Abstract
Piccolo and bassoon are highly homologous multidomain proteins of the presynaptic cytomatrix whose function is unclear. Here, we generated piccolo knockin/knockout mice that either contain wild-type levels of mutant piccolo unable to bind Ca2+ (knockin), ∼60% decreased levels of piccolo that is C-terminally truncated (partial knockout), or <5% levels of piccolo (knockout). All piccolo mutant mice were viable and fertile, but piccolo knockout mice exhibited increased postnatal mortality. Unexpectedly, electrophysiology and electron microscopy of piccolo-deficient synapses failed to uncover a major phenotype either in acute hippocampal slices or in cultured cortical neurons. To unmask potentially redundant functions of piccolo and bassoon, we thus acutely knocked down expression of bassoon in wild-type and piccolo knockout neurons. Despite a nearly complete loss of piccolo and bassoon, however, we still did not detect an electrophysiological phenotype in cultured piccolo- and bassoon-deficient neurons in either GABAergic or glutamatergic synaptic transmission. In contrast, electron microscopy revealed a significant reduction in synaptic vesicle clustering in double bassoon/piccolo-deficient synapses. Thus, we propose that piccolo and bassoon play a redundant role in synaptic vesicle clustering in nerve terminals without directly participating in neurotransmitter release.
Keywords: active zone, neurotransmitter release, synapse, vesicle docking, synaptogenesis
In presynaptic terminals, the active zone is associated with a cytoskeletal cytomatrix that extends into the presynaptic cytosol. Piccolo and bassoon are large (>400 kDa) multidomain proteins of the presynaptic cytomatrix that are present in all vertebrate synapses but are absent from invertebrates (1 –7). Piccolo and bassoon are composed of highly homologous zinc finger and coiled-coil sequences; in addition, piccolo contains an N-terminal glutamine-rich sequence, a C-terminal PDZ-domain, and two C-terminal C2-domains (referred to as the C2A- and C2B-domain) that are absent from bassoon (Fig. 1A). The piccolo C2A-domain includes an unusual Ca2+-binding site that is regulated by alternative splicing; this domain dimerizes and undergoes a conformational change upon Ca2+-binding (8, 9). The piccolo C2B-domain, conversely, does not bind Ca2+ but is alternatively spliced in its entirety (3).
Fig. 1.
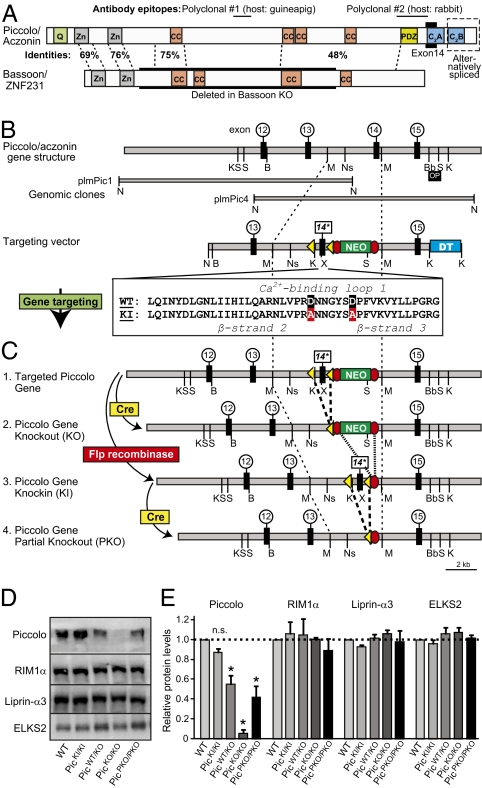
Structures of piccolo and bassoon, and generation of piccolo KO and KI mice. (A) Comparison of piccolo and bassoon domain structures. Homologous regions are indicated as % identity between piccolo and bassoon (Zn, zinc-finger domains; CC, predicted coiled-coil domains). Epitope locations for the two piccolo antibodies used are shown above the piccolo structure, and the C-terminal region in the piccolo C2A-domain that is encoded by exon 14, and targeted in our genetic experiments, is shown below the diagram, as is the even more C-terminal alternatively spliced area. For bassoon, the central region that is deleted in the bassoon KO (20) is boxed. (B) Generation of piccolo KO and KI mice. Genomic clones of piccolo containing exon 14 (plmPic1 and plmPic4) were used to generate a targeting vector that includes a neomycin gene resistance cassette for positive selection (NEO, flanked by flp recombination sites) and a diphtheria gene cassette for negative selection (DT). Exon 14 was flanked by loxP recombination sites and mutated to substitute the two Ca2+-binding aspartate residues in loop 1 for alanines [14* (9)]. (C) Mice containing the mutant piccolo gene were generated by homologous recombination (#1). Cre-recombinase-mediated excision of exon 14 causes suppression of piccolo expression (referred as piccolo KO mice; #2). Flp-recombinase-mediated excision of the neomycin resistance cassette reactivates piccolo expression, resulting in piccolo KI mice in which wild-type levels of piccolo with a mutant Ca2+-binding site in the C2A-domain are expressed (#3). Finally, combined flp- and cre-recombinase mediated excision of both the neomycin resistance cassette and exon 14 results in the partial expression of truncated piccolo lacking the C2A- and C2B-domains (#4). (D) Immunoblot analysis of the indicated active zone proteins in brain homogenates from wild-type (WT), homozygous KI (PicKI/KI), heterozygous KO (PicWT/KO), homozygous KO (PicKO/KO), and homozygous partial KO (PicPKO/PKO) mice. Blots were probed with 125I-labeled secondary antibodies, and signals were visualized with a phosphorimager. (E) Quantitation of active zone protein levels, as shown in D, in three independent experiments (mean ± SEM; n = 3; *, P < 0.05 by Student's t test; n.s., not significant).
