Abstract
Spermatogonial stem cells (SSCs) undergo self-renewal division to support spermatogenesis. Although several positive regulators of SSC self-renewal have been identified, little is known about the mechanisms that negatively regulate SSCs. Here we developed a novel transplantation assay for SSCs and demonstrate that p21 and p27 cyclin-dependent kinase inhibitors play critical roles in SSC self-renewal and differentiation. Overexpression of p21 or p27 abrogated proliferation of cultured SSCs in vitro, and their expression levels were downregulated by exogenous self-renewal signals. In contrast, no apparent defects were found in p21 or p27-deficient SSCs by spermatogonial transplantation. However, competitive spermatogonial transplantation with WT SSCs revealed that the loss of either gene causes distortion of germline transmission: p21-deficiency facilitated mutant offspring production, whereas germline transmission was limited by p27-deficiency. Serial transplantation also showed that the loss of p27, but not p21, decreases secondary colony formation, suggesting that appropriate amounts of p27 are necessary for sustaining SSC self-renewal. Thus, p21 and p27 cyclin-dependent kinase inhibitors play critical roles in germline transmission by regulating the balance between SSC self-renewal and differentiation, and competitive spermatogonial transplantation technique will be useful for analyzing subtle defects in spermatogenesis that are not evident by traditional spermatogonial transplantation.
Keywords: germline transmission, spermatogenesis, stem cell, cyclin-dependent kinase inhibitor, self-renewal
Spermatogenesis is a dynamic and complex process based on the self-renewal division of spermatogonial stem cells (SSCs). Very few SSCs are found in the testis (0.02–0.03% in a testis cell suspension), but SSCs proliferate throughout life to support spermatogenesis (1, 2). The balance between SSC self-renewal and pool size necessitates strict control, because excessive self-renewal or differentiation can cause male infertility. Although stem cells are relatively cytokine resistant in many tissues, glial cell line-derived neurotrophic factor (GDNF) promotes self-renewal division of SSCs (3). Changes in GDNF levels greatly impact on spermatogenesis in vivo: Excessive GDNF expression induces the accumulation of undifferentiated spermatogonia, whereas decreases in GDNF cause hypospermatogenesis and the gradual loss of SSCs (3). Thus, the regulation of SSC self-renewal is governed by a subtle balance between negative regulatory pathways that maintain mitotic quiescence and positive growth-promoting signals involving GDNF. Although we recently discovered that the Ras-cyclin D2 pathway acts downstream of GDNF to promote SSC self-renewal (4), little is known about how the balance between self-renewal and differentiation is maintained in vivo.
Cyclin-dependent kinase inhibitors (CDKIs) are good candidates for the negative regulation of SSC proliferation. Two families of CDKIs promote cell cycle withdrawal by blocking the activity of cyclin/CDK complexes: the Cip/Kip family, including p21, p27, and p57, and the INK4 family, including p15, p16, p18, and p19 (5). Whereas INK4 proteins bind to CDK4 or CDK6 and inhibit their activity, Cip/Kip proteins show a broader range of activities and interact with all cyclin/CDK complexes. Cip/Kip proteins are distinct from the INK4 family in that they also stimulate the formation of cyclin D/CDK4/6 complexes to promote proliferation (6). Importantly, Cip/Kip proteins have additional functions beyond regulating cell divisions and regulate differentiation. For example, enforced expression of p21 or p27 induces the differentiation of neuroblastoma cells and myelomonocytic leukemia cells (6). Although KO mice have been produced for Cip/Kip family genes, no apparent SSC phenotype has been reported, and the effects of these genes remain unclear (7, 8).
Because stem cells comprise only a small population and are defined retrospectively through the analysis of daughter cells, studies to analyze SSCs have been hampered by difficulties in distinguishing SSCs from committed spermatogonia. In 1994, however, a spermatogonial transplantation technique was developed (9). With this technique, SSCs can be detected by their ability to generate germ cell colonies after microinjection into seminiferous tubules of infertile recipient animals. Eventually, recipient animals can produce donor-derived offspring by mating with WT females. Because differentiated progenitor cells do not have self-renewal capacity, only SSCs can produce these results. Although this technique has been used extensively to study SSCs, the degree of SSC self-renewal and differentiation is difficult to evaluate by morphological analyses of germ cell colonies. Moreover, measurements of SSC numbers do not necessarily correlate with long-term functional capacities. In fact, declining fertility is reported after serial SSC transplantation, which raised question about whether transplanted SSCs are fully competitive as WT SSCs before transplantation (10).
