Abstract
In the developing central nervous system, the cell cycle clock plays a crucial role in determining cell fate specification. A second clock, the circadian oscillator, generates daily rhythms of cell cycle progression. Although these two clocks interact, the mechanisms linking circadian cell cycle progression and cell fate determination are still poorly understood. A convenient system to address this issue is the pineal organ of lower vertebrates, which contains only two neuronal types, photoreceptors and projection neurons. In particular, photoreceptors constitute the core of the pineal circadian system, being able to transduce daily light inputs into the rhythmical production of melatonin. However, the genetic program leading to photoreceptor fate largely remains to be deciphered. Here, we report a previously undescribed function for the homeobox gene Bsx in controlling pineal proliferation and photoreceptor fate in Xenopus. We show that Xenopus Bsx (Xbsx) is expressed rhythmically in postmitotic photoreceptor precursors, reaching a peak during the night, with a cycle that is complementary to the daily rhythms of S-phase entry displayed by pineal cells. Xbsx knockdown results in increased night levels of pineal proliferation, whereas activation of a GR-Xbsx protein flattens the daily rhythms of S-phase entry to the lowest level. Furthermore, evidence is presented that Xbsx is necessary and sufficient to promote a photoreceptor fate. Altogether, these data indicate that Xbsx plays a dual role in contributing to shape the profile of the circadian cell cycle progression and in the specification of pineal photoreceptors, thus acting as a unique link between these two events.
Keywords: homeobox, pineal organ, proliferation, differentiation, circadian
The correct balance between proliferation and differentiation is crucial to ensure the appropriate size of the different areas of the central nervous system and the proportionate generation of a remarkable variety of neuronal and glial cell types. In particular, in the retina and cerebral cortex, cell cycle exit of progenitors is strictly coordinated with cell fate specification following a temporal order that involves different competence stages (1, 2). An additional level of complexity in the control of cell proliferation was recently discovered with the observation that the circadian clock, which regulates metabolic and physiological rhythms, also generates daily rhythms of cell cycle progression. Indeed, several cell cycle regulators, including c-myc, cyclin D1, and Wee-1, are regulated in a circadian manner (3, 4). This results in S-phase entry of many cells at the end of the day or during the night, an evolutionarily conserved phenomenon that has been proposed to represent an adaptation of ancestral unicellular animals to reduce the risk of UV-induced DNA damage (5, 6).
Although significant progress has been made in understanding how the circadian clock and the cell cycle interact, it is still unclear what the molecular links are between the circadian control of cell proliferation and the generation of specific cell types.
A suitable system in which this issue can be addressed is the pineal organ, a dorsal diencephalic structure that plays a central role in the regulation of circadian rhythms. It is now clear, especially from studies on nonmammalian vertebrates, that the pineal organ shares many similarities, and apparently a common evolutionary origin, with the retina (7, 8). Compared with the retina, the pineal organ displays a simpler structure containing only two neuronal types, photoreceptors and projection neurons, which are generated from the same precursor and represent the functional homologues of retinal photoreceptors and ganglion cells, respectively (7, 9, 10). In particular, the photoreceptors constitute the core of the pineal circadian system, being able to transduce daily light inputs into the rhythmical production of melatonin. At the same time, the photoreceptors make contact with the projection neurons that innervate different areas of the brain. Despite the central role played by photoreceptors in pineal physiology, the mechanisms specifying this neuronal type are largely unknown. Studies in zebrafish have shown that the homeodomain transcription factor floating head (flh) and the proneural genes achaete/scute homolog 1a (ascl1a) and neurogenin (ngn) are required for pineal neurogenesis but are not involved in cell fate decision between photoreceptors and projection neurons (11). On the other hand, Notch plays a dual role in determining the pineal cell number and inhibiting the projection neuron fate (10). The lack of an instructive role for Notch in specifying the photoreceptor identity suggests the existence of positive signals that remain to be identified.
Here, we show that the homeobox gene Xenopus Bsx (Xbsx) (12) is expressed in photoreceptor precursors in a cyclical manner with a phase that is complementary to the daily rhythms of S-phase entry displayed by pineal cells. Our functional data indicate that Xbsx acts as a unique link between the rhythmical control of cell cycle progression and the specification of photoreceptors.
Results
Xbsx Demarcates the Early Pineal Territory and Is Expressed in Postmitotic Photoreceptor Precursors.
