Abstract
Multiple organs contribute to the development of peripheral insulin resistance, with the major contributors being skeletal muscle, liver, and adipose tissue. Because insulin resistance usually precedes the development of type 2 diabetes mellitus (T2DM) by many years, understanding the pathophysiology of insulin resistance should enable development of therapeutic strategies to prevent disease progression. Some subjects with mitochondrial genomic variants/defects and a subset of lean individuals with hereditary predisposition to T2DM exhibit skeletal muscle mitochondrial dysfunction early in the course of insulin resistance. In contrast, in the majority of subjects with T2DM the plurality of evidence implicates skeletal muscle mitochondrial dysfunction as a consequence of perturbations associated with T2DM, and these mitochondrial deficits then contribute to subsequent disease progression. We review the affirmative and contrarian data regarding skeletal muscle mitochondrial biology in the pathogenesis of insulin resistance and explore potential therapeutic options to intrinsically modulate mitochondria as a strategy to combat insulin resistance. Furthermore, an overview of restricted molecular manipulations of skeletal muscle metabolic and mitochondrial biology offers insight into the mitochondrial role in metabolic substrate partitioning and in promoting innate adaptive and maladaptive responses that collectively regulate peripheral insulin sensitivity. We conclude that skeletal muscle mitochondrial dysfunction is not generally a major initiator of the pathophysiology of insulin resistance, although its dysfunction is integral to this pathophysiology and it remains an intriguing target to reverse/delay the progressive perturbations synonymous with T2DM.
The aim of this review is to characterize the role of mitochondrial biology in the pathogenesis of skeletal muscle insulin resistance and to explore potential therapeutic options to intrinsically modulate mitochondria as a strategy to combat insulin resistance. After reviewing the literature we conclude that skeletal muscle mitochondrial dysfunction is not generally a major initiator of the pathophysiology of insulin resistance, although its dysfunction is integral to disease progression. Furthermore, the manipulation of mitochondrial function remains an intriguing target to reverse/delay the progressive perturbations synonymous with T2DM.
- I. Introduction
- A. The potential role of mitochondria in the pathophysiology of skeletal muscle insulin resistance
- B. A brief overview of mitochondrial regulation and function
- II. Skeletal Muscle Mitochondrial Biology in Insulin-Resistant and Diabetic Subjects
- A. Insulin-resistant offspring of T2DM parents and prediabetic subjects
- B. Established type 2 diabetes mellitus
- C. The polycystic ovary syndrome and insulin resistance
- D. Genetic linkage between mitochondrial biology and diabetes
- III. Mitochondrial Disruption in Association with Risk Factors Predisposing to Insulin Resistance
- A. Nutrient excess as a trigger of mitochondrial dysfunction in the pathophysiology of diabetes
- B. Physical inactivity orchestrated diminution in skeletal muscle oxidative metabolic capacity
- C. Senescence, insulin resistance, and muscle mitochondrial function
- IV. Molecular Manipulation of Mitochondrial Metabolism and Effects on Skeletal Muscle and Systemic Insulin Resistance
- A. Genetic disruption of the regulatory programs controlling skeletal muscle mitochondrial homeostasis and effects on insulin resistance
- B. Skeletal muscle fatty acid uptake and storage as putative mediators of insulin signaling
- C. Efficiency of fatty acid oxidation and insulin signaling
- D. Uncoupling of oxidative phosphorylation
- E. Glucose uptake and utilization defects in disruption of mitochondrial function
- V. Interventions to Improve Skeletal Muscle Mitochondrial Function and Effects on Insulin Signaling
- A. Pharmacological and biological activation of mitochondrial biogenesis
VI. Conclusions and Considerations
I. Introduction
A. The potential role of mitochondria in the pathophysiology of skeletal muscle insulin resistance
Understanding the integrated pathophysiology initiating the development of insulin resistance should broaden our capacity to identify novel therapeutic targets for the prevention and/or treatment of type 2 diabetes mellitus (T2DM). This biology remains incompletely characterized, in part, due to the interaction of multiple organ systems and numerous intracellular perturbations within these organs (including disrupted signaling and metabolic alterations) governing the development of insulin resistance (1). The complexity of this biology is further underscored by the progressive changes in the systemic milieu including the onset of hyperinsulinemia, elevated circulating free fatty acids and triglycerides, hyperglycemia, and the activation of systemic immune system during the development of T2DM (2).
Skeletal muscle is integral to the development of insulin resistance and is a major reservoir for postprandial glucose storage (3,4,5). In skeletal muscle, disruption of mitochondrial biology is evident in some insulin-resistant subjects years before they develop diabetes (6,7,8). Furthermore, the disruption in skeletal muscle mitochondria is recognized as a sine qua non in established T2DM (9,10). However, whether perturbations in this organelle are central to the pathophysiology of insulin resistance in skeletal muscle is robustly debated (11,12,13,14). Moreover, in light of the lack of macrovascular benefit of exclusively targeting glucose control in T2DM (15,16), and the strong association of elevated insulin levels with T2DM complications, the need to better understand the pathophysiology of insulin resistance is of immense clinical and therapeutic importance (17).
The fundamental questions that will be addressed in this review are: 1) whether the disruption of skeletal muscle mitochondria is a primary component in the pathophysiology of skeletal muscle insulin resistance; 2) conversely, whether mitochondrial dysfunction is rather a consequence of reduced aerobic activity and/or systemic factors in the pathophysiology of insulin resistance; 3) the potential roles/interactions of metabolic pathways within mitochondria in the development of insulin resistance; and 4) the adaptive local and systemic responses to the disruption of skeletal muscle mitochondrial and metabolic functioning.
B. A brief overview of mitochondrial regulation and function
The mitochondrial biogenesis regulatory program is defined as the intergenomic control of mitochondrial turnover, functional content, and number required to maintain diverse homeostatic demands across tissue types (reviewed in Refs. 18 and 19). In skeletal muscle, the relative abundance of mitochondria mirrors the skeletal muscle subtype with the most mitochondria in slow-twitch (type I fibers) and less mitochondria in fast-twitch (type II) fibers.
Mitochondrial biogenesis requires concordant and integrated regulation between the mitochondrial and nuclear genomes to generate the multisubunit complexes of the respiratory apparatus and the other proteins necessary for homeostatic mitochondrial functions. In skeletal muscle, these include: oxidative phosphorylation, calcium homeostasis, reactive oxygen species (ROS) biology, apoptosis and thermogenesis via mitochondrial uncoupling. The molecular conductors of intergenomic regulation of mitochondrial biogenesis include the nuclear regulatory proteins—nuclear respiratory factors 1 and 2, the cAMP response element binding protein, transcription factor A of mitochondria, and the mitochondrial transcription factor B isoforms, which together coordinately regulate genes encoding for the electron transfer chain (ETC) complexes (20,21,22,23). In turn, the upstream transcriptional coactivator peroxisome proliferator-activated receptor (PPAR) γ coactivator 1 (PGC1) α and β and the PGC1-related coactivator modulate these nuclear regulatory proteins to subsequently activate target genes encoding regulatory enzymes of oxidative phosphorylation (24,25,26) and genes that modulate antioxidant defenses, including nuclear-encoded mitochondrial antioxidant enzymes and the uncoupling proteins (UCPs) (27).
The biochemical/hormonal modulators of this program are not restricted to, but include ROS, nitric oxide, hypoxia, and thyroid hormones (28,29,30,31). Furthermore, substrate availability and insulin itself modulate the metabolic and molecular functioning of mitochondria, and these effects will be expanded upon in Section III. A (32,33,34).
Furthermore, autophagy has recently been shown to be integral to the catabolism of intracellular lipid stores (macrolipophagy) (35). Although not yet explored in skeletal muscle, this novel observation may be important in lipid overload-mediated mitochondrial dysfunction in skeletal muscle.
The contribution of mitochondrial dysfunction to the pathophysiology of insulin resistance and diabetes is not only robustly debated but also may be operational at various levels (12,36,37). These will be expanded on in context throughout the review. In brief, these include: 1) changes at the level of the mitochondrial genome (copy number/mutations), which may be instrumental in substrate utilization and ROS generation; 2) perturbation in the content and activity of the oxidative phosphorylation machinery within mitochondria, which may effect similar metabolic pathways; and 3) modifications in the efficiency of ATP production (i.e., mitochondrial coupling vs. the utilization of reducing equivalents in the generation of heat).
II. Skeletal Muscle Mitochondrial Biology in Insulin-Resistant and Diabetic Subjects
One hypothesis with respect to the role of the skeletal muscle mitochondria in the development of T2DM states that primary defects in skeletal muscle biogenesis and/or homeostasis disrupt substrate utilization, which leads to the generation of “toxic” fatty acid intermediates that disrupt insulin signaling (9,38,39,40). This mitochondrial deficit hypothesis implicates this organelle as primal in the pathophysiology of insulin resistance and T2DM (41) and is explored in Section II. A–D.
A. Insulin-resistant offspring of T2DM parents and prediabetic subjects
To evaluate whether perturbations in skeletal muscle mitochondrial biology are evident in subjects at risk for diabetes, investigators have studied offspring of subjects with T2DM. Lean offspring of diabetic patients have been shown to have a significant reduction in insulin-stimulated nonoxidative glucose metabolism in parallel with elevated intramyocellular lipid (IMCL) content (8). Compared with controls, these subjects also show reduced skeletal muscle ATP synthesis in response to insulin stimulation as measured by magnetic resonance spectroscopy (MRS) saturation transfer. These measurements reflect impaired baseline activity of mitochondrial oxidative phosphorylation (8,42). Insulin-resistant offspring of diabetic parents also show a reduction in skeletal muscle mitochondrial density and content (43). The lean offspring of diabetic parents in these studies may represent a narrowly selected cohort of individuals that do not necessarily reflect the early pathophysiology of T2DM in this heterozygous disease process. In addition, the measurement of basal oxidative phosphorylation flux does not reflect maximal oxidative capacity of skeletal muscle, and whether baseline perturbations are sufficient to initiate the development of skeletal muscle insulin resistance has been questioned (12,44).
Similarly, employing mRNA array analysis to investigate skeletal muscle gene expression profiles comparing normal control, offspring of diabetic subjects, and established T2DM subjects, the expression of nuclear respiratory factor-1, a key regulator of mitochondrial biogenesis, was reduced in diabetic patients, and the expression of the transcriptional coactivators PGC-1α and PGC-1β was down-regulated in nondiabetic individuals with a positive family history for diabetes (7). Remarkably, the top-ranked clusters of genes differentially expressed encode for mitochondrial metabolic processes, including proteins of mitochondrial inner membrane, respiratory chain complexes, ATP synthesis, fatty acid oxidation, tricarboxylic acid (TCA) cycle, and pyruvate kinase (7). These are all down-regulated in diabetic subjects compared with normal controls (7).
Interestingly, in subjects with normal cardiopulmonary function, maximal oxygen consumption (VO2max) correlates directly with skeletal muscle mitochondrial function (45,46). Studies show that insulin-resistant subjects with a family history of diabetes have diminished VO2max (47) and exercise endurance (48) compared with insulin-sensitive control subjects. These findings may even have clinical utility in predicting risk for diabetes as shown in a prospective study of 8633 nondiabetic men where subjects with the lowest cardiopulmonary fitness had a 3.7-fold increased risk of developing diabetes over the 6 yr study compared with the most fit subjects (49). However, the complexity of dissecting the interaction between skeletal muscle bioenergetics and insulin resistance is epitomized by these data in that these physical fitness findings could similarly support the predisposition to insulin resistance in more sedentary individuals (50).
Interestingly, overweight subjects with maintained insulin sensitivity exhibit increased lipid oxidation and the maintenance of normal myocellular lipid content (51). Conversely, nondiabetic extremely obese subjects often do exhibit insulin resistance (52) that is linked, in part, to the accumulation of fatty acid esters in skeletal muscle (53). Interestingly, here the skeletal muscle capacity to oxidize fatty acid substrates is significantly blunted (52,54). Whether this reduction in mitochondrial fatty acid β-oxidation (FAO) capacity in these individuals is a primary factor in the development of obesity and insulin resistance has not been resolved. However, at least in the short term, FAO perturbations are not restored by weight reduction. Here, after a greater than 45-kg weight reduction, insulin sensitivity was normalized without restoring defects in skeletal muscle FAO (52). Together, these studies may represent a continuum with initial adaptive mitochondrial reprogramming that becomes overwhelmed by progressive weight gain or may reflect distinct underlying genetic predispositions to fat-induced insulin resistance dependent on the adaptive capacity of mitochondrial function.
In summary, these studies show that in distinct subsets of individuals, i.e., subjects with a strong family history of T2DM or some individuals with severe obesity, perturbations in mitochondrial homeostasis and function are associated with insulin resistance. The relative contribution of these perturbations to the pathophysiological progression to established T2DM has not been definitely characterized.
B. Established type 2 diabetes mellitus
The study of skeletal muscle from diabetic individuals also shows the following: coordinate down-regulation of genes encoding oxidative phosphorylation enzymes (55); lower levels of the β-subunit of ATP synthase steady-state protein levels (56); diminished mitochondrial respiration (57); and evidence of reduced bioenergetic capacity, as illustrated by slower postexercise recovery of skeletal muscle high-energy phosphate stores compared with nondiabetic controls (58). These latter data have been confirmed, comparing overweight diabetic and nondiabetic subjects where in vivo skeletal muscle phosphocreatine recovery half-time after exercise is blunted in the diabetic subjects (59,60). Interestingly, in this cohort of obese subjects, the IMCL content was similar, comparing the diabetic and nondiabetic obese controls (60). Furthermore, in an established diabetic cohort we have shown that the VO2max is a strong indicator of the duration of diabetes and directly tracks the underlying skeletal muscle mitochondrial genomic content (61). Additional studies show that subjects with insulin resistance or T2DM have diminished type I oxidative muscle fiber content (62) and an increased skeletal muscle glycolytic to oxidative phosphorylation enzyme ratio (63). The analysis of direct respirometry to measure mitochondrial oxygen consumption in skeletal muscle aligns with this concept in that diabetic individuals have diminished complex I and II substrate-driven oxidative capacity in gastrocnemius muscle compared with controls when normalized to muscle mass; however, when normalized to mitochondrial genomic content, these respirometry differences were abolished (11). This study suggests that the function per mitochondria does not differ in diabetes, but rather that the overall skeletal muscle content of mitochondria is diminished. However, respiration in permeabilized isolated muscle mitochondria from obese diabetic subjects was reduced compared with obese nondiabetic controls when pyruvate/malate was used as substrate (57). Similarly, a study comparing basal and maximal stimulated respiration in isolated mitochondria from muscle samples of age- and BMI-matched diabetic patients, their normoglycemic first-degree relatives, and controls showed impaired mitochondrial respiration in diabetic patients compared with controls, thereby demonstrating reduced intrinsic mitochondrial function in diabetic muscle (59). These impairments seems to be independent of insulin sensitivity because first-degree relatives of diabetic patients showed a similar tendency toward impaired mitochondrial function in vivo and in vitro without any changes in glucose disposal.
An important factor in the interpretation of mitochondrial regulatory control and bioenergetic assessment in the skeletal muscle of diabetic subjects is the glycemic control and the levels of circulating insulin. Gene array analysis of skeletal muscle from diabetic individuals subjected to treatment withdrawal (poor glycemic control) and subsequent insulin therapy show down-regulation of genes encoding for mitochondrial oxidative phosphorylation when glycemic control is poor, with augmentation of these transcripts following insulin-mediated improved glycemic control (51,64). These effects of modulating glucose substrate on the measurement of mitochondrial regulatory programs is similarly suggested where differences in lipid availability and insulin sensitivity can modulate the rate of muscle ATP flux (51,65).
Another confounding factor that may not have been rigorously excluded in numerous human studies of mitochondrial biology comparing diabetic and nondiabetic subjects is body mass index (BMI). Relative adipose mass may have indirect effects on skeletal muscle mitochondrial function, and in some studies the BMI is significantly higher in T2DM subjects compared with controls (55,56). This potential confounding factor is, however, not uniform in that in other studies control and T2DM subjects have similar BMI ratios with differences in mitochondrial bioenergetic capacity (57,58,60).
