Abstract
Glucose-stimulated insulin secretion from the islet β-cell involves a sequence of metabolic events and an interplay between a wide range of signaling pathways leading to the generation of second messengers (e.g., cyclic nucleotides, adenine and guanine nucleotides, soluble lipid messengers) and mobilization of calcium ions. Consequent to the generation of necessary signals, the insulin-laden secretory granules are transported from distal sites to the plasma membrane for fusion and release of their cargo into the circulation. The secretory granule transport underlies precise changes in cytoskeletal architecture involving a well-coordinated cross-talk between various signaling proteins, including small molecular mass GTP-binding proteins (G proteins) and their respective effector proteins. The purpose of this article is to provide an overview of current understanding of the identity of small G proteins (e.g., Cdc42, Rac1, and ARF-6) and their corresponding regulatory factors (e.g., GDP/GTP-exchange factors, GDP-dissociation inhibitors) in the pancreatic β-cell. Plausible mechanisms underlying regulation of these signaling proteins by insulin secretagogues are also discussed. In addition to their positive modulatory roles, certain small G proteins also contribute to the metabolic dysfunction and demise of the islet β-cell seen in in vitro and in vivo models of impaired insulin secretion and diabetes. Emerging evidence also suggests significant insulin secretory abnormalities in small G protein knockout animals, further emphasizing vital roles for these proteins in normal health and function of the islet β-cell. Potential significance of these experimental observations from multiple laboratories and possible avenues for future research in this area of islet research are highlighted.
This review highlights our current understanding of the contributory roles of small G-proteins [e.g., Cdc42, Rac1 and ARF-6] and their regulatory factors in glucose-induced insulin secretion in the islet beta-cell. Potential mechanisms underlying activation of this class of signaling proteins by nutrient secretagogues of insulin are discussed. Further, modulatory roles of these small G-proteins in the onset of metabolic dysfunction in in vitro and in vivo models of impaired insulin secretion and diabetes are highlighted.
I. Introduction
II. The Three Major Classes of G Proteins in Insulin- Secreting β-Cells
- III. Role(s) of Posttranslational Modifications of Small G Proteins in Glucose-Stimulated Insulin Secretion
- A. G protein prenylation in insulin secretion
- B. G protein carboxylmethylation in insulin secretion
- C. G protein palmitoylation/acylation in insulin secretion
- IV. Regulation of Glucose-Stimulated Insulin Secretion by Small G Proteins
- A. Cdc42 in insulin secretion
- B. Rac1 in insulin secretion
- C. ARF-6 in insulin secretion
- V. Mechanism(s) of Regulation of Small G Proteins in the Islet β-Cell
- A. Regulation by biologically active lipid second messengers
- B. Regulation by novel histidine kinases
VI. A Model for Potential Cross-Talk between Cdc42, ARF-6, and Rac1 Leading to Glucose-Induced Insulin Secretion
VII. A Model for Regulation by Small G Proteins of Glucose-Induced ERK Activation in Insulin Gene Transcription and Insulin Secretion
- VIII. Regulation of the Metabolic Dysfunction of the Islet β-Cell by Small G Proteins
- A. The Rac1-NADPH oxidase-oxidative stress connection
- B. Small G proteins in the metabolic dysfunction induced by IL-1β
- C. Small G proteins in the metabolic dysfunction induced by GTP depletion
IX. Insulin Secretory Abnormalities in Small G protein Knockout Animal Models
- X. Insulin Secretory Defects in Diabetic Animals: Reversal by G Protein Agonists
- A. Effects of Mas on G protein activation and insulin secretion from isolated β-cells
- B. Reversal of insulin secretory defects in diabetic animals by analogs of Mas
XI. Conclusions and Future Directions
I. Introduction
Insulin secretion from pancreatic β-cells is regulated principally by the concentration of glucose in the interstitial fluid surrounding the islet cells. The principal signaling cascade of glucose-stimulated insulin secretion (GSIS) is initiated by the glucose transporter protein (e.g., Glut-2)-mediated entry of glucose into the β-cell. This is followed by an increase in the ATP/ADP ratio as a consequence of glucose metabolism within the β-cell. Such an increase in ATP levels culminates in the closure of ATP-sensitive potassium channels localized on the plasma membrane, resulting in membrane depolarization and facilitation of the influx of extracellular calcium due to opening of membrane-associated voltage-gated calcium channels. A net increase in the influx of extracellular calcium into the cytosolic compartment, in addition to the mobilization of intracellular calcium from its storage pools, has been shown to be critical for the transport of insulin-laden secretory granules to the plasma membrane for fusion and release of insulin into the circulation (1,2,3,4,5,6,7,8).
Despite a general understanding of some of the key steps involved in GSIS, the precise molecular and cellular mechanisms underlying the stimulus-secretion coupling of GSIS from the pancreatic β-cell remain only partially understood. In this context, it is widely accepted that GSIS is mediated largely via the generation of soluble second messengers, such as cyclic nucleotides, and hydrolytic products of phospholipases (PLases) A2, C, and D (1,2,3,4,5,6,7,8,9,10,11,12,13). It is becoming increasingly clear that changes in intracellular calcium concentrations also regulate activities of various enzymes within the β-cell, including protein kinases, phosphodiesterases, adenylyl cyclases, and PLases leading to insulin secretion (2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19). In addition to regulation by adenine nucleotides (e.g., ATP), earlier studies (20,21,22,23) have demonstrated the contributory roles for guanine nucleotides (i.e., GTP) in physiological insulin secretion. For example, using selective inhibitors of the GTP biosynthetic pathway [e.g., mycophenolic acid (MPA)], these studies have documented a permissive role for GTP in insulin secretion elicited by glucose (20,21,22). Although the precise mechanisms underlying the regulatory role(s) of GTP remain elusive, available evidence indicates that they might involve activation of one (or more) GTP-binding proteins (G proteins) endogenous to the β-cell (24,25,26).
In 1991, in a Perspective to Diabetes entitled “G proteins and modulation of insulin secretion,” Robertson et al. (27) reviewed potential roles of G proteins, specifically the stimulatory (Gs) and inhibitory (Gi) classes of heterotrimeric G proteins, in the modulation of insulin secretion. Therein, the authors stated that “the stage is set to pursue these initial observations in greater depth and ascertain whether G protein research will provide important new insights into normal and abnormal regulation of insulin secretion.” As will be seen from the discussion below, a significant amount of progress has been made since then to further understand the roles of these signaling proteins, specifically the small molecular mass G proteins, in islet function in general and GSIS in particular.
II. The Three Major Classes of G Proteins in Insulin-Secreting β-Cells
At least three classes of G proteins have been described in β-cells (24,25,26,27,28). The first group is heterotrimeric in nature and is comprised of α- (39–53 kDa), β- (∼37 kDa), and γ- (7–10 kDa) subunits. Typically, these proteins are involved in coupling membrane-associated receptors to their intracellular effectors such as ion channels, PLases, adenylyl cyclases, and phosphodiesterases (29,30,31). The α-subunits of the heterotrimeric G proteins undergo ADP-ribosylation catalyzed by ribosyl-transferases endogenous to bacterial toxins, such as cholera toxin and pertussis toxin (PTX). Apart from immunological characterization, bacterial toxin-mediated ribosylation of the α-subunits is also used as a tool for functional quantitation of heterotrimeric G proteins. The involvement of PTX-sensitive trimeric G proteins in insulin secretion was first demonstrated by Katada and Ui (32), who showed that treatment of rats with (or prior exposure of isolated islets to) PTX prevented the adrenergic inhibition of GSIS, suggesting the involvement of a PTX-sensitive mechanism(s) underlying secretion. These observations were confirmed by several investigators using isolated islets (33), intact pancreata (34), and insulin-secreting cells employing different adrenergic agents such as epinephrine or clonidine or, alternatively, the secretory inhibitors such as somatostatin and prostaglandin E2 (27). PTX-sensitive G proteins have been identified immunologically in clonal β-cells and primary islet cells (35,36). Furthermore, cholera toxin-sensitive trimeric G proteins (e.g., the Gs type) have also been identified in insulin-secreting β-cells (for review, see Refs. 37 and 38). It may be germane to point out that in addition to their classical association with the plasma membrane, previous studies from our laboratory in normal rat and human islets (39,40) and by Konrad et al. (41) in βTC3 cells have demonstrated localization of functionally active and regulable trimeric G proteins in the insulin-containing secretory granules.