The expression pattern of Xbsx is closely related to the expression of its mouse homologue (12). In situ hybridization analysis shows that Xbsx transcripts are first detected at midneurula stage (stage 16) in a few cells located bilaterally in the anterior neural plate (Fig. 1A). With the closure of the neural tube, these expression domains converge medially in the dorsal diencephalon at the level of the epiphysial anlage (Fig. 1 B–D). Xbsx expression in the pineal complex persists throughout the analyzed stages. At tailbud stage, Xbsx is also expressed in the hypothalamus and in the ventral telencephalon in an area possibly corresponding to the septum (Fig. 1E). The onset of Xbsx expression in the pineal territory occurs later than that of other homeobox genes such as Xrx1, Xotx5, and Xnot2, thus suggesting that Xbsx is not involved in the earliest events of pineal development.
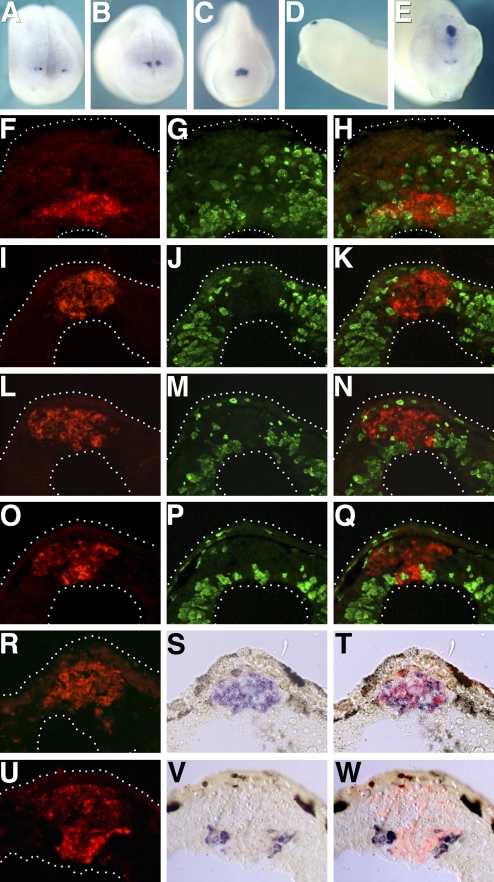
Fig. 1.
Xbsx expression during pineal organ development. Xbsx whole-mount in situ hybridization was performed at stage 16 (A), stage 19 (B), stage 22 (C), stage 32 (D), and stage 38 (E). Immunostaining for BrdU incorporation (G, J, M, and P; green staining), in situ hybridization for Xbsx (F, I, L, and O; red staining), and merge (H, K, N, and Q) were performed on cryostat sections at stage 24 (F–H), stage 32 (I–K), stage 37 (L–N), and stage 40 (O–Q). In situ hybridization for Xbsx (R, red staining), Xotx5 (S, blue staining), and merge (T) was performed on stage 37 cryostat sections. In situ hybridization for Xbsx (U, red staining), Hermes (V, blue staining), and merge (W) was performed on stage 37 cryostat sections.
To identify the pineal cells expressing Xbsx, we first tested whether this gene is expressed in proliferating or postmitotic cells. Analysis of BrdU incorporation as a marker of S-phase (Fig. 1 F–Q) as well as that of cyclin D1 expression to highlight cells in different phases of mitosis (Fig. S1) shows that Xbsx is expressed in postmitotic cells throughout pineal development. Because photoreceptors and projection neurons are the only two neuronal components of the pineal organ, we compared the expression of Xbsx with that of markers for these two cell types. Xbsx was found to colocalize with photoreceptor markers such as Xotx5 (Fig. 1 R–T) and to be complementary to Hermes, which demarcates projection neurons (Fig. 1 U–W). In particular, Xbsx is already expressed at stage 24 in postmitotic photoreceptor precursors that express Xotx5 (Fig. 1 F–H) but do not yet express the differentiation markers Interphotoreceptor retinoid-binding protein (IRBP) and Recoverin.
Xbsx Is Expressed in a Cyclical Manner in Light/Dark Conditions.
Because pineal photoreceptors represent a complete circadian system in lower vertebrates (8), we asked whether Xbsx expression might cycle over the 24 h. To this end, we performed in situ hybridization on brains dissected from tadpoles (stage 46) grown in a 12-h light/12-h dark (LD) cycle from the time of fertilization. Brains were collected at four time points, and the expression of Tph, which is known to undergo circadian variations (13), was used as a positive control. Xbsx expression was found to cycle similarly to Tph (Fig. 2 A and B), with the highest levels at zeitgeber time (ZT) 18 (ZT indicates hours after “lights on”: ZT0, lights on; ZT12, lights off), the lowest at ZT6, and intermediate levels at the other time points. To understand whether Xbsx expression is controlled by light or by the circadian oscillator, we tested the persistence of Xbsx rhythmicity in constant darkness (DD). Under these conditions, only genes regulated by the circadian clock, such as Tph and AANAT, maintain cyclical expression (13, 14). Because an initial exposure to LD cycles is required in zebrafish to establish clock-controlled rhythms of gene expression, we raised embryos for several days in LD conditions and subsequently for 2 days in the DD cycle. Embryos were collected during the second day of the DD cycle at 6-h intervals. Based on Tph expression, we found that, although 3 and 5 days of LD cycles are not sufficient, 7 days represents an adequate period for the entrainment of the circadian machinery. In fact, following this protocol, Tph expression in DD appears to be rhythmical, although its level is significantly lower than that observed in the LD cycle (Fig. 2 C and D). On the contrary, under the same conditions, Xbsx expression remains low and arrhythmical (Fig. 2 C and D). Altogether these data suggest a circadian clock-independent regulation for Xbsx.
