Abstract
To quantitatively investigate the effects of pentobarbital anesthesia on brain activity, brain metabolite concentrations and cerebral metabolic rate of glucose, in vivo proton MR spectra, and electroencephalography were measured in the rat brain with various doses of pentobarbital. The results show that (1) the resonances attributed to propylene glycol, a solvent in pentobarbital injection solution, can be robustly detected and quantified in the brain; (2) the concentration of most brain metabolites remained constant under the isoelectric state (silent electroencephalography) with a high dose of pentobarbital compared to mild isoflurane anesthesia condition, except for a reduction of 61% in the brain glucose level, which was associated with a 37% decrease in cerebral metabolic rate of glucose, suggesting a significant amount of “housekeeping” energy for maintaining brain cellular integrity under the isoelectric state; and (3) electroencephalography and cerebral metabolic activities were tightly coupled to the pentobarbital anesthesia depth and they can be indirectly quantified by the propylene glycol resonance signal at 1.13 ppm. This study indicates that in vivo proton MR spectroscopy can be used to measure changes in cerebral metabolite concentrations and cerebral metabolic rate of glucose under varied pentobarbital anesthesia states; moreover, the propylene glycol signal provides a sensitive biomarker for quantitatively monitoring these changes and anesthesia depth noninvasively.
Keywords: cerebral metabolic rate of glucose, CMRglc, brain activity, brain metabolism, propylene glycol
Sodium pentobarbital, one type of barbiturate, has been popularly used in clinic for treatment of seizures and preoperative sedation. It is also a common agent used for general anesthesia in clinical and animal research. Pentobarbital rapidly distributes to all tissues, with a higher concentration in brain, liver, and kidneys due to high lipid solubility. It can enhance the γ-aminobutyric acid receptor-coupled response, thereby directly depressing neuronal excitability and resulting in an anesthesia effect in the brain and vanishing consciousness (see a recent review article, Alkire et al. (1), and references therein). The suppression of neuronal activity is also accompanied by significant depression of cerebral metabolic activity due to the inhibition of nicotinamide adenine dinucleotide (NADH) oxidation in the respiratory chain, and a deep pentobarbital anesthesia can induce a complete suppression of brain electroencephalography (EEG) activity thereby reaching an isoelectric state. Therefore, the delivery of pentobarbital into a living body can lead to a variety of brain physiologic changes, including EEG activity, cerebral metabolites/metabolic rates, and cerebral blood flow, and all these changes are expected to be related to the pentobarbital concentration reached inside the brain. It is thus essential to seek a robust, reliable tool to noninvasively assess the brain pentobarbital concentration in vivo and, ultimately, to study the quantitative relationship between pentobarbital anesthesia depth and physiologic changes. In vivo proton (1H) magnetic resonance spectroscopy (MRS) could provide a suitable approach for fulfilling this task. Nevertheless, the detection sensitivity of in vivo proton MRS is still limited to directly measuring the brain pentobarbital due to a very low concentration under normal anesthesia conditions. For instance, a large dose of 70 mg pentobarbital per kg of body weight could induce a maximum brain pentobarbital concentration of approximately 300 µmol per kg brain tissue if one assumes that pentobarbital distributes uniformly in the body and it is not metabolized. Such a low concentration is barely detectable by in vivo proton MRS if the sampling time is inadequately long. However, to enhance the solubility of pentobarbital, the commercial available pentobarbital injection solution usually contains large amounts of water, ethanol (Eth) and propylene glycol (PG). It has been demonstrated that the 1H signal of Eth is not detectable in the rat brain with the pentobarbital anesthetic, presumably because of rapid metabolism of Eth in a living body (2). In contrast, the PG signal can be reliably detected by in vivo proton MRS (2–5). It is interesting to note that both pentobarbital and PG are chemically stable in the brain and primarily metabolized in the liver in which pentobarbital is oxidized and PG is converted to L-lactate (6). Therefore, the measure of the cerebral PG concentration could provide an indirect, sensitive index reflecting the pentobarbital concentration in the brain.
In the present study, we used the localized in vivo proton MRS and EEG to quantitatively study the relationships among the alterations in cerebral metabolites/metabolic rates and EEG activity levels in the rat brain under varied anesthesia depths, which were achieved by the application of isoflurane and two different doses of sodium pentobarbital. This study aims to address the following questions:
What is the implication of NMR signals of PG on quantification of brain glucose (Glc) concentration?
Do the brain metabolite concentrations change with varied anesthesia depth?
What is the quantitative relationship of neurometabolic coupling between brain EEG activity and cerebral metabolic rate?
Can the PG signal measured by in vivo proton MRS provide a unique biomarker for monitoring anesthesia depth and cerebral metabolic rate changes caused by pentobarbital?
MATERIALS AND METHODS
Pentobarbital Solution and Phantom Preparation
Sodium pentobarbital injection solution was purchased as a commercial product (Ovation Pharmaceuticals, Inc., Deerfield, IL). Each milliliter of sodium pentobarbital solution contains 50 mg sodium pentobarbital, 40% (volume-volume), PG and 10% (volume-volume) Eth. In order to identify NMR resonances resulted from anesthetic pentobarbital solution, which overlap with the resonances of brain metabolites, two phantom solutions were prepared. One phantom contained 5 mM sodium pentobarbital, which was directly diluted from the sodium pentobarbital injection solution, and another contained 1 mM Glc (Sigma-Aldrich, St. Louis, MO) in a saline solution (pH = 7.0). The temperature of the phantom solutions was maintained at 37 ± 0.5°C inside the magnet during NMR measurements.