In the present study, we report a unique SSC transplantation assay, in which stem cell function of a mutant donor is assayed by mixing with WT SSCs. Although spermatogonial transplantation failed to reveal abnormalities in p21 and p27 KO SSCs, p21 and p27 deficiencies have contrasting effects on germline transmission efficiency when they were mixed with WT SSCs. Moreover, serial transplantation showed decreased secondary colony production from p27 KO SSCs. This strategy of SSC analysis will be useful for comparing long-term SSC function and is capable of detecting subtle defects that escape detection by conventional transplantation technique.
Results
Effects of p21 and p27 CDKIs on Cultured SSCs.
To identify molecules that negatively regulate SSC proliferation, we used germline stem (GS) cells, cultured SSCs (11). The addition of GDNF and EGF and/or basic fibroblast growth factor (bFGF) induces SSC proliferation in vitro. Approximately 1–2% of the GS cells have SSC activity (4). GS cells from WT mice were infected with lentivirus vectors expressing candidate genes and Venus fluorescent marker gene under the control of an elongation factor2 promoter. Using this strategy, we found that overepression of p21 or p27 CDKIs compromises GS cell proliferation. When the transfected cells were analyzed 3 d after infection, significantly smaller numbers of Venus+ cells were found after CDKI overexpression in all six separate experiments (Fig. S1 A and B). In particular, very few germ cell clumps showed fluorescence after p21 overexpression, suggesting that the transfected cells proliferated more slowly than WT cells or died shortly after transfection. Some clumps, however, remained after p27 overexpression at 21 d, but significantly fewer clumps were observed as compared to controls (Fig. S1C). In contrast, cells transduced with an empty vector showed strong fluorescence and the cells could be passaged every 5–6 d. Flow cytometric analysis at 10 d postinfection also confirmed the reduction in Venus+ cells after CDKI overexpression. Cell cycle analyses also showed the growth suppression due to p21 overexpression (Fig. S1D).
We then examined whether p21 or p27 expression is influenced by exogenous cytokines. Real-time PCR analysis showed a significant reduction in p21 and p27 expression due to cytokine stimulation (Fig. S1E). The combination of EGF and bFGF showed a comparable effect as GDNF, and the addition of all cytokines suppressed the p21 and p27 levels to 25.8% and 19.0%, respectively. These in vitro results indicated that p21 and p27 CDKI levels are regulated by exogenous cytokines and also suggested that both genes may negatively regulate SSC proliferation in vivo.
SSC Activity of p21 and p27 KO Testis Cells After Spermatogonial Transplantation.
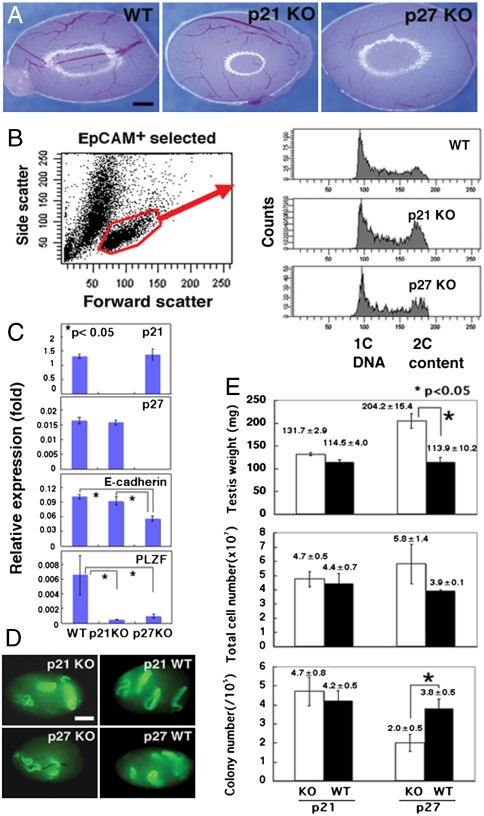
To examine the effects of p21 and p27 genes in vivo, we analyzed testes of p21 and p27 KO mice. Both mutant mice survived to birth and matured into adults. Whereas p21 KO mice appeared normal, p27 KO mice exhibited multiple organ hyperplasia and females were infertile due to poor ovarian follicle development (7, 8). In contrast, both p21 and p27 KO males produced spermatozoa and were fertile, suggesting normal spermatogenesis. However, whereas testes from p21 KO mice were comparable to those of WT mice, testes from p27 KO mice were significantly larger (Fig. 1A). Although flow cytometry showed normal size of p21 and p27 KO spermatogonia, cell cycle analyses revealed that EpCAM+ spermatogonia from both mutants proliferated more actively than those of WT mice, and significantly more cells were found in the G2/M phase (Fig. 1B). However, whereas real-time PCR analyses showed no compensatory upregulation of p21 or p27 in either type of KO mice, expression of E-cadherin or promyelocytic leukemia zinc finger (PLZF), markers for undifferentiated spermatogonia (12–14), was significantly reduced (Fig. 1C), suggesting that undifferentiated spermatogonia in these mice comprise relatively smaller population.