Although there are numerous studies supporting the concept that a reduction in mitochondrial oxidative function and capacity are operational in subjects with established T2DM, this paradigm is by no means uncontested. Contrarian results are seen in American subjects of Asian Indian ancestry. Despite being more insulin resistant, these Asian Indian T2DM subjects have a substantially higher skeletal muscle mitochondrial genomic content and basal skeletal muscle oxidative phosphorylation capacity compared with nondiabetic Americans of European descent (66). Moreover, in these Americans of Asian Indian descent, the mitochondrial oxidative phosphorylation capacity exceeds their European counterparts irrespective of the insulin sensitivity status of the Indians (66). It is interesting to note that the higher incidence of insulin resistance found in Asian-Indian men is associated with increased hepatic triglyceride content (67). Whether their skeletal muscle mitochondrial capacity represents an adaptation to compensate for altered hepatic lipid metabolism is an intriguing concept that has not been directly explored.
The variability in mitochondrial content and function in these different studies of subjects with established T2DM may reflect: 1) the polygenic nature of T2DM with varying susceptibility to similar environmental triggers; 2) distinct stages in the progression of this pathophysiology that may range from initial adaptive to more chronic maladaptive changes; and 3) the skeletal muscle mitochondrial biology in the context of the homeostasis of the other peripheral organs that together coordinate systemic insulin sensitivity.
C. The polycystic ovary syndrome and insulin resistance
The polycystic ovary syndrome (PCOS), which affects 5–10% of reproductive-age women, is an endocrine disorder associated with insulin resistance and increased risk for the development of T2DM (68). These subjects have skeletal muscle insulin resistance (69), and disruption in skeletal muscle mitochondrial biology is beginning to be implicated in this disease process (70). Compared with age-matched nondiabetic subjects, women with the PCOS have reduced skeletal muscle expression of nuclear genes encoded for mitochondrial oxidative metabolism as well as reduced expression of the mitochondrial biogenesis regulatory coactivator PGC-1α (70). Moreover, the administration of the insulin-sensitizing thiazolidinedione pioglitazone, which has been shown to directly activate the mitochondrial biogenesis program in C2C12 myocytes (71), augments skeletal muscle expression of genes encoding for mitochondrial oxidative phosphorylation proteins in PCOS subjects (72). Despite these promising findings, the augmentation of the mitochondrial biogenesis program and the enhancement of skeletal muscle mitochondrial function has not been a uniform response to thiazolidinedione therapy. These discrepancies are reviewed in Section V. Furthermore and similarly to the cohort of insulin-resistant offspring of T2DM parents (7), these PCOS subjects exhibit both down-regulation of the mitochondrial biogenesis regulatory program and insulin resistance. However, the interaction between these two observations in this pathophysiology has not been established.
Moreover, as with offspring of subjects with T2DM, young otherwise healthy women with PCOS show a significant reduction in VO2max compared with age- and BMI-matched controls (73). This latter observation has, however, been questioned in a smaller study (74).
D. Genetic linkage between mitochondrial biology and diabetes
A genetic link is evident between mitochondrial DNA mutations and the development of T2DM in the maternally inherited diabetes and deafness (MIDD) syndrome (75). Whether mutations in the mitochondrial genome in skeletal muscle contribute toward insulin resistance in these subjects is an intriguing concept that has not been established. If this were the case, it would be expected in MIDD individuals with a high level of skeletal muscle mitochondrial genomic heteroplasmy. Furthermore, and although not proven to be causative or directly linked to mitochondrial dysfunction, the disruption in skeletal muscle glucose disposal and insulin resistance presaging pancreatic β-cell dysfunction has been shown in a cohort of subjects with MIDD (76).
A recent case report in a 37-yr-old subject with the mitochondrial MELAS syndrome (myopathy, encephalopathy, lactic acidosis, and stroke-like episodes) similarly shows reduced basal and insulin-stimulated skeletal muscle rates of ATP synthesis and recovery of high-energy phosphate stores after exercise as measured by MRS (77). Although these data do not diminish the contribution of pancreatic β-cell dysfunction as a result of mitochondrial abnormalities in this subject, they do support defective skeletal mitochondrial energetic function and capacity as a component of this subject’s impaired insulin sensitivity (77).
A relationship between mitochondrial genome DNA (mtDNA) variants and the risk of T2DM has also recently been suggested (78). Combining genetic and computational analysis, it has been calculated that approximately 20% of subjects with T2DM have mtDNA mutations (79), compared with approximately 6% in the general population (80). Although, the direct investigation of these mutations or variants in human disease requires further investigation, the role of the mtDNA in the risk for diabetes has recently been explored using elegant molecular genetic transfer techniques in rats (81). Here, the mitochondrial genome from Brown Norway rats was used to replace the mitochondrial genome of the spontaneously hypertensive rat, thereby generating a conplastic strain (81). Because this strain is genetically identical to the spontaneously hypertensive rat progenitor strain, with the exception of differences in their mtDNA, different phenotypes can be attributed directly to their mitochondrial genome. Using this approach, natural variants in mitochondrial encoded ETC proteins were identified that diminished glucose disposal in response to glucose loading (81). However, whether these variants directly modulate skeletal muscle mitochondrial biology and insulin resistance has not been directly explored.
III. Mitochondrial Disruption in Association with Risk Factors Predisposing to Insulin Resistance
The opposing hypothesis states that mitochondrial dysfunction is predominantly a consequence of risk factors associated with T2DM, rather than a primary initiator of this pathophysiology (10). This hypothesis does not preclude a subsequent role of mitochondrial dysfunction as a component of a “vicious cycle” exacerbating the consequences of insulin resistance and T2DM. The data supporting a more distal role of mitochondria in the pathophysiology of insulin resistance is discussed here.
A. Nutrient excess as a trigger of mitochondrial dysfunction in the pathophysiology of diabetes
The prevalence of T2DM is increasing rapidly in modern societies and is thought to result, in large part, from excess food consumption (82). In peripheral tissues, this excess influx of nutrients leads to ectopic lipid deposition, ROS damage, and organ dysfunction and is conceptually defined singly or in combination as glucotoxicity in response to hyperglycemia and/or lipotoxicity in response to excess fat (83).
Glucotoxicity itself has been shown to promote cell damage in cells that have a low capacity to resist glucose uptake, such as endothelial and neuronal cells (84). The cellular pathways evoked to explain glucotoxicity in these cell types result in the oversupply of electron carriers NAD(P)H and FADH2 to the mitochondria, thereby facilitating reverse electron flow at complex III of the ETC, resulting in increased mitochondrial superoxide production (84,85). Skeletal muscle attenuates excess glucose uptake via down-regulation of glucose transporters, and the role of glucotoxicity per se in the disruption of skeletal muscle mitochondrial function has not been well characterized. However, severe hyperglycemia has recently been suggested to reversibly inhibit mitochondrial respiration in skeletal muscle cultures from human subjects (86).
Severe hyperglycemia in association with hypoinsulinemia in rodents with streptozotocin-induced diabetes has been used to investigate skeletal muscle metabolic gene regulation. In these studies, several genes involved in fatty acid uptake including hormone-sensitive lipase, fatty acid binding protein 4, and carnitine palmitoyltransferase 1 (CPT-1), as well as markers of oxidative stress, such as catalase and superoxide dismutase, were induced 2 or 4 wk after the development of diabetes (87,88). The up-regulation of antioxidant encoding genes is consistent with the prior finding that these gene regulatory programs are induced as an adaptive response to prevent ROS-mediated cell damage and the progression of diabetes (89). Remarkably, whereas genes involved in oxidative stress, lipid transport, and signal transduction were significantly overrepresented among up-regulated genes in hyperglycemic mouse and rat skeletal muscle, several genes involved in oxidative phosphorylation such as cytochrome oxidase and ATP synthase subunits were significantly down-regulated (87,90). These findings support an emerging concept of a disassociation between various fat catabolic cycles and the ETC. This idea is expanded upon in Section IV.C, where the effects of excess fat on synchronous mitochondrial metabolic cycles are described (91).
The mechanisms whereby excess fat can promote mitochondrial oxidative damage are being extensively investigated. What is clear is that excess fat in the diet results in extraadipocyte fat deposition, including accumulation of IMCLs in skeletal muscle. This process is evident in lean healthy individuals fed a short-term high-fat diet (92). Intracellular fatty acids form lipid peroxides in the presence of ROS, and these in turn have been shown to oxidatively damage mitochondria (93,94). It is mechanistically feasible that IMCLs may contribute to perturbations in mitochondrial functioning in diabetes with specific relevance in obesity and aging (95). However, IMCL accumulation per se is not sufficient to evoke mitochondrial damage because their accumulation is evident in endurance athletes who concurrently have robust oxidative-phosphorylation capacity (96,97) and are generally insulin sensitive (98). A distinction between these groups is that increased IMCL content is associated with lipid peroxidation in obese subjects, in contrast to skeletal muscle IMCL accumulation without elevated lipid peroxidation in endurance athletes (99). The mechanism explaining this paradox is hypothesized to be that fat-induced obesity is associated with an inability to catabolize the excess skeletal muscle IMCL and therefore predispose to lipid peroxide formation (100). The investigation of this paradox is inferred in studies associating skeletal muscle IMCL accumulation with genes encoding fat metabolism programs in response to endurance training vs. high-fat feeding (92,101). These gene expression profile studies show an induction of genes encoding for FAO in endurance training (101) and an up-regulation of genes encoding for fatty acid storage proteins in sedentary subjects fed a high-fat diet (92). It has also been shown that high endurance athletes uncouple oxidative phosphorylation in skeletal muscle, which would support enhanced FAO and diminished oxidative stress (96,102). Uncoupling of oxidative phosphorylation in this context is where “exercise-mediated regulatory events” increase proton leak across the inner mitochondrial membrane without generating additional ATP. This reduces the electrochemical gradient across the mitochondrial inner membrane, which results in increased respiration, in an attempt to maintain the membrane potential. A consequence of this could be increased use of substrate, e.g., fatty acids (futile cycling) and a reduction in ROS generation due to the modest reduction in the inner mitochondrial membrane potential (103).
The direct effect of fatty acids on skeletal muscle mitochondrial ATP generating capacity has also been explored in mitochondria isolated from skeletal muscle biopsies from normal healthy volunteers. In an albeit ex vivo study, the fatty acid metabolite palmitoyl carnitine itself suppresses mitochondrial ATP production (104). Additionally, primary skeletal muscle cultures from T2DM subjects show decreased insulin-activated citrate synthase activity vs. controls (105). In the control subjects, this activation of citrate synthase is abolished by the presence of palmitate (105). In a separate study, volunteers fed an isoenergetic high-fat diet for 3 d showed down-regulation of genes encoding for the mitochondrial biogenesis regulators PGC-1α and -β and for genes encoding mitochondrial oxidative phosphorylation enzymes (33). Similarly, in healthy volunteers, the acute infusion of lipids promotes insulin resistance with attenuation of glucose uptake/phosphorylation in concert with reduced muscle glycogen synthesis, glucose oxidation, and the ATP synthase flux as measured by MRS (106,107). Furthermore, the accumulation of IMCL content and toxic intermediates inhibits insulin signaling via the activation of protein kinases (38,39,40,43). This inhibition of myocyte insulin signaling may in turn attenuate the mitochondrial biogenesis regulatory program and mitochondrial bioenergetics (71). Together, these studies suggest that multiple mechanisms may be operational whereby increased circulating free fatty acids and IMCL deposition directly modulate mitochondrial regulatory and respiratory functions.
The temporal effects of high dietary fat consumption on skeletal muscle mitochondrial energetics and on glucose tolerance have been investigated in rats (108). Here, after 24 h of high-fat feeding, basal skeletal muscle mitochondrial ATP synthesis rates, as measured by 31P MRS saturation transfer, were markedly depressed. Continued high-fat feeding then resulted in a restoration of the rate of ATP synthesis and a cessation in IMCL accumulation over the next few weeks. However, from approximately 4 wk onward, this dietary intervention results in progressive deterioration in ATP synthesis and a concordant increase in IMCL accumulation. Interestingly, switching back to a chow diet at the 4-wk time-point reverses the mitochondrial bioenergetic defects, IMCL accumulation, and glucose tolerance. This study supports the concept of parallel deterioration in skeletal muscle mitochondrial function with enhanced IMCL content and reduced glucose tolerance. Moreover, this study suggests a degree of plasticity in the system that enables reversibility, at least within an acute time span (108). This reversibility in mitochondrial function may additionally require numerous interventions, as illustrated in a recent study where dietary weight loss alone in human subjects did not restore skeletal muscle respiratory capacity, but the combination of dietary restriction and exercise does have salutary effects on respiratory capacity (109). A potential reason why the combination of exercise and weight loss can be synergistic in improving skeletal muscle mitochondrial functioning may include the finding that dietary restriction alone preferentially diminishes adipose tissue and hepatic lipid stores with a relative paucity of effect on skeletal muscle (110). The concept that elevated lipids can alter the molecular control of mitochondrial biogenesis has also been shown in humans, where muscle biopsies were performed before and either 6 or 48 h into a lipid infusion (111,112). Here, the acute increase in circulating free fatty acids resulted in down-regulation of transcripts encoding for PGC-1α and PGC-1β and for mitochondrial and nuclear-encoded mitochondrial genes. In contrast, a 5-d high-fat diet in healthy volunteers failed to show any effect on mitochondrial function or expression of oxidative phosphorylation gene expression (113). The modulation of mitochondrial regulatory programs and respiratory function in response to high-fat feeding is therefore not unequivocal and may reflect in part the overall dietary content, genetic background, age, and degree of mitochondrial plasticity. This discrepancy is illustrated on the one hand by the significant down-regulation of genes encoding for oxidative phosphorylation proteins in skeletal muscle in association with a modest reduction in mitochondrial enzyme activities in high-fat fed c57BL mice (33). Conversely, fat-fed insulin-resistant C57BL/6J mice, Wistar rats, obese Zucker rats, and db/db mice all show increased fatty acid oxidation and up-regulation of PGC-1α and oxidative phosphorylation genes in skeletal muscle (32,114,115). Similarly, raising free fatty acid levels in rats by a high-fat diet and concomitant heparin injections for 3 wk induced mitochondrial biogenesis that was reflected by an increase in mitochondrial DNA copy number, enzymes of the respiratory chain, and fatty acid oxidation pathway in skeletal muscle (116). Interestingly, an 8-wk high-fat diet in Wistar rats did not change skeletal muscle mitochondrial respiration, ETC protein content, or citrate synthase activity despite significant intramyocellular triglyceride accumulation, but it led to a reduction of mitochondrial copy number a and up-regulation of PGC-1α protein level (117). The thought process here is that these changes may be adaptive, at least in the short term, to compensate for the increased availability of lipids in these models (13,32) and may reflect a response to the deficit in leptin signaling in the Zucker rats and the db/db mice. The effect of fatty acids on mitochondrial biology and insulin signaling is additionally dependent, in part, on fatty acid saturation and chain length. Experimental data using a reductionist approach demonstrate the presence of polyunsaturated fatty acid channel lipids toward inert triglyceride storage and complete FAO and, conversely, that saturated fats preferentially generate “toxic” diacylglycerol (DAG) and promote insulin resistance (118,119,120). Furthermore, long-chain saturated fatty acids, but not medium-chain saturated fats, promote insulin desensitization in skeletal muscle cells (121). In addition, the short-chain fatty acid butyrate was recently demonstrated to prevent the development of diet-induced insulin resistance, possibly via induction of mitochondrial biogenesis (122). In summary, the role of fat in disrupting skeletal muscle mitochondrial regulation and function appears to depend on: 1) an individual’s underlying genetic capacity to up-regulate FAO to compensate for excess fat exposure; (2) the chronicity and the composition of the fat exposure; and 3) the activity status of the individual subject spanning from a sedentary lifestyle to one with high-intensity training.