Multiple functions were suggested for piccolo and bassoon, but no analyses of synapses deficient in both were performed. Piccolo and bassoon may mediate formation of nascent active zones from precursor vesicles that bud from the trans-Golgi network (10 –12). In vitro binding and transfection experiments suggested that both interact with the active zone protein ELKS (6, 13, 14), that bassoon binds to CtBPs (1, 15) and dynein light chains (16), and that piccolo binds to profilin (3), Abp1 (2), Pra1 (2), GIT1 (17), cAMP GEFII (5), RIM2α (5), and L-type calcium channels (18). Based on these interactions, bassoon may function in retrograde axonal transport (16) and active zone organization (13, 17), whereas piccolo may participate in neurotransmitter release (13) or insulin secretion (5, 18). Although many of these protein interactions could be important, some of these interactions may not be physiologically relevant. For instance, it is unlikely that piccolo functionally interacts with RIM2α during insulin secretion (18) because insulin-secreting cells do not express RIM2α, and the deletion of RIM2α does not impair insulin secretion (19). Bassoon knockout (KO) mice are viable at birth, but exhibit debilitating epileptic seizures (20). Surprisingly, no morphological changes were observed in synapses of bassoon KO mice, whereas electrophysiological recordings in autapses revealed inactivation of a subset of synapses (20). In contrast to the proposed activator function of bassoon, piccolo was suggested based on RNAi studies to function as an inhibitor of neurotransmitter release (21). Here, we have examined the functions of piccolo and bassoon by creating piccolo mutant mice and then knocking down expression of bassoon on top of the piccolo deficiency. Our data suggest that piccolo and bassoon function as tethering proteins that mediate efficient synaptic vesicle clustering, but that neither piccolo nor bassoon has a direct role in either synapse formation or neurotransmitter release.
Results
Generation of Piccolo Knockin (KI) and KO Mice.
We isolated genomic clones that contain the 3′ end of the murine piccolo gene (Fig. 1B), including exon 14, which specifies loop 1 of the C2A-domain Ca2+-binding site (3). We used these genomic clones to construct a targeting vector in which the Ca2+-ligating aspartate residues of the loop 1 Ca2+-binding sites of the C2A-domain were mutated to alanines, thereby abolishing Ca2+-binding to the C2A-domain (Fig. 1B) (9). In the targeting vector, we also flanked exon 14 with loxP sites to allow its conditional deletion with cre recombinase, and inserted a neomycin resistance cassette flanked by frt sites (NEO) into the adjacent 3′ intron for positive selection and a diphtheria toxin gene cassette (DT) next to the short 3′ arm of the vector for negative selection (Fig. 1B).
We used the targeting vector for homologous recombination in embryonic stem cells by means of standard procedures (22) and obtained mice containing the targeted mutant piccolo gene (#1; Fig. 1C). Three different mouse lines were generated from the initially targeted piccolo mutant mice by removing exon 14 and/or the neomycin resistance cassette (#2–4 in Fig. 1C). These three lines are piccolo KO mice in which exon 14 is deleted but the neomycin resistance cassette remains (#2), piccolo KI mice in which the neomycin resistance cassette is deleted but the mutant exon 14 remains (#3), and piccolo partial KO mice in which both exon 14 and the neomycin resistance cassette are deleted (#4). All of these mice were viable and fertile, although piccolo KO mice exhibited increased mortality (see below).
We next measured the levels of piccolo and of the active zone proteins RIM1α, liprin-3α, and ELKS2 in the piccolo mutant mice by quantitative immunoblotting with 125I-labeled secondary antibodies (Fig. 1 D and E). In homozygous mutant piccolo KI mice (#3, Fig. 1C), the levels of mutant piccolo were not significantly decreased compared to wild-type littermate controls (Fig. 1E). In piccolo KO mice (#2), in contrast, piccolo levels were decreased to <5% of wild type (Fig. 1E and Table S1). Moreover, no truncated piccolo protein was detected (Fig. S1). It is likely that the destabilizing effect of the exon 14 deletion on the piccolo mRNA and the inhibitory effect of the remaining neomycin resistance gene cassette together completely suppress piccolo expression. Consistent with this interpretation, piccolo partial KO mice (which lack both exon 14 and the neomycin resistance gene cassette; #4 in Fig. 1C) contain levels of truncated piccolo protein that are ∼40% of wild-type levels (Fig. 1E).