The second group of G proteins (which is the main focus of this review) is comprised of a large set of monomeric G proteins (17–30 kDa in size), which have been shown to play key regulatory roles in protein sorting and trafficking of secretory vesicles in many cell types (42). A growing body of evidence in multiple cell types, including the islet β-cell (see Section III), indicates that this family of G proteins undergoes posttranslational modifications, such as isoprenylation and methylation at their C-terminal cysteine residues (often referred to as the CAAX motif; see Section III and Refs. 42,43,44,45,46). Interestingly, however, unlike the heterotrimeric G proteins, the majority of small G proteins (with some possible exceptions; e.g., Rho and Cdc42) retain their ability to bind to GTP even after SDS-PAGE and nitrocellulose transfer. The currently available evidence on the localization and regulatory roles of various small G proteins in islet function and potential mechanism(s) of regulation of these signaling proteins, specifically by insulin secretagogues, is described in Sections IV–VI.
The third group of G proteins are the elongation factors and Tau proteins, which are involved in protein synthesis. Although these proteins are characterized extensively in other cell types, little is understood with regard to the functional regulation of this class of G proteins in endocrine cells in general and islets in particular. In Section II, I will describe the existing evidence in support of the viewpoint that small G proteins (e.g., Cdc42 and Rac1) undergo posttranslational modifications and that they are requisite for GSIS to occur.
III. Role(s) of Posttranslational Modifications of Small G Proteins in Glucose-Stimulated Insulin Secretion
As stated above, most small G proteins (and the γ-subunits of trimeric G proteins) undergo a series of posttranslational modifications at their C-terminal CAAX motifs, where C represents cysteine, A is an aliphatic amino acid, and X is the terminal amino acid (25). The first set of these modification steps constitutes the incorporation of either a farnesyl [i.e., a 15-carbon derivative of mevalonic acid (MVA)] or geranylgeranyl (i.e., a 20-carbon derivative of MVA) group via a thioether linkage to the C-terminal cysteine of candidate proteins. Following this, the three amino acids after the farnesylated/geranylgeranylated cysteine are removed by the Ras-converting enzyme 1-mediated proteolysis, thereby exposing the carboxylate anion of the prenylated cysteine (25,47). This site is then methylated by the isoprenylcysteine-O-carboxyl methyltransferase, which transfers a methyl group onto the carboxylate anion. Several earlier studies have demonstrated that the carboxylmethylation (CML) increases the hydrophobicity of the modified protein due to neutralization of the carboxylate anion. In addition to these modifications, certain G proteins undergo fatty acylation at cysteine residues, which are upstream to the prenylated cysteine. Typically, palmitate is incorporated into the cysteine; this step is catalyzed by the palmitoyl transferases. It is felt that palmitoylation (or acylation) provides a firm anchoring for the modified proteins into the cell membrane for optimal interaction with their respective effector proteins (25,47,48). Section III.A will provide a brief overview of potential contributory roles for G proteins in insulin secretion, which is based on the evidence derived from their requisite posttranslational prenylation, CML, and palmitoylation.
A. G protein prenylation in insulin secretion
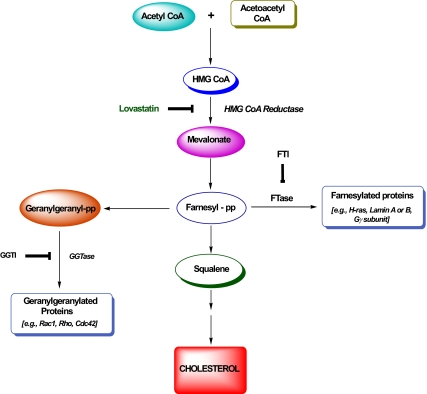
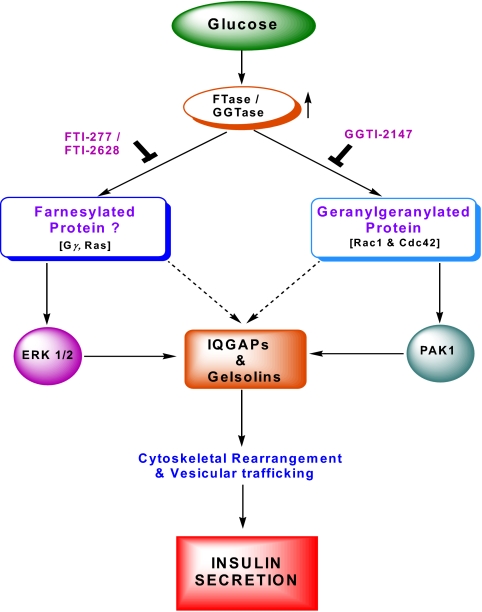
Both farnesyl and geranylgeranyl pyrophosphates are generated from acetyl-coenzyme A (CoA) and acetoacetyl-CoA in the cholesterol biosynthetic pathway (Fig. 1). These are incorporated into candidate proteins by farnesyltransferases (FTases) and geranylgeranyltransferases (GGTases), respectively. Some of the farnesylated proteins include Ras, nuclear lamins (A and B), and the γ-subunits of trimeric G proteins. Examples of geranylgeranylated proteins include Cdc42, Rac1, and Rho (Fig. 1). At least three distinct prenylating enzymes have been described in the literature (43,44,45,46). The FTase and GGTase-1 are often referred to as CAAX prenyl transferases because their substrate proteins share a conserved CAAX motif at their C-terminal region. The GGTase-II (also referred to as the Rab GGTase) prenylates the Rab subfamily of proteins at a different motif and hence is termed a non-CAAX prenyl transferases (43,44,45,46). FTase, GGTase-I, and GGTase-II are heterodimeric (i.e., consisting of α- and β-subunits) in nature. It should be noted that both FTase and GGTase-I share a common α-subunit (48 kDa) and different β-subunits with Mr of 46 kDa (FTase-β) and 43 kDa (GGTase-1β). The α-subunit is the regulatory subunit, whereas the β-subunit confers substrate specificity. The molecular sizes of GGTase-II α- and β-subunits are reported to be 60 and 38 kDa, respectively (43,44,45,46). Using Western blot methods and overexpression of inactive mutants, we have previously reported evidence for the localization and involvement of this signaling pathway in GSIS in isolated islet β-cells (Ref. 49; also see Section VII).
Figure 1.
Schematic representation of the biosynthesis of farnesyl and geranyl pyrophosphates. HMG-CoA is synthesized from acetyl-CoA and acetoacetyl-CoA; this step is catalyzed by HMG-CoA-synthetase. HMG-CoA-reductase catalyzes the conversion of HMG-CoA to MVA, which is the precursor for cholesterol biosynthesis. MVA also serves as the precursor molecule for the biosynthesis of farnesyl pyrophosphate (farnesyl-pp) and geranylgeranyl pyrophosphates (geranylgeranyl-pp). These MVA derivatives, in turn, are incorporated into candidate substrate proteins via the prenylation reaction catalyzed by FTase and GGTase. Due to the paucity of specific inhibitors for FTase/GGTase, initial studies used LOVA to decipher the roles for protein prenylation in insulin secretion. Follow-up studies using more specific inhibitors of these enzymes further confirmed the novel roles of these lipid modification steps in insulin secretion (25,47).