The temporal pathophysiology of combined glucose and lipotoxicity-mediated mitochondrial dysfunction was recently delineated in mice exposed to a high-fat/high-sucrose diet (89). Mice showed skeletal muscle insulin resistance and evidence of oxidative stress 1 month into the diet, but only disrupted mitochondrial biogenesis, loss of mitochondrial structural integrity, and respiratory functioning after more prolonged high-fat/high-sucrose dietary exposure (89). Interestingly, antioxidant therapy administration to streptozotocin-induced hyperglycemic mice shows diminished skeletal muscle ROS generation and the restoration of mitochondrial integrity (89). In parallel, excess palmitate or glucose administration to muscle cells induces ROS generation and disruption of the mitochondrial biogenesis regulatory program and metabolic functioning (89). This phenotype is similarly evoked in muscle cells exposed to the combination of excess glucose and insulin (71).
Overnutrition can also include excess protein intake and excess circulating amino acids (hyperaminoacidemia). Amino acid excess is clearly linked to the development of insulin resistance (123); however, whether this modulates skeletal muscle mitochondrial biology appears not to have been explored to date.
The role of modulating dietary intake in reversing insulin resistance in skeletal muscle has been alluded to with respect to obesity and excess IMCL. Additionally, one dietary intervention that has been shown to up-regulate the skeletal muscle mitochondrial biogenesis regulatory program is caloric restriction (124,125). The caloric intake from this diet is deficient of caloric requirements, resulting in low-level biological stress (126). This stress is termed hormesis, and stress-activated programs responsive to this up-regulate the mitochondrial biogenesis regulatory program in skeletal muscle (30,109). Here, the mitochondrial biogenesis program is orchestrated in part by the up-regulation of nutrient-sensing deacetylase SIRT1, which in turn activates skeletal muscle PGC-1α (127). Caloric restriction has been shown to improve insulin sensitivity in human subjects (128), although here the direct effects on skeletal muscle mitochondrial metabolism do not appear to have been directly investigated. Furthermore, salutary effects of caloric restriction on adipose tissue, the liver, and pancreas are probably also operational in improving insulin sensitivity.
B. Physical inactivity orchestrated diminution in skeletal muscle oxidative metabolic capacity
Physical inactivity is also a well-established risk factor for the development of insulin resistance and T2DM (50,129,130). Physical inactivity is similarly associated with a reduced VO2max (131,132), diminished skeletal muscle oxidative enzymes (132,133), and decreased skeletal muscle lipoprotein lipase activities (134,135). Together, these physical inactivity-mediated reductions in oxidative metabolic capacity may directly limit skeletal muscle metabolism of circulating nutrients. This in turn is a feasible mechanism whereby primary physical inactivity could precede mitochondrial down-regulation in the promotion of insulin resistance. This hypothesis is further supported where increased physical activity restores insulin sensitivity, enhances skeletal muscle mitochondrial bioenergetic capacity, and concomitantly up-regulates the mitochondrial biogenesis regulatory program (136,137,138,139,140). The augmentation of VO2max, skeletal muscle mitochondrial content, and induction of oxidative phosphorylation enzymes is also evident when modest exercise is coupled to dietary restriction and weight loss (141). Increased physical activity has also been shown to delay the onset and/or progression of insulin resistance in large prospective clinical studies (142,143). These observations are not universal in that skeletal muscle ATP recovery in response to skeletal muscle exertion has been shown to be similar in well-controlled diabetic subjects compared with age- and BMI-matched control subjects that undergo similar and modest daily activity (144). Interestingly, in these same individuals, a modest increase in exercise resulted in improved lipid oxidation without enhancement of skeletal muscle maximal ATP generation as measured by MRS (144).
An interesting study was performed in rats separated by low or high intrinsic aerobic capacity and then bred over 11 generations (145). Rats with low aerobic capacity have diminished skeletal muscle expression of genes encoding for mitochondrial biogenesis regulatory proteins and oxidative phosphorylation enzymes in parallel with features of insulin resistance including hyperglycemia, hyperinsulinemia, and hypertriglyceridemia (145). Moreover, in rats fed a high-fat diet, exercise has been shown to reverse skeletal muscle insulin resistance (146). In mice, endurance training also activates the skeletal muscle mitochondrial biogenesis program, enhances mitochondrial oxidative capacity, and improves glucose tolerance (136). The duration and intensity of exercise may also play a role in modeling glucose tolerance and mitochondrial biology. This is illustrated where high-intensity interval training and continuous moderate-intensity exercise have the same ameliorative effects on weight loss and fat mass in rats. However, the high-intensity training showed greater benefit with respect to improved glucose tolerance and in the induction of skeletal muscle mitochondrial biogenesis (147).
C. Senescence, insulin resistance, and muscle mitochondrial function
Skeletal muscle mass and strength diminish at approximately 5% per decade, starting in the fourth decade of life (140,148). The loss of muscle mass is termed sarcopenia and is associated with a disproportionate reduction in type II vs. type I fibers (148). In parallel, the majority of evidence supports a parallel down-regulation in skeletal muscle mitochondrial oxidative capacity and function (reviewed in Ref. 149). In the context of the data presented to date, these age-associated skeletal muscle changes should associate with progressive insulin resistance. This is indeed supported by epidemiological evidence showing diminished glucose tolerance with aging (150,151,152) and impaired aging-associated skeletal muscle insulin-stimulated glucose uptake (153). In addition, the study of age-associated mitochondrial biology and glucose tolerance shows a temporal decline in skeletal muscle mitochondrial DNA content and ATP production in parallel with increasing mitochondrial oxidative damage and glucose intolerance (154). These age-associated changes are probably dependent in large part on the level of physical activity because the reduced skeletal muscle mitochondrial genomic copy number and ATP-generating capacity is clearly demonstrated in sedentary elderly individuals compared with young sedentary controls and is less pronounced in trained elderly individuals (155). Finally, comparing lean healthy young and elderly individuals, the elderly subjects are more insulin-resistant and show diminished skeletal muscle insulin-stimulated glucose uptake and mitochondrial oxidative phosphorylation capacity (95). An intriguing and alternative mechanism promoting the rise in skeletal muscle insulin resistance with aging was noted in a rat study in which older rats showed increased mitochondrial efficiency and thermodynamic coupling with a diminution in fatty acid-induced proton leak (156). This in turn is proposed to lead to a decrease in fat utilization, increased IMCL accumulation, and lipotoxicity. Interestingly in this regard, in insulin-resistant elderly human subjects with similar fat mass and BMI to younger subjects, skeletal muscle IMCL content is increased (95). An additional factor that may be operational in the down-regulation of skeletal muscle adaptive programs with aging is that the activation of the nutrient-sensing mediator of mitochondrial biogenesis, i.e., AMP kinase (AMPK), via either pharmacological treatment or exercise, is blunted in association with impaired mitochondrial biogenesis regulatory programming in old vs. young rats (157).
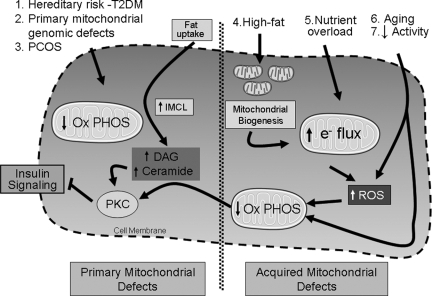
Figure 1 shows a schematic representation of the pathophysiology associated with primary skeletal muscle mitochondrial deficits evident before the onset of T2DM and secondary deficits in response to environmental cues. In light of gene polymorphism studies and genome-wide association studies implicating proteins modulating mitochondrial function as diabetogenic candidates (158,159,160), it is intriguing to postulate whether individual subjects have distinct genetic susceptibility to environmental cues in the development of insulin resistance.
Figure 1.
Mitochondrial role in the development of insulin resistance and T2DM. This schematic shows primary mitochondrial defects to the left of the hatched line in the center of the cell and the development of mitochondrial deficits in response to environmental cues and aging to the right of the hatched line. In primary disruption in the mitochondrial metabolic capacity, FAO is diminished, fat intermediates accumulate and DAG, which appears to be the primary intermediate that then activates protein kinase C isoforms. These in turn phosphorylate and inactivate numerous kinase substrates in the insulin signaling pathway. The reduced insulin sensitivity exacerbates the metabolic perturbations by reducing glucose uptake and possibly by further down-regulation of the mitochondrial biogenesis program. The etiologies of primary mitochondrial defects are labeled 1–3. The etiologies of secondary disruption of mitochondrial dysfunction are labeled 4–7. High-fat diet can promote mitochondrial biogenesis; alternatively nutrient overload, which may include both glucose and fats, enhances both lipid intermediates that facilitate oxidative damage and impair insulin signaling. Furthermore, the nutrient overload presents excess reducing equivalents to the ETC that can result in increased ROS generation. The oxidative damage, in turn, disrupts the mitochondrial oxidative capacity, which then recapitulates the phenotype of primary mitochondrial deficits promoting insulin resistance. Ox PHOS, Mitochondrial oxidative phosphorylation.
IV. Molecular Manipulation of Mitochondrial Metabolism and Effects on Skeletal Muscle and Systemic Insulin Resistance
The studies described to date suggest that the role of the mitochondria in the development of insulin resistance can be primary or secondary, depending on the subject’s underlying genetic predisposition and environmental factors. Because the integration between cellular and mitochondrial metabolism is complex, the review of both reductionist and in vivo animal studies can be used to further dissect out the role of skeletal muscle mitochondria in the pathophysiology of skeletal muscle and systemic insulin sensitivity/resistance. The ability to genetically modulate these programs in mice and, more specifically, in a skeletal muscle-restricted manner, creates a testable biological system to either validate or question the hypotheses generated by clinical studies. It is interesting to note that numerous genetic modulations of mitochondrial metabolic processes have divergent effects when comparing skeletal muscle to systemic insulin sensitivity. To contextualize these, the direct skeletal muscle effects will be initially described and then the systemic effects of these same skeletal muscle-specific perturbations will be examined. The composite of these effects also illustrates the complexity of the interaction between multiple organs in the pathophysiology of insulin resistance.
A. Genetic disruption of the regulatory programs controlling skeletal muscle mitochondrial homeostasis and effects on insulin resistance
Mitochondrial homeostasis is controlled via complex intergenomic regulatory programs as described in Section I. Studies in skeletal muscle cell lines show that transient overexpression of the “master regulator” of mitochondrial biogenesis, PGC-1α (26), increases mitochondrial content and respiration (27,71) in parallel with enhancing glucose uptake and insulin sensitivity (71,161). The direct transient electrotransfection of PGC-1α into rat skeletal muscle similarly improves mitochondrial function and insulin sensitivity (162). However, chronic and/or robust restricted overexpression of this transcriptional coactivator in skeletal muscle of transgenic mice has a somewhat complex phenotype including systemic insulin resistance (163). As one would postulate, these mice do have increased expression of genes controlling mitochondrial oxidative metabolism but also show decreased glucose transporter isoform 4 (GLUT4) expression and reduced glucose uptake (163). A second skeletal muscle PGC-1α transgenic mouse line confirms these effects in parallel with evidence of increased IMCL accumulation that appears to result from increased fatty acid uptake in excess of the capacity for FAO (164). Given this phenotype, mice with skeletal muscle-restricted PGC-1α depletion were generated (165,166). These mice show down-regulation of the mitochondrial biogenesis transcriptional machinery with parallel down-regulation of TCA cycle and ETC enzyme activities (165) and a shift from oxidative to glycolytic muscle fibers (165,166). In addition, these mice are resistant to high-fat diet-induced weight gain, and they generate more heat and have higher oxygen consumption—all factors compatible with diminished mitochondrial efficiency (165). The sum of these changes results in the preservation of systemic insulin sensitivity and again shows that it is the summation of adaptive and maladaptive changes after robust modulation of mitochondrial oxidative phosphorylation that determines the local and systemic insulin responsiveness.
An intriguing inflammatory program is also induced in these mice, where IL-6 production is up-regulated in PGC-1α-depleted skeletal muscle. Interestingly, the direct administration of IL-6 to pancreatic islet cells suppresses glucose-stimulated insulin secretion (165). Similarly in diabetic subjects with known down-regulation of genes encoding for mitochondrial oxidative proteins (55), the transcript levels of IL-6 and the cytokine TNFα are inversely correlated to the levels of PGC-1α (165). Together, these findings support an intriguing concept that diabetes-associated disruption in skeletal muscle metabolism may provoke skeletal muscle cytokine-mediated cross-talk with the pancreas as an adaptive process to retard the development of insulin resistance. Skeletal muscle production of cytokines (myokines) to mediate systemic effects is discussed in greater depth in the section reviewing modulation of glucose metabolism in skeletal muscle (Section IV. E).
This insulin responsiveness to chronic skeletal muscle manipulation of PGC-1α may, in part, reflect the broad array of transcriptional targets under the control of PGC-1α (26) and additionally reflect the plasticity of tissues to adapt to energy pathway modulations by the utilization of alternate substrates. To dissect this out further, genetic manipulation of distinct downstream transcriptional targets of PGC-1α has been investigated. The restricted skeletal muscle deletion of the mitochondrial transcriptional activator transcription factor A of mitochondria shows a phenotype similar to PGC-1α knockdown. These mice have progressive deterioration of skeletal muscle respiratory chain function in parallel with increasing skeletal muscle glucose uptake and glucose tolerance (167). Another target of PGC-1α that has been specifically characterized for insulin resistance in skeletal muscle is the overexpression of PPARα (168). Similarly to the PGC-1α transgenic mice, the PPARα transgenics have increased FAO, with repression of GLUT4 and diminished insulin-sensitive skeletal muscle glucose uptake (168). The insulin resistance that develops in these mice is associated with diminution in AMPK activity.
Taken together, these genetic studies directly modulating the transcriptional control of mitochondrial biogenesis and fatty acid oxidation show that chronic manipulation of these programs initiates adaptive and maladaptive responses that, in turn, modulate glucose uptake with a concordant effect on systemic insulin sensitivity.
The complexity of this paradigm is further confirmed in mice with skeletal muscle-restricted ablation of the mitochondrial flavoprotein apoptosis-inducing factor (AIF). This protein functions to maintain the integrity of the mitochondrial respiratory apparatus (169), and its genetic depletion results in a progressive disruption of mitochondrial respiratory function with the longer term disruption in organ integrity (170,171). Interrogation of insulin sensitivity was therefore investigated relatively early in this temporal progression to mitochondrial dysfunction in skeletal muscle-restricted AIF-ablated mice (172). These mice show significant albeit modest reductions in skeletal muscle mitochondrial respiration, in ETC gene transcripts, and ATP levels. In parallel activation of the metabolic sensor, AMPK and up-regulation of glucose metabolism were evident in association with improved systemic glucose tolerance and insulin sensitivity. Moreover, these mice had no evidence of altered global nutrient utilization (similar respiratory quotient), activity, and energy expenditure (172). In isolation, this metabolic phenotype is inconsistent with a primary contribution of skeletal muscle mitochondrial disruption in the development of insulin resistance. However, the adaptations operational in these mice include enhanced glucose utilization and reduced fat mass (172), features that are not present in the majority of human subjects with T2DM. Another interesting observation in this study is that AIF depletion does not result in the generation of excess ROS. This additionally distinguishes this mitochondrial disruption genetic model from that associated with insulin resistance and diabetes (84,89).