Piccolo KO Moderately Impairs Mouse Growth and Survival but Does Not Detectably Alter Neurotransmitter Release.
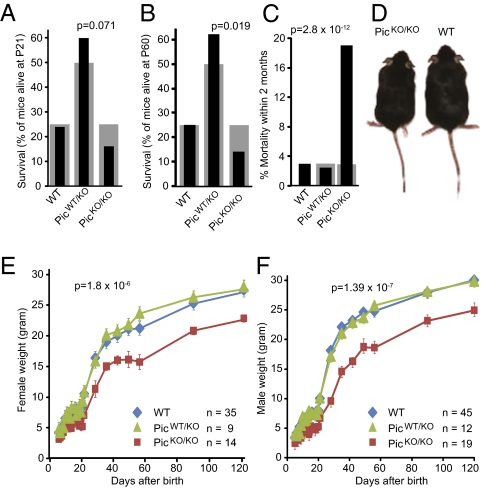
We analyzed the weight and survival of piccolo KO mice by following the offspring of heterozygous matings. Homozygous piccolo KO mice exhibit a modest but significant increase in postnatal mortality that becomes manifest at P60 (Fig. 2 A and B) and leads to the death of ∼20% of homozygous KO mice (Fig. 2 C and D). No obvious seizures were noted. Systematic weight measurements uncovered a significant decrease in size in male and female piccolo KO mice that is only present in homozygous, not heterozygous, piccolo KO mice (Fig. 2 E and F).
Fig. 2.
Survival and weight of piccolo KO mice. (A and B) Survival of the offspring of crosses between heterozygous piccolo KO mice (PicWT/KO), plotted as a function of genotype and analyzed at P21 (A) or P60 (B). The actual (black bars) and expected (gray bars, based on Mendelian inheritance) distribution of genotypes for wild-type (WT), heterozygous (PicWT/KO), and homozygous KO (PicKO/KO) mice are shown. The indicated P values were calculated using a χ-test comparing observed and expected distributions (n = 133). (C) Plot of the observed mortality of littermate wild-type (WT), heterozygous (PicWT/KO), and homozygous piccolo KO (PicKO/KO) mice at P60. Indicated P value was calculated using a χ-test (n = 133). (D) Representative image of littermate male wild-type (WT) and piccolo KO (PicKO/KO) mice. (E and F) Weight curves of littermate female (E) and male (F) piccolo wild-type (WT), heterozygous (PicWT/KO), and homozygous piccolo KO (PicKO/KO) mice. Data shown are mean ± SEM; indicated P values were calculated using a two-tailed paired t test (n, number of mice in each group).
Using hippocampal slice physiology, we analyzed synaptic transmission at the Schaffer collateral to CA1 pyramidal cell synapse but failed to uncover major abnormalities. Specifically, paired-pulse facilitation and posttetanic potentiation were indistinguishable between synapses from wild-type and littermate KO mice, demonstrating that short-term plasticity was unchanged (Fig. S2 A and B). Use-dependent depression during a 14-Hz stimulus train exhibited a small increase in depression at later stages in the train (25th/1st response ratio: WT 1.03 ± 0.04, n = 5 mice, 10 slices; KO 0.92 ± 0.03, n = 5 mice, 10 slices; P = 0.04918), but the modest extent of the effect precludes assigning to it major significance (Fig. S2C). Overall, compared to other synaptic KO mice [e.g., those of synapsins (23, 24)], the piccolo KO mice do not exhibit a dramatic synaptic phenotype.
Designing an shRNA for Knockdown of Bassoon.
To characterize the effect of deleting piccolo and bassoon on presynaptic terminals, we developed an RNAi-dependent knockdown of bassoon in cultured neurons. We used a previously developed lentiviral strategy that allows expression of shRNAs in all neurons in a culture (25), and assessed the effectiveness of a given shRNA by quantitative rt-PCR, immunoblotting, and immunocytochemistry (Fig. S3). We identified one shRNA that did not impair neuronal viability but decreased the bassoon mRNA levels by >80% and abolished the bassoon immunoblotting signal. Expression of this shRNA in wild-type and piccolo KO neurons largely abrogated bassoon immunoreactivity but had no apparent effect on synapse density (Fig. S3).
To examine the effect of the piccolo and bassoon deficiency on synapse composition, we cultured cortical neurons from littermate wild-type and piccolo KO mice, and infected the wild-type culture with control lentivirus and the piccolo KO culture with lentivirus expressing the bassoon shRNA. We then measured by quantitative immunoblotting the levels of 12 synaptic proteins in the two sets of neurons but failed to uncover a significant change induced in any protein by the piccolo/bassoon deficiency (Fig. S4 A and B). Moreover, the solubility of active zone proteins did not exhibit a major change (Fig. S4C), indicating that the composition of the presynaptic terminal is not dramatically altered by abrogating piccolo and bassoon expression.
Piccolo and Bassoon Are Dispensable for Synaptic Transmission.