Through the use of pharmacological (e.g., generic as well as structure-specific) inhibitors, numerous earlier studies have demonstrated regulatory roles for protein prenylation in physiological insulin secretion (47). The initial studies that examined possible roles of protein prenylation in islet function used lovastatin (LOVA), an inhibitor of the biosynthesis of MVA, which is a precursor for the biosynthesis of farnesyl and geranylgeranyl pyrophosphates (Fig. 1). These studies demonstrated that preincubation of isolated β-cells with LOVA resulted in a significant accumulation of nonprenylated proteins in the soluble fraction, with a concomitant decrease in their abundance in the membrane fraction (48,50). Under these conditions, LOVA significantly inhibited GSIS from normal rat islets (48) and bombesin- and vasopressin-mediated insulin secretion from HIT-T15 cells (50). Based on these data, it was speculated that inhibition of protein prenylation in β-cells results in selective accumulation of unprenylated G proteins in the soluble compartment, possibly interfering with the interaction of these proteins with their respective effector proteins, which may be necessary for insulin release. Several other generic inhibitors of protein prenylation have also been tested to further assess the roles for prenylation in GSIS (for review, see Ref. 47). However, more recent studies have used a novel class of prodrug inhibitors, such as 3-allyl/vinyl-farnesols and 3-allyl/vinyl geranylgeraniols with a greater specificity of inhibition of FTases and GGTases, respectively. These two classes of inhibitors significantly reduced glucose- and calcium-stimulated insulin secretion from β-cells (51). The degree of inhibition was much greater than what was demonstrable in the presence of LOVA in isolated rat islets, suggesting that they are much more site-specific than the classical hydroxymethylglutaryl CoA (HMG-CoA) reductase blockers. In a manner akin to LOVA, the above prodrug inhibitors significantly influenced the subcellular distribution of small G proteins, as evidenced by a considerable degree of accumulation of the unprenylated proteins in the cytosolic fraction, with a concomitant decrease in their abundance in the membrane fraction (51). Together, data accrued from the pharmacological as well as molecular biological investigations implicate essential roles for protein prenylation in insulin secretion. It should be noted, however, that a considerable degree of work is still needed, especially in the area of identification and regulation by insulin secretagogues of the prenylating enzymes within the islet β-cell.
B. G protein carboxylmethylation in insulin secretion
Available evidence suggests regulatory roles for G protein CML in insulin secretion. Experimental data also suggest that the CML of prenylated cysteine is regulated acutely by glucose and other insulin secretagogues in the pancreatic β-cell (24,52). The carboxyl methyltransferase, which has been characterized in insulin-secreting cells (53), mediates the incorporation of a methyl group onto the carboxylate anion of the prenylated cysteine via an ester linkage in the presence of S-adenosyl methionine. Previous studies have demonstrated that stimulatory concentrations of glucose transiently (within 15–30 sec) augmented the CML of two small G proteins (e.g., Cdc42 and Rap1) in rat islets and clonal β-cells. Such stimulatory effects of glucose were blocked by acetyl farnesyl cysteine (AFC), a specific inhibitor of prenylated cysteine methylation (24). Studies by Leiser et al. (52) have also shown glucose- and calcium-mediated stimulation of the CML of Rap1 in insulin-secreting cells, which was attenuated by AFC. Furthermore, a requirement for endogenous GTP for glucose-stimulated CML of these islet G proteins was demonstrated in isolated rat islets because depletion of endogenous GTP (using MPA) markedly reduced the ability of glucose to stimulate the CML of islet G proteins (24). Together, these findings using structure-specific inhibitors implicate the CML of specific G proteins in insulin secretion. However, whereas the pharmacological data are encouraging, no data are available to date to further support these conclusions via additional experimental manipulations, including the molecular biological approaches involving the dominant-negative mutants as well as small interfering RNAs (siRNAs) specific for the prenyl cysteine carboxyl methyltransferases.
C. G protein palmitoylation/acylation in insulin secretion
As in the case of prenylation and CML, earlier studies have suggested that palmitoylation of specific proteins may be necessary for GSIS. Typically, palmitate is incorporated into these proteins via a thioester linkage at cysteine residues upstream of the prenylated and methylated cysteine (54,55). This signaling step is felt to promote the interaction between candidate G proteins and their effector proteins. Cerulenin (CLN), a known inhibitor of protein acylation, was shown to reduce GSIS from isolated rat islets (48); these findings were further confirmed by several studies (56,57,58,59). Interestingly, CLN failed to inhibit insulin secretion facilitated by nonnutrient secretagogues, such as a membrane-depolarizing concentration of potassium, activators of protein kinase A, or mastoparan (Mas) (56,57). In addition, studies by Deeney et al. (58) have implicated the protein palmitoylation step in insulin secretion from pancreatic β-cells elicited by long-chain fatty acids. These investigators have suggested regulatory roles of protein palmitoylation/acylation in fusion of insulin-containing secretory granules with the plasma membrane. It is important to note that the inhibitory effects of CLN (specifically, at higher concentrations and over longer periods of incubation) on protein acylation are rather nonspecific because CLN can inhibit fatty acid, sterol, and protein synthesis. 2-Bromo-palmitate has also been used to study the roles of protein acylation in cellular function (59). More specific CLN analogs have been reported previously (60) and remain to be investigated in the islet β-cell in the context of GSIS.
Together, findings reviewed above suggest that specific β-cell G proteins undergo posttranslational modifications and that inhibition of such signaling steps leads to reduced GSIS. Future studies will be needed to determine the potential reversible nature of these modification steps in the islet β-cell. For example, very little is known with regard to the identity and regulation of “de-prenylases,” carboxylmethyl esterases, and palmitoylesterases in the islet β-cell. In this context, we previously reported localization of a demethylase, which hydrolyzes the C-terminal leucine methyl esters of the catalytic subunit of protein phosphatases (61) and the cysteine methyl esterases of the Gγ-subunits of trimeric G proteins (62) in β-cells. It remains to be determined whether the Gγ-methylesterase catalyzes the demethylation of small molecular mass G proteins (e.g., Cdc42 and Rac1) as well.
Lastly, it may be germane to point out that bacterial toxins have been used as tools to study roles of small G proteins in insulin secretion. These toxins specifically monoglucosylate and inactivate G proteins with reliable specificity (63). For example, Clostridium difficile toxins A or B monoglucosylate (at threonine residues) and inactivate Rho, Rac, and Cdc42 (but not Ras or Rab). The data from previous studies involving the use of these toxins suggested that Cdc42, Rap, and Rac1 might be involved in physiological insulin secretion (63); these findings, which are compatible with pharmacological inhibitor data (51), are suggestive of regulatory roles for specific G proteins (e.g., Cdc42, Rac1, and Rap) in GSIS. Section IV will review the direct experimental evidence from multiple laboratories to implicate the small G proteins, specifically the Rho subfamily of guanosine triphosphatases (GTPases) in insulin secretion.
IV. Regulation of Glucose-Stimulated Insulin Secretion by Small G Proteins
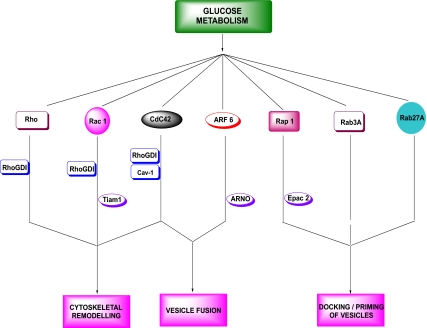
Based on the existing evidence on the functional regulation of islet β-cell function, the small G proteins can be divided into three major groups. The first group consists of Rho, Rac1, Cdc42, and the ADP-ribosylation factor-6 (ARF-6) (Fig. 2). Convincing experimental evidence from multiple laboratories suggests important roles for these proteins in the cytoskeletal remodeling and vesicle fusion in the pancreatic β-cell (49,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79). The second group of small G proteins consists of Rap1 (24,52,80), Rab3A (81,82,83,84,85,86,87), and Rab27 (88,89,90,91,92,93,94,95). In general, the Rab GTPases are associated with the secretory granules and have been shown to play regulatory roles in the priming and docking of insulin-laden secretory granules at the plasma membrane (Fig. 2) (24,52,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96). Compared with the GTPases belonging to the first group (e.g., Cdc42 and Rac1), the potential mechanisms underlying the activation of Rab proteins by physiological insulin secretagogues is relatively less understood. Furthermore, it is unknown whether Rab G proteins require posttranslational prenylation for their optimal function and whether inhibition of Rab GGTase-II results in attenuated insulin secretion. It should be noted, however, that as in the case of Cdc42, Rap1 has been shown to be activated transiently by glucose (24). It also undergoes CML, which is activated by both glucose and KCl (24,52). It has also been implicated in cAMP-sensitive signaling pathways involving exchange proteins directly activated by cAMP (Epac)-like GDP/GTP exchange factors (GEFs). Epacs have also been shown to play key regulatory roles in various cellular signaling steps involved in β-cell function, including calcium-induced calcium mobilization, insulin secretion, β-cell growth, and proliferation (97,98,99,100,101). The third group of small GTPases (not described in Fig. 2) comprises relatively less studied proteins, including Rab2 (96), Rhes (102,103,104), and Rem2 (105). In addition, RalA, a small G protein, appears to elicit direct regulatory effects in exocytosis via its direct interaction with the exocyst complex (106).