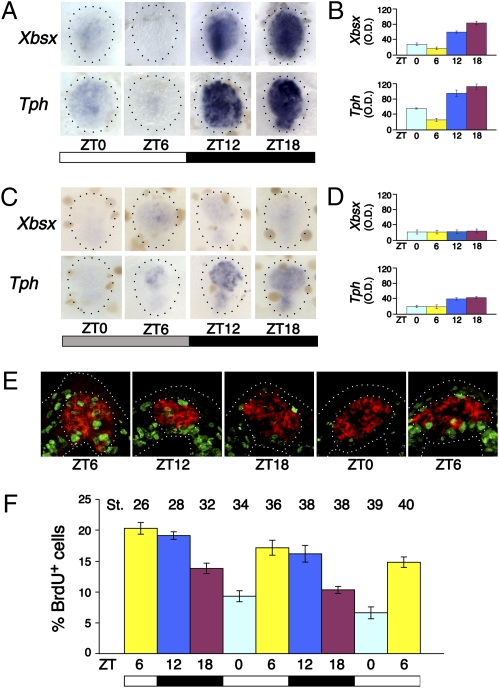
Fig. 2.
Daily rhythms of Xbsx expression and S-phase entry of pineal cells. (A and C) Whole-mount in situ hybridization for Xbsx and Tph was performed on dissected stage 46 brains. Embryos were kept in an LD (A) or DD (C) cycle and collected at the indicated time points (ZT0, lights on; ZT12, lights off). Quantification was determined by optical density (O.D.) of Xbsx and Tph in situ hybridization signal for embryos kept in LD (B) or DD (D) condition. The peak-to-trough statistical difference was determined by the Student's t test: Xbsx, P = 0.006; Tph, P = 0.002 (B) and Xbsx, P = 0.21; Tph, P = 0.008 (D). (E) Representative cryostat sections showing BrdU incorporation (green staining) in the pineal organ at five time points between stage 26 and stage 36. The Xotx5-positive area (red staining) is circled. (F) Quantification of BrdU-labeled cells present in the Xotx5-positive area. At each time point, the mean percentage per pineal organ of BrdU-positive nuclei over the total number of nuclei was plotted against ZT time. The embryonic stage (St.) corresponding to each time point is indicated on the top. Data represent pooled results from three independent experiments. The peak-to-trough statistical difference was determined by the Student's t test: P = 0.001. The number of cells counted is shown in Table S1. In this and the following figures, the white and black bars indicate the light and dark phases, respectively. The gray bar in DD experiments indicates a dark phase at the time when control embryos are in the light phase.
Daily Rhythmicity in Pineal Cell Cycle Progression.
Daily rhythms of cell cycle progression have been documented in a wide variety of organisms (4, 15, 16). To assess whether entry in S-phase is regulated by light during Xenopus pineal development, embryos kept in the LD cycle were treated with BrdU at 6-h intervals for 2 days starting from stage 26. At this initial stage the pineal organ evaginates from the roof of the diencephalon and becomes functional, beginning to produce melatonin rhythmically (17).
To normalize for variations in proliferation occurring at different stages of development, we calculated the percentage of BrdU-positive cells over the total number of cells for each time point. In particular, we analyzed BrdU incorporation in the Xotx5-positive area, thus focusing the analysis on photoreceptor precursors. Our data show that pineal cell proliferation levels are characterized by a cyclical profile during a period of 24 h (Fig. 2 E and F). In particular, the highest percentage of BrdU-positive nuclei was observed at ZT6, whereas the lowest percentage was detected at ZT0. Embryos analyzed at ZT12 and ZT18 show decreasing intermediate percentages of S-phase nuclei. In accordance with data from other biological systems (16, 15), S-phase entry in the pineal organ displays a peak in the second half of the day and in the first part of the night. These effects are independent of the embryonic stage and appear to be linked to the daily progression of time.
Xbsx Is Necessary to Block S-Phase Entry During the Night.