Given previous suggestions of essential functions for piccolo and bassoon in synaptic terminals (6, 16, 21), we anticipated that the piccolo- and bassoon-deficient synapses should exhibit major phenotypes. Thus, we set out to perform an extensive analysis of these synapses that measures spontaneous and evoked responses in both excitatory and inhibitory synapses, as well as short-term plasticity and the readily releasable pool (RRP) of vesicles by application of hypertonic sucrose (26). To be comprehensive, we employed cultured neurons from littermate wild-type and piccolo KO mice, and compared neurons that were infected with control lentivirus with those infected with lentivirus expressing the bassoon shRNA. All recordings were performed in the whole-cell patch-clamp configuration using a standard protocol (27) and examining neurons whose genotype was unknown to the experimentalist (Fig. 3 and Fig. S5).
Fig. 3.
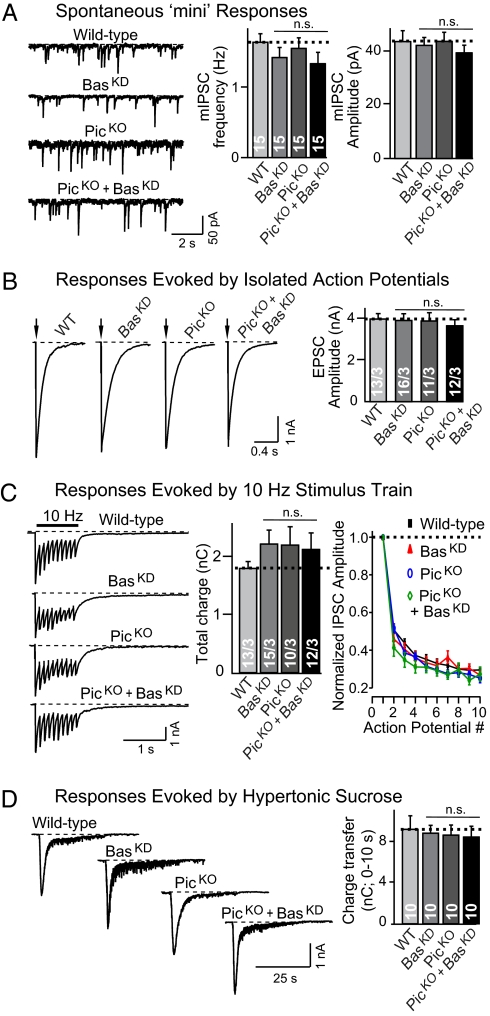
Inhibitory synaptic transmission in piccolo- and bassoon-deficient neurons. All experiments were performed on cortical neurons cultured from littermate wild-type and piccolo KO mice, and infected with lentiviruses. WT and BasKD, cortical neurons cultured from wild-type mice and infected with control lentiviruses or lentivirus expressing the bassoon shRNA, respectively; PicKO and PicKO+BasKD, cortical neurons cultured from piccolo KO mice and infected with control lentiviruses or lentivirus expressing the bassoon shRNA, respectively. Neurons were infected twice (at DIV2 and at DIV8) and analyzed at DIV14–DIV16 by voltage-clamp whole-cell recordings. (A) Spontaneous miniature inhibitory postsynaptic currents (mIPSCs) monitored in the presence of 1 μM tetrodotoxin, 10 μM CNQX, and 50 μM APV. Representative traces are shown on the left, and summary graphs of the mIPSC frequency and amplitudes are shown on the right. (B) Inhibitory postsynaptic currents (IPSCs) evoked by isolated action potentials elicited by a local electrode in 20 μM CNQX and 50 μM APV (27). Representative traces are shown on the left, and summary graphs of the IPSC amplitudes are shown on the right. (C) IPSCs evoked by a 10-Hz, 1-s stimulus train in the presence of 10 μM CNQX and 50 μM APV. Representative traces are shown on the left, summary graphs of the total charge transfer induced by the train are shown in the center, and the degree of synaptic depression as a function of the action potential number is plotted on the right. (D) IPSCs evoked by a 30-s application of 0.5 M sucrose in the presence of 1 μM tetrodotoxin, 20 μM CNQX, and 50 μM APV to measure the size of the readily releasable pool (RRP). Representative traces are depicted on the left, and summary graphs of the total synaptic charge transfer during the first 10 s (which measures the RRP) are shown on the right. All data shown are mean ± SEM; numbers in bars list the total number of neurons recorded in at least three independent experiments. P-value calculations using Student's t test revealed that none of the manipulations induced a statistically significant change in synaptic responses (n.s., nonsignificant).
To our great surprise, we observed no significant changes in either excitatory or inhibitory synaptic transmission in any of the three deficiency states (piccolo KO, bassoon RNAi, or double deficiency). In particular, we did not detect a change in spontaneous mini release or in evoked release elicited either by isolated action potentials or stimulus trains (Fig. 3 A–C and Fig. S5 A–C). Moreover, we observed no significant change in short-term synaptic plasticity (Fig. 3C and Fig. S5C) or in the size of the RRP (Fig. 3D and Fig. S5D), consistent with the lack of a change in total synaptic charge transfer during the 10-Hz stimulus train. Thus, abrogating piccolo and bassoon expression from synapses does not appear to change the basic parameters of synaptic transmission.