Figure 2.
Modulatory roles of various classes of small G proteins and their regulatory proteins/factors in insulin secretion. Small G proteins, such as Rac1, Cdc42, and ARF-6 (and potentially Rho) regulate cytoskeletal remodeling and vesicular fusion in the islet. Rab3A, Rab27A, and Rap1 are implicated docking and priming of secretory vesicles at the exocytotic sites. Glucose-mediated activation of some of these signaling proteins is under the fine control of various regulatory proteins including GDIs (e.g., Rho GDI and Cav-1) and GEFs (Tiam1, ARNO, and Epac2).
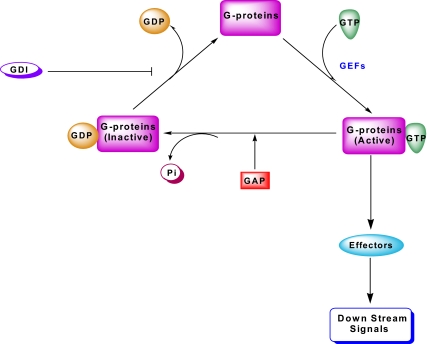
It should be noted that, in a manner akin to the heterotrimeric G proteins, small G proteins cycle between their inactive (GDP-bound) and active (GTP-bound) conformations, which are tightly controlled by various G protein regulatory proteins/factors (Fig. 3). Three major types of such regulatory proteins/factors have been described for small G proteins. The first group is comprised of GEFs, which facilitate the conversion of the GDP-bound (inactive) forms to their GTP-bound (active) forms. The second group of regulatory factors are the GDP-dissociation inhibitors (GDIs), which prevent the dissociation of GDP from G proteins, and hence are considered “inhibitory” in the G protein activation cascade. The third group represents the GTPase-activating proteins (GAPs); these proteins promote the conversion of the active (GTP-bound) G proteins to their respective inactive (GDP-bound) conformations by activating the intrinsic GTPase function of candidate G proteins to complete the GTP hydrolytic cycle (Fig. 3). The currently available evidence on the identity, subcellular distribution, functional activation, and potential roles of specific small G proteins (e.g., Cdc42, Rac1, and ARF-6) and their regulatory proteins/factors in the function of the pancreatic β-cell, including GSIS, is summarized briefly in this section.
Figure 3.
Guanine nucleotide regulatory proteins/factors involved in the activation-deactivation cycle of G proteins. The small G proteins (e.g., Cdc42 and Rac1) in their GDP-bound (inactive) conformation remain associated with their respective GDIs. The principal role of GDIs is to prevent dissociation of GDP from the corresponding G protein. After the receipt of the appropriate signal, the G protein/GDI complex dissociates, thereby facilitating GTP/GDP exchange mediated by various guanine nucleotide exchange factors (GEFs). The GTP-bound, functionally active G protein, in turn, regulates its effector proteins and downstream signaling steps leading to cellular (de)activation. GTP bound to these G proteins is hydrolyzed by the GTPase activity intrinsic to the candidate G protein, to GDP yielding the inactive conformation of the G protein. Under specific conditions, the GTPase activity can be stimulated by additional regulatory factors, such as the GAP.
A. Cdc42 in insulin secretion
Published evidence by several researchers (64,65,66,67,68,69,70,71), including our own (Madison, WI, and Detroit, MI), Li and co-workers (Geneva, Madison, WI, and Singapore), Thurmond and co-workers (Indianapolis, IN), and Regazzi and colleagues (Geneva) contributed significantly toward the current understanding of regulatory roles of Cdc42 in insulin secretion. Regazzi et al. (64) first described localization of Cdc42 in insulin-secreting clonal β (RINm5F and HIT-T15) cells. Subsequent immunological and confocal microscopic evidence further supported localization of Cdc42 in clonal β-cells, normal rat islets, and human islets (24,65). Earlier studies have also demonstrated a significant translocation of cytosolic Cdc42 to the membrane fraction in lysates incubated with GTPγS, suggesting that GTP-bound conformation (and activation) is necessary for its membrane association (24). It was also demonstrated that Cdc42 remains associated with Rho-GDI in clonal β-cells and that exposure of isolated β-cells to a prenylation inhibitor prevented association of Cdc42 with Rho-GDI, resulting in selective accumulation of this protein (64). These data provided the first supporting evidence to implicate prenylation in the functional regulation of Cdc42 in the islet β-cell. Further support for this postulation that posttranslational modifications of C-terminal cysteine of specific G proteins is necessary for their activation was obtained in our studies demonstrating that GTP (in broken cells) and glucose (in intact cells) increased the CML of Cdc42 in rodent and human islets and clonal β-cells. The CML resulted in its translocation to the membrane fraction. Glucose-induced CML and activation of Cdc42 was also shown to be rapid (within 15–30 sec) and transient (24). Such stimulatory effects of glucose were blocked completely by mannoheptulose, which is an inhibitor of glucose metabolism. Furthermore, 3-O-methyl glucose, a transportable, but nonmetabolizable analog of glucose, failed to activate Cdc42. AFC, a specific inhibitor of CML, markedly attenuated glucose-induced phosphoinositide turnover and insulin secretion. Lastly, the ability of glucose to activate Cdc42 was markedly reduced after intracellular depletion of GTP (using MPA); it was restored by coprovision of guanosine (24). Together, these data provided the first direct evidence to implicate glucose-derived metabolic signals in the activation of Cdc42 in insulin-secreting cells.
A series of more recent investigations from the Thurmond’s laboratory provided substantial support for involvement of Cdc42 in GSIS. A study published in 2003 presented the first evidence to suggest that glucose promotes actin cytoskeletal rearrangements and insulin secretion via a signaling step involving the cycling of Cdc42 between the inactive GDP-bound form to its active GTP-bound conformation (68). The time course for such activation-deactivation cycle of Cdc42 was shown to within 3 min after exposure to glucose (68)—data compatible with the transient nature of the activation of this protein by glucose (24). Furthermore, these investigators presented evidence to indicate that deactivation of Cdc42 involves a posttranslational glucosylation step (68). Follow-up investigations from this laboratory (71) provided a detailed time line for the activation of Cdc42 and its downstream signaling events in clonal MIN6 cells and mouse islets in the cascade of events leading to insulin secretion. Using siRNA-mediated knockdown approaches, they demonstrated that glucose-mediated transient activation of Cdc42 leads to activation of its effector protein PAK1, which is followed by activation of Rac1 within 15 min after exposure to glucose. Depletion of endogenous Cdc42 or PAK1 elicited a marked reduction in the ability of glucose to activate Rac1 and insulin secretion. Together, these studies provide compelling evidence in support of a signaling pathway involving Cdc42, PAK1, and Rac1 in GSIS (see Section IV). In addition to its ability to regulate cytoskeletal remodeling, Cdc42 has also been implicated in the vesicle fusion of insulin-laden secretory granules involving soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein (SNARE protein) complexes. For example, Cdc42 and the SNARE protein [vesicle-associated membrane protein 2 (VAMP2)] were shown to be associated in the secretory granule fraction and translocated to the plasma membrane after stimulation by glucose. Glucose increased the activation of Cdc42, culminating in the translocation of the Cdc42/VAMP2 complex to the plasma membrane for association with Syntaxin 1A to promote granule fusion and insulin release (69). Due to the scope of this article, these aspects of Cdc42 biology in islet β-cell function are not discussed further, but the reader can consult a recent review article by Wang and Thurmond (26) for additional insights into this regulatory mechanism(s).