Fig. 1.
Phenotypic and functional analyses of p21 or p27 KO testis cells. (A) Appearance of mutant testes. Note the larger size of the p27 KO testis. (B) Cell cycle analysis of EpCAM+ spermatogonia. Note the enhanced proliferation of p21 and p27 KO cells. WT mice were used as a control. (C) Real-time PCR analyses of p21, p27, E-cadherin, and PLZF expression. Expression of E-cadherin or PLZF was significantly downregulated in the mutant mice. (D) Macroscopic appearance of the recipient testes. Green fluorescence indicates donor cell colonization. (E) Testis weight (Upper, n = 3), total cell number after enzymatic digestion (Middle, n = 3), and colony number (Lower, n = 15–17). Asterisks denote significant differences compared to the control (P < 0.01). Bar = 1 mm (A and D).
To study the function of SSCs, we used spermatogonial transplantation (Fig. 1D). Donor testis cells were marked by mating the KO animals with a transgenic mouse line C57BL6/Tg14(act-EGFP-OsbY01) (green mice) that ubiquitously expresses enhanced green fluorescent protein (EGFP). Preliminary transplantation of mutant cells into nonablated WT testis did not result in colonization, thus suggesting that mutant SSCs do not have an enhanced ability to compete for the niche that is occupied by WT SSCs. We then transplanted the mutant cells into empty tubules to quantify SSC number. In three separate experiments, the same number of mutant and WT testis cells were transplanted into adult WBB6F1-W/Wv(W) mice that were deficient for spermatogenesis due to c-kit gene defects (15). Two months after transplantation, the recipient mice were sacrificed and their testes were analyzed for colonization under UV light.
Both p21 and p27 KO donor cells produced germ cell colonies that were similar to those of WT mice. We found no apparent abnormalities in the length and the morphology of the colonies, which suggested normal proliferation and differentiation of transplanted SSCs (Fig. 1D). The p21 KO cells and WT cells produced 4.7 ± 0.8 and 4.2 ± 0.5 colonies of donor-derived spermatogenesis/105 cells injected, respectively, and this difference was not statistically significant (Fig. 1E). In contrast, the p27 KO cells produced significantly smaller numbers of colonies as compared to WT cells, and the number of colonies generated was 2.0 ± 0.5 and 3.8 ± 0.5/105 cells injected for p27 KO cells and WT cells, respectively. Because each colony was derived from a single transplanted SSC (16, 17), these results indicated that the SSC concentration in p27 KO mice was significantly lower than that of WT mice. Although the total number of SSCs per testis (cell recovery × concentration of SSCs in the injected cell suspension determined by transplantation) was increased for the WT background (1482 vs. 1160 for WT and p27 KO cells), the difference was not significant. These results show that both p21 and p27 KO testis contain similar numbers of SSCs.
Transmission Distortion by Competitive Spermatogonial Transplantation.
Although these transplantation experiments showed no apparent SSC abnormalities, we speculated that subtle imbalances in SSC self-renewal and differentiation might not be evident in a morphological analysis of germ cell colonies using a simple transplantation assay. To overcome this problem, we cotransplanted WT and mutant cells into the same recipient, and analyzed cell differentiation potential by confirming the genotype of the offspring (Fig. 2A). We collected single cell suspensions from p21 or p27 KO pup testes that were enriched for SSCs due to the absence of differentiated cells (18). Testis cells from each mutant mouse were mixed at a 1∶1 ratio with those from control WT mice of the same age. The mixed testis cells were then transplanted into 5–10 day old W recipient pups to produce offspring from the transplanted SSCs. Previously, we showed that immature recipients are superior to adult recipients in restoring fertility due to the enhanced SSC colonization (18). Recipient males were housed with two or three WT B6 females, at least 4 weeks after transplantation. Two separate experiments were performed for p21 KO mice, whereas three experiments were carried out for p27 KO mice. About 3 × 105 cells were transplanted into each recipient testis.