Collectively, the studies to date targeting nuclear regulatory control of mitochondrial metabolic functioning lend caution to the capacity to directly modulate these regulatory programs in skeletal muscle to alleviate insulin resistance. However, this paradigm is countered by the effects of skeletal muscle-restricted modulation of PPARδ (also termed PPARβ). Although expressed ubiquitously, this PPAR isoform is most abundant in skeletal muscle (173) and promotes FAO and skeletal muscle fiber type switching to enhance type I oxidative fiber development (174,175). Skeletal muscle-restricted genetic ablation of PPARδ exhibits a functional switch in skeletal muscle fiber type toward lower oxidative capacity with age-associated increase in systemic insulin resistance and glucose intolerance in association with increasing fat mass (176). Interestingly, the absence of PPARδ in skeletal muscle results in down-regulation of genes encoding for the mitochondrial biogenesis regulatory proteins and of components of the ETC (176). The central role of PPARδ in skeletal muscle oxidative metabolism and in the control of insulin sensitivity is confirmed after the generation of a skeletal muscle-specific transgenic mouse harboring the PPARδ transgene (177). These mice show enhanced type I fibers in association with an up-regulation of the mitochondrial biogenesis regulatory program, oxygen consumption, and a markedly enhanced capacity for endurance exercise. Moreover, even in the absence of exercise, these mice are resistant to high-fat-induced weight gain and significantly more glucose tolerant compared with wild-type control mice (177).
B. Skeletal muscle fatty acid uptake and storage as putative mediators of insulin signaling
Excess lipid uptake and impaired disposal may account, in part, for skeletal muscle lipid accumulation and the development of insulin resistance and diabetes. The molecular regulatory programs orchestrating the partitioning of fat into energy production, synthetic pathways, and storage in skeletal muscle are actively being investigated and will be discussed in the context of the modulation of insulin resistance.
The uptake of skeletal muscle fatty acids can be modulated by the overexpression or disruption of transport mechanisms. In skeletal muscle-restricted lipoprotein lipase knockout mice, fat uptake is diminished in parallel with enhanced skeletal muscle insulin sensitivity (178). Interestingly, the contribution of multiple organs in the pathophysiology of insulin resistance is reiterated here in that the diminution in skeletal muscle fat uptake associates with increased liver, heart, and adipose tissue fat uptake, with a resultant exacerbation of overall systemic insulin resistance (178).
The major transport proteins facilitating skeletal muscle fat uptake include the insulin-responsive CD36 and fatty acid transport proteins (179,180). To our knowledge, skeletal muscle-specific ablation of fatty acid transporters or skeletal muscle-restricted transgenic mice encoding for these proteins have not been generated. The whole body knockout of CD36 results in diminished fat uptake in skeletal muscle (181). At baseline, these mice show lower fasting glucose and insulin levels compared with controls and have enhanced glucose tolerance with lower skeletal muscle glycogen and triglyceride content (182). These mice illustrate the complex interaction between molecular defects and diet in that the CD36 null mice had similar susceptibility to high-fat-induced insulin resistance but greater insulin resistance to high fructose compared with control mice (182).
The mechanism whereby accumulation of fatty intermediates in promoting insulin resistance is due to the accumulation of “toxic” lipid intermediates including DAG and ceramide is shown in Fig. 1. The elevated DAG activates protein kinase Cε, a serine/threonine kinase that then binds to the insulin receptor and inhibits its tyrosine kinase activity (183). Normally, the neutralization of DAG is orchestrated by the microsomal enzyme acyl-coenzyme A (CoA):DAG acyltransferase (DGAT), which catalyzes the final step in triglyceride synthesis by linking DAG to long-chain acyl-CoA. It is proposed that the exercise-induced “athletes paradox” of increased IMCL stores and improved insulin sensitivity results from the up-regulation of muscle-enriched DGAT1 isoform that converts DAG intermediate into neutral stored triglyceride (184). This hypothesis has been validated in transgenic mice where skeletal muscle-specific induction of DGAT1 increased triglyceride stores and protected these mice from high-fat diet-induced insulin resistance (185). In parallel, using primary myocytes, the knockdown of DGAT1 exacerbated fat-induced insulin resistance, and its overexpression ameliorated this detrimental effect (185). The complex interplay between the multiple peripheral tissues and pathways in regulating whole body insulin sensitivity is again illustrated in that the whole body knockout of DGAT1 resulted in a paradoxical improvement in insulin sensitivity, which is thought to result from these mice having increased energy expenditure in association with the up-regulation of genes encoding for UCPs (186,187). The role of UCPs is addressed separately in Section IV. D.
C. Efficiency of fatty acid oxidation and insulin signaling
As described, after import into myocytes, fatty acids are esterified into fatty acyl-CoA and partitioned into storage, synthetic, or catabolic pathways. To generate energy, fatty acyl-CoA enters the mitochondria. The mitochondria exquisitely coordinate FAO, the TCA cycle, and the ETC to optimize ATP production (188,189). The synchronization of these fatty acid catabolic pathways in response to high fat has recently been questioned in a series of elegant studies. In skeletal muscle mitochondria isolated from rats exposed to high fat, different metabolic efficiency is noted in skeletal muscle fiber types with complete FAO in type I oxidative fibers and incomplete FAO in mitochondria isolated from fast-twitch type II fibers (138). Interestingly, it was also shown that high-fat feeding promotes incomplete FAO with a diminution in the generation of CO2 as the end-product. Conversely, exercise augments complete FAO to generate appropriate amounts of CO2. In myocyte cultures, these investigators go on to show that the overexpression of PGC-1α enhances complete FAO (138). In a further study using targeted metabolomic screening to identify these metabolic intermediates, high-fat diet increased the rate of FAO but depleted TCA organic acid intermediates, thereby rendering the TCA cycle limited in the completion of oxidative phosphorylation. This concept is linked to insulin resistance in cultured myocytes, where fat-induced insulin resistance is prevented by the inhibition of fatty acid mitochondrial uptake by the introduction of the CPT-1 inhibitor etomoxir (190). A caveat of this observation is that the reduction in FAO by etomoxir may activate AMPK, which in its own right could enhance insulin sensitivity. Collectively, these studies show that the combination of skeletal muscle fat overload coupled to a relatively diminished mitochondrial oxidative capacity due to limitations in TCA cycle metabolic intermediates appears to coalesce to promote insulin resistance.
Of note, the exact interplay between accumulation of lipid intermediates in the cytosol and the rate and completeness of FAO requires additional investigation. The balance between these programs is further illustrated where the import of fatty acids into the mitochondria via the overexpression of CPT-1 not only increased FAO but also prevented fatty acid-induced insulin resistance in myotubes and after electrotransfer of CPT-1 into rat skeletal muscle in vivo (191,192).
In stark contrast to this, the systemic knockout of malonyl-CoA decarboxylase, the enzyme that degrades the natural CPT-1 inhibitor malonyl-CoA, prevents skeletal muscle mitochondrial fat uptake and FAO in parallel with the accumulation of IMCL (190). In this mouse model in response to a high-fat diet, the knockout mice have lower systemic fasting blood glucose levels and are more glucose and insulin tolerant than the wild-type controls (190). Interestingly, this phenotype may be influenced, in part, by the fact that the malonyl-CoA decarboxylase−/− mice show an enhanced capacity for glucose oxidation, at least in the heart under stress conditions (193). Whether this is operational in other tissues such as the liver and skeletal muscle appears not to have been directly studied at this time.
D. Uncoupling of oxidative phosphorylation
Excess intramyocellular fat appears instrumental in the development of skeletal muscle insulin resistance and in the disruption of mitochondrial function. A potential strategy to dissipate this program could be via the promotion of “inefficient” oxidative phosphorylation. This counterintuitive process could deplete lipid stores via the generation of heat, and if the change in efficiency is modest, lipid depletion may be achievable without a significant reduction in ATP generation (194,195). This cellular process results from the leak of protons across the inner mitochondrial membrane into the matrix and is termed uncoupled mitochondrial respiration. The inner mitochondrial membrane UCPs are thought to be major facilitators of uncoupled respiration and may modulate the pathophysiology of diabetes (103,196,197).
UCP3 is the skeletal muscle-enriched isoform (198,199), and the skeletal muscle UCP3 protein levels are down-regulated in T2DM subjects (200) and conversely increased in response to exercise training (201). Furthermore, insulin-sensitizing exercise and lifestyle modifications restore skeletal muscle UCP3 levels in patients with T2DM, supporting a link between insulin sensitivity and regulation of this mitochondrial inner membrane protein (202). Additionally, endurance-trained individuals exhibit increased skeletal muscle uncoupled respiration (96). The role of UCP3 in skeletal muscle biology has not been definitively established, although data show that these proteins modestly uncouple oxidative phosphorylation in parallel with preferential oxidation of fatty acids (203), diminish skeletal muscle ROS generation (102,204), and are required in pathological thermogenesis (204). To test whether the induction of UCP3 could modulate insulin sensitivity, a skeletal muscle transgenic mouse line harboring the human UCP3 gene was generated (205). These mice are hyperphagic and lean with diminished adipose tissue mass, a phenotype consistent with uncoupled mitochondrial oxidative phosphorylation. Further investigation of these mice shows that they are protected against fat-induced insulin resistance and have lower intracellular DAG levels with improved insulin-responsive signal transduction compared with controls (206). An additional signaling network that probably contributes to the insulin-sensitizing effects of UCP3 overexpression is the resultant activation of AMPK shown to be present after up-regulation of UCP3 in skeletal muscle (207). The long-term response to a high-fat diet has recently been explored in these mice compared with wild-type control and to whole body UCP3 knockout mice (208). The skeletal muscle UCP3 transgenic mice are relatively resistant to weight gain in response to long-term high-fat exposure, and the UCP3−/− mice gain more weight compared with both controls and the transgenic mice. Interestingly, the enhanced glucose tolerance noted in transgenic mice in response to short-term high-fat exposure disappeared after exposure to this diet for 4 months (208). These data suggest that this adaptive mechanism can be overwhelmed by persistent fat loading. Mice with muscle-specific depletion of UCP3 have to our knowledge not been generated, and the whole body UCP3−/− mice show a paradoxical improvement in glucose tolerance similar to that achieved with the skeletal muscle UCP3 transgene (208). The possible adaptive mechanisms operational in these UCP3−/− mice have not been extensively explored.
Similarly, the ectopic overexpression of the dominant brown adipose tissue thermoregulatory UCP1 in skeletal muscle also evokes resistance to high-fat diet-induced insulin resistance in a dose-dependent manner, resulting in increased sensitivity with higher expression levels (22). In a second study, this group showed that robust overexpression of UCP1 in skeletal muscle perturbs skeletal muscle mitochondrial architecture and content, promotes IMCL accumulation, and increases the activities of AMPK and hexokinase with the concurrent up-regulation of GLUT4 (209).
In addition, when skeletal muscle UCP1 transgenic mice are crossed with genetically obese mice, diabetes and hypertension are reversed (210). Together, these UCP studies further support the concept that partial uncoupling of respiration in skeletal muscle may be useful to improve lipid-induced insulin resistance and even possibly the longer term sequelae of diabetes and vascular pathology. A putative mechanism for this ameliorative effect may stem in part from the activation of AMPK and the enhancement of glucose uptake present in these transgenic mice (211). It has not been established whether additional mechanisms, including altered mitochondrial efficiency and/or attenuation of ROS generation, are also operational.
E. Glucose uptake and utilization defects in disruption of mitochondrial function
As illustrated by prior mouse studies, a systemic adaptive response to disrupted fat metabolism is associated with activation of AMPK, accompanied by increased glucose uptake and metabolism with improved insulin sensitivity. These data suggest interdependence between glucose and fat metabolism in the development of insulin resistance and/or diabetes. Because genetic studies have also been undertaken to modulate skeletal muscle glucose biology, these are briefly explored to focus on the consequences of these perturbations on mitochondrial metabolism and insulin resistance.
GLUT4, the insulin-sensitive glucose transporter, is expressed in skeletal muscle (212), and the translocation of GLUT4 to the skeletal myocyte membrane, which facilitates glucose uptake, is impaired in insulin resistance and diabetes (213,214). Whole body genetic depletion of GLUT4 impairs skeletal muscle insulin sensitivity and glucose uptake (215,216) without altering systemic glucose tolerance (215). Adaptations associated with GLUT4 depletion include improved hepatic triglyceride clearance, increased skeletal muscle mitochondrial mass, and augmented skeletal muscle fat uptake and oxidation (217). In contrast, GLUT4 haploinsufficient mice (GLUT4+/−) show systemic insulin resistance and diabetes (218). Crossing these GLUT4 +/− mice with a fast-twitch skeletal muscle-restricted GLUT4 transgene prevents insulin resistance and diabetic complications (219). Taken together, these studies show that the direct inhibition of glucose uptake as an initial insult promotes skeletal muscle mitochondrial biogenesis, fat uptake, and oxidation (217) and that restricted rescue of glucose uptake in skeletal muscle can ameliorate the diabetic phenotype of GLUT4 knockout mice.
Augmenting skeletal muscle glucose uptake and utilization via activation of insulin signaling may also promote enhanced insulin sensitivity. This was explored by transient induction of constitutively activated Akt1 in skeletal muscle (220). Before the activation of this transgene, the mice were fed a high-fat/high-sucrose diet for 8 wk and then continued on this diet with or without transgene activation for an additional 4 wk. Transient activation of Akt1 improved glucose tolerance in parallel with an attenuation in hepatic steatosis. Gene expression data support increased skeletal muscle glucose utilization with a concordant induction of genes encoding for FAO in the liver (220). Although the activation of Akt1 did not increase physical activity or change food consumption, the activation of Akt1 was associated with increased VO2max and diminished fat mass (220). This adaptive utilization of fat by the liver is reminiscent of extramyocyte adaptations identified in the PGC-1α and GLUT4 knockout mice (165,217). In contrast to the PGC-1α knockout mice, IL-6 was not modified in the skeletal muscle after conditional activation of Akt1 (220). However, the concept that muscle-secreted factors or “myokines” may be operational in response to skeletal muscle stressors to coordinate systemic metabolic activities is beginning to be explored (221). The conditional skeletal muscle Akt1 mouse was therefore employed to identify potential candidate myokines, and the first two identified include fibroblast growth factor 21 and follistatin-like 1 factor (222,223). Their roles in modulating metabolism and insulin sensitivity are beginning to be explored, and fibroblast growth factor 21 has recently been shown to improve insulin sensitivity in the pancreas, adipocytes, and liver (224,225,226).
The composite of the studies where skeletal muscle metabolic pathways are genetically manipulated has significantly contributed to our knowledge with respect to metabolic interactions, mitochondrial biology, and local and systemic insulin resistance. The broad concepts can be summarized as follows:
In general, the long-term genetic modulation of regulatory programs directing mitochondrial biogenesis gives rise to paradoxical findings, in part due to the wide array of targets of these regulatory proteins and due to adaptive systemic responses to counter the disruption of skeletal muscle mitochondrial function.
However, specific modulation of PPARδ is completely consistent with improving glucose tolerance and insulin sensitivity in parallel with enhanced mitochondrial content and higher oxidative muscle fiber type.
Disruption in the storage of neutral lipids in the myocyte itself will disrupt insulin signaling and therefore glucose tolerance.
Incomplete FAO can arise from excessive fat import into the mitochondria, and this, too, can evoke insulin resistance.
Mitochondrial reprogramming to uncouple oxidative phosphorylation, at least in the short term, can improve insulin sensitivity in part by the energy-futile fat catabolism.
Enhancing skeletal muscle glucose oxidation either directly, or in response to the disruption of FAO can ameliorate insulin sensitivity.
A novel concept identified after the genetic disruption in skeletal muscle metabolic programs is that skeletal muscle itself possesses both innate adaptive programs and endocrine responses capable of secreting myokines that, in turn, modulate whole body fuel disposal, pancreatic islet cell insulin secretion, and systemic insulin sensitivity.
Finally, although the knowledge gained from these mouse models has significantly expanded our insight into the interaction of mitochondrial biology with insulin signaling pathways and glucose control, we need to be cognizant of the fact that these are murine models with genetic alterations in orders of magnitude different from the more modest alternations in mitochondrial programs in the insulin-resistant patient.