1. Regulatory factors for Cdc42 in the islet β-cell
Very little is known to date about the identity of GEFs for Cdc42 in the islet β-cell. However, recently published evidence from three laboratories provides support for the localization and regulation of GDIs in the pancreatic β-cell (64,70,73,107,108,109). The GDIs are known to: 1) prevent dissociation of GDP from specific G proteins; 2) inhibit the intrinsic and GTPase-activating protein-catalyzed hydrolysis of GTP; 3) sequester back specific G proteins (e.g., Cdc42, Rac, and Rho) from their membranous sites, thereby inhibiting their interaction with their respective effector proteins; and 4) regulate spatial determination in the actin cytoskeletal control (110,111,112,113). Earlier studies by Regazzi et al. (64) suggested the presence of GDI (e.g., Rho-GDI) in insulin-secreting RINm5F and HIT-T15 cells. In addition to classical GDIs (e.g., Rho-GDI), it appears that scaffolding proteins, such as caveolin-1 (Cav-1), also subserve the roles of GDI (70). For example, recent observations from the Thurmond laboratory have reported that Cav-1 acts as the GDI for Cdc42 in insulin-secreting cells (70). Under basal conditions, Cav-1 remains associated with Cdc42-VAMP2-bound granules just beneath the plasma membrane in insulin-secreting cells. Exposure of these cells to stimulatory glucose results in the dissociation of Cav-1 from Cdc42-VAMP2 complexes. Time course measurements suggested a close relationship between dissociation of Cav-1/Cdc42-VAMP2 complex and Cdc42 activation (GTP-bound conformation). Based on these and other supporting data, these investigators proposed that Cav-1 functions as a GDI for Cdc42 in β-cells to maintain Cdc42 in an inactive state and regulating basal secretion in the absence of stimuli. They also proposed that Cav-1 may contribute to the specific targeting of granules to “active sites” of exocytosis organized by caveolae within the plasma membrane (70).
B. Rac1 in insulin secretion
Using Clostridium difficile toxins A and B, which irreversibly monoglucosylate and inactivate specific G proteins (e.g., Cdc42 and Rac1), we first reported a potential role for Rac1 in GSIS (63). In the same study, it was also demonstrated that Rac1 undergoes posttranslational CML, which was also stimulated by stimulatory glucose concentrations in a variety of insulin-secreting cells. Follow-up studies by Li et al. (72) documented essential roles for Rac1 in GSIS. They demonstrated that exposure of insulin-secreting cells to glucose resulted in translocation of cytosolic Rac1 to the plasma membrane fraction. Moreover, glucose activated Rac1 (i.e., GTP-bound conformation) under those conditions as determined by the PAK1 pull-down assay. Based on the data accrued in these studies, these investigators concluded that glucose-induced activation and membrane translocation of Rac1 takes place within 15 min of exposure to glucose. Further evidence for a role of Rac1 in GSIS came from studies involving dominant-negative mutant of Rac1 (N17Rac1). Expression of this inactive mutant in INS-1 cells resulted in significant morphological changes and disappearance of F-actin structures, culminating in reduction of GSIS. Based on these observations, it was proposed that Rac1 activation may be necessary for the recruitment of secretory granules through actin cytoskeletal reorganization and remodeling (72).
Further support for the overall hypothesis that Rac1 activation is necessary for GSIS came from experiment approaches involving the use of highly specific pharmacological inhibitors of geranylgeranylation. For example, geranylgeranyltransferase inhibitor (GGTI)-2147, a protein geranylgeranylation inhibitor (Fig. 1), significantly increased the cytosolic accumulation of Rac1 and inhibited GSIS in INS 832/13 cells. In addition, overexpression of an inactive mutant of the regulatory α-subunit of protein prenyltransferase, which catalyzes the incorporation of geranylgeranyl groups into the C-terminal cysteine of Rac1 (Fig. 1) markedly attenuated glucose-induced, but not KCl-induced, insulin secretion in these cells, suggesting that glucose-mediated activation of protein prenyltransferases and the subsequent prenylation of Rac1 are necessary for GSIS (49). In addition, siRNA-mediated depletion of endogenous Rac1 expression in INS 832/13 cells markedly reduced GSIS in these cells (S. Ismail and A. Kowluru, unpublished observations).
In support of the above observations are studies by Asahara et al. (74) demonstrating impaired glucose tolerance and hypoinsulinemia in Rac1-null (βRac1−/−) mice. Consistent with data described above, only glucose-induced, but not KCl-induced, insulin secretion was inhibited significantly in islets from βRac1−/− mice. The β-cell mass or islet density remained unchanged in these mice. siRNA-mediated knockdown of Rac1 in INS-1 cells also resulted in a significant defect in glucose-induced, but not KCl-induced, insulin secretion. Based on these findings, it was concluded that Rac1 plays a key regulatory role in insulin secretion primarily by regulating cytoskeletal organization (74). Lastly, Greiner et al. (75) provided evidence to suggest that Rac1-null mice exhibited marked alterations in islet morphogenesis. The β-cell spreading and migration were markedly attenuated. Cell-cell contact E-cadherin was increased in Rac1-null mice. Actin remodeling and cell spreading induced by betacellulin was also not seen in the transgenic islets. This is the first study to suggest a role for Rac1 in islet morphogenesis (75). Taken together, the above-described findings from multiple laboratories involving pharmacological and molecular biological tools as well as knockout animal models provide compelling evidence for novel regulatory roles for Rac1 in islet function, including GSIS.
1. Regulatory factors for Rac1 in the islet β-cell
Several lines of published evidence support the localization of a number of GEFs in the islet β-cell (97,98,114,115,116,117,118,119,120,121,122,123,124,125). In reconstitution studies involving purified subcellular fractions of β-cells, we first reported (114) localization of GEFs in isolated β-cells. In the GEF activity determinations, which involved GTPγS35-binding assays, a significant stimulation of this activity by biologically active lipids [e.g., arachidonic acid (AA) and lysophospholipids] was observed, thus providing the first evidence for G protein activation by these lipid second messenger molecules in the pancreatic β-cell (114). More recently, novel regulatory roles of T-lymphoma invasion and metastasis1 (Tiam1), a GEF for Rac1, in GSIS from a variety of insulin-secreting cells have also been reported (125). Western blot analysis indicated that Tiam1 is predominantly cytosolic in distribution. NSC23766, a specific inhibitor of Tiam1-mediated activation of Rac1, but not Cdc42 or Rho, markedly attenuated glucose-induced, but not KCl-induced, insulin secretion in INS 832/13 cells and normal rat islets. Furthermore, NSC23766 significantly reduced glucose-induced activation and membrane association of Rac1 in INS 832/13 cells and rat islets. The pharmacological data were further confirmed by molecular biological approaches. For example, the siRNA-mediated knockdown of Tiam1 markedly inhibited glucose-induced membrane trafficking and activation of Rac1 in INS 832/13 cells. Based on the immunological, pharmacological, and molecular biological data, it was concluded that Tiam1 represents a GEF for Rac1 in the pancreatic β-cell and that Tiam1/Rac1 signaling pathway is involved in glucose-induced, but not KCl-induced, insulin secretion (125).