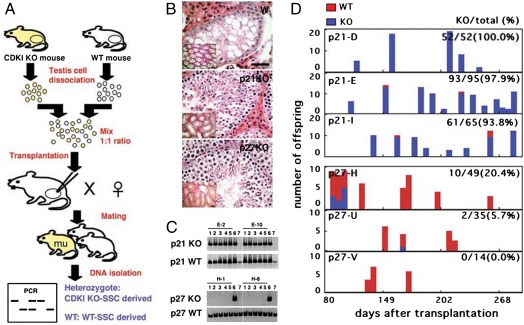
Fig. 2.
Competitive spermatogonial transplantation. (A) Experimental procedure. Two populations of testis cells, one from a WT mouse and the other from a KO mouse, were mixed at a 1∶1 ratio and transplanted into W mice to produce offspring. Tail DNA of the F1 offspring were analyzed by PCR for genotyping. (B) Normal appearing spermatogenesis in the recipient testes. (C) Genotyping by PCR. (Upper) Offspring from recipient E, which were transplanted with a mixture of p21 KO and WT testis cells. Analyses of the second and tenth littermates are shown (Lanes 1–5). Lane 6: p21 heterozygous; lane 7: WT control tails. All F1-derived offspring showed p21 mutant bands. (Lower) Offspring from recipient H, which were transplanted with a mixture of p27 KO and WT testis cells. Analyses of the second and tenth littermates are shown (Lanes 1–5). Lane 6: p21 heterozygous ; lane 7: WT control tails. None of the offspring showed p27 mutant bands. (D) Temporal analysis of offspring. Bar = 50 μm, (B); 100 μm (B, Inset).
Within 3 months after transplantation, 3/8 and 3/9 recipient males that received p21 or p27 KO SSCs, respectively, became fertile (Table S1). Histological analyses of both types of recipients confirmed normal spermatogenesis (Fig. 2B). When the offspring genotypes were confirmed by genomic PCR using tail DNA, transmission rate distortion of the donor haplotype was noted in both experiments (Fig. 2 C and D). For p21 experiments, 212 offspring were produced from the three recipients, and the animals were maintained as long as 304 d after transplantation. PCR analysis revealed that 206/212 offspring contained the neo gene. All of these offspring were heterozygous for the p21 gene, because the recipient males were mated with WT females. The percentage of these heterozygous males was 97.2 ± 1.8% from each recipient. In contrast, for p27 experiments, a total of 98 offspring were produced during 205 d from the three recipients that received p27 KO testis cell transplantation. WT offspring, however, were predominantly produced from the recipients, and only 12/98 offspring were heterozygous for the p27 gene. Although the overall percentage of heterozygous offspring was 12.2%, one of the recipient males produced as many as 10 heterozygous offspring. Moreover, these offspring were born within 98 d after transplantation, and no heterozygous offspring were produced from this male up to 195 d.
Impact of p21 and p27 Deficiencies in Self-Renewal Divisions of SSCs.
To directly examine the impact of p21- and p27-deficiency on SSC self-renewal, we performed serial transplantation (Fig. 3A). In normal testes, SSCs are kept under constant pressure to differentiate and produce sperm. The most generally accepted hypothesis is that SSCs undergo only two types of cell division: They produce either two stem cells (self-renewal division) or two progenitor cells (differentiating division) (1). Each division occurs at about the same frequency. After transplantation, however, SSCs are thought to undergo self-renewal divisions more frequently than differentiation divisions, and thus increase their numbers (19). Because the number of colonies in the recipient testis indicates the number of SSCs that initially colonized the testis, the number of SSCs that were produced by subsequent divisions may be determined by transplantation into another testis. We collected testis cells from WT and mutant donors that contained the EGFP transgene. After dissecting out colonies in each recipient testis at 10 weeks posttransplantation, the tubules were dissociated into single cells and suspended in 15–21 μl of injection medium. The number of cells recovered from the three types of recipients ranged from 0.4 - 3.0 × 106 cells, with an average of 1.6 × 106 cells. Differences among donors were not significant. Approximately 4 μl of the cell suspension was microinjected into three secondary recipient testes.