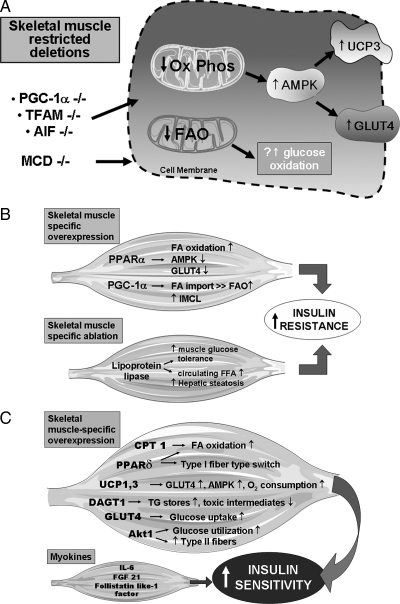
Figure 2 schematizes the major observations after skeletal muscle-restricted molecular modulation of metabolism and mitochondrial function and illustrates the adaptive and maladaptive sequelae of these distinct perturbations.
Figure 2.
Adaptive and maladaptive consequences of the molecular modulation of skeletal muscle mitochondrial biology determine overall insulin resistance. A, Skeletal muscle-restricted knockdown of mitochondrial regulatory proteins that exhibit adaptive augmentation of glycolysis, glucose oxidation, and possibly uncoupled respiration resulting in the overall improvement in insulin sensitivity and resilience to fat-induced lipid accumulation. B, Skeletal muscle-restricted overexpression of mitochondrial regulatory proteins and the skeletal muscle-restricted knockdown of a fatty acid transporter that result in the exacerbation of insulin resistance. C, Induction of various regulatory and functional mitochondrial proteins that improve insulin sensitivity. The myokines secreted by skeletal muscle are in response to the skeletal muscle-restricted deletion of PGC-1α and inducible Akt1, respectively. The myokines then moderate additional peripheral metabolic tissues to enhance insulin sensitivity. TFAM, Transcription factor A of mitochondria; FGF, fibroblast growth factor; FFA, free fatty acid; FA, fatty acid; Ox PHOS, mitochondrial oxidative phosphorylation.
V. Interventions to Improve Skeletal Muscle Mitochondrial Function and Effects on Insulin Signaling
As discussed in this review, specific restricted molecular targeting of skeletal muscle mitochondrial metabolism enhances resistance to dietary-induced insulin resistance and diabetes (177,185,190,191,206,210,220). However, it is technically challenging to administer lifestyle changes or noninvasive systemic therapeutics to specifically target skeletal muscle. Nevertheless, this limitation is not insurmountable in that the pathophysiology of insulin resistance extends beyond skeletal muscle to incorporate interactions between multiple metabolic tissues. The adaptive and maladaptive metabolic programming within these tissues, on balance, either promotes or prevents the development of systemic insulin resistance and diabetes. Thus, both skeletal muscle weighted interventions (e.g., exercise) and therapeutic strategies that function in part through modulation of skeletal muscle mitochondrial metabolism are probably valid approaches to ameliorate insulin sensitivity. Moreover, in light of the disappointing long-term cardiovascular outcomes from multicenter clinical studies exclusively targeting glucose reduction (15,16), an emphasis on improving systemic insulin sensitivity through metabolism-based therapy to reduce intracellular lipid accumulation and to enhance glucose utilization may mean more mechanism-directed approaches to therapy and this, in part, can be directed at skeletal muscle. In this section, we explore potential novel pharmacological and biological targets to modulate skeletal muscle mitochondrial bioenergetic capacity as a component of the overall strategy to prevent, reverse, and/or delay T2DM.
A. Pharmacological and biological activation of mitochondrial biogenesis
The biological interplay between skeletal muscle insulin resistance and mitochondria has also been addressed by evaluating whether therapeutic interventions to modulate mitochondrial biology can augment skeletal muscle metabolic capacity and concordantly improve systemic insulin sensitivity.
In adipose tissue, it has clearly been established that the thiazolidinedione class of diabetic agents promotes mitochondrial biogenesis (227,228). This augmentation of mitochondrial oxidative capacity may play a role in the insulin-sensitizing effects of this class of compound (229). The thiazolidinediones predominantly activate PPARγ, although they do have varying degrees of activity against other PPAR isoforms (230). In C2C12 muscle cells, the thiazolidinedione pioglitazone directly activates the mitochondrial biogenesis regulatory program, and this induction of mitochondrial energetic capacity appears to be integrally linked to improved insulin sensitivity (71). Furthermore, primary skeletal myocytes grown from T2DM subjects, which show diminished mitochondrial FAO compared with nondiabetic controls, also exhibit reversal of these mitochondrial FAO defects in response to the administration of thiazolidinedione troglitazone (231). An additional study in primary human skeletal muscle from T2DM subjects shows thiazolidinedione-mediated improvement in FAO with enhanced fat uptake in parallel with up-regulation of the fatty acid transporter CD36 (232). In diabetic animal models, the dose of pioglitazone required to enhance skeletal muscle glucose uptake is shown to be higher than required to mediate ameliorative effects in the liver (233). The in vivo effects of thiazolidinediones on skeletal muscle have been assessed in numerous human studies. Pioglitazone up-regulates the skeletal muscle mitochondrial oxidative phosphorylation gene expression profile in association with improving insulin sensitivity and glucose tolerance in young women with PCOS (72). Rosiglitazone has been shown to up-regulate skeletal muscle PGC-1α and PPARδ with concordant induction of oxidative phosphorylation enzyme activity in parallel with insulin sensitization in diabetic subjects (234), albeit without induction of mitochondrial oxidative phosphorylation protein content or a diminution in IMCL content (234). Furthermore, in newly diagnosed T2DM, the administration of rosiglitazone directly enhances skeletal muscle insulin sensitivity as assessed by glucose uptake (235). In contrast, we and others have demonstrated that in established diabetic subjects, the improvement of glycemic control in response to rosiglitazone appears to be independent of muscle mitochondrial content/activity (61,236). Interestingly, in our study rosiglitazone-mediated improvement in ETC protein content and citrate synthase activity was evident only in those subjects with relatively preserved VO2max and mitochondrial copy numbers (61). One possibility of these divergent findings could be that to enhance skeletal muscle mitochondrial biogenesis, the intervention with a thiazolidinedione is required earlier in the course of insulin resistance. This hypothesis is consistent with the diminished capacity to augment skeletal muscle mitochondrial biogenesis in aging rats (157). Of note, and in keeping with the multiorgan coordination of insulin resistance, thiazolidinediones additionally promote insulin sensitivity by decreasing hepatic steatosis (237,238). From a clinical perspective, the use of thiazolidinediones to prevent the onset of diabetes in subjects at risk has been demonstrated (239). However, the long-term use of these agents is beginning to be questioned in the light of studies suggesting detrimental effects of these agents on coronary artery disease and with respect to bone density (240,241).
In light of the very promising effects in the skeletal muscle-specific PPPARδ transgenic mice, investigators have studied the insulin-sensitizing effects of the PPARδ agonist GW501516 (242). In wild-type mice placed on a high-fat diet, a 2-month treatment period with GW501516 resulted in reduction in weight gain, increased type 1 skeletal muscle fiber type, and improved glucose tolerance compared with vehicle-treated controls (177). This ameliorative effect has also been shown in db/db mice and is thought to occur at least in part via the promotion of skeletal muscle FAO (175). A recent proof of concept study in human subjects showed that a 2-wk treatment of insulin-resistant subjects with GW501516 did improve insulin sensitivity in parallel with induction of CPT-1 gene expression in skeletal muscle (243). As recently demonstrated in human skeletal muscle cells, the responsible mechanism mediating these beneficial effects of GW501516 involves both PPARδ- and AMPK-dependent and -independent pathways (244).
Because caloric restriction improves insulin resistance and enhances mitochondrial function, studies have been performed using caloric restriction mimetics to evaluate their efficacy in moderating this pathophysiology. A central mediator of the ameliorative effects of caloric restriction is via activation of the NAD+-dependent deacetylase SIRT1 (245), which in turn activates PGC-1α (127,246). Resveratrol, a polyphenolic compound extracted from grape skins, increases SIRT1 activity (247) and has been administered to mice in parallel with high-fat feeding (248,249). Resveratrol markedly attenuated weight gain in association with induction of mitochondrial biogenesis in skeletal muscle and brown adipose tissue in the high-fat-fed mice (249). In parallel, the resveratrol-treated mice show increased endurance capacity, higher oxygen consumption, and improved glucose tolerance (249). The effect of resveratrol extends beyond activation of SIRT1 and includes both antioxidant properties and the activation of AMPK (250,251,252,253). These later mechanisms may also be operational in the ameliorative insulin-sensitizing effect of resveratrol in response to fat overload, although these pathways have not been as well characterized in this context. The dose of resveratrol used in mice is not feasible in humans (252,254), although a study to address the effects of lower doses in humans has been initiated (Clinicaltrials.gov Identifier, NCT00823381).
To directly assess the insulin-sensitizing effect of SIRT1, numerous small molecule activators of this sirtuin have been identified and studied (255,256). Here, too, these compounds improve insulin sensitivity in parallel with induction of skeletal muscle glucose tolerance and with the improvement of skeletal muscle mitochondrial capacity (255,256). Although, the direct link to mitochondria has not been established, it is also important to note that SIRT1 has also been shown to enhance insulin sensitivity in insulin- resistant myotubes via repression of the protein-tyrosine phosphatase 1B (257).
Interestingly, it has recently been observed that levels of MUP-1, a lipocalin-like secreted low molecular weight protein that is predominantly produced by the liver, are diminished in dietary and genetic obese mice (258). Replacement of MUP-1 via osmotic minipump improves glucose tolerance with salutary effects on skeletal muscle without modification of hepatic gluconeogenesis or hepatic insulin signaling (258). The ameliorative effects on skeletal muscle include up-regulation of the mitochondrial biogenesis program, increased mitochondrial oxidative capacity, diminished IMCL accumulation, improved insulin signaling in parallel with enhanced activity, and an elevation in the core temperature (258). Whether this protein represents a “hepatokine” that the liver secretes to enhance skeletal muscle bioenergetic capacity is an intriguing new concept that may have therapeutic potential.
Table 1, highlights the therapeutic strategies showing promise in the parallel improvement in skeletal muscle mitochondrial function and insulin resistance and identifies additional potential salutary effects on other peripheral metabolic tissues.
Table 1.
Therapeutic insulin-sensitizing interventions that modulate mitochondrial and metabolic functioning in skeletal muscle, adipose tissue, and the liver
| Intervention | Skeletal muscle effects | Adipose effects | Hepatic effects |
|---|---|---|---|
| Caloric restriction | Increase mitochondrial biogenesis (125) | Decrease adipocyte mass (261) | Diminish steatosis (262) |
| Intensive exercise | Increase mitochondrial biogenesis, increase muscle mass–oxidative capacity, increase DGAT1 (136,137,138,139,140,184) | Decrease adipocyte mass (261) | |
| Thiazolidinediones | Promote mitochondrial FAO and fat uptake (231,232) | Increase lipid uptake and metabolism (227) | Diminish steatosis (237,238) |
| GW501516 | Induction of type I fibers and oxidative capacity (175,177,244) | Diminish steatosis (243) | |
| Resveratrol | Increase mitochondrial biogenesis (249) | Increase mitochondrial biogenesis (249) | |
| SIRT1 activators | Increase mitochondrial oxidative capacity and glucose uptake (255,256) | No change in mitochondrial oxidative capacity (256) | |
| MUP-1 | Increase mitochondrial biogenesis and oxidative capacity (258) | No weight reduction (258) |
VI. Conclusions and Considerations
T2DM represents a composite of systemic manifestations and pathological sequelae resulting from a heterogeneous mix of pathophysiological events that modulate skeletal muscle and other organs including the liver, adipocytes, pancreas, brain, and immune system. The disruption of skeletal muscle mitochondria as an early and probable initiating event in insulin resistance appears to be restricted to: 1) individuals with a strong hereditary predisposition to T2DM that develop insulin resistance at a young age; 2) women with PCOS; 3) some individuals with mitochondrial genomic defects; and 4) in the development of insulin resistance in lean elderly individuals.
In a plurality of T2DM subjects, mitochondrial dysfunction appears to be a direct manifestation of nutrient excess and physical inactivity, environmental factors that initiate a vicious cycle that further exacerbates the metabolic perturbations of T2DM in obese and or sedentary individuals. Whether these individuals have underlying genetic susceptibility to mitochondrial dysfunction is beginning to be explored (158,159,160). The complexity of the role of skeletal muscle mitochondrial biology in the pathophysiology of diabetes is further suggested, where the induction of skeletal muscle mitochondrial function can represent a more acute adaptive response to nutrient load (32,115) and/or possibly an adaptive response to metabolic defects that initiate in other metabolic tissues, as may be operational in insulin-resistant individuals of Asian-Indian descent (66,67).
The complexity of mitochondrial dysfunction incorporates interplay between substrate uptake, storage, and catabolism as well as efficient and balanced interaction of the various metabolic pathways within the mitochondrion itself. At the organ level, the adaptive and maladaptive metabolic interaction of various insulin-responsive tissues has also been shown to be operational in the systemic insulin sensitivity status (259,260). The summative effect of these intertissue interactions collectively defines the overall systemic tolerance to glucose and insulin sensitivity.
The isolated genetic modulation of skeletal muscle mitochondrial/metabolic functioning in experimental studies shows that the disruption in skeletal muscle mitochondrial/energetic integrity additionally results in the induction of secretory myokines that function to modulate the biology of other organ systems as a component of the adaptive program to prevent perturbations in overall fuel handling by the body.
These emerging data support a hypothesis that multiple adaptive metabolic and mitochondrial programs in peripheral metabolically active tissues need to be overwhelmed to progress to systemic insulin resistance. Although this review has focused on the metabolic and mitochondrial functioning in skeletal muscle, in all probability, the homeostatic control across all metabolic tissues integrates in this pathophysiology.
In conclusion, we propose that understanding the regulatory control of mitochondrial homeostasis may generate therapeutic options to modify the development of and reversal of insulin resistance. Moreover, in light of the capacity to overwhelm adaptive modulations by persistent overnutrition, the global strategy to reverse insulin resistance would appear to require a multipronged approach including tackling the underlying deficits and to prevent the environmental stressors promoting progression to diabetes.
Acknowledgments
We thank Christopher C. Dimond for establishing the Reference Manager database.
Footnotes
All the investigators on this review are funded by the Division of Intramural Research of the National Heart Lung and Blood Institute of the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 27, 2009
Abbreviations: AIF, Mitochondrial flavoprotein apoptosis-inducing factor; AMPK, AMP kinase; BMI, Body mass index; CoA, coenzyme A; CPT-1, carnitine palmitoyl transferase 1; DAG, diacylglycerol; DGAT, acyl-CoA:DAG acyltransferase; ETC, electron transfer chain; FAO, mitochondrial fatty acid β-oxidation; GLUT4, glucose transporter isoform 4; IMCL, intramyocellular lipid; MIDD, maternally inherited diabetes and deafness (syndrome); MRS, magnetic resonance spectroscopy; mtDNA, mitochondrial genome DNA; PCOS, polycystic ovary syndrome; PGC-1α, PPAR γ coactivator 1α; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; TCA, tricarboxylic acid; T2DM, type 2 diabetes mellitus; UCP, uncoupling protein; VO2max, maximal oxygen consumption.