More recent studies have provided additional evidence for a regulatory role for Rho-GDI in GSIS (108). For example, using several independent experimental approaches (e.g., Triton X-114 phase partition, coimmunoprecipitation, and sucrose density gradient centrifugation methods), a potential coexistence (and association) of GDI with Rac1 was demonstrated in pancreatic β-cells. Furthermore, overexpression of the wild-type GDI significantly inhibited glucose-induced, but not KCl- or Mas-induced, insulin secretion in these cells, suggesting a negative modulatory role for GDI in physiological insulin secretion. Such a conclusion was further supported by additional data which demonstrated that siRNA-mediated knockdown of endogenous GDI resulted in a significant increase in GSIS (108). Together, these findings suggested an inhibitory role for GDI in the glucose metabolic signaling cascade, which may be relevant for GSIS. Based on the experimental evidence available up until now, it is proposed that GDIs play a negative modulatory role in the cascade of events leading to GSIS. It is accomplished primarily by retaining the candidate G proteins (e.g., Cdc42 and Rac1) in their GDP-bound conformation. Additional roles for GDIs have been proposed in the preservation of cell viability (presumably by modulating specific G protein functions) under normal conditions. For example, recent studies by Park et al. (109) indicated a significant down-regulation of Rho-GDI expression, increase in JNK activity, and cell death in β-cells exposed to MPA; such defects were prevented in cells overexpressing Rho-GDI. Further investigations are needed to precisely define the signaling mechanisms underlying such “cytoprotective” roles of Rho-GDI.
C. ARF-6 in insulin secretion
It is well established that the ARF family of small G proteins plays a key regulatory role in membrane trafficking. Among these, ARF-6 is well studied and has been shown to regulate several cellular events including cell motility, vesicle transport, and cortical actin rearrangements. In addition, ARF-6 has been shown to activate several enzymes of lipid metabolism including phospholipase-D (PLD) and phosphatidylinositol 4-phosphate 5-kinase type 1γ to generate fusogenic lipids such as phosphatidic acid (PA) and PIP2, respectively (126,127,128,129,130). Previous observations from multiple laboratories demonstrated potential involvement of PLD activation in the signaling mechanisms leading to insulin secretion (10,13,131,132,133,134). Therefore, it is likely that ARF-6 might represent one of the GTPases whose activation may be necessary for insulin granule trafficking to the plasma membrane and their eventual fusion with the plasma membrane for the exocytosis of insulin. Indeed, published evidence appears to support such a hypothesis.
Using the Triton phase partitioning assay, Regazzi et al. (76) first reported the localization and the hydrophilic nature of ARF in RINm5F and HIT-T15 cells. More recent studies by Lawrence and Birnbaum (77) provided compelling evidence in support of regulatory roles of ARF-6 in insulin secretion. These investigators demonstrated that an inactive mutant of ARF-6 (T27N) markedly reduced glucose-, potassium-, and GTPγS-induced insulin secretion from MIN6 cells. ARF-6 (T27N) also reduced depolarization-dependent generation of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], which coincided with the time course for inhibition of insulin secretion. Lastly, inhibition of PI(4,5)P2-mediated signaling events via overexpression of a lipid-binding protein culminated in depolarization-induced insulin secretion in a manner akin to the ARF-6 (T27N) effects, suggesting a specific role for ARF-6 in the maintenance of intracellular PI(4,5)P2 levels and insulin secretion (77). Subsequent studies by Grodnitzky et al. (78) have reported localization of ARF-1 and ARF-6 in HIT-T15 cells. They demonstrated that overexpression of ARF-6 (T27N) or siRNA-mediated knockdown of endogenous ARF-6 markedly reduced somatostatin-mediated activation of PLD. Overexpression of wild-type ARF-6 potentiated somatostatin-mediated PLD activation.
1. Regulatory factors for ARF-6 in the islet β-cell
Very little is known about the existence of putative regulatory factors for ARF-6 in the islet β-cell. Findings in other cell types suggest that the activation of ARF-6 is mediated through the intermediacy of GEFs, such as cytohesin/Arf nucleotide binding site opener (ARNO) (135,136,137). Preliminary immunological data from our laboratory suggest localization of ARNO in normal rat islets and INS 832/13 cells. Furthermore, siRNA-mediated depletion of endogenous ARNO markedly attenuated GSIS in INS 832/13 cells, further implicating ARF-6/ARNO signaling steps in GSIS (B. Jayaram, S. Ismail and A. Kowluru, manuscript in preparation). It still remains to be verified whether the ARNO-mediated activation of ARF-6 is essential for the regulation of PLD in the pancreatic β-cell. In addition to ARNO, Grodnitzky et al. (78) have reported localization EFA6A, another GEF for ARF-6 in insulin-secreting cells. Their data suggested that EFA6A, but not ARNO, is required for somatostatin-mediated activation of PLD. It was concluded that somatostatin elicits its PLD stimulatory effects in an EFA6A/ARF-6-sensitive manner. A significant amount of work is still needed to further evaluate roles for ARF-6 in islet function, but the available data thus far appear to support our working model proposed in Section VI.
The data reviewed above clearly implicate Cdc42, ARF-6, and Rac1 in GSIS. In addition, published evidence also supports localization of and modulation by specific regulatory proteins/factors of Cdc42, Rac1, and ARF-6 in the islet β-cell. These include GEFs (e.g., Tiam1 for Rac1; ARNO for ARF-6; and Epacs for Rap1) and GDIs (e.g., Rho GDI for Rac1 and Cav-1 for Cdc42). It should be noted that little is understood with regard to the identity and properties of GAPs for Cdc42, ARF-6, and Rac1 in islet β-cell. Bouzakri et al. (138) recently reported a novel role for AS160, a Rab-GAP in insulin secretion, cell proliferation, and survival of human and mouse β-cells. Future studies will need to determine the identity of putative GAPs for Rho-GTPases.
Finally, potential roles of Rho G protein (Fig. 2) in insulin secretion remain to be confirmed further. Using Clostridial C3 exoenzyme, which selectively inactivates Rho G protein via ADP-ribosylation, we failed to see any clear effects of Rho inhibition on glucose- and KCl-induced insulin secretion from pancreatic β-cells (63). However, more recent studies by Hammar et al. (79) have demonstrated that inhibition of Rho and Rho-associated kinase in primary β-cells culminates in a significant increase in cell spreading, actin depolymerization, and GSIS. Future studies will further evaluate regulatory roles for this Rho-sensitive signaling pathway in islet function. Section V will review the existing experimental evidence in support of two potential mechanisms for the regulation of GEF/GDI functions in the pancreatic β-cell leading to small G protein activation and insulin secretion. The first involves the regulation of these functions by biologically active lipid hydrolytic products of PLases, and the second involves regulation of these functions by a novel class of histidine kinases, which have been identified and characterized in the islet β-cell.
V. Mechanism(s) of Regulation of Small G Proteins in the Islet β-Cell
A. Regulation by biologically active lipid second messengers
Numerous investigations have implicated regulatory roles for endogenous PLase activation in the stimulus secretion coupling of GSIS. In a Perspectives to Diabetes entitled “The pancreatic islet as Rubik’s Cube. Is phospholipid hydrolysis a piece of the puzzle?” Stewart Metz first reviewed the available evidence to suggest control of insulin secretion by biologically active lipid hydrolytic products (10). This topic was revisited and the proposal was validated by Vincent Poitout recently (13). In our original studies on the identification and characterization of various small G proteins in the pancreatic β-cell, we quantitated the GTP-binding and hydrolytic activities in purified subcellular fractions from normal rat islets and clonal β-cell preparations (65,114). Three specific GTPase activities with varying degrees of affinity for GTP (i.e., high- and low-affinity GTPases) were detected in islet subcellular fractions; most notably, two of these were enriched in the secretory granules. The regulation of GTPase activity in subcellular fractions of normal rat islets by insulinotropic lipids followed a rank order similar to their insulin-releasing capacity. For example, AA, lysophosphatidylcholine (LPC), or PA inhibited the GTPase activities significantly (by 60–80%) in islet homogenates and in individual purified subcellular fractions. Less insulinotropic fatty acids, such as linoleic acid and oleic acid, inhibited GTPase to a lesser degree, whereas lysophosphatidic acid, phosphatidylcholine, or palmitic acid, which do not acutely promote secretion, elicited minimal effects. Similar inhibitory effects of these lipids were also noticed in subcellular fractions isolated from human islets. Moreover, in reconstitution assays involving purified subcellular fractions, a significant potentiation of the GEF activity (up to 2-fold) by biologically active lipids, including AA, PA, and LPC, was demonstrable (114). Based on these findings, it was proposed that the insulinotropic nature of the lipids might, in part, be due to their ability to maintain G proteins in their GTP-bound (active) configuration by increasing GTP binding (via the GEF activity) and decreasing its hydrolysis (see Fig. 3 for a schematic representation of the GTP hydrolytic cycle). These studies, therefore, comprised the first evidence for the regulation by biologically active lipids of β-cell G proteins at a locus distal to plasma membrane events (i.e., on secretory granules) and provided a potential mechanism whereby the activation of islet endogenous PLases might culminate in insulin exocytosis.