Animal Preparation
Unfasted, adult male Sprague-Dawley rats (280–390 g) were divided into two groups for MR (n = 8) and EEG (n= 3) measurements with the same animal preparation procedures under three anesthesia states. The rat femoral artery and vein were catheterized for blood sampling, physiologic monitoring, and sodium pentobarbital infusion. Animal rectal temperature was maintained at 37 ± 0.5°C throughout the entire experiment. The first anesthesia state was achieved by inhalation of 2% (volume-volume) isoflurane mixed with nitrous oxide/oxygen (3:2). After the initial MR or EEG measurements, anesthesia was turned to a deeper state by switching the isoflurane anesthetic to the sodium pentobarbital injection solution with 30 mg/kg bolus followed by continuous infusion of 30 mg/kg/h (defined as low-dose pentobarbital, or Low-PB). There was a waiting period (~90 min) before performing MR or EEG measurements until animal physiology approached a stable condition and the concentration of isoflurane was below 0.1% (volume-volume) to minimize the residual effects of isoflurane. The last anesthesia state was obtained by increasing the pentobarbital infusion rate to 70 mg/kg/h after the Low-PB experiments to completely suppress brain EEG signal, which took approximately 40 min after the high-dose pentobarbital infusion (High-PB). Animal surgical procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
MR Measurement
MR experiments were carried out at a 9.4-T/31-cm horizontal magnet (Magnex Scientific, Abingdon, UK) equipped with Varian INOVA console (Varian, Palo Alto, CA). Anatomic MR imaging and in vivo proton MRS were acquired using an elliptical surface coil with a long axis of 2 cm and a short axis of 1.2 cm. Scout images were obtained using a turbo fast low-angle shot MRI sequence (7). The localized 1H spectra were acquired using the point-resolved spectroscopy approach (8) with pulse repetition time/echo time = 3000/13 ms and 128 averages. An MRS voxel of 4 × 4× 4 mm3 mainly covering the cortical region and a portion of subcortical region symmetrically along the brain central fissure line was selected and shimmed on using FASTMAP algorithm (9). Outer volume suppression was performed using the amplitude of radiofrequency field-insensitive selective train to obliterate signal method (10).
EEG Measurement
Two electrodes were utilized to record the EEG signals using a commercial machine (Grass, Astro-Med Inc., West Warwick, RI). One was put on the rat nose to serve as a reference and the other was inserted into the rat somatosensory cortex through a small hole in the skull (2-mm depth from the surface of the skull, 3 mm posterior to bregma, and 3 mm from the brain midline) (11). The filtered EEG signal (0.0–30 Hz) was sampled at a rate of 1000 Hz. Home-written software based on the Shannon spectral entropy method was used to quantify EEG activity via the calculation of Shannon spectral entropy index (SEI) (11,12). The recorded EEG signal was divided into epochs of 10-sec duration, and then SEI was calculated for each epoch in the 0.3–30 Hz frequency range. The EEG SEI values were obtained by averaging SEI data points of 20 min for each physiologic condition. Three rats were used for EEG measurements. The obtained EEG results were then pooled together with the EEG results of nine rats from our previous publication (11), aiming to study the correlation between brain spontaneous EEG activity level to the cerebral metabolic rate of adenosine triphosphate (ATP) under the same physiologic conditions. In the present study, the averaged EEG results (n = 12) were applied to correlate with the changes in brain metabolites, metabolic rate, and the PG concentration under varied anesthesia depths.
In Vivo Proton MRS Quantification
In vivo 1H spectra of rat brain were analyzed by the LC-Model fitting method (Stephen Provencher Inc., Toronto, Canada) in the frequency domain (4,13). The basis set for LCModel was simulated using density matrix simulations (14) and chemical-shifts and J-couplings reported in the literature (15). Two different basis sets were utilized for the LCModel fitting: the first one containing the brain metabolites and macromolecules (MM); the second one containing brain metabolites, MM and PG. The MM spectrum was measured experimentally in vivo by using the same pulse sequence and parameters except by adding an inversion-recovery preparation and adjusting the inversion recovery time to null signal from metabolites (16,17). The detailed approach of using LCModel spectral fitting for metabolite quantification in the presence of PG signal has been described previously (2). The reported concentrations of cerebral metabolites and PG (mM) were relative to the total creatine/phosphocreatine (tCr) signal at 3.01 ppm ([tCr] = 8 mM) under all the anesthesia conditions. The peak integral ratios between the NMR resonances of PG at 1.13 ppm and creatine/phosphocreatine at 3.01 ppm were manually measured using Varian software package.
Quantification of the Cerebral Metabolic Rate of Glucose
The cerebral metabolic rate of glucose (CMRglc) was calculated according to the standard Michaelis-Menten Glc transport equation (18,19) as described by Eq. 1:
| [1] |
where, [Gi] is the brain Glc concentration which was measured by in vivo proton MRS and quantified by the LCModel fitting; [G0] is the blood plasma Glc concentration which was measured using the blood sampling and quantified by Glc analyzer (Accu-Chek, Roche Diagnostics, Madison, WI); Tmax is the maximal velocity of Glc transport; KT is the half-maximum transport constant for Glc transport. A wide range of KT from 3.3 to 8.8 mM had been reported in a number of publications (20–25), and it was assumed to be a constant of 6.1 mM (the median value calculated based on the literature values) in the present study. When [Gi] is at the steady-state, i.e., , Eq. 2 can be deduced from Eq. 1 as the following:
| [2] |
Thereby, Eq. 2 can be used to determine the ratio of CM-Rglc/Tmax, an index reflecting the relative CMRglc when Tmax is treated as a constant.
Data and results are reported as mean ± standard deviation.
RESULTS
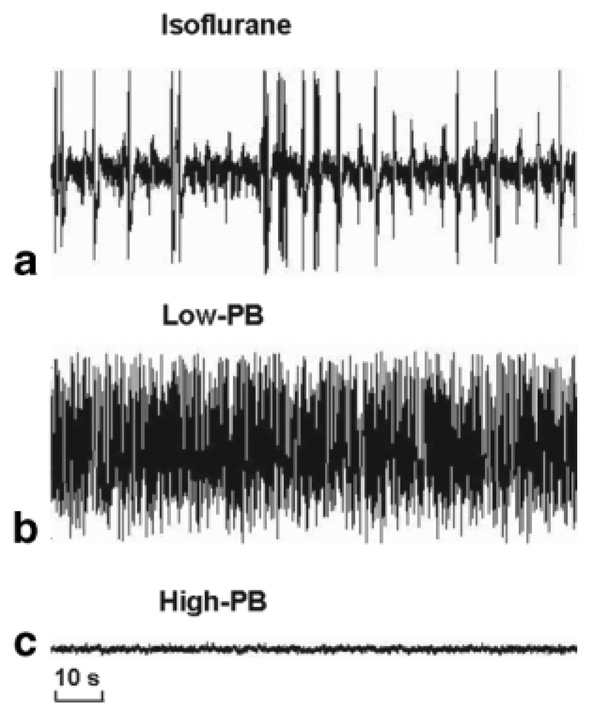
Figure 1 illustrates the time courses of EEG measurements from a representative rat under three anesthesia states. The EEG firing pattern was characterized by intensive bursts under the light isoflurane anesthesia condition (Fig. 1a; SEI = 0.70). The EEG bursts were substantially suppressed under the mild anesthesia condition of Low-PB (Fig. 1b; SEI = 0.66), and then EEG activity became silent (Fig. 1c) under the deep anesthesia condition of High-PB leading to an isoelectric state and a smallest SEI value (SEI = 0.39). With deepening anesthesia, the averaged SEI of EEG gradually decreased from 0.72 ± 0.06 (n = 12) under light isoflurane anesthesia to 0.64 ± 0.05 under Low-PB anesthesia and to 0.43 ± 0.09 under the isoelectric state (High-PB). These results indicate a tight correlation between depth of anesthesia and spontaneous EEG brain activity.