The cyclical expression of Xbsx and its exclusion from proliferating pineal cells raised the hypothesis that this gene might play a role in controlling S-phase rhythmicity of pineal photoreceptor precursors. To address this issue, we performed BrdU incorporation in embryos injected with an antisense morpholino oligonucleotide that specifically targets Xbsx (MoXbsx) (Fig. S2A). We found that compared with control embryos, MoXbsx-injected embryos display an increase in pineal BrdU-positive cells exclusively at ZT18 and ZT0, whereas no significant change is observed for ZT6 and ZT12 (Fig. 3 A and B). These results are consistent with a specific interference of the cyclical Xbsx expression that reaches the highest level during the night. The attenuation of MoXbsx effects on the second analyzed day (corresponding to the fifth day after the injection) is likely attributable to a decrease in stability and/or availability of the injected morpholino. Thus, Xbsx appears to contribute to the rhythmicity of pineal cell proliferation, specifically inhibiting S-phase entry during the night.
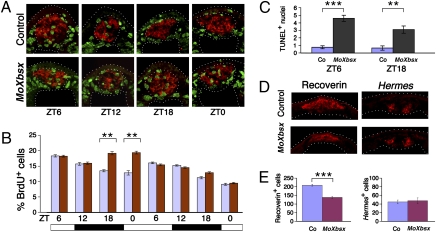
Fig. 3.
Xbsx knockdown leads to increased S-phase entry during the night and to a reduction of pineal photoreceptors. (A) Representative cryostat sections showing BrdU incorporation (green staining) in control- and MoXbsx-injected embryos during 24 h between stage 26 and stage 34. The Xotx5-positive area (red staining) is circled. (B) Quantification of BrdU-labeled cells present in the Xotx5-positive area of control-injected (blue bars) and MoXbsx-injected (red bars) embryos at eight time points. At each time point, the mean percentage per pineal organ of BrdU-positive nuclei over the total number of nuclei is plotted against ZT time. Data represent pooled results from three independent experiments. The number of cells counted is shown in Table S1. (C) Average number of TUNEL-positive nuclei in the Xotx5-positive area per pineal organ for embryos collected at ZT6 and ZT18, corresponding to stage 36 and stage 38, respectively. Data represent pooled results from three independent experiments. Six control (Co)- and six MoXbsx-injected embryos were analyzed per time point in each experiment. (D and E) Analysis of pineal cell types in control- and MoXbsx-injected stage 42 tadpoles. (D) Representative cryostat sections showing expression of markers for photoreceptors (Recoverin) and projection neurons (Hermes). (E) Average numbers of cells positive for Recoverin and Hermes per pineal organ. Data represent pooled results from three independent experiments. For each marker, four control (Co)-injected and four MoXbsx-injected embryos were analyzed per time point in each experiment. Asterisks indicate statistical differences as determined by the Student's t test: **P < 0.01; ***P < 0.001. Error bars indicate SEM.
Xbsx Knockdown Results in Reduction of Pineal Photoreceptors.
Many physiological processes, including proper tissue development and homeostasis, require a balance between apoptosis and cell proliferation. We therefore decided to evaluate the level of pineal apoptosis during development of MoXbsx-injected embryos. TUNEL assays performed at ZT6 and ZT18 show that Xbsx knockdown causes an increase of pineal apoptotic cells in the Xotx5-positive area with respect to control embryos at both time points (Fig. 3C). Thus, inhibition of Xbsx induces an increase in both S-phase entry and cell death. To assess the consequence of these opposite actions, we analyzed the effects of MoXbsx injection on the generation of differentiated pineal cell types. To this aim, we used Recoverin and Hermes as specific markers to identify differentiated pineal photoreceptors and projection neurons, respectively (Fig. 3 D and E). Embryos injected with MoXbsx are characterized by a 25% reduction of photoreceptor cells compared with control injected embryos. Conversely, Xbsx knockdown does not significantly affect the genesis of pineal projection neurons. The specificity of this effect is supported by the efficient rescue of photoreceptors observed in embryos coinjected with MoXbsx and synthetic RNA encoding a hormone-inducible GR-Xbsx protein (Fig. S2B). Taken together, the loss of function data suggests that Xbsx is required for the proper cell cycle exit and differentiation of pineal photoreceptor precursors and the generation of mature photoreceptors.
Xbsx Gain of Function Inhibits Entry in S-Phase of Pineal Photoreceptor Precursors and Promotes Their Differentiation.