Piccolo- and Bassoon-Deficient Synapses Exhibit a Decrease in Synaptic Vesicle Clustering.
Finally, we analyzed synapses in cultured piccolo- and bassoon-deficient neurons by quantitative electron microscopy (EM). Only asymmetric, presumably excitatory synapses were examined because only these synapses can be unequivocally identified by EM. In the quantitative analyses, we compared two sets of neuronal cultures from littermate wild-type and piccolo KO mice, one set without lentiviral infection and a second set that was infected with either control lentivirus or lentivirus expressing the bassoon shRNA (Fig. 4 A–F).
Fig. 4.
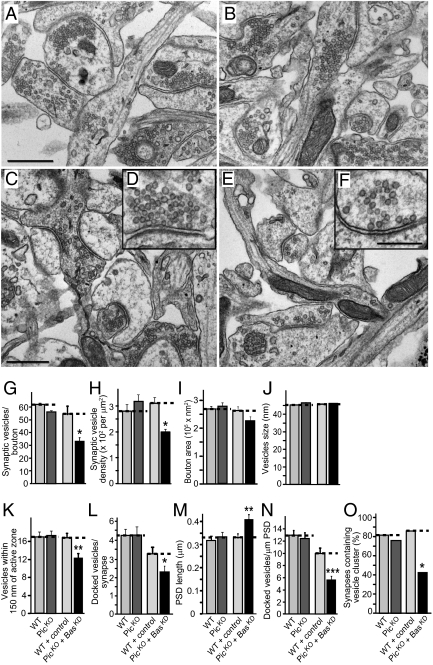
Ultrastructure of synapses lacking piccolo and bassoon. (A–F) Representative electron micrographs of synapses in cortical neurons that were cultured from wild-type mice and infected with control lentiviruses (Wild-type; A, C, and D), and from littermate piccolo KO mice and infected with lentivirus expressing bassoon shRNA (PicKO + BasKD; B, E, and F). Neurons were infected twice (at DIV2 and at DIV8) and analyzed at DIV14. [Scale bars: C (for A–C), 600 nm; F (for E and F), 400 nm.] (G–O) Quantifications of synaptic parameters in anonymized electron micrographs of cultured cortical neurons. Two sets of neurons were analyzed separately: neurons from littermate wild-type and piccolo KO mice that were not infected with lentiviruses (WT, wild-type neurons; PicKO, piccolo KO neurons), and neurons from wild-type mice that were infected with control lentivirus (WT + control) or from littermate piccolo KO mice that were infected with lentivirus expressing the bassoon shRNA (PicKO + BasKD). (G) Average number of synaptic vesicles per bouton. (H) Average density of synaptic vesicles in the terminal. (I) Average bouton area. (J) Average size of synaptic vesicles. (K) Average number of synaptic vesicles within 150 nm of the active zone. (L) Average number of docked vesicles per active zone. (M) Average PSD length. (N) Average number of docked vesicles per PSD length. (O) Percentage of presynaptic terminals containing a vesicle cluster. Parameters are shown as mean ± SEM (n = 75 synapses) from a single experiment that was independently repeated once for the double-deficient comparison with comparable results (Fig. S5). Statistical significance was assessed by Student's t test (n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
Synapses from piccolo KO and littermate wild-type controls mice exhibited no significant difference. In contrast, synapses deficient in both bassoon and piccolo displayed a striking phenotype, suggesting that vesicle clustering was impaired (Fig. 4O). Specifically, the number of vesicles in nerve terminals was decreased ∼40% (Fig. 4G), and the overall density of vesicles per terminal area was lowered ∼33% in double-deficient synapses (Fig. 4H). The sizes of presynaptic terminals or of synaptic vesicles, however, were unchanged (Fig. 4 I and J). In addition to overall vesicle numbers, the number of vesicles close to the active zone (Fig. 4K), or docked at the active zone (Fig. 4L), was lowered, whereas the size of the postsynaptic density (PSD) was increased (∼25%; Fig. 4M), possibly as a compensatory event, resulting in a ∼50% overall decrease in the number of docked vesicles per micrometer of PSD (Fig. 4N). Finally, the proportion of synapses containing vesicles clusters was dramatically decreased (>50%; Fig. 4O).
Despite the differences between culture sets, for most parameters the absolute values of the EM results are similar (Fig. 4). The most variable parameter was vesicle docking, which is thus the most difficult to evaluate and presumably the most sensitive to fixation methods. Thus, we repeated the EM analysis of the double-deficient neurons in an independent set of experiments and obtained quantitatively similar results (Fig. S5). In summary, whereas deletion of either piccolo or bassoon by itself seems to have little effect on the structure of the synapse (Fig. 4) (2020), deletion of both causes a redistribution of synaptic vesicles with a loss of synaptic vesicle clusters.
