10.1016/0022-2836(67)90025-3. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Halvorson H. O. Method for restricting incorporation of radioactivity from 3 H-thymidine into deoxyribonucleic acid only during outgrowth of spores of Bacillus cereus T. J Bacteriol. 1972 Feb;109(2):599–605. doi: 10.1128/jb.109.2.599-605.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Ikeda Y. The dormant spore-specific state of DNA and macromolecular synthesis in spheroplasts of Bacillus subtilis spores. Biochim Biophys Acta. 1969 Apr 22;179(2):429–438. doi: 10.1016/0005-2787(69)90051-3. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y., Saito H., Ikeda Y. The similarity of DNA from dormant spores of Bacillus subtilis and that from vegetative cells. Biochim Biophys Acta. 1969 Feb 18;174(2):752–754. doi: 10.1016/0005-2787(69)90304-9. [DOI] [PubMed] [Google Scholar]
- Soska J., Lark K. G. Regulation of nucleic acid synthesis in Lactobacillus acidophilus R-26. Biochim Biophys Acta. 1966 Jun 22;119(3):526–539. doi: 10.1016/0005-2787(66)90129-8. [DOI] [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. I. Ordered enzyme synthesis. J Bacteriol. 1968 Feb;95(2):469–478. doi: 10.1128/jb.95.2.469-478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. II. Relationship between ordered enzyme synthesis and deoxyribonucleic acid replication. J Bacteriol. 1968 Feb;95(2):479–489. doi: 10.1128/jb.95.2.479-489.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanooka H., Sakakibara Y. Radioresistant nature of the transforming activity of DNA in bacterial spores. Biochim Biophys Acta. 1968 Jan 29;155(1):130–142. doi: 10.1016/0005-2787(68)90343-2. [DOI] [PubMed] [Google Scholar]
- Tanooka H., Terano H., Otsuka H. Increase of thymidine, thymidylate and deoxycytidine kinase activites during germination of bacterial spores. Biochim Biophys Acta. 1971 Jan 1;228(1):26–37. doi: 10.1016/0005-2787(71)90543-0. [DOI] [PubMed] [Google Scholar]
- Tanooka H. Ultraviolet resistance of DNA in spore spheroplasts of Bacillus subtilis as measured by the transforming activity. Biochim Biophys Acta. 1968 Sep 24;166(2):581–583. doi: 10.1016/0005-2787(68)90248-7. [DOI] [PubMed] [Google Scholar]
- Terano H., Tanooka H., Kadota H. Germination-induced repair of single-strand breaks of DNA in irradiated Bacillus subtilis spores. Biochem Biophys Res Commun. 1969 Sep 24;37(1):66–71. doi: 10.1016/0006-291x(69)90881-x. [DOI] [PubMed] [Google Scholar]
- Torriani A., Levinthal C. Ordered synthesis of proteins during outgrowth of spores of Bacillus cereus. J Bacteriol. 1967 Jul;94(1):176–183. doi: 10.1128/jb.94.1.176-183.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKE R. G. SEQUENTIAL REPLICATION OF DNA IN SYNCHRONOUSLY GERMINATING BACILLUS SUBTILIS SPORES. Biochem Biophys Res Commun. 1963 Sep 10;13:67–70. doi: 10.1016/0006-291x(63)90164-5. [DOI] [PubMed] [Google Scholar]
- Wake R. G. A study of the possible extent of synthesis of repair DNA during germination of Bacillus subtilis spores. J Mol Biol. 1967 Apr 28;25(2):217–234. doi: 10.1016/0022-2836(67)90139-8. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA H., O'SULLIVAN A., SUEOKA N. SEQUENTIAL REPLICATION OF THE BACILLUS SUBTILIS CHROMOSOME. 3. REGULATION OF INITIATION. Proc Natl Acad Sci U S A. 1964 Oct;52:973–980. doi: 10.1073/pnas.52.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of Bacillus subtilis chromosome. I. Comparison of marker frequencies in exponential and stationary growth phases. Proc Natl Acad Sci U S A. 1963 Apr;49:559–566. doi: 10.1073/pnas.49.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG I. E., FITZ-JAMES P. C. Pattern of synthesis of deoxyribonucleic acid in Bacillus cereus growing synchronously out of spores. Nature. 1959 Feb 7;183(4658):372–373. doi: 10.1038/183372a0. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H. DNA synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1476–1483. doi: 10.1073/pnas.53.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]