To analyze the role of Xbsx in pineal development further, we performed gain-of-function experiments. The effects observed on ectopic expression of a hormone-inducible GR-Xbsx protein are essentially opposite to the ones described for MoXbsx injection. In fact, comparison between dexamethasone-treated (i.e., activated) and untreated (control) GR-Xbsx-injected embryos shows that Xbsx overexpression leads to a decrease in BrdU incorporation at ZT6, ZT12, and ZT18, thus sensibly flattening the daily rhythms of cell cycle progression (Fig. 4 A and B). TUNEL assays show that activation of GR-Xbsx does not affect apoptosis in the Xotx5-positive area (Fig. 4C). Moreover, analysis of pineal cell types in GR-Xbsx-injected embryos shows that Xbsx induces a 29% increase in pineal photoreceptors, whereas it does not affect the generation of projection neurons (Fig. 4 D and E). Altogether, these results show that Xbsx is sufficient to prevent entry in S-phase of pineal photoreceptor precursors and to promote photoreceptor specification.
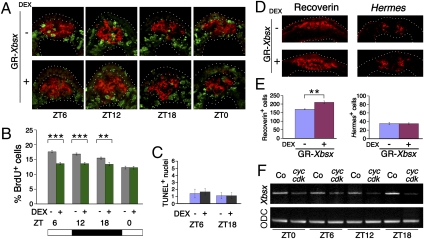
Fig. 4.
Overexpression of Xbsx reduces S-phase entry and increases the number of pineal photoreceptors. (A) Representative cryostat sections showing BrdU incorporation (green staining) at four time points in embryos injected with GR-Xbsx and treated or untreated with dexamethasone (DEX). The Xotx5-positive area (red staining) is circled. (B) Quantification of BrdU-labeled cells present in the Xotx5-positive area of embryos injected with GR-Xbsx and untreated (gray bars) or treated (green bars) with DEX. At each time point, the mean percentage per pineal organ of BrdU-positive nuclei over the total number of nuclei is plotted against ZT time. Data represent pooled results from three independent experiments. The number of cells counted is shown in Table S1. **P < 0.01; ***P < 0.001. (C) Average number of TUNEL-positive nuclei in the Xotx5-positive area per pineal organ for embryos collected at ZT6 and ZT18, corresponding to stage 36 and stage 38, respectively. Data represent pooled results from three independent experiments. Six DEX-treated and six untreated GR-Xbsx-injected embryos were analyzed per time point in each experiment. (D and E) Analysis of pineal cell types in stage 42 tadpoles. Embryos were injected with GR-Xbsx and allowed to develop in the presence or absence of DEX. (D) Representative cryostat sections showing expression of markers for photoreceptors (Recoverin) and projection neurons (Hermes). (E) Average numbers of cells positive for Recoverin and Hermes per pineal organ. Data represent pooled results from three independent experiments. For each marker, four DEX-treated and four untreated GR-Xbsx-injected embryos were analyzed per time point in each experiment. Asterisks indicate statistical differences as determined by the Student's t test: **P < 0.01. Error bars indicate SEM. (F) RT-PCR analysis to evaluate the effects of cyclin A2/cdk2 overexpression on Xbsx expression. Control and injected embryos were collected at the indicated time points. ODC, ornithine decarboxylase.
Cell Proliferation Inhibits Xbsx Expression.
Because gain- and loss-of-function experiments indicate that Xbsx is necessary and sufficient to repress pineal cell proliferation, we decided to analyze how Xbsx expression is affected by high levels of proliferation. This was achieved by overexpressing cyclin A2 together with cdk2. Under these conditions, Xbsx expression is repressed at all the analyzed time points, thus indicating a mutual antagonism between cell proliferation and Xbsx activity (Fig. 4F).
Discussion
Xbsx Is Expressed in a Cyclical Manner in Pineal Photoreceptor Precursors.
The photosensitive pineal organ of lower vertebrates has been proposed to reflect an ancient form of the vertebrate retina (8). In particular, the retina and the pineal organ share genetic pathways involved in the control of photoreception, phototransduction, and melatonin production. Moreover, the developing pineal organ expresses a set of transcription factors that is known to direct retina development (18) and includes Pax6, Rx1, Six3, Otx2, Otx5, Lhx3, and ET. Although the signals that provide specific instructions leading to a pineal rather than retinal fate have not been clearly dissected yet, there are also rare examples of genes displaying selective expression in the pineal organ but not in the retina. These include pinopsin and exo-rhodopsin, two pineal-specific opsins (8), and the transcription factor Not2 [denominated floating head (flh) in zebrafish], which is expressed in precursors of both photoreceptors and projection neurons (9). The homeodomain-containing transcription factor Xbsx is another example of this small subset of pineal-specific proteins. Compared with Not2, Xbsx shows the peculiarity of being expressed exclusively in photoreceptor precursors and is characterized by cyclical expression. What does control Xbsx pineal expression? Xbsx is activated in the pineal territory later than Xotx5, Xnot2, and Xrx1. Moreover, a survey of a 5-kb region located upstream of the mouse Bsx gene highlights the presence of three Crx and two Otx2 binding sites, two transcription factors involved in mammalian pinealocyte specification and differentiation (19–21). Interestingly, Crx belongs to the Otx gene family and is considered to be the homologue of Xenopus Otx5. Altogether, these observations suggest that Bsx might function downstream of Otx5/Crx, Otx2, Not2, and Rx1 in events that follow the initial pineal territory specification. However, because none of the above-mentioned genes is expressed rhythmically, other factors should be responsible for Xbsx cyclical expression. Xbsx rhythmicity is lost in embryos kept in DD conditions, suggesting that this gene is controlled by the alternation of light and dark rather than by the circadian oscillator. A similar behavior, although with opposite rhythmicity, was described for Xper2 expression in the Xenopus retina (22). Xbsx cyclical expression is consistent with a model in which light promotes the accumulation of an activator that reaches a threshold, or becomes otherwise active, at the end of the day, thus being able to stimulate the transcription of Xbsx during the night. Alternatively, Xbsx could be continuously transcribed, but inhibitory factors present during the daytime might degrade or destabilize its mRNA. Putative inhibitory factors could be represented by rhythmically expressed deadenilases (similar to Nocturnin but with an opposite phase of expression) (23) and micro-RNAs (24).