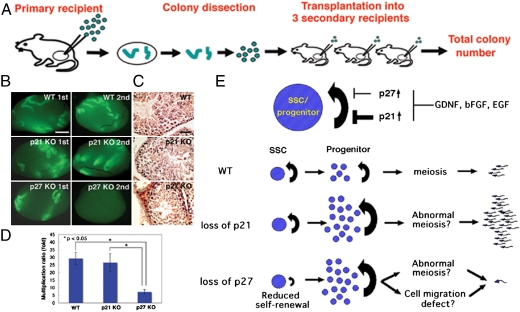
Fig. 3.
Serial transplantation. (A) Experimental procedure. Colonies in the primary recipients were dissected out at 10 weeks after transplantation, and a portion of them was transplanted into the three testes of the secondary recipients. (B, C) Macroscopic (B) and histological (C) appearance of the recipient testes. Green fluorescence indicates donor cell colonization. Note the decrease in secondary colony numbers from p27 KO testis cells. (D) The degree of secondary colony formation, indicated by the ratio of SSC number between the two time points. Asterisks denote significant differences compared to the control (P < 0.01). (E) Summary of results and models for transmission distortion of p21 and p27 SSCs. (Upper) Upregulated p21 or p27 inhibits SSC/progenitor cell proliferation. Exogenous cytokines downregulate p21 and p27 expression. (Lower) Loss of p21 does not influence SSC self-renewal, but likely enhances progenitor cell proliferation. In contrast, loss of p27 decreased SSC self-renewal but may enhance progenitor cell proliferation. However, these progenitor cells have defects in meiosis and/or cell migration, both of which may have caused decreased spermatogenesis efficiency. Bar = 1 mm (B); 100 μm (C).
Analysis of the secondary recipient testes at 2 months after transplantation revealed that significantly fewer SSCs were produced from the p27 KO donor testis cells (Fig. 3 B and C). Although 13/14 transplantations showed colony number increases (total regenerated colony number—primary colony number used for transplantation) in experiments using WT and p21 KO mice, only 5/14 transplantations showed increase in experiments using p27 KO mice, indicating that the SSCs in p27 KO mice produced smaller numbers of secondary colonies. Assuming that 10% of the SSCs can colonize (19), and that each colony is produced by one SSC (16, 17), the multiplication of colony numbers (total regenerated colony number × 10/primary colony number used for serial transplantation) were 7.2 ± 1.6 (n = 14) and 29.3 ± 4.0 (n = 14) for p27 KO cells and WT cells, respectively (Table S2). The difference between p27 KO and WT cells was significant. In contrast, p21 KO cells produced comparable numbers of secondary colonies with WT cells, and the average number of colonies per primary colony was 26.6 ± 5.9 (n = 14). No significant difference was observed in the self-renewal capacity of SSCs between p21 KO and WT cells. The doubling times of the SSCs during the 10-week period were 14.4, 14.8, and 24.6 d for WT, p21 KO, and p27 KO SSCs, respectively (Fig. 3D).
Discussion
Spermatogonial transplantation technique provided the first functional assay for SSCs. It has been used to detect SSCs and determine whether abnormal spermatogenesis is caused by germ cell defects or their respective microenvironments. This technique is also useful for assessing SSC numbers quantitatively. For example, when a mixture of two testis cell populations were transplanted at a 1∶1 ratio, recipient testes contained a near 1∶1 ratio of colonies with each of the two genotypes (16). However, spermatogenesis is not completely normal after transplantation, and multiple abnormalities that are associated with transplantation, such as increased apoptosis or missing layers of germ cells (20), raise questions about the efficiency and quality of spermatogenesis after transplantation. In this study, we developed a competitive spermatogonial transplantation technique to provide a selective pressure to identify SSCs with high capacity for competitive long-term repopulation. In its concept and design, the technique is somewhat similar to competitive repopulation technique in hematopoietic stem cells (HSCs) (21). Forced competition against WT SSCs allowed direct functional comparisons between the two donors in a quantitative manner under consistent microenvironmental stimuli. Using this method, growth factors, nutrient conditions, and systemic environments such as hormonal levels are provided equally for both donors in the same recipient animal. This was particularly important in this study, because abnormalities are reported for both Sertoli and Leydig cells in p27 KO mice (22, 23). Furthermore, although periodic sperm sampling from the same individual is difficult in mice, this technique allows for the monitoring donor cell dynamics over a long period. Thus, competitive spermatogonial transplantation will be useful for functional analyses of spermatogenesis.