Recently, McDonald et al. (73) presented additional support for the hypothesis that activation of small G proteins can be modulated by biologically active lipids. Incubation of β-cell lysates with PIP2, PA, phosphatidylcholine, and phosphatidylserine significantly promoted trafficking of cytosolic Rac1 to the membrane fraction. Lysophosphatidic acid, but not LPC or lysophosphatidylserine, also promoted translocation and membrane association of Rac1. AA, diacylglycerol, calcium, and cAMP exerted no clear effects on Rac1 translocation to the membrane. Together, these findings are suggestive of a potential possibility that generation of biologically active lipids, known to occur in the glucose-stimulated β-cell, may mediate targeting of Rac1 to the membrane for optimal interaction with its putative effector proteins leading to GSIS (73).
B. Regulation by novel histidine kinases
Emerging evidence from several laboratories implicates novel regulatory mechanisms for the activation of heterotrimeric as well as small G proteins mediated by histidine kinases (for a recent review, see Ref. 19). For example, Otsuki et al. (139) have reported interaction between Tiam1, a GEF for Rac1, and nucleoside diphosphate kinase (NDPK; nm23-H1) in 293T human embryonal kidney cells. A significant inhibition of Tiam1-induced formation of GTP-bound Rac1 and c-Jun kinase activation was noticed in cells overexpressing nm23-H1. Furthermore, forced overexpression of wild-type nm23-H1, but not its kinase-deficient mutant, converted the GDP-bound forms of Rac1, Cdc42, and Rho-A to their active GTP-bound forms in vitro. Based on these findings, the authors concluded that nm23-H1 plays a modulatory role in the Tiam1-induced activation of Rac1 (139). Along these lines, in support of a G protein-regulating property for nm23 are the observations from the Kahn laboratory (140) suggesting a unique interaction between the Ras-related protein associated with diabetes (Rad) and nm23 protein. They noticed a GAP activity intrinsic to nm23 toward Rad because an antiserum directed against nm23 completely depleted Rad GAP activity in human skeletal muscle preparations. Interestingly, nm23 also exhibited GEF activity because it promoted GTP loading onto Rad in the presence of ATP and GDP; such effects are mediated via the NDPK activity. Thus, these findings suggest novel roles for nm23 in the effects of Rad on glucose metabolism. It remains to be seen whether the nm23 class of proteins exerts GEF/GAP-like properties on G proteins in the islet β-cell.
Recent experimental evidence further supports the hypothesis that nm23-H1 plays novel regulatory roles in GSIS (141). For example, forced expression of the wild-type nm23-H1, but not its kinase-deficient mutant (H118F), markedly potentiated GSIS in INS 832/13 cells, without affecting basal secretion. These data indicate that signals derived from glucose metabolism are needed for the potentiating effects of nm23-H1 (141). Unpublished evidence from our laboratory suggests that glucose promotes translocation of the cytosolic nm23-H1 to the membrane fraction in INS 832/13 cells under conditions in which glucose promoted membrane association of Cdc42 and Rac1. Future investigations in this area will define potential roles for nm23-H1-like histidine kinases in the regulation of small G protein function, specifically at the level of the various regulatory steps involved in the G protein activation-deactivation cycle (Fig. 3). In summary, data reviewed above suggest localization of Cdc42, Rac1, and ARF-6 and their regulatory factors/proteins in insulin-secreting cells. Furthermore, it appears that the lipid second messengers of glucose metabolism elicit stimulatory effects on these G proteins culminating in insulin secretion.
VI. A Model for Potential Cross-Talk between Cdc42, ARF-6, and Rac1 Leading to Glucose-Induced Insulin Secretion
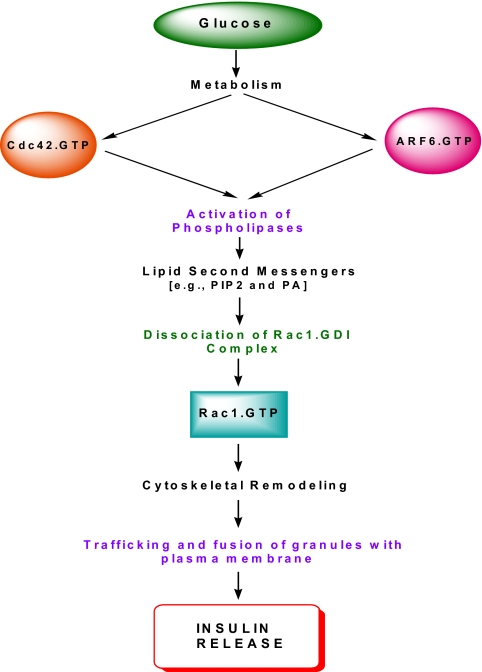
Based on the available experimental evidence reviewed above, a model for a potential cross-talk between Cdc42, Rac1, and ARF-6 leading to GSIS is proposed in Fig. 4. Glucose metabolism leads to a selective increase in intracellular GTP, which in turn leads to transient stimulation of the CML (within 30 sec) (24) and optimal activation (within 3 min) (71) of Cdc42. The activated Cdc42, in turn, promotes catalytic stimulation of at least two effector proteins, namely phospholipase-C (24) and PAK1 (71). Activation of phospholipase-C leads to the generation of biologically active lipids, which promote the dissociation of Rho-GDI/Rac1-GDP complex (73). Furthermore, PAK1 activation, in turn, leads to phosphorylation of Rho-GDI to facilitate the dissociation of GDI/Rac1-GDP complex (113). The GDP-Rac1 (inactive form) is converted to its active GTP-bound conformation through the intermediacy of Tiam1 (125). Based on time course assays conducted in the laboratories of the author, Li (72), and Thurmond (71), it appears that glucose-mediated activation of Rac1 takes place within 15 min of exposure. The activated Rac1 and Cdc42 promote the trafficking/translocation of secretory granules to the membrane via their effects on actin cytoskeletal rearrangement/remodeling.
Figure 4.
A model for potential cross-talk between ARF-6, Cdc42, and Rac1 leading to GSIS. Glucose metabolism leads to activation of Cdc42 and ARF-6, which, in turn, culminates in the activation of lipid-metabolizing enzymes within the β-cell. The lipid second messengers (e.g., PIP2 and PA) generated from PLase activation promote dissociation of Rac1 from the Rac1/GDI complex, paving the way for Rac1 activation (GTP-Rac1) mediated by Tiam1, a GEF specific for Rac1. Activation of Rac1 promotes cytoskeletal remodeling, which facilitates the trafficking of insulin-laden secretory granules to the plasma membrane for their fusion and exocytotic secretion of insulin. It should be noted that whereas previous studies have provided evidence to suggest that glucose-mediated activation of Cdc42 is upstream to Rac1 activation, the time course for ARF-6 activation by glucose remains to be determined in cognate cellular preparations.