Xbsx Couples the Daily Rhythmicity of Pineal Cell Cycle Progression and the Specification of Pineal Photoreceptors.
Recent studies have implicated components of the circadian clock in the transcriptional control of cell cycle regulatory genes (4). In this work, we investigated the control of cell proliferation and cell fate specification in the pineal organ, which offers the advantage of being composed of only two neuronal types. Focusing on photoreceptor precursors, we found that they display daily rhythms of S-phase entry throughout pineal development, reaching a peak during the latter part of the light phase and the first part of the dark period. Interestingly, Xbsx is expressed in BrdU-negative cells, and the phase of its rhythmical expression is complementary to the daily phase of proliferation (Fig. 5A). Moreover, functional experiments indicate that Xbsx expression inversely correlates to cell proliferation. In fact, Xbsx knockdown raises the level of BrdU-positive cells specifically during the night, whereas Xbsx overexpression leads to a flattening of cell proliferation to the lowest level. Conversely, when cell proliferation is maintained at high levels by overexpressing cyclin A2/cdk2, Xbsx expression is drastically repressed. Accordingly, studies performed in cell cultures show that Bsx transcription is activated during retinoic acid-induced neuronal differentiation (25). Although the daily rhythms of cell proliferation are controlled by the circadian clock, light plays a central role in entraining and maintaining significant amplitude of S-phase rhythmicity (15). Moreover, light may exert independent effects on pacemaker rhythmical outputs, as was recently shown for control of the nocturnal release of melatonin (26). Bsx, whose expression does not appear to be under the control of circadian rhythms, could play an essential role in synchronizing photoreceptor differentiation with the end of the daily proliferative phase.
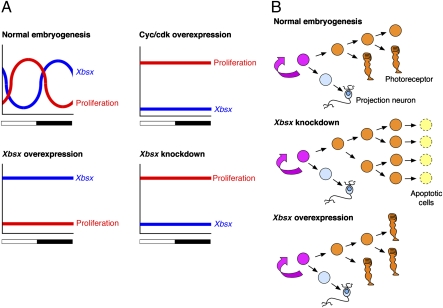
Fig. 5.
Working model for Xbsx function in cell proliferation and differentiation of photoreceptor precursors. (A) Schematic summary of the relation between Xbsx expression and pineal cell proliferation in WT and manipulated embryos. (B) Model of action for Xbsx. The pink cell represents the common progenitor of projection neurons and photoreceptors. It is assumed that photoreceptor precursors undergo a limited number of asymmetrical cell divisions. Xbsx knockdown prevents cell cycle exit of photoreceptor precursors that eventually undergo apoptosis. Xbsx overexpression increases cell cycle exit of photoreceptor precursors and promotes their differentiation.
Cell fate determination during pineal development was recently addressed in zebrafish (10). In particular, it was shown that photoreceptors and projection neurons are born simultaneously and that although reduction of Notch activity promotes the projection neuron fate, constitutive activation of Notch is not sufficient to induce photoreceptors. This led to postulation of the existence of a still unidentified photoreceptor-inducing signal. Our data add significant information to this model, indicating that Bsx could be an effector of such a signal. Indeed, when Xbsx is knocked down, we observe both stimulation of cell proliferation and increased apoptosis, which results in a decrease of differentiated photoreceptors. Apoptosis may be attributable to the activation of excessive proliferation (27) or to the lack of an appropriate differentiation signal. These data are consistent with the hypothesis that Xbsx is required for cell cycle exit and/or differentiation of photoreceptor precursors (Fig. 5B). According to this hypothesis, in the absence of Xbsx, photoreceptor progenitors do not exit the cell cycle, as suggested by the increased BrdU incorporation in MoXbsx-injected embryos, but, lacking appropriate specification cues, would eventually die. This model is also supported by Xbsx gain-of-function experiments that show a reduction of BrdU incorporation but no effect on apoptosis and an increase of pineal photoreceptors. In both gain-of-function and loss-of-function experiments, the number of projection neurons remains unchanged compared with control embryos, indicating that Xbsx functions exclusively in the lineage of photoreceptor precursors.