References
- Reaven GM 1995 Pathophysiology of insulin resistance in human disease. Physiol Rev 75:473–486 [DOI] [PubMed] [Google Scholar]
- Fernández-Real JM, Pickup JC 2008 Innate immunity, insulin resistance and type 2 diabetes. Trends Endocrinol Metab 19:10–16 [DOI] [PubMed] [Google Scholar]
- Karlsson HK, Ahlsén M, Zierath JR, Wallberg-Henriksson H, Koistinen HA 2006 Insulin signaling and glucose transport in skeletal muscle from first-degree relatives of type 2 diabetic patients. Diabetes 55:1283–1288 [DOI] [PubMed] [Google Scholar]
- Meyer C, Dostou JM, Welle SL, Gerich JE 2002 Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am J Physiol Endocrinol Metab 282:E419–E427 [DOI] [PubMed] [Google Scholar]
- Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG 1990 Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 322:223–228 [DOI] [PubMed] [Google Scholar]
- Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI 2007 Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ 2003 Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI 2004 Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI 2005 Mitochondrial dysfunction and type 2 diabetes. Science 307:384–387 [DOI] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Roden M, Kooi ME, Hesselink MK, Schrauwen P 2007 Muscular mitochondrial dysfunction and type 2 diabetes mellitus. Curr Opin Clin Nutr Metab Care 10:698–703 [DOI] [PubMed] [Google Scholar]
- Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F 2007 Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50:790–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO 2009 Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am J Clin Nutr 89:463S–466S [DOI] [PubMed] [Google Scholar]
- Kraegen EW, Cooney GJ, Turner N 2008 Muscle insulin resistance: a case of fat overconsumption, not mitochondrial dysfunction. Proc Natl Acad Sci USA 105:7627–7628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabøl R, Boushel R, Dela F 2006 Mitochondrial oxidative function and type 2 diabetes. Appl Physiol Nutr Metab 31:675–683 [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Miller ME, Byington RP, Goff Jr DC, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm Jr RH, Probstfield JL, Simons-Morton DG, Friedewald WT 2008 Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F 2008 Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572 [Google Scholar]
- Goodarzi MO, Psaty BM 2008 Glucose lowering to control macrovascular disease in type 2 diabetes: treating the wrong surrogate end point? JAMA 300:2051–2053 [DOI] [PubMed] [Google Scholar]
- Clementi E, Nisoli E 2005 Nitric oxide and mitochondrial biogenesis: a key to long-term regulation of cellular metabolism. Comp Biochem Physiol A Mol Integr Physiol 142:102–110 [DOI] [PubMed] [Google Scholar]
- McLeod CJ, Pagel I, Sack MN 2005 The mitochondrial biogenesis regulatory program in cardiac adaptation to ischemia—a putative target for therapeutic intervention. Trends Cardiovasc Med 15:118–123 [DOI] [PubMed] [Google Scholar]
- Cotney J, Wang Z, Shadel GS 2007 Relative abundance of the human mitochondrial transcription system and distinct roles for h-mtTFB1 and h-mtTFB2 in mitochondrial biogenesis and gene expression. Nucleic Acids Res 35:4042–4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig RP, Scacco S, Scarpulla RC 2000 Sequential serum-dependent activation of CREB and NRF-1 leads to enhanced mitochondrial respiration through the induction of cytochrome c. J Biol Chem 275:13134–13141 [DOI] [PubMed] [Google Scholar]
- Li B, Holloszy JO, Semenkovich CF 1999 Respiratory uncoupling induces δ-aminolevulinate synthase expression through a nuclear respiratory factor-1-dependent mechanism in HeLa cells. J Biol Chem 274:17534–17540 [DOI] [PubMed] [Google Scholar]
- Scarpulla RC 2002 Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene 286:81–89 [DOI] [PubMed] [Google Scholar]
- Huss JM, Torra IP, Staels B, Giguère V, Kelly DP 2004 Estrogen-related receptor α directs peroxisome proliferator-activated receptor α signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol 24:9079–9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss JM, Kelly DP 2004 Nuclear receptor signaling and cardiac energetics. Circ Res 95:568–578 [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM 1999 Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124 [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM 2003 Bioenergetic analysis of peroxisome proliferator-activated receptor γ coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. J Biol Chem 278:26597–26603 [DOI] [PubMed] [Google Scholar]
- Lee HC, Yin PH, Lu CY, Chi CW, Wei YH 2000 Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem J 348:425–432 [PMC free article] [PubMed] [Google Scholar]
- Lynn EG, Lu Z, Minerbi D, Sack MN 2007 The regulation, control, and consequences of mitochondrial oxygen utilization and disposition in the heart and skeletal muscle during hypoxia. Antioxid Redox Signal 9:1353–1361 [DOI] [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO 2003 Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299:896–899 [DOI] [PubMed] [Google Scholar]
- Weitzel JM, Iwen KA, Seitz HJ 2003 Regulation of mitochondrial biogenesis by thyroid hormone. Exp Physiol 88:121–128 [DOI] [PubMed] [Google Scholar]
- Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO 2008 High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105:7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR 2005 A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54:1926–1933 [DOI] [PubMed] [Google Scholar]
- Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS 2003 Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100:7996–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ 2009 Autophagy regulates lipid metabolism. Nature 458:1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Ghani MA, DeFronzo RA 2008 Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Curr Diab Rep 8:173–178 [DOI] [PubMed] [Google Scholar]
- Szendroedi J, Roden M 2008 Mitochondrial fitness and insulin sensitivity in humans. Diabetologia 51:2155–2167 [DOI] [PubMed] [Google Scholar]
- Adams 2nd JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ 2004 Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53:25–31 [DOI] [PubMed] [Google Scholar]
- Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI 1999 Free fatty acid-induced insulin resistance is associated with activation of protein kinase C θ and alterations in the insulin signaling cascade. Diabetes 48:1270–1274 [DOI] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G 2002 Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51:2005–2011 [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Shulman GI 2006 Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55(Suppl 2):S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Shulman GI 2005 Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2:e233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI 2005 Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115:3587–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp GJ 2008 The interpretation of abnormal 31P magnetic resonance saturation transfer measurements of Pi/ATP exchange in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab 294:E640–E642 [DOI] [PubMed] [Google Scholar]
- Daussin FN, Zoll J, Ponsot E, Dufour SP, Doutreleau S, Lonsdorfer E, Ventura-Clapier R, Mettauer B, Piquard F, Geny B, Richard R 2008 Training at high exercise intensity promotes qualitative adaptations of mitochondrial function in human skeletal muscle. J Appl Physiol 104:1436–1441 [DOI] [PubMed] [Google Scholar]
- Daussin FN, Zoll J, Dufour SP, Ponsot E, Lonsdorfer-Wolf E, Doutreleau S, Mettauer B, Piquard F, Geny B, Richard R 2008 Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: relationship to aerobic performance improvements in sedentary subjects. Am J Physiol Regul Integr Comp Physiol 295:R264–R272 [DOI] [PubMed] [Google Scholar]
- Nyholm B, Mengel A, Nielsen S, Skjaerbaek C, Møller N, Alberti KG, Schmitz O 1996 Insulin resistance in relatives of NIDDM patients: the role of physical fitness and muscle metabolism. Diabetologia 39:813–822 [DOI] [PubMed] [Google Scholar]
- Berntorp K, Lindgärde F 1985 Impaired physical fitness and insulin secretion in normoglycaemic subjects with familial aggregation of type 2 diabetes mellitus. Diabetes Res 2:151–156 [PubMed] [Google Scholar]
- Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN 1999 The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med 130:89–96 [DOI] [PubMed] [Google Scholar]
- Booth FW, Laye MJ, Lees SJ, Rector RS, Thyfault JP 2008 Reduced physical activity and risk of chronic disease: the biology behind the consequences. Eur J Appl Physiol 102:381–390 [DOI] [PubMed] [Google Scholar]
- Perseghin G, Scifo P, Danna M, Battezzati A, Benedini S, Meneghini E, Del Maschio A, Luzi L 2002 Normal insulin sensitivity and IMCL content in overweight humans are associated with higher fasting lipid oxidation. Am J Physiol Endocrinol Metab 283:E556–E564 [DOI] [PubMed] [Google Scholar]
- Thyfault JP, Kraus RM, Hickner RC, Howell AW, Wolfe RR, Dohm GL 2004 Impaired plasma fatty acid oxidation in extremely obese women. Am J Physiol Endocrinol Metab 287:E1076–E1081 [DOI] [PubMed] [Google Scholar]
- Cooney GJ, Thompson AL, Furler SM, Ye J, Kraegen EW 2002 Muscle long-chain acyl CoA esters and insulin resistance. Ann NY Acad Sci 967:196–207 [DOI] [PubMed] [Google Scholar]
- Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA 2000 Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279:E1039–E1044 [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC 2003 PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273 [DOI] [PubMed] [Google Scholar]
- Højlund K, Wrzesinski K, Larsen PM, Fey SJ, Roepstorff P, Handberg A, Dela F, Vinten J, McCormack JG, Reynet C, Beck-Nielsen H 2003 Proteome analysis reveals phosphorylation of ATP synthase β-subunit in human skeletal muscle and proteins with potential roles in type 2 diabetes. J Biol Chem 278:10436–10442 [DOI] [PubMed] [Google Scholar]
- Mogensen M, Sahlin K, Fernström M, Glintborg D, Vind BF, Beck-Nielsen H, Højlund K 2007 Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56:1592–1599 [DOI] [PubMed] [Google Scholar]
- Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K 2003 Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 107:3040–3046 [DOI] [PubMed] [Google Scholar]
- Phielix E, Schrauwen-Hinderling VB, Mensink M, Lenaers E, Meex R, Hoeks J, Kooi ME, Moonen-Kornips E, Sels JP, Hesselink MK, Schrauwen P 2008 Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes 57:2943–2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Jeneson JA, Backes WH, van Echteld CJ, van Engelshoven JM, Mensink M, Schrauwen P 2007 Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia 50:113–120 [DOI] [PubMed] [Google Scholar]
- Pagel-Langenickel I, Schwartz DR, Arena RA, Minerbi DC, Johnson DT, Waclawiw MA, Cannon 3rd RO, Balaban RS, Tripodi DJ, Sack MN 2007 A discordance in rosiglitazone mediated insulin sensitization and skeletal muscle mitochondrial content/activity in type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol 293:H2659–H2666 [DOI] [PubMed] [Google Scholar]
- Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Järvinen H, Christin L, Secomb TW, Bogardus C 1987 Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 80:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau JA, Kelley DE 1997 Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 83:166–171 [DOI] [PubMed] [Google Scholar]
- Sreekumar R, Halvatsiotis P, Schimke JC, Nair KS 2002 Gene expression profile in skeletal muscle of type 2 diabetes and the effect of insulin treatment. Diabetes 51:1913–1920 [DOI] [PubMed] [Google Scholar]
- Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, Nowotny P, Wolzt M, Waldhausl W, Roden M 2007 Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 4:e154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, Guo ZK, Sreekumar R, Irving BA 2008 Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes 57:1166–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, Cobelli C, Shulman GI 2006 Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci USA 103:18273–18277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrmann DA 2005 Polycystic ovary syndrome. N Engl J Med 352:1223–1236 [DOI] [PubMed] [Google Scholar]
- Corbould A, Kim YB, Youngren JF, Pender C, Kahn BB, Lee A, Dunaif A 2005 Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab 288:E1047–E1054 [DOI] [PubMed] [Google Scholar]
- Skov V, Glintborg D, Knudsen S, Jensen T, Kruse TA, Tan Q, Brusgaard K, Beck-Nielsen H, Højlund K 2007 Reduced expression of nuclear-encoded genes involved in mitochondrial oxidative metabolism in skeletal muscle of insulin-resistant women with polycystic ovary syndrome. Diabetes 56:2349–2355 [DOI] [PubMed] [Google Scholar]
- Pagel-Langenickel I, Bao J, Joseph JJ, Schwartz DR, Mantell BS, Xu X, Raghavachari N, Sack MN 2008 PGC-1α integrates insulin signaling, mitochondrial regulation, and bioenergetic function in skeletal muscle. J Biol Chem 283:22464–22472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov V, Glintborg D, Knudsen S, Tan Q, Jensen T, Kruse TA, Beck-Nielsen H, Højlund K 2008 Pioglitazone enhances mitochondrial biogenesis and ribosomal protein biosynthesis in skeletal muscle in polycystic ovary syndrome. PLoS ONE 3:e2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio Jr F, Palomba S, Giallauria F, Colao A, Vigorito C 2007 Impaired cardiopulmonary parameters in young women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 66:152–153 [DOI] [PubMed] [Google Scholar]
- Cosar E, Köken G, Sahin FK, Akgün L, Uçok K, Genç A, Yilmazer M 2008 Resting metabolic rate and exercise capacity in women with polycystic ovary syndrome. Int J Gynaecol Obstet 101:31–34 [DOI] [PubMed] [Google Scholar]
- Maassen JA, Jahangir Tafrechi RS, Janssen GM, Raap AK, Lemkes HH, 't Hart LM 2006 New insights in the molecular pathogenesis of the maternally inherited diabetes and deafness syndrome. Endocrinol Metab Clin North Am 35:385–396, x–xi [DOI] [PubMed] [Google Scholar]
- Lindroos MM, Majamaa K, Tura A, Mari A, Kalliokoski KK, Taittonen MT, Iozzo P, Nuutila P 2009 m. 3243A>G mutation in mitochondrial DNA leads to decreased insulin sensitivity in skeletal muscle and to progressive β-cell dysfunction. Diabetes 58:543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendroedi J, Schmid AI, Meyerspeer M, Cervin C, Kacerovsky M, Smekal G, Gräser-Lang S, Groop L, Roden M 2009 Impaired mitochondrial function and insulin resistance of skeletal muscle in mitochondrial diabetes. Diabetes Care 32:677–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC 2005 A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39:359–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Cui J, Gavras H, Schwartz F 2003 A novel class of tests for the detection of mitochondrial DNA-mutation involvement in diseases. Am J Hum Genet 72:1515–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin JA, Saunier JL, Niederstätter H, Strouss KM, Sturk KA, Diegoli TM, Brandstätter A, Parson W, Parsons TJ 2009 Investigation of heteroplasmy in the human mitochondrial DNA control region: a synthesis of observations from more than 5000 global population samples. J Mol Evol 68:516–527 [DOI] [PubMed] [Google Scholar]
- Pravenec M, Hyakukoku M, Houstek J, Zidek V, Landa V, Mlejnek P, Miksik I, Dudová-Mothejzikova K, Pecina P, Vrbacky M, Drahota Z, Vojtiskova A, Mracek T, Kazdova L, Oliyarnyk O, Wang J, Ho C, Qi N, Sugimoto K, Kurtz T 2007 Direct linkage of mitochondrial genome variation to risk factors for type 2 diabetes in conplastic strains. Genome Res 17:1319–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain P, Kawar B, El Nahas M 2007 Obesity and diabetes in the developing world—a growing challenge. N Engl J Med 356:213–215 [DOI] [PubMed] [Google Scholar]
- Krebs M, Roden M 2004 Nutrient-induced insulin resistance in human skeletal muscle. Curr Med Chem 11:901–908 [DOI] [PubMed] [Google Scholar]
- Brownlee M 2005 The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625 [DOI] [PubMed] [Google Scholar]
- Brownlee M 2001 Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820 [DOI] [PubMed] [Google Scholar]
- Rabøl R, Højberg PM, Almdal T, Boushel R, Haugaard SB, Madsbad S, Dela F 2009 Effect of hyperglycemia on mitochondrial respiration in type 2 diabetes. J Clin Endocrinol Metab 94:1372–1378 [DOI] [PubMed] [Google Scholar]
- Knoll KE, Pietrusz JL, Liang M 2005 Tissue-specific transcriptome responses in rats with early streptozotocin-induced diabetes. Physiol Genomics 21:222–229 [DOI] [PubMed] [Google Scholar]
- Willsky GR, Chi LH, Liang Y, Gaile DP, Hu Z, Crans DC 2006 Diabetes-altered gene expression in rat skeletal muscle corrected by oral administration of vanadyl sulfate. Physiol Genomics 26:192–201 [DOI] [PubMed] [Google Scholar]
- Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J 2008 Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechoor VK, Patti ME, Ueki K, Laustsen PG, Saccone R, Rauniyar R, Kahn CR 2004 Distinct pathways of insulin-regulated versus diabetes-regulated gene expression: an in vivo analysis in MIRKO mice. Proc Natl Acad Sci USA 101:16525–16530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Koves TR 2007 Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 α: implications for metabolic disease. Appl Physiol Nutr Metab 32:874–883 [DOI] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Moonen-Kornips E, Schaart G, Mustard KJ, Hardie DG, Saris WH, Nicolay K, Schrauwen P 2005 Intramyocellular lipid content and molecular adaptations in response to a 1-week high-fat diet. Obes Res 13:2088–2094 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Kewalramani G, Yuen G, Pulinilkunnil T, An D, Innis SM, Allard MF, Wambolt RB, Qi D, Abrahani A, Rodrigues B 2006 Induction of mitochondrial nitrative damage and cardiac dysfunction by chronic provision of dietary omega-6 polyunsaturated fatty acids. Free Radic Biol Med 41:1413–1424 [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E 1998 Peroxidative damage to cardiac mitochondria: cytochrome oxidase and cardiolipin alterations. FEBS Lett 424:155–158 [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI 2003 Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befroy DE, Petersen KF, Dufour S, Mason GF, Rothman DL, Shulman GI 2008 Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proc Natl Acad Sci USA 105:16701–16706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibom R, Hultman E, Johansson M, Matherei K, Constantin-Teodosiu D, Schantz PG 1992 Adaptation of mitochondrial ATP production in human skeletal muscle to endurance training and detraining. J Appl Physiol 73:2004–2010 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, He J, Watkins S, Kelley DE 2001 Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86:5755–5761 [DOI] [PubMed] [Google Scholar]
- Russell AP, Gastaldi G, Bobbioni-Harsch E, Arboit P, Gobelet C, Dériaz O, Golay A, Witztum JL, Giacobino JP 2003 Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: a case of good vs. bad lipids? FEBS Lett 551:104–106 [DOI] [PubMed] [Google Scholar]
- Schrauwen P 2007 High-fat diet, muscular lipotoxicity and insulin resistance. Proc Nutr Soc 66:33–41 [DOI] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Hesselink MK, Moonen- Kornips E, Schaart G, Kooi ME, Saris WH, Schrauwen P 2006 Short-term training is accompanied by a down regulation of ACC2 mRNA in skeletal muscle. Int J Sports Med 27:786–791 [DOI] [PubMed] [Google Scholar]
- Lu Z, Sack MN 2008 ATF-1 is a hypoxia-responsive transcriptional activator of skeletal muscle mitochondrial-uncoupling protein 3. J Biol Chem 283:23410–23418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack MN 2006 Mitochondrial depolarization and the role of uncoupling proteins in ischemia tolerance. Cardiovasc Res 72:210–219 [DOI] [PubMed] [Google Scholar]
- Abdul-Ghani MA, Muller FL, Liu Y, Chavez AO, Balas B, Zuo P, Chang Z, Tripathy D, Jani R, Molina-Carrion M, Monroy A, Folli F, Van Remmen H, DeFronzo RA 2008 Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab 295:E678–E685 [DOI] [PubMed] [Google Scholar]
- Ortenblad N, Mogensen M, Petersen I, Højlund K, Levin K, Sahlin K, Beck-Nielsen H, Gaster M 2005 Reduced insulin-mediated citrate synthase activity in cultured skeletal muscle cells from patients with type 2 diabetes: evidence for an intrinsic oxidative enzyme defect. Biochim Biophys Acta 1741:206–214 [DOI] [PubMed] [Google Scholar]
- Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhäusl W, Roden M 2006 Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes 55:136–140 [PubMed] [Google Scholar]
- Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI 1996 Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97:2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent D, Didier L, Yerby B, Yerby B, Deacon R, Gao J 2007 Diet-induced modulation of mitochondrial activity in rat muscle. Am J Physiol Endocrinol Metab 293:E1169–E1177 [DOI] [PubMed] [Google Scholar]
- Toledo FG, Menshikova EV, Azuma K, Radiková Z, Kelley CA, Ritov VB, Kelley DE 2008 Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 57:987–994 [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI 2005 Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54:603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeks J, Hesselink MK, Russell AP, Mensink M, Saris WH, Mensink RP, Schrauwen P 2006 Peroxisome proliferator-activated receptor-γ coactivator-1 and insulin resistance: acute effect of fatty acids. Diabetologia 49:2419–2426 [DOI] [PubMed] [Google Scholar]
- Richardson DK, Kashyap S, Bajaj M, Cusi K, Mandarino SJ, Finlayson J, DeFronzo RA, Jenkinson CP, Mandarino LJ 2005 Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J Biol Chem 280:10290–10297 [DOI] [PubMed] [Google Scholar]
- Brøns C, Jensen CB, Storgaard H, Hiscock NJ, White A, Appel JS, Jacobsen S, Nilsson E, Larsen CM, Astrup A, Quistorff B, Vaag A 2009 Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol 587:2387–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde J, Mohren R, van den Berg S, Boekschoten M, Dijk KW, de Groot P, Müller M, Mariman E, Smit E 2008 Short-term high fat-feeding results in morphological and metabolic adaptations in the skeletal muscle of C57BL/6J mice. Physiol Genomics 32:360–369 [DOI] [PubMed] [Google Scholar]
- Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ 2007 Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 56:2085–2092 [DOI] [PubMed] [Google Scholar]
- Garcia-Roves P, Huss JM, Han DH, Hancock CR, Iglesias-Gutierrez E, Chen M, Holloszy JO 2007 Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci USA 104:10709–10713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeks J, Briedé JJ, de Vogel J, Schaart G, Nabben M, Moonen-Kornips E, Hesselink MK, Schrauwen P 2008 Mitochondrial function, content and ROS production in rat skeletal muscle: effect of high-fat feeding. FEBS Lett 582:510–516 [DOI] [PubMed] [Google Scholar]
- Coll T, Eyre E, Rodríguez-Calvo R, Palomer X, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M 2008 Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem 283:11107–11116 [DOI] [PubMed] [Google Scholar]
- Dimopoulos N, Watson M, Sakamoto K, Hundal HS 2006 Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J 399:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Pinnamaneni SK, Eo SJ, Cho IH, Pyo JH, Kim CK, Sinclair AJ, Febbraio MA, Watt MJ 2006 Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol 100:1467–1474 [DOI] [PubMed] [Google Scholar]
- Hommelberg PP, Plat J, Langen RC, Schols AM, Mensink RP 2009 Fatty acid-induced NF-κB activation and insulin resistance in skeletal muscle are chain length dependent. Am J Physiol Endocrinol Metab 296:E114–E120 [DOI] [PubMed] [Google Scholar]
- Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J 2009 Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58: 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M, Krssak M, Bernroider E, Anderwald C, Brehm A, Meyerspeer M, Nowotny P, Roth E, Waldhäusl W, Roden M 2002 Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 51:599–605 [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA 2008 A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE 3:e2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E 2007 Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med 4:e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Finkel T 2004 Ageing and the mystery at Arles. Nature 429:149–152 [DOI] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P 2007 Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J 26:1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO 2004 Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA 101:6659–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE 1991 Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 338:774–778 [DOI] [PubMed] [Google Scholar]
- Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH 1992 A prospective study of exercise and incidence of diabetes among US male physicians. JAMA 268:63–67 [PubMed] [Google Scholar]
- Coyle EF, Martin 3rd WH, Sinacore DR, Joyner MJ, Hagberg JM, Holloszy JO 1984 Time course of loss of adaptations after stopping prolonged intense endurance training. J Appl Physiol 57:1857–1864 [DOI] [PubMed] [Google Scholar]
- Houston ME, Bentzen H, Larsen H 1979 Interrelationships between skeletal muscle adaptations and performance as studied by detraining and retraining. Acta Physiol Scand 105:163–170 [DOI] [PubMed] [Google Scholar]
- Henriksson J, Reitman JS 1977 Time course of changes in human skeletal muscle succinate dehydrogenase and cytochrome oxidase activities and maximal oxygen uptake with physical activity and inactivity. Acta Physiol Scand 99:91–97 [DOI] [PubMed] [Google Scholar]
- Bey L, Hamilton MT 2003 Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol 551:673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MT, Areiqat E, Hamilton DG, Bey L 2001 Plasma triglyceride metabolism in humans and rats during aging and physical inactivity. Int J Sport Nutr Exerc Metab 11 Suppl:S97–S104 [DOI] [PubMed] [Google Scholar]
- Chow LS, Greenlund LJ, Asmann YW, Short KR, McCrady SK, Levine JA, Nair KS 2007 Impact of endurance training on murine spontaneous activity, muscle mitochondrial DNA abundance, gene transcripts, and function. J Appl Physiol 102:1078–1089 [DOI] [PubMed] [Google Scholar]
- Kang J, Robertson RJ, Hagberg JM, Kelley DE, Goss FL, DaSilva SG, Suminski RR, Utter AC 1996 Effect of exercise intensity on glucose and insulin metabolism in obese individuals and obese NIDDM patients. Diabetes Care 19:341–349 [DOI] [PubMed] [Google Scholar]
- Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM 2005 Peroxisome proliferator-activated receptor-γ co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280:33588–33598 [DOI] [PubMed] [Google Scholar]
- Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH 2006 Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 61:534–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS 2003 Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52:1888–1896 [DOI] [PubMed] [Google Scholar]
- Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE 2007 Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 56:2142–2147 [DOI] [PubMed] [Google Scholar]
- Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Li H, Jiang Y, An Y, Shuai Y, Zhang B, Zhang J, Thompson TJ, Gerzoff RB, Roglic G, Hu Y, Bennett PH 2008 The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 371:1783–1789 [DOI] [PubMed] [Google Scholar]
- Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S 2005 The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 142:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenell MI, Hollingsworth KG, Lim EL, Taylor R 2008 Increased daily walking improves lipid oxidation without changes in mitochondrial function in type 2 diabetes. Diabetes Care 31:1644–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisløff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernström M, Rezaei K, Lee SJ, Koch LG, Britton SL 2005 Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307:418–420 [DOI] [PubMed] [Google Scholar]
- Yaspelkis 3rd BB, Lessard SJ, Reeder DW, Limon JJ, Saito M, Rivas DA, Kvasha I, Hawley JA 2007 Exercise reverses high-fat diet-induced impairments on compartmentalization and activation of components of the insulin-signaling cascade in skeletal muscle. Am J Physiol Endocrinol Metab 293:E941–E949 [DOI] [PubMed] [Google Scholar]
- Haram PM, Kemi OJ, Lee SJ, Bendheim MØ, Al-Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM, Wisløff U 2009 Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res 81:723–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J 1995 Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50 Spec No:11–16 [DOI] [PubMed] [Google Scholar]
- Karakelides H, Nair KS 2005 Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol 68:123–148 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA 1981 Glucose intolerance and aging. Diabetes Care 4:493–501 [DOI] [PubMed] [Google Scholar]
- Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, Sullivan L, D'Agostino RB, Nathan DM 2008 Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care 31:1991–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Suzuki T, Horiuchi N 1982 Development of diabetes in Japanese subjects with impaired glucose tolerance: a seven year follow-up study. Diabetologia 22:154–157 [DOI] [PubMed] [Google Scholar]
- Rowe JW, Minaker KL, Pallotta JA, Flier JS 1983 Characterization of the insulin resistance of aging. J Clin Invest 71:1581–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS 2005 Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 102:5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS 2008 Endurance exercise as a countermeasure for aging. Diabetes 57:2933–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossa S, Mollica MP, Lionetti L, Crescenzo R, Tasso R, Liverini G 2004 A possible link between skeletal muscle mitochondrial efficiency and age-induced insulin resistance. Diabetes 53:2861–2866 [DOI] [PubMed] [Google Scholar]
- Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI 2007 Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5:151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso I, Luan J, Sandhu MS, Franks PW, Crowley V, Schafer AJ, O'Rahilly S, Wareham NJ 2006 Meta-analysis of the Gly482Ser variant in PPARGC1A in type 2 diabetes and related phenotypes. Diabetologia 49:501–505 [DOI] [PubMed] [Google Scholar]
- Franks PW, Barroso I, Luan J, Ekelund U, Crowley VE, Brage S, Sandhu MS, Jakes RW, Middelberg RP, Harding AH, Schafer AJ, O'Rahilly S, Wareham NJ 2003 PGC-1α genotype modifies the association of volitional energy expenditure with VO2max. Med Sci Sports Exerc 35:1998–2004 [DOI] [PubMed] [Google Scholar]
- O'Rahilly S, Barroso I, Wareham NJ 2005 Genetic factors in type 2 diabetes: the end of the beginning? Science 307:370–373 [DOI] [PubMed] [Google Scholar]
- Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM 2001 Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA 98:3820–3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton CR, Nickerson JG, Lally J, Han XX, Holloway GP, Glatz JF, Luiken JJ, Graham TE, Heikkila JJ, Bonen A 2008 Modest PGC-1α overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. J Biol Chem 283:4228–4240 [DOI] [PubMed] [Google Scholar]
- Miura S, Kai Y, Ono M, Ezaki O 2003 Overexpression of peroxisome proliferator-activated receptor γ coactivator-1α down-regulates GLUT4 mRNA in skeletal muscles. J Biol Chem 278:31385–31390 [DOI] [PubMed] [Google Scholar]
- Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, Zhang D, Cline GW, Handschin C, Lin J, Petersen KF, Spiegelman BM, Shulman GI 2008 Paradoxical effects of increased expression of PGC-1α on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci USA 105:19926–19931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM 2007 Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic β cell crosstalk. J Clin Invest 117:3463–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM 2007 Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1α muscle-specific knock-out animals. J Biol Chem 282:30014–30021 [DOI] [PubMed] [Google Scholar]
- Wredenberg A, Freyer C, Sandström ME, Katz A, Wibom R, Westerblad H, Larsson NG 2006 Respiratory chain dysfunction in skeletal muscle does not cause insulin resistance. Biochem Biophys Res Commun 350:202–207 [DOI] [PubMed] [Google Scholar]
- Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere LL, Holloszy JO, Semenkovich CF, Kelly DP 2005 A potential link between muscle peroxisome proliferator- activated receptor-α signaling and obesity-related diabetes. Cell Metab 1:133–144 [DOI] [PubMed] [Google Scholar]
- Vahsen N, Candé C, Brière JJ, Bénit P, Joza N, Larochette N, Mastroberardino PG, Pequignot MO, Casares N, Lazar V, Feraud O, Debili N, Wissing S, Engelhardt S, Madeo F, Piacentini M, Penninger JM, Schägger H, Rustin P, Kroemer G 2004 AIF deficiency compromises oxidative phosphorylation. EMBO J 23:4679–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joza N, Oudit GY, Brown D, Bénit P, Kassiri Z, Vahsen N, Benoit L, Patel MM, Nowikovsky K, Vassault A, Backx PH, Wada T, Kroemer G, Rustin P, Penninger JM 2005 Muscle-specific loss of apoptosis-inducing factor leads to mitochondrial dysfunction, skeletal muscle atrophy, and dilated cardiomyopathy. Mol Cell Biol 25:10261–10272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G 1999 Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441–446 [DOI] [PubMed] [Google Scholar]
- Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G, Esterbauer H, Kozlov A, Kahn CR, Kroemer G, Rustin P, Burcelin R, Penninger JM 2007 Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 131:476–491 [DOI] [PubMed] [Google Scholar]
- Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, Desvergne B 2001 Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology 142:4195–4202 [DOI] [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX 2004 PPARs and the complex journey to obesity. Nat Med 10:355–361 [DOI] [PubMed] [Google Scholar]
- Lee CH, Olson P, Hevener A, Mehl I, Chong LW, Olefsky JM, Gonzalez FJ, Ham J, Kang H, Peters JM, Evans RM 2006 PPARδ regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci USA 103:3444–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, Desvergne B, Wahli W, Chambon P, Metzger D 2006 PGC1α expression is controlled in skeletal muscles by PPARβ, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab 4:407–414 [DOI] [PubMed] [Google Scholar]
- Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM 2004 Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol 2:e294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Knaub LA, Jensen DR, Young Jung D, Hong EG, Ko HJ, Coates AM, Goldberg IJ, de la Houssaye BA, Janssen RC, McCurdy CE, Rahman SM, Soo Choi C, Shulman GI, Kim JK, Friedman JE, Eckel RH 2009 Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes 58:116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpeleijn E, Pelsers MM, Soenen S, Mensink M, Bouwman FG, Kooi ME, Saris WH, Glatz JF, Blaak EE 2008 Insulin acutely upregulates protein expression of the fatty acid transporter CD36 in human skeletal muscle in vivo. J Physiol Pharmacol 59:77–83 [PubMed] [Google Scholar]
- Wu Q, Ortegon AM, Tsang B, Doege H, Feingold KR, Stahl A 2006 FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol Cell Biol 26:3455–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn CT, Knapp Jr FF, Febbraio M, Beets AL, Silverstein RL, Abumrad NA 2000 Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 275:32523–32529 [DOI] [PubMed] [Google Scholar]
- Hajri T, Han XX, Bonen A, Abumrad NA 2002 Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest 109:1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI 2007 Inhibition of protein kinase Cε prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest 117:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Miyazaki H, Nakatani T, Kai Y, Kamei Y, Miura S, Tsuboyama-Kasaoka N, Ezaki O 2002 Up-regulation of SREBP-1c and lipogenic genes in skeletal muscles after exercise training. Biochem Biophys Res Commun 296:395–400 [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH 2007 Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest 117:1679–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Smith SJ, Ladha Z, Jensen DR, Ferreira LD, Pulawa LK, McGuire JG, Pitas RE, Eckel RH, Farese Jr RV 2002 Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest 109:1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese Jr RV 2000 Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet 25:87–90 [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE 2002 Regulation of fat metabolism in skeletal muscle. Ann NY Acad Sci 967:217–235 [DOI] [PubMed] [Google Scholar]
- Kiens B 2006 Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev 86:205–243 [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM 2008 Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7:45–56 [DOI] [PubMed] [Google Scholar]
- Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, Cooney GJ, Febbraio MA, Kraegen EW 2009 Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes 58:550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdomo G, Commerford SR, Richard AM, Adams SH, Corkey BE, O'Doherty RM, Brown NF 2004 Increased β-oxidation in muscle cells enhances insulin-stimulated glucose metabolism and protects against fatty acid-induced insulin resistance despite intramyocellular lipid accumulation. J Biol Chem 279:27177–27186 [DOI] [PubMed] [Google Scholar]
- Dyck JR, Hopkins TA, Bonnet S, Michelakis ED, Young ME, Watanabe M, Kawase Y, Jishage K, Lopaschuk GD 2006 Absence of malonyl coenzyme A decarboxylase in mice increases cardiac glucose oxidation and protects the heart from ischemic injury. Circulation 114:1721–1728 [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB 2005 The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol 6:248–261 [DOI] [PubMed] [Google Scholar]
- Stuart JA, Brindle KM, Harper JA, Brand MD 1999 Mitochondrial proton leak and the uncoupling proteins. J Bioenerg Biomembr 31:517–525 [DOI] [PubMed] [Google Scholar]
- Brand MD, Esteves TC 2005 Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab 2:85–93 [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Hoeks J, Hesselink MK 2006 Putative function and physiological relevance of the mitochondrial uncoupling protein-3: involvement in fatty acid metabolism? Prog Lipid Res 45:17–41 [DOI] [PubMed] [Google Scholar]
- Bézaire V, Hofmann W, Kramer JK, Kozak LP, Harper ME 2001 Effects of fasting on muscle mitochondrial energetics and fatty acid metabolism in Ucp3(−/−) and wild-type mice. Am J Physiol Endocrinol Metab 281:E975–E982 [DOI] [PubMed] [Google Scholar]
- Boss O, Hagen T, Lowell BB 2000 Uncoupling proteins 2 and 3: potential regulators of mitochondrial energy metabolism. Diabetes 49:143–156 [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Mensink M, Schaart G, Moonen-Kornips E, Sels JP, Blaak EE, Russell AP, Hesselink MK 2006 Reduced skeletal muscle uncoupling protein-3 content in prediabetic subjects and type 2 diabetic patients: restoration by rosiglitazone treatment. J Clin Endocrinol Metab 91:1520–1525 [DOI] [PubMed] [Google Scholar]
- Jones TE, Baar K, Ojuka E, Chen M, Holloszy JO 2003 Exercise induces an increase in muscle UCP3 as a component of the increase in mitochondrial biogenesis. Am J Physiol Endocrinol Metab 284:E96–E101 [DOI] [PubMed] [Google Scholar]
- Mensink M, Hesselink MK, Borghouts LB, Keizer H, Moonen-Kornips E, Schaart G, Blaak EE, Schrauwen P 2007 Skeletal muscle uncoupling protein-3 restores upon intervention in the prediabetic and diabetic state: implications for diabetes pathogenesis? Diabetes Obes Metab 9:594–596 [DOI] [PubMed] [Google Scholar]
- García-Martinez C, Sibille B, Solanes G, Darimont C, Macé K, Villarroya F, Gómez-Foix AM 2001 Overexpression of UCP3 in cultured human muscle lowers mitochondrial membrane potential, raises ATP/ADP ratio, and favors fatty acid vs. glucose oxidation. FASEB J 15:2033–2035 [DOI] [PubMed] [Google Scholar]
- Mills EM, Banks ML, Sprague JE, Finkel T 2003 Pharmacology: uncoupling the agony from ecstasy. Nature 426:403–404 [DOI] [PubMed] [Google Scholar]
- Clapham JC, Arch JR, Chapman H, Haynes A, Lister C, Moore GB, Piercy V, Carter SA, Lehner I, Smith SA, Beeley LJ, Godden RJ, Herrity N, Skehel M, Changani KK, Hockings PD, Reid DG, Squires SM, Hatcher J, Trail B, Latcham J, Rastan S, Harper AJ, Cadenas S, Buckingham JA, Brand MD, Abuin A 2000 Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature 406:415–418 [DOI] [PubMed] [Google Scholar]
- Choi CS, Fillmore JJ, Kim JK, Liu ZX, Kim S, Collier EF, Kulkarni A, Distefano A, Hwang YJ, Kahn M, Chen Y, Yu C, Moore IK, Reznick RM, Higashimori T, Shulman GI 2007 Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest 117:1995–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen P, Hardie DG, Roorda B, Clapham JC, Abuin A, Thomason-Hughes M, Green K, Frederik PM, Hesselink MK 2004 Improved glucose homeostasis in mice overexpressing human UCP3: a role for AMP-kinase? Int J Obes Relat Metab Disord 28:824–828 [DOI] [PubMed] [Google Scholar]
- Costford SR, Chaudhry SN, Crawford SA, Salkhordeh M, Harper ME 2008 Long-term high-fat feeding induces greater fat storage in mice lacking UCP3. Am J Physiol Endocrinol Metab 295:E1018–E1024 [DOI] [PubMed] [Google Scholar]
- Han DH, Nolte LA, Ju JS, Coleman T, Holloszy JO, Semenkovich CF 2004 UCP-mediated energy depletion in skeletal muscle increases glucose transport despite lipid accumulation and mitochondrial dysfunction. Am J Physiol Endocrinol Metab 286:E347–E353 [DOI] [PubMed] [Google Scholar]
- Gates AC, Bernal-Mizrachi C, Chinault SL, Feng C, Schneider JG, Coleman T, Malone JP, Townsend RR, Chakravarthy MV, Semenkovich CF 2007 Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab 6:497–505 [DOI] [PubMed] [Google Scholar]
- Neschen S, Katterle Y, Richter J, Augustin R, Scherneck S, Mirhashemi F, Schürmann A, Joost HG, Klaus S 2008 Uncoupling protein 1 expression in murine skeletal muscle increases AMPK activation, glucose turnover, and insulin sensitivity in vivo. Physiol Genomics 33:333–340 [DOI] [PubMed] [Google Scholar]
- Charron MJ, Brosius 3rd FC, Alper SL, Lodish HF 1989 A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci USA 86:2535–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB, Rossetti L, Lodish HF, Charron MJ 1991 Decreased in vivo glucose uptake but normal expression of GLUT1 and GLUT4 in skeletal muscle of diabetic rats. J Clin Invest 87:2197–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King PA, Horton ED, Hirshman MF, Horton ES 1992 Insulin resistance in obese Zucker rat (fa/fa) skeletal muscle is associated with a failure of glucose transporter translocation. J Clin Invest 90:1568–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz EB, Stenbit AE, Hatton K, DePinho R, Charron MJ 1995 Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature 377:151–155 [DOI] [PubMed] [Google Scholar]
- Stenbit AE, Burcelin R, Katz EB, Tsao TS, Gautier N, Charron MJ, Le Marchand-Brustel Y 1996 Diverse effects of Glut 4 ablation on glucose uptake and glycogen synthesis in red and white skeletal muscle. J Clin Invest 98:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranalletta M, Jiang H, Li J, Tsao TS, Stenbit AE, Yokoyama M, Katz EB, Charron MJ 2005 Altered hepatic and muscle substrate utilization provoked by GLUT4 ablation. Diabetes 54:935–943 [DOI] [PubMed] [Google Scholar]
- Stenbit AE, Tsao TS, Li J, Burcelin R, Geenen DL, Factor SM, Houseknecht K, Katz EB, Charron MJ 1997 GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat Med 3:1096–1101 [DOI] [PubMed] [Google Scholar]
- Tsao TS, Stenbit AE, Factor SM, Chen W, Rossetti L, Charron MJ 1999 Prevention of insulin resistance and diabetes in mice heterozygous for GLUT4 ablation by transgenic complementation of GLUT4 in skeletal muscle. Diabetes 48:775–782 [DOI] [PubMed] [Google Scholar]
- Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, Hamilton JA, Ouchi N, LeBrasseur NK, Walsh K 2008 Fast/glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab 7:159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Akerström TC, Nielsen AR, Fischer CP 2007 Role of myokines in exercise and metabolism. J Appl Physiol 103:1093–1098 [DOI] [PubMed] [Google Scholar]
- Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K 2008 FGF21 is an Akt-regulated myokine. FEBS Lett 582:3805–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K 2009 Adipokines, myokines and cardiovascular disease. Circ J 73:13–18 [DOI] [PubMed] [Google Scholar]
- Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Rydén M 2008 FGF21 attenuates lipolysis in human adipocytes—a possible link to improved insulin sensitivity. FEBS Lett 582:1725–1730 [DOI] [PubMed] [Google Scholar]
- Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J 2006 Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55:2470–2478 [DOI] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Véniant MM 2009 Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Homko C, Mozzoli M, Showe LC, Nichols C, Cheung P 2005 Thiazolidinediones upregulate fatty acid uptake and oxidation in adipose tissue of diabetic patients. Diabetes 54:880–885 [DOI] [PubMed] [Google Scholar]
- Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S 2004 Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest 114:1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogacka I, Ukropcova B, McNeil M, Gimble JM, Smith SR 2005 Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. J Clin Endocrinol Metab 90:6650–6656 [DOI] [PubMed] [Google Scholar]
- Sakamoto J, Kimura H, Moriyama S, Odaka H, Momose Y, Sugiyama Y, Sawada H 2000 Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun 278:704–711 [DOI] [PubMed] [Google Scholar]
- Cha BS, Ciaraldi TP, Park KS, Carter L, Mudaliar SR, Henry RR 2005 Impaired fatty acid metabolism in type 2 diabetic skeletal muscle cells is reversed by PPARγ agonists. Am J Physiol Endocrinol Metab 289:E151–E159 [DOI] [PubMed] [Google Scholar]
- Wilmsen HM, Ciaraldi TP, Carter L, Reehman N, Mudaliar SR, Henry RR 2003 Thiazolidinediones upregulate impaired fatty acid uptake in skeletal muscle of type 2 diabetic subjects. Am J Physiol Endocrinol Metab 285:E354–E362 [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh H, Yano W, Ogata H, Tokuyama K, Takamoto I, Mineyama T, Ishikawa M, Moroi M, Sugi K, Yamauchi T, Ueki K, Tobe K, Noda T, Nagai R, Kadowaki T 2006 Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J Biol Chem 281:8748–8755 [DOI] [PubMed] [Google Scholar]
- Mensink M, Hesselink MK, Russell AP, Schaart G, Sels JP, Schrauwen P 2007 Improved skeletal muscle oxidative enzyme activity and restoration of PGC-1 α and PPAR β/δ gene expression upon rosiglitazone treatment in obese patients with type 2 diabetes mellitus. Int J Obes (Lond) 31:1302–1310 [DOI] [PubMed] [Google Scholar]
- Hällsten K, Virtanen KA, Lönnqvist F, Sipilä H, Oksanen A, Viljanen T, Rönnemaa T, Viikari J, Knuuti J, Nuutila P 2002 Rosiglitazone but not metformin enhances insulin- and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetes. Diabetes 51:3479–3485 [DOI] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Mensink M, Hesselink MK, Sels JP, Kooi ME, Schrauwen P 2008 The insulin-sensitizing effect of rosiglitazone in type 2 diabetes mellitus patients does not require improved in vivo muscle mitochondrial function. J Clin Endocrinol Metab 93:2917–2921 [DOI] [PubMed] [Google Scholar]
- Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E, Grimaldi A, Poynard T 2008 Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 135:100–110 [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Gerrard J, Dalla Man C, Firbank MJ, Lane A, English PT, Cobelli C, Taylor R 2008 Pioglitazone decreases fasting and postprandial endogenous glucose production in proportion to decrease in hepatic triglyceride content. Diabetes 57:2288–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR 2006 Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368:1096–1105 [DOI] [PubMed] [Google Scholar]
- Psaty BM, Furberg CD 2007 Rosiglitazone and cardiovascular risk. N Engl J Med 356:2522–2524 [DOI] [PubMed] [Google Scholar]
- Wan Y, Chong LW, Evans RM 2007 PPAR-γ regulates osteoclastogenesis in mice. Nat Med 13:1496–1503 [DOI] [PubMed] [Google Scholar]
- Oliver Jr WR, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM 2001 A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA 98:5306–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risérus U, Sprecher D, Johnson T, Olson E, Hirschberg S, Liu A, Fang Z, Hegde P, Richards D, Sarov-Blat L, Strum JC, Basu S, Cheeseman J, Fielding BA, Humphreys SM, Danoff T, Moore NR, Murgatroyd P, O'Rahilly S, Sutton P, Willson T, Hassall D, Frayn KN, Karpe F 2008 Activation of peroxisome proliferator-activated receptor (PPAR)δ promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes 57:332–339 [DOI] [PubMed] [Google Scholar]
- Krämer DK, Al-Khalili L, Guigas B, Leng Y, Garcia-Roves PM, Krook A 2007 Role of AMP kinase and PPARδ in the regulation of lipid and glucose metabolism in human skeletal muscle. J Biol Chem 282:19313–19320 [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA 2004 Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305:390–392 [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P 2005 Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434:113–118 [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA 2003 Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425:191–196 [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA 2006 Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J 2006 Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127:1109–1122 [DOI] [PubMed] [Google Scholar]
- Doré S 2005 Unique properties of polyphenol stilbenes in the brain: more than direct antioxidant actions; gene/protein regulatory activity. Neurosignals 14:61–70 [DOI] [PubMed] [Google Scholar]
- Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M 2008 SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 283:20015–20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SH, Montminy M 2006 In vino veritas: a tale of two sirt1s? Cell 127:1091–1093 [DOI] [PubMed] [Google Scholar]
- Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA 2006 Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55:2180–2191 [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Jirousek M 2008 Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs 9:371–378 [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J 2008 Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8:347–358 [DOI] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH 2007 Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450:712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q 2007 SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 6:307–319 [DOI] [PubMed] [Google Scholar]
- Hui X, Zhu W, Wang Y, Lam KS, Zhang J, Wu D, Kraegen EW, Li Y, Xu A 2009 Major urinary protein-1 increases energy expenditure and improves glucose intolerance through enhancing mitochondrial function in skeletal muscle of diabetic mice. J Biol Chem 284:14050–14057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, Steinberg HA, Chaibub Neto E, Kleinhanz R, Turner S, Hellerstein MK, Schadt EE, Yandell BS, Kendziorski C, Attie AD 2008 A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res 18:706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI 2007 The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA 104:12587–12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO 2006 One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci 61:943–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, Lefevre M, Rood JC, Williamson DA, Ravussin E 2008 Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 16:1355–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]