FIG. 1.
EEG time courses with distinct spike patterns measured from a representative rat brain with (a) dominant burst activity under isoflurane anesthesia, (b) suppressed burst activity under low-dose pentobarbitol (Low-PB) anesthesia, and (c) isoelectric activity under high-dose pentobarbitol (High-PB) anesthesia.
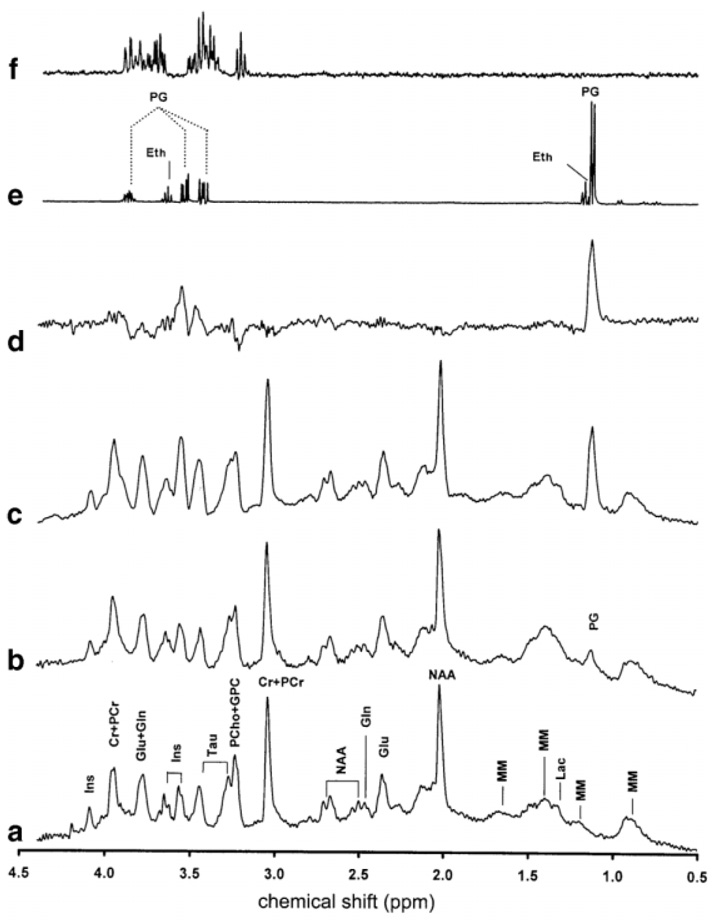
Figure 2 shows the in vivo 1H spectra obtained under the same anesthesia conditions from a representative rat brain. Beyond the NMR resonance peak at 1.13 ppm, which was elevated with the increased dose of sodium pentobarbital, the main spectral difference appeared in the range of 3.1 to 4.0 ppm, as demonstrated in the difference spectrum (Fig. 2d) between the spectra acquired under light isoflurane anesthesia (Fig. 2a) and deepest High-PB anesthesia (Fig. 2c). To identify these resonances, the phantom solutions were studied using the same proton MRS pulse sequence and parameters as that applied to the rat brain. Fig. 2e shows the 1H spectrum acquired from 5 mM sodium pentobarbital phantom solution, in which the detected resonance peaks are primarily attributed to the solvents of PG and Eth; and Fig. 2f shows the 1H spectrum acquired from 1 mM Glc phantom solution, showing intense resonance peaks which are overlapped with the PG resonance peaks between 3.1 and 4.0 ppm.
FIG. 2.
Localized in vivo 1H spectra acquired from a rat brain under three anesthesia conditions: (a) isoflurane, (b) Low-PB, and (c) High-PB. (d) Difference spectrum between (c) and (a). (e) Phantom 1H spectrum from 5-mM sodium pentobarbital solution. (f) Phantom 1H spectrum from 1-mM Glc solution. The spectra (a-d) were plotted with the same vertical scale. The different vertical scales were used for spectra (e) and (f) compared to spectra (a-d). Ins, myo-inositol; Cr, creatine; PCr, phosphocre-atine; Glc, glucose; Gln, glutamate; Tau, taurine; PCho, phosphocholine; GPC, glycerophosphocholine; NAA, N-acetyl-aspartate; Lac, lactate; PG, propylene glycol; MM, macromolecule; Eth, ethanol.
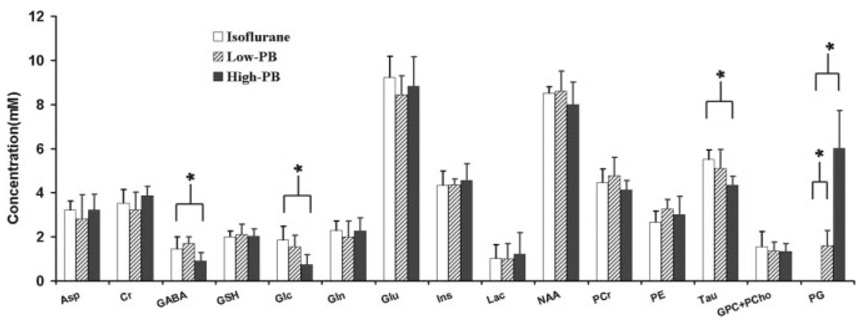
Table 1 and Fig. 3 summarize the results of the concentrations of cerebral metabolites and PG under various anesthesia conditions, as well as the corresponding LCModel fitting results and fitting errors for the individual animals (n = 8).
Table 1.