The remarkable conservation of the Bsx expression pattern between the mouse model and Xenopus suggests a conservation of function. Thus far, studies on Bsx knockout mice have focused on the hypothalamic role played by this gene in controlling the homeostasis of energy balance (28, 29). Nonetheless, it was noticed that these mice display pineal gland hypoplasticity (28). Because the mammalian pinealocyte and the nonmammalian pineal photoreceptor appear to have evolved from a common ancestral photoreceptor cell (8), it is tempting to speculate that the role in pineal photoreceptor specification we observe in Xenopus might be conserved among vertebrates. To be tested, this hypothesis awaits a cell type-specific analysis of the pineal gland in Bsx knockout mice as well as comparative studies of Bsx function in other vertebrate model systems.
In conclusion, this work provides insights into the molecular mechanisms coordinating the daily rhythms of cell proliferation and the generation of specific cell types. Our results highlight a previously undescribed essential role for Bsx in orchestrating these two events during the specification of pineal photoreceptors. Because endogenous circadian oscillators regulate cell proliferation in most cells, further studies are expected to identify additional factors linking circadian cell cycle exit to specific cell fates.
Materials and Methods
In situ hybridization, immunostaining, embryo microinjection, and BrdU incorporation were performed as previously described (30–32). TUNEL staining was performed on tissue cryosections using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon) and following the manufacturer's protocol. The Xbsx antisense morpholino and control morpholino were obtained from Gene Tools, LLC. Injections and embryo manipulations during the dark phase were performed under red safe lights. More details are described in SI Text.
Supplementary Material
Acknowledgments
We thank Debora Angeloni, Federico Cremisi, Irma Nardi, Paolo Sassone-Corsi, Gianluca Tosini, and Robert Vignali for discussion and helpful suggestions. We are grateful to Carla B. Green for the gift of the Tph plasmid. This work was supported by Cofinanziamento PRIN-Universita’ di Pisa (prot. 2007TEFM7M), Telethon (Grant no. GGP07275) and Ministero Affari Esteri (NFNS 30370453).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000854107/DCSupplemental.
References
- 1.Ohnuma S, Harris WA. Neurogenesis and the cell cycle. Neuron. 2003;40:199–208. doi: 10.1016/s0896-6273(03)00632-9. [DOI] [PubMed] [Google Scholar]
- 2.Andreazzoli M. Molecular regulation of vertebrate retina cell fate. Birth Defects Res., Part C. 2009;87:284–295. doi: 10.1002/bdrc.20161. [DOI] [PubMed] [Google Scholar]
- 3.Okamura H. Clock genes in cell clocks: Roles, actions, and mysteries. J Biol Rhythms. 2004;19:388–399. doi: 10.1177/0748730404269169. [DOI] [PubMed] [Google Scholar]
- 4.Hunt T, Sassone-Corsi P. Riding tandem: Circadian clocks and the cell cycle. Cell. 2007;129:461–464. doi: 10.1016/j.cell.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Roenneberg T, Foster RG. Twilight times: Light and the circadian system. Photochem Photobiol. 1997;66:549–561. doi: 10.1111/j.1751-1097.1997.tb03188.x. [DOI] [PubMed] [Google Scholar]
- 6.Nikaido SS, Johnson CH. Daily and circadian variation in survival from ultraviolet radiation in Chlamydomonas reinhardtii. Photochem Photobiol. 2000;71:758–765. doi: 10.1562/0031-8655(2000)071<0758:dacvis>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Ekström P, Meissl H. Evolution of photosensory pineal organs in new light: The fate of neuroendocrine photoreceptors. Philos Trans R Soc London B. 2003;358:1679–1700. doi: 10.1098/rstb.2003.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mano H, Fukada Y. A median third eye: Pineal gland retraces evolution of vertebrate photoreceptive organs. Photochem Photobiol. 2007;83:11–18. doi: 10.1562/2006-02-24-IR-813. [DOI] [PubMed] [Google Scholar]
- 9.Masai I, et al. floating head and masterblind regulate neuronal patterning in the roof of the forebrain. Neuron. 1997;18:43–57. doi: 10.1016/s0896-6273(01)80045-3. [DOI] [PubMed] [Google Scholar]
- 10.Cau E, Quillien A, Blader P. Notch resolves mixed neural identities in the zebrafish epiphysis. Development. 2008;135:2391–2401. doi: 10.1242/dev.013482. [DOI] [PubMed] [Google Scholar]