Discussion
The presynaptic active zone is an electron-dense structure composed of proteins such as RIMs, Munc13s, α-liprins, and ELKS that together participate in the docking, priming, and fusion of synaptic vesicles (28). Piccolo and bassoon are large multidomain proteins that extend from the active zone into the presynaptic cytomatrix (29). Various protein interactions and functions have been ascribed to piccolo and bassoon, but to date no analysis of the combined loss-of-function states of these proteins is available. As a first step toward such an analysis, we have now generated mutant mice that either lack piccolo expression or express mutant piccolo containing two amino acid substitutions in the Ca2+-binding site of the C2A-domain, rendering it unable to bind Ca2+ (Fig. 1). Moreover, we produced efficient shRNAs that decrease bassoon expression by >75% (Fig. S3). We then analyzed the function of the piccolo/bassoon proteins by studying synapses that are deficient in either piccolo, bassoon, or both. Our data provide three key observations: (i) Deletion of piccolo caused a small but significant survival phenotype and decreased the weight of mice, demonstrating that piccolo is important but not essential (Fig. 1 D and E). (ii) Synapses deficient in both piccolo and bassoon exhibited an apparently normal protein composition (Fig. S4), but an abnormal ultrastructure with decreased density, clustering, and docking of synaptic vesicles but an increased PSD size (Fig. 4 and Fig. S6). (iii) Despite their strong ultrastructural phenotype, piccolo/bassoon-deficient synapses display no major release phenotype, including no change in spontaneous release, evoked release, short-term plasticity, or the readily releasable pool of glutamatergic or GABAergic synapses (Fig. 3 and Fig. S5)
Based on these results, our overall conclusion is that piccolo and bassoon, in contrast to the core active zone proteins Munc13, RIM, ELKS, and α-liprins, are peripheral organizers of synaptic vesicles with only a minor role in neurotransmitter release. This conclusion agrees with the lack of evolutionary conservation of piccolo and bassoon, but appears to be at odds with some of the previous studies on these fascinating proteins. For example, shRNA-mediated knockdown of piccolo suggested that piccolo performs a unique function mediated by synapsin-1, and that it couples the mobilization of synaptic vesicles in the reserve pool to events within the active zone, even though the piccolo knockdown did not alter synaptic ultrastructure (21). However, we did not observe a major change in neurotransmitter release in piccolo KO mice (or the piccolo/bassoon double deficiency state), whereas such a change is detectable in synapsin-1 KO mice (23). This discrepancy may be due to the approach: we directly measured synaptic transmission and the size of functional synaptic vesicle pools electrophysiologically in both piccolo (this study) and synapsin-1 KO (23, 24) mice, whereas the piccolo KD was examined by FM-dye destaining (21).
Could we have missed a hidden phenotype that may not be apparent in the generic analyses we performed? It is likely that bassoon and piccolo perform as yet undefined essential functions in specialized uncommon synapses that were not examined in our electrophysiological and ultrastructural studies. For example, although synapse structure in bassoon KO mice is normal, in retinal ribbon synapses the bassoon KO caused detachment of ribbons from the plasma membrane (30). Floating ribbons appear to be manifestations of synapse dysfunction and degeneration (31 –34), suggesting that floating ribbons in bassoon KO mice are indicative of a major dysfunction of the ribbon synapses. Similarly, it is possible that nonclassical presynaptic terminals, such as those formed by catecholaminergic varicosities that contain bassoon (7), may exhibit more severe functional deficits upon piccolo/bassoon deletions.
It is interesting to compare the piccolo/bassoon deficiency phenotype with that of synapsin KO mice (23, 24). In both cases, synaptic vesicles in nerve terminals are lost. However, the synapsin double KO enhances synaptic vesicle clustering and docking, and synapsin-deficient vesicles form a denser and smaller cluster in front of the active zone (24). Moreover, synaptic vesicle protein levels are decreased ∼50%, consistent with a loss of total vesicle numbers (35). In contrast, in piccolo/bassoon-deficient synapses, the vesicle density is decreased uniformly throughout the nerve terminal, including a loss of vesicles near the active zone and of docked vesicles at the active zone (Fig. 4). At the same time, the levels of synaptic vesicle proteins are not altered (Fig. S4). Another difference between the two deficiency states is that the synapsin double KO leads to prominent changes in short-term synaptic plasticity (23, 24), whereas the piccolo/bassoon deficiency phenotype exhibits no major electrophysiological changes (Fig. 3 and Fig. S5).
Thus, in comparison with the synapsin double KO phenotype, the most plausible hypothesis to account for the piccolo/bassoon deficiency phenotype is that piccolo and bassoon normally function as protein rails to recruit and tether synaptic vesicles in the presynaptic cytomatrix. As a result, the loss of piccolo and bassoon leads to a loss of vesicle clustering without changes in release because vesicle clustering is not rate-limiting for release. This hypothesis is consistent with the requirement for piccolo and bassoon in mouse survival, despite the lack of a direct effect of their deletion on neurotransmitter release, as one can assume that the cumulative effect of a moderate disorganization of nerve terminals throughout the brain could lead to an overall failure of brain function even in the absence of a dramatic release phenotype.
Materials and Methods
Generation of Knockin (KI) and KO Mice.