Metabolite and PG Concentrations Quantified by the LCmodel Spectral Fitting in the Rat Brain Under Three Anesthesia Conditions and Fitting Errors (n = 8)
| Metabolites | Isofluranea |
Low-PBa |
High-PBa |
|||
|---|---|---|---|---|---|---|
| Concentration | CRLB | Concentration | CRLB | Concentration | CRLB | |
| Asp | 3.21 ± 0.42 | 23 ± 9% | 2.82 ± 1.08 | 21 ± 7% | 3.22 ± 0.71 | 24 ± 14% |
| Cr | 3.53 ± 0.61 | 23 ± 15% | 3.23 ± 0.79 | 34 ±11% | 3.87 ± 0.42 | 22 ± 8% |
| GABA | 1.46 ± 0.55 | 25 ± 9% | 1.70 ± 0.30 | 20 ± 6% | 0.92 ± 0.36 | 52 ± 38% |
| GSH | 2.01 ± 0.25 | 11 ± 4% | 2.09 ± 0.49 | 13 ± 6% | 2.03 ± 0.32 | 21 ± 22% |
| Glc | 1.87 ± 0.61 | 29 ± 19% | 1.56 ± 0.52 | 33 ± 12% | 0.73 ± 0.47 | 174 ± 132% |
| Gln | 2.30 ± 0.42 | 17 ± 5% | 2.00 ± 0.71 | 20 ± 9% | 2.26 ± 0.59 | 19 ± 5% |
| Glu | 9.23 ± 0.96 | 4 ± 1% | 8.45 ± 0.86 | 5 ± 1% | 8.82 ± 1.33 | 10 ± 12% |
| Ins | 4.34 ± 0.64 | 8 ± 2% | 4.37 ± 0.24 | 11 ± 3% | 4.57 ± 0.76 | 11 ± 5% |
| Lac | 1.02 ± 0.62 | 25 ± 12% | 1.00 ± 0.69 | 33 ± 15% | 1.22 ± 0.67 | 29 ± 11% |
| NAA | 8.51 ± 0.29 | 4 ± 1% | 8.62 ± 0.90 | 4 ± 1% | 7.99 ± 1.02 | 4 ± 1% |
| PCr | 4.47 ± 0.61 | 8 ± 3% | 4.67 ± 0.83 | 6 ± 2% | 4.13 ± 0.42 | 9 ± 3% |
| PE | 2.66 ± 0.52 | 22 ± 15% | 3.26 ± 0.43 | 18 ± 10% | 3.01 ± 0.82 | 16 ± 7% |
| Taurine | 5.50 ± 0.43 | 6 ± 2% | 5.11 ± 0.84 | 7 ± 2% | 4.33 ± 0.40 | 10 ± 3% |
| GPC+PCho | 1.54 ± 0..71 | 18 ± 9% | 1.35 ± 0.42 | 21 ± 5% | 1.33 ± 0.36 | 21 ± 5% |
| PG | 1.60 ± 0.60 | 23 ± 10% | 6.01 ± 1.73 | 6 ± 2% | ||
The LCModel basis including metabolites and MM was applied to fit 1H MRS data from the rats anesthetized with isoflurane. The LCModel basis including metabolites, MM, and PG was applied to fit 1H MRS data from the rats anesthetized with pentobarbital.
FIG. 3.
Results of brain metabolites and PG concentrations analyzed by the LCModel fitting under three anesthesia conditions (n= 8). The asterisk (*) indicates a statistically significant difference between two measurements (P < 0.05). The LCModel basis including metabolites and MM was used to fit the 1H spectra acquired from the animals anesthetized with isoflurane. The LCModel basis including metabolites, MM, and PG was applied to fit the 1H spectra acquired from the animals anesthetized with pentobarbital.
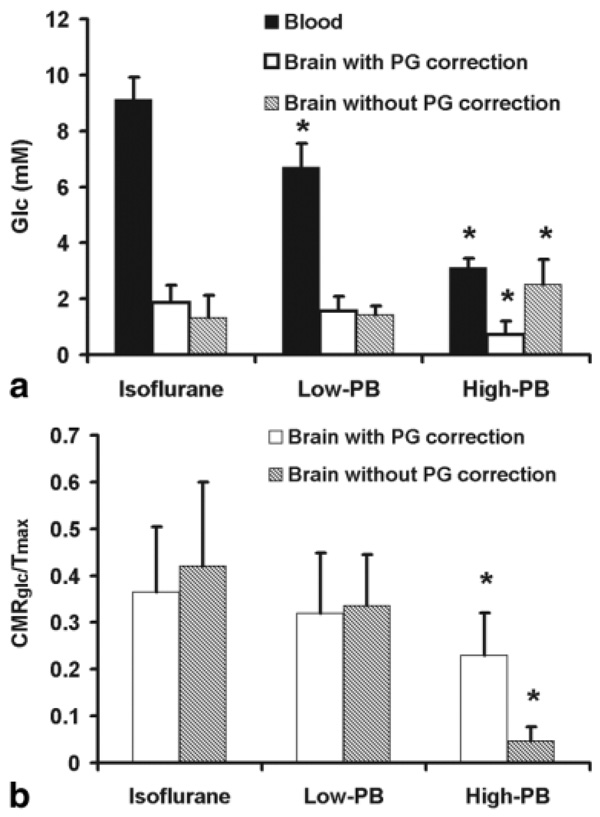
Figure 4a displays the Glc concentrations measured in the blood and the brain tissue under the three states of anesthesia. These data were used to calculate the relative CMRglc change according to Eq. 2 under two conditions, with and without PG resonance correction in the LCModel fitting, and the results are shown in Fig. 4b.
FIG. 4.
(a) Brain and blood Glc concentrations and (b) relative CMRglc measured under three anesthesia conditions (n = 8). Brain Glc concentrations and relative CMRglc were obtained by the two different LCModel fitting basis sets with (open bars) and without (shaded bars) correction of the PG signal contaminations, respectively. The asterisk (*) indicates a statistically significant difference as compared to the isoflurane anesthesia condition.