- 11.Cau E, Wilson SW. Ash1a and Neurogenin1 function downstream of Floating head to regulate epiphysial neurogenesis. Development. 2003;130:2455–2466. doi: 10.1242/dev.00452. [DOI] [PubMed] [Google Scholar]
- 12.Cremona M, Colombo E, Andreazzoli M, Cossu G, Broccoli V. Bsx, an evolutionary conserved Brain Specific homeoboX gene expressed in the septum, epiphysis, mammillary bodies and arcuate nucleus. Gene Expression Patterns. 2004;4:47–51. doi: 10.1016/s1567-133x(03)00151-0. [DOI] [PubMed] [Google Scholar]
- 13.Green CB, Cahill GM, Besharse JC. Regulation of tryptophan hydroxylase expression by a retinal circadian oscillator in vitro. Brain Res. 1995;677:283–290. doi: 10.1016/0006-8993(95)00166-n. [DOI] [PubMed] [Google Scholar]
- 14.Gothilf Y, et al. Zebrafish serotonin N-acetyltransferase-2: Marker for development of pineal photoreceptors and circadian clock function. Endocrinology. 1999;140:4895–4903. doi: 10.1210/endo.140.10.6975. [DOI] [PubMed] [Google Scholar]
- 15.Dekens MP, et al. Light regulates the cell cycle in zebrafish. Curr Biol. 2003;13:2051–2057. doi: 10.1016/j.cub.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo T, et al. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–259. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 17.Green CB, Liang MY, Steenhard BM, Besharse JC. Ontogeny of circadian and light regulation of melatonin release in Xenopus laevis embryos. Brain Res Dev Brain Res. 1999;117:109–116. doi: 10.1016/s0165-3806(99)00109-1. [DOI] [PubMed] [Google Scholar]
- 18.Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–5167. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, et al. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19:1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- 20.Li X, et al. A pineal regulatory element (PIRE) mediates transactivation by the pineal/retina-specific transcription factor CRX. Proc Natl Acad Sci USA. 1998;95:1876–1881. doi: 10.1073/pnas.95.4.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishida A, et al. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- 22.Besharse JC, Zhuang M, Freeman K, Fogerty J. Regulation of photoreceptor Per1 and Per2 by light, dopamine and a circadian clock. Eur J Neurosci. 2004;20:167–174. doi: 10.1111/j.1460-9568.2004.03479.x. [DOI] [PubMed] [Google Scholar]
- 23.Baggs JE, Green CB. Nocturnin, a deadenylase in Xenopus laevis retina: A mechanism for posttranscriptional control of circadian-related mRNA. Curr Biol. 2003;13:189–198. doi: 10.1016/s0960-9822(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 24.Pegoraro M, Tauber E. The role of microRNAs (miRNA) in circadian rhythmicity. J Genet. 2008;87:505–511. doi: 10.1007/s12041-008-0073-8. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Kim JB, Han YM. REST is a key regulator in brain-specific homeobox gene expression during neuronal differentiation. J Neurochem. 2007;103:2565–2574. doi: 10.1111/j.1471-4159.2007.04947.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz MD, et al. Dissociation of circadian and light inhibition of melatonin release through forced desynchronization in the rat. Proc Natl Acad Sci USA. 2009;106:17540–17545. doi: 10.1073/pnas.0906382106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–334. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 28.McArthur T, Ohtoshi A. A brain-specific homeobox gene, Bsx, is essential for proper postnatal growth and nursing. Mol Cell Biol. 2007;27:5120–5127. doi: 10.1128/MCB.00215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakkou M, et al. A role for brain-specific homeobox factor Bsx in the control of hyperphagia and locomotory behavior. Cell Metab. 2007;5:450–463. doi: 10.1016/j.cmet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Casarosa S, et al. Xrx1 controls proliferation and multipotency of retinal progenitors. Mol Cell Neurosci. 2003;22:25–36. doi: 10.1016/s1044-7431(02)00025-8. [DOI] [PubMed] [Google Scholar]
- 31.D'Autilia S, et al. Cloning and developmental expression of the Xenopus homeobox gene Xvsx1. Dev Genes Evol. 2006;216:829–834. doi: 10.1007/s00427-006-0109-0. [DOI] [PubMed] [Google Scholar]
- 32.Gestri G, et al. Six3 functions in anterior neural plate specification by promoting cell proliferation and inhibiting Bmp4 expression. Development. 2005;132:2401–2413. doi: 10.1242/dev.01814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.