We isolated genomic clones containing the 3′ end of the piccolo gene and constructed a targeting vector for homologous recombination by standard procedures (22 –24) (Fig. 1B). Two point mutations (D4668A, D4674A) known to inhibit Ca2+-binding (8) were introduced into exon 14 encoding part of the C2A-domain of piccolo by mutagenesis, and exon 14 was flanked with loxP sites to allow excision by cre recombinase. In addition, we inserted into the intron 14 a neomycin gene cassette for positive selection after homologous recombination and flanked the neomycin gene cassette with frt sites to allow excision of the neomycin cassette by flp recombinase. Finally, we placed a diphtheria toxin gene adjacent to the short arm of the vector for negative selection. Mutant mice were generated by homologous recombination experiments with R1 embryonic stem cells and genotyped as described in SI Materials and Methods.
Neuronal Cultures.
Neurons were cultured from the cortex of newborn wild-type of various KO mice as described in refs. 25 and 27. Briefly, neurons were dissociated by papain digestion, plated on polylysine-coated glass coverslips, and cultured in modified Eagle's medium (Invitrogen) supplemented with B27 (Invitrogen), glucose, transferrin, FBS, and Ara-C (Sigma).
Designing Bassoon shRNAs.
To construct bassoon shRNA lentivirus, we designed oligos targeting five bassoon mRNA sequences and cloned them into an shRNA-expressing lentivirus (L307) (25). Among five constructs tested, one produced a knockdown of the bassoon mRNA of >75%, without causing neuronal cell death (sequence of sense strand: GCCAGAGAACAACTTCTCCAA). Recombinant lentiviruses were produced as described in ref. 25. Neurons cultured in 24-well at high density were infected at DIV2 and DIV8 (250 μL of conditioned cell medium per well). All experiments were performed after ensuring nearly 100% infection of neurons as estimated by GFP expression. shRNA effectiveness was measured using quantitative real-time PCR (Applied Biosystems), with GAPDH and β-actin as controls. To estimate protein levels, Triton-X insoluble fractions from infected cultured neurons were probed for bassoon and RIM1.
Electron Microscopy.
Cortical neurons cultured on coverslips and infected with lentiviruses as described above were fixed for 30 min at 4 °C in 2% glutaraldehyde buffered with 0.1 M Na-phosphate (pH 7.4). Coverslips were rinsed twice in buffer and incubated for 30 min at room temperature in 0.5% OsO4. After rinsing with distilled water, coverslips were stained en bloc with 2% aqueous uranyl acetate for 15 min, dehydrated in ethanol, and embedded in poly/bed 812 epoxy resin (Polysciences) for 24 h. Sections (60 nm) were poststained with uranyl acetate and lead citrate, and viewed with an FEI Tecnai transmission electron microscope at 120 kV of accelerating voltage. Digital images were acquired with an Olympus SIS Morada CCD camera. Random anonymized images were quantified using Metamorph software (Molecular Devices) as described in ref. 22.
Immunocytochemistry.
Neurons attached to glass coverslips were analyzed by double immunofluorescence labeling using Alexa 546- and Alexa 633-conjugated secondary antibodies as described in ref. 35.
Protein Quantitation.
Primary neuronal cultures (DIV14-16) were either directly solubilized in boiling sample buffer or harvested in Triton X-100, and the Triton-insoluble fraction was obtained by centrifugation and resuspended in sample buffer. For comparisons, cultures from littermate mice were used. Proteins were analyzed by SDS/PAGE and quantitative immunoblotting by using 125I-labeled secondary antibodies and PhosphorImager (Molecular Dynamics) detection with MAP2 as internal standards (24).
Electrophysiology.
To monitor synaptic responses in cultured hippocampal neurons, whole-cell patch-clamp recordings were obtained with neurons at DIV14-16 as described in ref. 27. For hippocampal slice physiology, acute slices from young adult mice were analyzed as described in ref. 19.
Supplementary Material
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Fellowship GE1042 (to S.H.G.) and a grant from the NIDA (P.E.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1002307107/DCSupplemental.