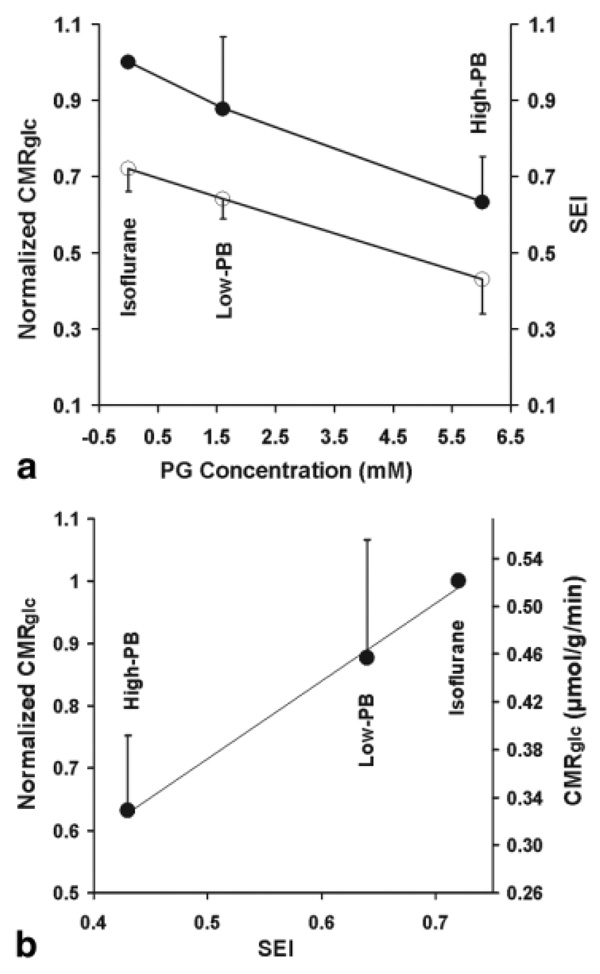
The PG concentration measured under various anesthesia states was used to correlate to the brain EEG activity quantified by SEI, as well as to the relative CMRglc, which was normalized to the value measured under the light isoflurane anesthesia condition. These correlations as shown in Fig. 5a suggest tight coupling relationships among brain PG concentration and EEG activity level, as well as cerebral metabolic rate across a wide range of pentobarbital anesthesia depth. Fig. 5b shows a tight correlation between the relative CMRglc and the basal EEG activity level under a wide range of anesthesia depth. It also indicates that the CMRglc value was reduced by 37% at the isoelectric state (High-PB) compared to the light isoflurane anesthesia condition.
FIG. 5.
(a) Correlation of brain PG concentration to SEI of EEG activity (open circles) and normalized CMRglc (full circles) under varied anesthesia conditions. (b) Correlation of SEI versus normalized and absolute CMRglc.
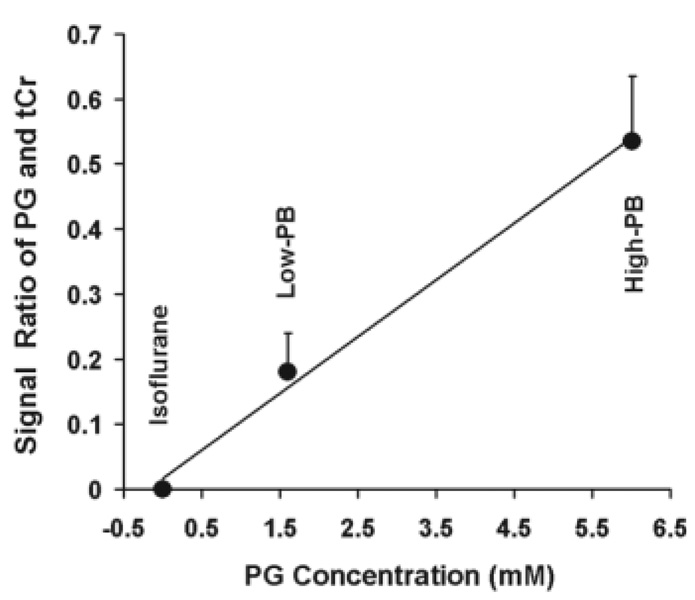
Figure 6 displays a linear relationship between the signal intensity ratio of PG at 1.13 ppm and tCr at 3.01 ppm and the PG concentration quantified by LCModel fitting.
FIG. 6.
Correlation of peak integral ratios of PG at 1.13 ppm and tCr at 3.01 ppm versus the absolute PG concentrations quantified by LCModel.
DISCUSSION
Quantification of Cerebral Glc Under Pentobarbital Anesthesia
Due to low pentobarbital concentration and rapid Eth consumption in the rat brain, these two compounds are almost invisible in the in vivo 1H spectra of brain acquired in the present study; in contrast, the PG solvent provides intense proton signals across a wide range of chemical shift (2,4,5). In the present study, we found that the presence of PG signal significantly influences quantification of lactate (Lac), Glc and glutathione, which have low concentrations (~1 mM) and which resonances partially overlap with the PG resonances in the rat brain under deep pentobarbital anesthesia (High-PB). The concentrations of those metabolites were overestimated by the LCModel fitting if the PG resonance peaks were not considered and corrected in LCModel fitting. One example of Glc quantification shown in Fig. 4a suggests a very large error (> 2-fold) in quantifying [Glc] under the High-PB condition if the PG contribution was not taken into consideration.
Another challenge faced by the quantification of absolute metabolite concentration is how to choose an internal concentration reference using a physiologically stable metabolite. The common practice is to use [tCr] as a standard reference, which was assumed as a constant of 8 mM under all the anesthesia conditions in this study. However, this quantification approach can become problematic if [tCr] changes under various anesthesia conditions. We have tested this question using the results from the present study and found no statistical differences between the tCr peak (3.01 ppm) integral measured under the light isoflurane anesthesia and that under Low-PB, as well as High-PB, condition (P > 0.05; n = 8, unpaired two-tail t test). This is evident from the following quantification results:
where, Stcr is the integral of tCr resonance peak quantified by the Varian software package. The results indicate that [tCr] remains constant under a wide range of physiologic conditions and, thus, the tCr resonance peak provides a reliable and stable internal reference for in vivo proton MRS quantification of other brain metabolites.