Using this technique, we found transmission distortion of mutant SSCs with contrasting results: p21-deficiency facilitated the production of mutant offspring, whereas it was severely limited by p27-deficiency. In contrast, p21 and p27 overexpression inhibited GS cell proliferation. These results suggested that p21 and p27 levels are important in maintaining normal proliferation of SSCs and/or progenitor cells. In fact, studies of other self-renewing tissues also suggested the involvement of CDKIs in regulating the stem cell quiescence and pool size. In general, p21 is thought to act on quiescent stem cells, whereas p27 is a progenitor-specific inhibitor for repopulation efficiency (24). During hematopoiesis, for example, whereas p21 governs cell cycle entry of HSCs, p27 does not affect HSC number, cell cycling, or self-renewal but has an impact on the cell cycle of progenitors (24). Likewise, p21-deficiency influences the number and proliferation of neural stem cells (NSCs) (25). Although mutant animals initially have increased numbers of NSCs due to excessive proliferation, the levels decrease as they age due to exhaustion.
Recent studies using male germ cells also suggest that p21 plays an important role in SSCs: Atm-deficient undifferentiated spermatogonia upregulates p21, which is responsible for cell cycle arrest upon DNA damage (26). Suppression of p21 can partially restore SSC activity in Atm KO mice, but its normal function remains unclear. In this study, we showed that p21 levels are regulated by exogenous cytokines and that ectopic overexpression of p21 leads to growth inhibition. In contrast, p21-deficiency does not alter SSC number or self-renewal activities, which suggested that transmission distortion was caused by defects in more differentiated cells. On the other hand, reduced PLZF expression suggested smaller size of undifferentiated spermatogonia population, and these conflicting observations make it difficult to explain why germline transmission occurred predominantly from p21 KO cells. Nevertheless, given the increased mitotic activity of whole spermatogonia population, we speculate that more differentiated type of spermatogonia, such as type A1–4 or B spermatogonia, are proliferating more actively and caused transmission distortion by increasing the population size or differentiating faster. Alternatively, it may result from an advantage of p21 KO germ cells to progress through meiosis; p21 is most strongly expressed in pachytene spermatocytes and spermatids in normal testis.
On the other hand, p27-deficiency had a direct effect on SSC. We initially assumed that p27-deficiency would not influence germline transmission, because Sertoli cells have been hypothesized as being responsible for the large testis phenotype of p27 KO mice: p27 has been detected only in Sertoli cells and germ cells were thought to be normal (27). However, offspring were rarely produced from p27 KO cells after competitive transplantation, which suggested defects in germ cells. Although the possibility of Sertoli cell colonization cannot be totally excluded, another study also showed the important role of p27 in germ cells: mice deficient in Skp2, which mediates ubiquitin-dependent degradation of p27, exhibited a progressive loss in spermatogenic cells (28). Furthermore, disruption of p27 in these mice restored fertility, suggesting that testicular hypoplasia of Skp2 mutant mice is attributable to the antiproliferative effects of p27 accumulation. Therefore, although p27 has not been detected at protein levels in the germline, these results suggest that germ cells also contribute to the large testis phenotype of p27 KO mice, and that it probably has an important influence on the fate decision of SSCs.
Although p27 KO mice show a comparable number of SSCs per testis, our serial transplantation experiments showed reduced secondary colony formation from p27 KO SSCs. It is possible that loss of p27 might have accelerated senescence/differentiation. However, because p27 KO mice remain fertile for long-term and produce significantly more sperm than WT mice (23), we rather speculate that decreases in p27 levels in SSCs may enhance the production of progenitor cells by increasing the relative frequency of differentiating divisions. On the other hand, we also showed using GS cells that p27 overexpression compromises proliferation and that increase in self-renewal factors decreases p27 mRNA levels. Besides transcriptional regulation, p27 is also regulated by protein degradation (29). We recently found that self-renewal signals promote the export of p27 from the GS cell nucleus (4). Therefore, appropriate levels of p27, as well as its cellular location, are required for undergoing self-renewal divisions, and this appears to be regulated in a sophisticated manner by changes in the local self-renewal factor levels in the seminiferous tubules. Disturbance in this regulation may cause abnormalities in SSC self-renewal.