References
- 1.tom Dieck S, et al. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. J Cell Biol. 1998;142:499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenster SD, et al. Piccolo, a presynaptic zinc finger protein structurally related to bassoon. Neuron. 2000;25:203–214. doi: 10.1016/s0896-6273(00)80883-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, et al. Aczonin, a 550-kD putative scaffolding protein of presynaptic active zones, shares homology regions with Rim and Bassoon and binds profilin. J Cell Biol. 1999;147:151–162. doi: 10.1083/jcb.147.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin GM, et al. Comparative genomics of the eukaryotes. Science. 2000;287:2204–2215. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujimoto K, et al. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.Rim2. Piccolo complex in cAMP-dependent exocytosis. J Biol Chem. 2002;277:50497–50502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- 6.Inoue E, et al. ELKS, a protein structurally related to the active zone protein CAST, is involved in Ca2+-dependent exocytosis from PC12 cells. Genes Cells. 2006;11:659–672. doi: 10.1111/j.1365-2443.2006.00970.x. [DOI] [PubMed] [Google Scholar]
- 7.Juranek J, et al. Differential expression of active zone proteins in neuromuscular junctions suggests functional diversification. Eur J Neurosci. 2006;24:3043–3052. doi: 10.1111/j.1460-9568.2006.05183.x. [DOI] [PubMed] [Google Scholar]
- 8.Gerber SH, Garcia J, Rizo J, Südhof TC. An unusual C(2)-domain in the active-zone protein piccolo: implications for Ca(2+) regulation of neurotransmitter release. EMBO J. 2001;20:1605–1619. doi: 10.1093/emboj/20.7.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia J, Gerber SH, Sugita S, Südhof TC, Rizo J. A conformational switch in the Piccolo C2A domain regulated by alternative splicing. Nat Struct Mol Biol. 2004;11:45–53. doi: 10.1038/nsmb707. [DOI] [PubMed] [Google Scholar]
- 10.Zhai RG, et al. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 11.Shapira M, et al. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 2003;38:237–252. doi: 10.1016/s0896-6273(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 12.Dresbach T, et al. Assembly of active zone precursor vesicles: obligatory trafficking of presynaptic cytomatrix proteins Bassoon and Piccolo via a trans-Golgi compartment. J Biol Chem. 2006;281:6038–6047. doi: 10.1074/jbc.M508784200. [DOI] [PubMed] [Google Scholar]
- 13.Takao-Rikitsu E, et al. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. J Cell Biol. 2004;164:301–311. doi: 10.1083/jcb.200307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohara-Imaizumi M, et al. ELKS, a protein structurally related to the active zone-associated protein CAST, is expressed in pancreatic beta cells and functions in insulin exocytosis: interaction of ELKS with exocytotic machinery analyzed by total internal reflection fluorescence microscopy. Mol Biol Cell. 2005;16:3289–3300. doi: 10.1091/mbc.E04-09-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jose M, et al. Investigating interactions mediated by the presynaptic protein bassoon in living cells by Foerster's resonance energy transfer and fluorescence lifetime imaging microscopy. Biophys J. 2008;94:1483–1496. doi: 10.1529/biophysj.107.111674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fejtova A, et al. Dynein light chain regulates axonal trafficking and synaptic levels of Bassoon. J Cell Biol. 2009;185:341–355. doi: 10.1083/jcb.200807155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, et al. The GIT family of proteins forms multimers and associates with the presynaptic cytomatrix protein Piccolo. J Biol Chem. 2003;278:6291–6300. doi: 10.1074/jbc.M212287200. [DOI] [PubMed] [Google Scholar]
- 18.Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem. 2004;279:7956–7961. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- 19.Schoch S, et al. Redundant functions of RIM1α and RIM2α in Ca2+-Triggered Neurotransmitter Release. EMBO J. 2006;25:5852–5863. doi: 10.1038/sj.emboj.7601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altrock WD, et al. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 2003;37:787–800. doi: 10.1016/s0896-6273(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 21.Leal-Ortiz S, et al. Piccolo modulation of Synapsin1a dynamics regulates synaptic vesicle exocytosis. J Cell Biol. 2008;181:831–846. doi: 10.1083/jcb.200711167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber SH, et al. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science. 2008;321:1507–1510. doi: 10.1126/science.1163174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosahl TW, et al. Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell. 1993;75:661–670. doi: 10.1016/0092-8674(93)90487-b. [DOI] [PubMed] [Google Scholar]
- 24.Rosahl TW, et al. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- 25.Maximov A, Tang J, Yang X, Pang ZP, Südhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 27.Maximov A, Pang ZP, Tervo DG, Südhof TC. Monitoring synaptic transmission in primary neuronal cultures using local extracellular stimulation. J Neurosci Methods. 2007;161:75–87. doi: 10.1016/j.jneumeth.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 29.Cases-Langhoff C, et al. Piccolo, a novel 420 kDa protein associated with the presynaptic cytomatrix. Eur J Cell Biol. 1996;69:214–223. [PubMed] [Google Scholar]
- 30.Dick O, et al. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 31.Brandstatter JH, Shaw SR, Meinertzhagen IA. Invagination of presynaptic ribbons in the fly's optic lobe following loss of their target neuron. Proc Biol Sci. 1991;245:13–22. doi: 10.1098/rspb.1991.0082. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz F, et al. CSPalpha-deficiency causes massive and rapid photoreceptor degeneration. Proc Natl Acad Sci USA. 2006;103:2926–2931. doi: 10.1073/pnas.0510060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossman GH, Pauer GJ, Narendra U, Peachey NS, Hagstrom SA. Early synaptic defects in Tulp1−/− mice. Invest Ophthalmol Vis Sci. 2009;50:3074–3083. doi: 10.1167/iovs.08-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reim K, et al. Aberrant function and structure of retinal ribbon synapses in the absence of complexin 3 and complexin 4. J Cell Sci. 2009;122:1352–1361. doi: 10.1242/jcs.045401. [DOI] [PubMed] [Google Scholar]
- 35.Han W, et al. N-glycosylation is essential for vesicular targeting of synaptotagmin 1. Neuron. 2004;41:85–99. doi: 10.1016/s0896-6273(03)00820-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.