Alterations in Cerebral Metabolites Under Deep Anesthesia
The cerebral metabolism is tightly correlated to brain function and neuronal activity (26). Generally, increased brain activities require higher brain energy demands, resulting in a decrease of Glc concentration and possibly an increase of Lac concentration in the brain (19,27,28). In addition, an increase in brain activity also leads to increased neurotransmitter cycling rates (24,29), which may ultimately change the concentrations of neurotransmitters such as glutamate/glutamine and γ-aminobutyric acid (28,30,31). For instance, the transient increases in the concentrations of lactate and glutamate, accompanied with the tendency of concentration decrease in Glc, have been observed in the human visual cortex during sustained visual stimulation (19,27,28,32,33). It was also reported that there are significant changes in a number of brain metabolites and neurotransmitters, including the ratio of PCr versus Cr, γ-aminobutyric acid, glutamine, and glutamate, as well as neurotransmission cycling rate at near-freezing body temperature in hibernating mammals (31). In the present study, the results based on the LCModel fitting of each animal proton MRS data (Table 1 and Fig. 3) reveal that besides a significant increase of PG concentration when the pentobarbital dose was increased, three compounds, γ-aminobutyric acid, taurine, and Glc, were found to be significantly decreased (P < 0.05, unpaired two-tail t test) at the isoelectric state as compared to other two anesthesia states (isoflurane and Low-PB). The concentrations of γ-aminobutyric acid and Glc are relatively low in the brain, resulting in low signal-to-noise ratios and large LCModel fitting errors, indicated by the high values of Cramer-Rao lower bounds, in particular, under the isoelectric condition (Table 1). To reduce the uncertainty of spectral fitting results, the spectra were summed from all rat measurements, respectively, for the three anesthesia conditions (isoflurane, Low-PB, High-PB), and each summed spectrum from each of the three groups was fitted with LCModel again. When doing so, only the Glc and PG changes were found to be statistically significant and had the same trends as those found in individual-animal analysis, as shown in Table 1 and Fig. 3.
Interestingly, there were no statistically significant changes in the concentrations of Lac and neurotransmitters such as glutamate/glutamine while brain activity was significantly depressed, especially at the isoelectric state, in which the spontaneous EEG activity is completely suppressed. Therefore, one should be cautious to interpret the concentration changes associated with brain bioenergetic changes. On the other hand, the cerebral metabolic rates (e.g., CMRglc) may provide a more informative measure to evaluate brain activity and energy changes in the anesthetized rat model.
Alterations in CMRglc Under Various Anesthesia Conditions and Neurometabolic Coupling
The brain Glc concentration, [Gi], gradually decreased from the isoflurane to High-PB anesthesia state only if the PG resonance peaks between 3.1 and 4.0 ppm were accounted for in the LCModel fitting (see Figs. 3 and 4). [Gi] showed an increase under deep anesthesia condition if the contaminations from the PG resonance peaks were not corrected in LCModel fitting. This quantification error could result in an overestimated [Gi] (Fig. 4a) and consequently to an underestimated CMRglc value at the isoelectric condition leading to a large decrease of 89% in the measured CMRglc as compared to that measured under the isoflurane anesthesia condition (Fig. 4b). In contrast, the percentage of CMRglc decrease became 37% after the correction of PG contribution.
The observation of reduction in the brain Glc concentration when the brain EEG activity was suppressed under the isoelectric condition in the present study seemingly contradicts with other brain activation studies showing a decreased brain Glc concentration when the brain activity level was increased by brain stimulation (19,27,28,34). However, this apparent discrepancy can be explained by the blood plasma Glc concentration change, which was substantially decreased under the isoelectric condition in the present study (Fig. 4a). Since the brain Glc concentration is tightly coupled to the plasma Glc concentration and is regulated by the Glc transportation across the blood-brain barrier according to Eqs. 1 and 2, a significant reduction in plasma Glc level could lead to a large decrease in brain Glc concentration, as observed in the present study.
Several lines of evidence support our observation of brain Glc reduction under the isoelectric condition. First, it has been shown that a concentration gradient between the plasma and brain Glc concentrations was preserved under the deep pentobarbital anesthesia condition, showing a much larger [G0] than that of [Gi] with a [G0]/[Gi] ratio about 3 to 4 (24). The measured [G0]/[Gi] ratios under isoflurane (4.9 ± 1.0), Low-PB (4.3 ± 1.0), and High-PB (4.3 ± 1.6) conditions in the present study are in line with this range. Second, we have applied the quantitative relationship between steady-state [G0] and [Gi] as reported in the literature (24,35) to estimate the values of [Gi] based on the [G0] values measured in the present study, and the estimated [Gi] values were 2.3 mM, 1.3 mM, and 0.7 mM under the isoflurane, Low-PB, and High-PB anesthesia condition, respectively, which are in good agreement with our LCModel fitting results with PG correction (Table 1 and Fig. 3). Third, the measured ratios of CMRglc/Tmax were from 0.3 to 0.4 for the isoflurane and Low-PB anesthesia conditions. They are similar to the value of CMRglc/Tmax = 0.38, measured under α-chloralose anesthesia conditions (35).
One important finding from the present study is that the CMRglc value remained 63% under the isoelectric condition compared to the isoflurane anesthesia condition (Fig. 5b). This result suggests a significant amount of brain energy in the absence of brain spike activity; and this “housekeeping” energy is essential for maintaining cellular integrity in the brain. Note that the CMRglc measurement in the present study reflects the total metabolic rate, which includes oxidative and nonoxidative Glc metabolism in both neuronal and glial cells. The reduction in total (or oxidative) CMRglc in the rat brain by deep pentobarbital anesthesia was reported to be 40 ~ 64% compared to nitrous oxide analgesia, light α-chloralose anesthesia, or the awake conditions (24,36,37). This metabolic rate reduction is consistent with other measurements showing a 61% reduction in the cerebral metabolic rate of oxygen (38) and a 48% reduction in the cerebral metabolic rate of ATP under the isoelectric condition compared to the light isoflurane condition (11).
However, one should be cautious when quoting the “housekeeping” energy as the percentage of the total brain energy budget since the value of total brain energy depends on the brain state referred. In the present study, the reference brain state was based on the light isoflurane anesthesia condition, in which the Glc metabolic rate has been significantly suppressed compared to an awake condition. For instance, cerebral metabolic rate of oxygen was reduced 35% in the dog brain under anesthesia with 2% end-tidal isoflurane concentration compared to an awake state (39). Similarly, CMRglc was found to reduce 41% in the rat cortical regions covering the motor, visual, auditory, and somatosensory cortices under anesthesia with 1.5% end-tidal isoflurane concentration (averaged CMRglc = 0.53 µmol/g/min) compared to an awake state (averaged CMRglc = 0.90 µmol/g/min) (40). To consider this reduction (assuming approximately 41%) effect in the cerebral metabolic rates between the isoflurane and awake conditions, the “housekeeping” brain energy left under the isoelectric state determined by CMRglc measurement in our study became ~37% in comparison with that in the awake brain. In the absolute quantification scale, the CMRglc value in the rat under the isoelectric condition was estimated to be 0.33 µmol/g/min, which was in an excellent agreement with the values of oxidative CMRglc measured by in vivo 13C MRS under the isoelectric condition with High-PB (24,37) and the autoradiographic result of total CMRglc from 0.26 to 0.33 µmol/g/min in the sensory cortices (41). It has been suggested that a substantial portion of this Glc metabolic activity under the isoelectric condition may be attributed from the glial cell (24,37). Moreover, there is a decent agreement (in both absolute and relative scales) between the total (oxidative plus nonoxidative) CMRglc values estimated by this study and by autoradiographic measurements under the isoelectric condition (40,41) and the oxidative CMRglc values measured by in vivo 13C MRS (24,37) and other indirect measurements (11,38). This similarity suggests that a significant amount of “housekeeping” brain energy is produced by oxidative Glc metabolism.