Besides regulating mitosis, p27 is involved in meiosis. Testes from p27 KO mice have a significant number of abnormal leptotene spermatocytes that cannot enter the meiotic prophase (27). Strikingly, some of these spermatocytes attempted to carry out mitotic divisions instead of entering into prophase. Furthermore, p27 KO cells may have impaired migratory activity: p27 binds to RhoA and inhibits its activation by interfering with the interaction between RhoA and its activators (30). Impaired migratory activity can interfere with several steps of spermatogenesis, such as the migration of preleptotene spermatocytes through the blood–testis barrier. Collectively, these factors may have decreased the efficiency of spermatogenesis and caused transmission distortion despite the expanded pool of progenitor cells (Fig. 3E).
Our study revealed a critical role for p21 and p27 CDKIs in regulating germline transmission from SSCs. Although the small population size of SSCs makes it difficult to study their dynamics, competitive spermatogonial transplantation techniques have proved to be more sensitive in detecting subtle abnormalities in spermatogenesis in a quantitative manner. Moreover, serial transplantation techniques were instrumental in analyzing effects on SSC self-renewal. This strategy of analyzing the dynamics of SSCs in vivo can be extended to different mouse mutants, and further analysis of CDKIs will help to determine how self-renewal and differentiation are differentially regulated in SSCs.
Materials and Methods
Animals and Transplantation.
The p21 KO mice were purchased from the Jackson laboratory, and p27 KO mice were kindly provided by K. Nakayama (Kyushu University). Both strains of mice were maintained on a B6 background. In some experiments, we also used a Green mouse that expressed the EGFP gene ubiquitously to mark the donor cells (M. Okabe, Osaka University). For spermatogonial transplantation to quantify SSC number, testis cells were collected from 4–6 week old mice. Donor cells were collected by two-step enzymatic digestion using collagenase and trypsin (both from Sigma). Dissociated cells were introduced into the seminiferous tubules of pup (5–10 d old) or adult (4–6 weeks old) W mice (Japan SLC) via an efferent duct. To produce offspring, testis cells were obtained from 5–10 d old mice. The Institutional Animal Care and Use Committee of Kyoto University approved all of the animal experimentation protocols.
Cell Culture and Transfection.
GS cells were cultured on mouse embryonic fibroblasts (MEFs) as described previously using StemPro-34 SFM (Invitrogen) (11). For real-time PCR, the cells were cultured on laminin (20 μg/mL; BD Biosciences) to avoid contamination of MEF-derived RNA. For lentivirus transduction, mouse cDNAs encoding p21 or p27 (both from Addgene) were cloned into CSII-EF-IRES-Venus, and virus particles were produced by transient transfection of 293T cells, as described previously (4). All infections were conducted on MEFs by centrifuging at 3000 × g for 1 h at 32 °C in the presence of 10 μg/mL polybrene (Sigma). GS cells were infected overnight at a multiplicity of infection of 35 for overnight (4 × 104 cells/cm2 in 6-well plate).
PCR.
Total RNA was isolated using Trizol reagent, and first strand cDNA was synthesized using Superscript™ II (both from Invitrogen). Real-time PCR was performed using the StepOnePlus™ Real-Time PCR system and Power SYBR Green PCR Master Mix (Applied Biosystems) with the PCR primers listed in Table S3. All transcript levels were normalized to those of Hprt1. The PCR conditions were 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, and 60 °C for 1 min. Experiments were performed on at least two independent samples, and each PCR was run in triplicate. Genotypes of p21 and p27 KO mice were determined using the PCR primers listed in Table S4.
Analysis of the Recipient Testes.
In experiments using Green mice, SSC colonization was determined by observation of fluorescence under UV light. Colonies were defined as germ cell clusters longer than 0.1 mm occupying the entire circumference of the tubule. For the morphological examination, testes were processed for paraffin sectioning and counterstained with hematoxylin and eosin.
Cell Cycle Analysis.
Testis cells were selected by rat antimouse EpCAM antibody (G8.8; BD Bioscience) using procedures as previously described (26). EpCAM+ cells were then incubated with Hoechst 33342 (Sigma) at 12.5 μg/mL for 45 min at 37 °C and suspended in phosphate-buffered saline/1% fetal calf serum containing 1 μg/mL propidium iodide (PI; Sigma). Cells were analyzed on a FACSAria2 equipped with a 375-nm UV laser (BD Biosciences). Dead cells and nonspermatogonial cells were gated out by high PI staining and forward scatter.
Statistical Analysis.