The relatively small portion of “housekeeping” brain energy estimated in this study is consistent with the notion that the majority of brain energy is used to support brain activity and neuronal signaling in a resting and awake brain (11,26,42,43). Moreover, CMRglc is tightly correlated to SEI under varied anesthesia conditions (Fig. 5b). This result indicates a tight neurometabolic coupling across a wide range of brain physiologic conditions, although SEI only provides a relative measure of spontaneous brain activity level.
This coupling relation can be quantified by a linear approximation as described by Eq. 3:
| [3] |
In Eq. [3] and Fig. 5b, the CMRglc values were relative to the isoelectric CMRglc value (= 0.33 mol/g/min), which was estimated in the present study and reported in the literature (24).
It is interesting to note that if [Gi] was quantified without using PG correction in the LCModel fitting, it could lead to an overestimated [Gi], ultimately, an underestimated CMRglc under the isoelectric condition with a large reduction of 89% compared to the isoflurane anesthesia condition (Fig. 4b). This could result in an unreasonably smaller “housekeeping” energy of ~6% compared to the awake condition. These comparisons indicate again the importance of correcting PG contaminations for reliable quantification of the brain Glc concentration after the application of pentobarbital injection solution.
PG as a Biomarker Reflecting Anesthesia Depth, Brain Activity, and Bioenergetics
The PG concentration can approach up to 6 mM in the rat brain under the High-PB anesthesia condition (Table 1 and Fig. 3). This unique solvent provides an intense, stable, well-resolved resonance peak at 1.13 ppm, which can be robustly detected by in vivo proton MRS and readily quantified by using LCModel fitting or manual integration (Fig. 6). Therefore, the brain PG concentration can be conveniently measured and quantified in a clinic MRI/MRS scanner.
Our results clearly indicate that the brain PG concentration is tightly correlated to the spontaneous EEG activity level quantified by SEI, as well as to the cerebral metabolic activity level quantified by relative CMRglc (Fig. 5a). These findings lead to the conclusion that the PG resonance peak at 1.13 ppm closely reflects the pentobarbital concentration in the brain, and it provides a reliable and quantitative biomarker to link the levels of both EEG and brain metabolic activities, as well as anesthesia depth with varied pentobarbital dosage.
CONCLUSIONS
We used in vivo proton MRS to measure the changes in the concentration of cerebral metabolites and the rate of Glc utilization under various depths of pentobarbital anesthesia. The results suggest that (1) there is a close relationship between brain PG solvent and pentobarbital content; (2) the PG resonance peak at 1.13 ppm can serve a sensitive biomarker for noninvasively monitoring varied pentobar-bital anesthetic depth and associated changes in brain bioenergetics and EEG activity; (3) it is critical to correct the PG signal contributions in spectral quantification, in particular, for those metabolites having resonance peaks between 3.1 and 4.0 ppm; (4) the brain EEG and cerebral metabolic activities are tightly coupled to one another, as well as to the anesthesia depth; and (5) there is a significant amount of “housekeeping” brain energy under the isoelectric state for maintaining cellular integrity.
ACKNOWLEDGMENTS
The authors thank Dr. Kamil Ugurbil for his support. This work was in part supported by the W. M. Keck Foundation.
Grant sponsor: National Institutes of Health; Grant numbers: NS39043, NS41262, P41 RR08079, and P30 NS057091.
REFERENCES
- 1.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iltis I, Marjanska M, Du F, Koski DM, Zhu XH, Ugurbil K, Chen W, Henry PG. 1H MRS in the rat brain under pentobarbital anesthesia: accurate quantification of in vivo spectra in the presence of propylene glycol. Magn Reson Med. 2008;59:631–635. doi: 10.1002/mrm.21502. [DOI] [PubMed] [Google Scholar]
- 3.Lundbom NM, Manner T, Komu M, Peltola O, Leino KA, Kirvela OA. Barbiturate anesthesia and brain proton spectroscopy. AJNR Am J Neu-roradiol. 1999;20:1543–1546. [PMC free article] [PubMed] [Google Scholar]
- 4.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 5.Oakden WK, Noseworthy MD. Propylene glycol is essential in the LCModel basis set for pediatric 1H–MRS. J Comput Assist Tomogr. 2005;29:136–139. doi: 10.1097/01.rct.0000145860.13963.2f. [DOI] [PubMed] [Google Scholar]
- 6.Knodell RG, Spector MH, Brooks DA, Keller FX, Kyner WT. Alterations in pentobarbital pharmacokinetics in response to parenteral and enteral alimentation in the rat. Gastroenterology. 1980;79:1211–1216. [PubMed] [Google Scholar]
- 7.Haase A, Frahm J, Matthaei D, Hanicke W, Merboldt KD. FLASH imaging. Rapid NMR imaging using low flip angle pulses. J Magn Reson. 1986;67:258–266. doi: 10.1016/j.jmr.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 9.Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 10.de Graaf RA, Luo Y, Garwood M, Nicolay K. B1-insensitive, single-shot localization and water suppression. J Magn Reson B. 1996;113:35–45. doi: 10.1006/jmrb.1996.0152. [DOI] [PubMed] [Google Scholar]
- 11.Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U|S|A. 2008;105:6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruhn J, Lehmann LE, Ropcke H, Bouillon TW, Hoeft A. Shannon entropy applied to the measurement of the electroencephalographic effects of desflurane. Anesthesiology. 2001;95:30–35. doi: 10.1097/00000542-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 14.Henry PG, Marjanska M, Walls JD, Valette J, Gruetter R, Ugurbil K. Proton-observed carbon-edited NMR spectroscopy in strongly coupled second-order spin systems. Magn Reson Med. 2006;55:250–257. doi: 10.1002/mrm.20764. [DOI] [PubMed] [Google Scholar]