Results are presented as the mean ± SEM. Data were analyzed using a Student’s t test. Significant differences in the CDKI expression and serial transplantation were determined by a Tukey’s honestly significant differences multiple comparisons test.
Supplementary Material
Acknowledgments.
We thank Ms. Y. Ogata for technical assistance. This research was supported by the Genome Network Project; Japan Science and Technology Agency; Program for Promotion of Basic and Applied Researches for Innovation in Biooriented Industry; the Ministry of Health, Labour, and Welfare; Senri Life Science Foundation; Novartis Foundation (Japan) for the Promotion of Science; Astellas Foundation for Research on Metabolic Disorders; and the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914448107/DCSupplemental.
References
- 1.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- 2.Meistrich ML, van Beek MEAB. In: Cell and Molecular Biology of the Testis. Desjardins C, Ewing LL, editors. New York: Oxford Univ Press; 1993. pp. 266–295. [Google Scholar]
- 3.Meng X, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, et al. Genetic reconstruction of mouse spermatogonial stem cell self-renewal in vitro by Ras-Cyclin D2 Activation. Cell Stem Cell. 2009;5:76–86. doi: 10.1016/j.stem.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg RA. In: The Biology of Cancer. Weinberg RA, editor. New York: Garland; 2007. pp. 255–306. [Google Scholar]
- 7.Brugarolas J, et al. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama K, et al. Mice lacking p27 (Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitatry tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 9.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24:1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanatsu-Shinohara M, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 12.Tokuda M, Kadonaga Y, Kurahashi H, Marunouchi T. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol Reprod. 2007;76:130–141. doi: 10.1095/biolreprod.106.053181. [DOI] [PubMed] [Google Scholar]
- 13.Costoya JA, et al. Essentiial role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- 14.Buaas FW, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 15.Geissler EN, Ryan MA, Housman DE. The dominant white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Ebata KT, Nagano MC. Genetic analysis of the clonal origin of regenerating mouse spermatogenesis following transplantation. Biol Reprod. 2003;69:1872–1878. doi: 10.1095/biolreprod.103.019273. [DOI] [PubMed] [Google Scholar]
- 17.Kanatsu-Shinohara M, et al. Clonal origin of germ cell colonies after spermatogonial transplantation in mice. Biol Reprod. 2006;75:68–74. doi: 10.1095/biolreprod.106.051193. [DOI] [PubMed] [Google Scholar]
- 18.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc Natl Acad Sci USA. 2001;98:6186–6191. doi: 10.1073/pnas.111158198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagano M, Avarbock MR, Brinster RL. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod. 1999;60:1429–1436. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parreira GG, et al. Development of germ cell transplants in mice. Biol Reprod. 1998;59:1360–1370. doi: 10.1095/biolreprod59.6.1360. [DOI] [PubMed] [Google Scholar]
- 21.Harison DE. Competitive repopulation: A new assay for long-term stem cell functional capacity. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 22.Lin H, et al. Increased proliferation but decreased steroidogenic capacity in Leydig cells from mice lacking cyclin-dependent kinase inhibitor 1B. Biol Reprod. 2009;80:1232–1238. doi: 10.1095/biolreprod.108.074229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holsberger DR, et al. Cell-cycle inhibitors p27Kip1 and p21Cip regulate murine Sertoli cell proliferation. Biol Reprod. 2005;72:1429–1436. doi: 10.1095/biolreprod.105.040386. [DOI] [PubMed] [Google Scholar]
- 24.Cheng T. Cell cycle inhibitors in normal and tumor stem cells. Oncogene. 2004;23:7256–7266. doi: 10.1038/sj.onc.1207945. [DOI] [PubMed] [Google Scholar]
- 25.Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takubo K, et al. Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell. 2008;2:170–182. doi: 10.1016/j.stem.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Beumer TL, et al. Regulatory role of p27kip1 in the mouse and human testis. Endocrinoloogy. 1999;140:1834–1840. doi: 10.1210/endo.140.4.6638. [DOI] [PubMed] [Google Scholar]
- 28.Fotovati A, Nakayama K, Nakayama KI. Impaired germ cell development due to compromised cell cycle progression in Skp2-deficient mice. Cell Div. 2006;1:4. doi: 10.1186/1747-1028-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Susaki E, Nakayama KI. Multiple mechanisms for p27kip1 translocation and degradation. Cell Cycle. 2007;6:3015–3020. doi: 10.4161/cc.6.24.5087. [DOI] [PubMed] [Google Scholar]
- 30.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.