- 15.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Hwang JH, Graham GD, Behar KL, Alger JR, Prichard JW, Rothman DL. Short echo time proton magnetic resonance spectroscopic imaging of macromolecule and metabolite signal intensities in the human brain. Magn Reson Med. 1996;35:633–639. doi: 10.1002/mrm.1910350502. [DOI] [PubMed] [Google Scholar]
- 17.Pfeuffer J, Tkac I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time 1H NMR spectra of the rat brain. J Magn Reson. 1999;141:104–120. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- 18.Lund-Andersen A. Transport of glucose from blood to brain. Physiol Rev. 1979;59:305–352. doi: 10.1152/physrev.1979.59.2.305. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Novotny E, Zhu XH, Rothman D, Shulman RG. Localized 1H NMR measurement of glucose consumption in human brain during visual stimulation. Proc Natl Acad Sci U|S|A. 1993;90:9896–9900. doi: 10.1073/pnas.90.21.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gjedde A, Wienhard K, Heiss WD, Kloster G, Diemer NH, Herholz K, Pawlik G. Comparative regional analysis of 2-fluorodeoxyglucose and methylglucose uptake in brain of four stroke patients. With special reference to the regional estimation of the lumped constant. J Cereb Blood Flow Metab. 1985;5:163–178. doi: 10.1038/jcbfm.1985.23. [DOI] [PubMed] [Google Scholar]
- 21.Mori K, Maeda M. Use of 3H methylglucose and 14C iodoantipyrine to determine kinetic parameters of glucose transport in rat brain. Am J Physiol. 1997;272(1 pt 2):R163–R171. doi: 10.1152/ajpregu.1997.272.1.R163. [DOI] [PubMed] [Google Scholar]
- 22.Choi IY, Lee SP, Kim SG, Gruetter R. In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J Cereb Blood Flow Metab. 2001;21:653–663. doi: 10.1097/00004647-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 23.LaManna JC, Harik SI. Regional comparisons of brain glucose influx. Brain Res. 1985;326:299–305. doi: 10.1016/0006-8993(85)90039-3. [DOI] [PubMed] [Google Scholar]
- 24.Choi IY, Lei H, Gruetter R. Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: on the correlation of total glucose consumption with glutamatergic action. J Cereb Blood Flow Metab. 2002;22:1343–1351. doi: 10.1097/01.WCB.0000040945.89393.46. [DOI] [PubMed] [Google Scholar]
- 25.Gjedde A, Rasmussen M. Pentobarbital anesthesia reduces blood-brain glucose transfer in the rat. J Neurochem. 1980;35:1382–1387. doi: 10.1111/j.1471-4159.1980.tb09013.x. [DOI] [PubMed] [Google Scholar]
- 26.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 27.Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, Howseman A, Hanstock C, Shulman RG. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U|S|A. 1992;88:5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangia S, Tkac I, Gruetter R, Van de Moortele PF, Maraviglia B, Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab. 2007;27:1055–1063. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- 29.Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutama-tergic neuronal activity. Proc Natl Acad Sci U|S|A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu S, Yang J, Li CQ, Zhu W, Shen J. Metabolic alterations in focally activated primary somatosensory cortex of alpha-chloralose-anesthe-tized rats measured by 1H MRS at 11.7 T. Neuroimage. 2005;28:401–409. doi: 10.1016/j.neuroimage.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Henry PG, Russeth KP, Tkac I, Drewes LR, Andrews MT, Gruetter R. Brain energy metabolism and neurotransmission at near-freezing temperatures: in vivo 1H MRS study of a hibernating mammal. J Neurochem. 2007;101:1505–1515. doi: 10.1111/j.1471-4159.2007.04514.x. [DOI] [PubMed] [Google Scholar]
- 32.Sappey-Marinier D, Deicken RF, Fein G, Calabrese G, Hubesch B, Van Dyke C, Dillon WP, Davenport L, Meyerhoff DJ, Weiner MW. Alterations in brain phosphorus metabolite concentrations associated with areas of high signal intensity in white matter at MR imaging. Radiology. 1992;183:247–256. doi: 10.1148/radiology.183.1.1549681. [DOI] [PubMed] [Google Scholar]
- 33.Tuunanen PI, Murray IJ, Parry NR, Kauppinen RA. Heterogeneous oxygen extraction in the visual cortex during activation in mild hypoxic hypoxia revealed by quantitative functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2006;26:263–273. doi: 10.1038/sj.jcbfm.9600186. [DOI] [PubMed] [Google Scholar]
- 34.Sappey-Marinier D, Calabrese G, Fein G, Hugg JW, Biggins C, Weiner MW. Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1992;12:584–592. doi: 10.1038/jcbfm.1992.82. [DOI] [PubMed] [Google Scholar]
- 35.Lei H, Gruetter R. Effect of chronic hypoglycaemia on glucose concentration and glycogen content in rat brain: a localized 13C NMR study. J Neurochem. 2006;99:260–268. doi: 10.1111/j.1471-4159.2006.04115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koga K, Miura I. A measurement of cerebral glucose uptake rate by 31P MRS. Biochem Biophys Res Commun. 1988;157:1258–1263. doi: 10.1016/s0006-291x(88)81010-6. [DOI] [PubMed] [Google Scholar]
- 37.Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyder F, Kennan RP, Kida I, Mason GF, Behar KL, Rothman D. Dependence of oxygen delivery on blood flow in rat brain: a 7 tesla nuclear magnetic resonance study. J Cereb Blood Flow Metab. 2000;20:485–498. doi: 10.1097/00004647-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Stullken EH, Jr, Milde JH, Michenfelder JD, Tinker JH. The nonlinear responses of cerebral metabolism to low concentrations of halothane, enflurane, isoflurane, and thiopental. Anesthesiology. 1977;46:28–34. doi: 10.1097/00000542-197701000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Ori C, Dam M, Pizzolato G, Battistin L, Giron G. Effects of isoflurane anesthesia on local cerebral glucose utilization in the rat. Anesthesiology. 1986;65:152–156. doi: 10.1097/00000542-198608000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Sakabe T, Tsutsui T, Maekawa T, Ishikawa T, Takeshita H. Local cerebral glucose utilization during nitrous oxide and pentobarbital anesthesia in rats. Anesthesiology. 1985;63:262–266. doi: 10.1097/00000542-198509000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]