It is also proposed that ARF-6 activation is necessary for the initiation of the events leading to Rac1 activation. As depicted in Fig. 4, glucose-induced ARNO-mediated activation of ARF-6 leads to the stimulation of islet endogenous PLases (e.g., PLD) and phosphatidylinositol kinases, which in turn leads to the generation of biologically active lipids (e.g., PIP2 and PA). These lipid second messengers have been shown to promote the dissociation of Rac1 from the GDI/Rac1 complex in the pancreatic β-cell (73). This hypothesis of the role of ARF-6 in insulin exocytosis is further supported by recent studies by Bèglè et al. (142) demonstrating that ARF-6 promotes the generation of fusogenic lipids for calcium-evoked secretion in neuroendocrine cells. siRNA-mediated depletion of ARF-6 in PC12 cells markedly attenuated PLD activation, PA formation, and secretagogue-induced exocytosis. Moreover, expression of an ARF-6, which is insensitive to siRNA, maximally rescued secretion in ARF-6 depleted cells. Therefore, based on these data, it appears that ARF-6 represents an upstream signaling protein, the activation of which leads to stimulation of endogenous PLD and subsequent release of fusogenic lipids, which in turn promotes fusion of insulin-containing secretory granules with the plasma membrane for the secretion of insulin. This postulation of regulation of PLD by ARF-6 remains to be verified in the context of insulin secretion in the islet β-cell. The reader is referred to a recent review by Bader and Vitale (143) for additional mechanistic details on the potential involvement of PLD in calcium-regulated exocytosis from chromaffin cells.
In summary, we propose that glucose metabolism leads to the activation of and interplay between Cdc42, ARF-6, and Rac1 leading to insulin secretion. It should be noted that several details/steps in this signaling pathway remain to be verified, including the potential identity of a GEF for Cdc42 and GAP activities for Cdc42 and Rac1. The precise identity of the phosphoprotein substrates for PAK1 (besides Rho-GDI) remain to be addressed further. Along these lines, evidence described below suggests that, in addition to PAK1, ERK might represent one of the target proteins whose activation is under the control of small G proteins in the pancreatic β-cell.
VII. A Model for Regulation by Small G Proteins of Glucose-Induced ERK Activation in Insulin Gene Transcription and Insulin Secretion
Original studies from several laboratories suggested that activation of ERK represents a key signaling event in islet function including glucose-induced insulin gene transcription and β-cell proliferation (11,144,145,146,147,148,149,150,151,152,153,154). These studies indicated that the effects of glucose and other secretagogues involve the intermediacy of several G proteins. For example, Ehses et al. (150) reported that glucose-dependent insulinotropic peptide increased ERK activation in INS 832/13 cells in a cAMP-dependent fashion and that such stimulatory effects are mediated by Rap1, but not Gβγ subunits of trimeric G proteins. Gomez et al. (153) have demonstrated that glucagon-like peptide-1 (GLP-1), in the presence of stimulatory concentrations of glucose, activates ERK in a protein kinase A- and calmodulin kinase-sensitive manner in MIN6 cells. They also reported that such effects required calcium efflux through L-type voltage-gated calcium channels but do not require the activation of Rap1, Ras, or Raf-1 in these signaling steps mediated by glucose and GLP-1.
Arnette et al. (145) reported activation of ERK1/2 by glucose and GLP-1 in INS-1 cells. FK506, a calmodulin antagonist, cyclosporine, or thapsigargin inhibited glucose and GLP-1-induced ERK activation. Inactive mutants of Ras or Raf-1 markedly attenuated glucose-induced activation of ERK, suggesting regulatory roles of the Ras/Raf-1 signaling pathway in glucose-induced ERK activation. Interestingly, in contrast to these findings, studies by Briaud et al. (151) reported that adenoviral-mediated expression of dominant-negative Ras inhibited IGF-I-mediated, but not glucose-induced, activation of ERK in INS-1 cells. The underlying reasons for these differences between the two studies on the involvement of Ras in glucose-mediated effects remain unclear. Studies from Trümper et al. (152) demonstrated that both GLP-1 and glucose modulate ERK activation in a Rap-1/B-Raf-sensitive, but not Ras/Raf-1-sensitive, manner in human islets. Using specific, but structurally distinct inhibitors of FTases, we have recently demonstrated that glucose-induced activation of ERK and insulin secretion were markedly attenuated by inhibitors of G protein farnesylation [e.g., farnesyltransferase inhibitor (FTI)-277 or -2628] in INS 832/13 cells and normal rat islets, implicating the requirement for a farnesylated protein in glucose-mediated effects on ERK activation and insulin secretion (155). Therefore, it appears that the effects of glucose and GLP-1 on insulin gene transcription and insulin secretion may, in part, be mediated via activation of the ERK signaling pathway involving one (or more) small G proteins including Rap1. Potential roles of Ras or the Gγ-subunit of trimeric G proteins in these signaling events still remain to be verified.
It should also be pointed out that whereas the above discussion is focused primarily on G protein-mediated ERK-sensitive regulation of insulin gene transcription and insulin secretion, it also appears that ERK activation may be necessary for cytoskeletal rearrangement, including its interactions with various scaffolding proteins such as IQ motif-containing GTPase-activating protein 1 (IQGAPs) and gelsolins (154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173). For example, IQGAP proteins have been shown to participate in a variety of cellular functions including cytoskeletal organization, cell adhesion, and the control of numerous intracellular signaling pathways involving the Rho subfamily of G proteins (e.g., Cdc42 and Rac1) (158,159,160,161). The IQGAP protein structure is comprised of calmodulin-binding IQ motifs with homology to the classical GAP. At least three forms of IQGAPs are identified. Of these, the IQGAP1 has been shown to bind to a variety of proteins including F-actin, microtubule-interacting proteins, E-cadherin, β-catenin, calmodulin, and small G proteins (e.g., Cdc42 and Rac1).
Along these lines, studies by Nauert et al. (164) identified an IQGAP1/A-kinase anchoring protein 79 complex in insulin-secreting cells. A clear association between these two proteins was established through two independent experimental approaches. In the first, using RINm5F lysates, they demonstrated copurification of IQGAP1 with protein kinase A by cAMP-affinity chromatography. In the second approach, they were able to detect protein kinase A activity in immunopreciptates of β-cell lysates using an anti-IQGAP1 antiserum. Data from these studies have also suggested direct association between the A- kinase-anchoring protein 79 and the C-terminal region of IQGAP1 (164). Rittmeyer et al. (165) also reported dual regulatory roles for IQGAP1 in regulated exocytosis. Based on a series of mutational analysis, these investigators suggested vital roles for IQGAP1 in the events leading to septin-exocyst localization and organization. These studies also provided additional insights into the interaction of Cdc42 with IQGAP1 and suggested novel roles for IQGAP1 as a regulator of exocytosis in β-cells (165). It also seems that IQGAP1 plays a vital role in the intercellular adhesion of β-cells via interaction with menin. Recent findings of Yan et al. (166) suggested that ectopic expression of menin promotes islet cell adhesion with a concomitant reduction in cell migration in pretumor β-cells. Further evidence from experiments involving various recombinant fragments of IQGAP1 suggested a strong binding of menin at the C-terminal fragment (amino acids 956–1657) of IQGAP1 (166). Taken together, it appears that IQGAP1 exerts regulatory role(s) in islet function via its interactions with various small G proteins, including Cdc42 and Rac1. This represents a new area of investigation because very little is known with regard to the localization and functional regulation of other IQGAPs (e.g., IQGAP3) in islet β-cells. For example, IQGAP3 has been shown to regulate cell proliferation through the Ras/ERK signaling pathways (167) and has also been identified as a target protein for Rac1 and Cdc42 in the regulation of neurite growth (168).
In addition to IQGAPs, certain members of gelsolin family have been implicated in cytoskeletal rearrangements (169,170,171). As reviewed by Silacci et al. (169) recently, the gelsolin superfamily of proteins is comprised of at least seven different proteins, including gelsolin, adservin, villin, capG, advillin, supervillin, and flightless. In addition to the control of cytoskeletal architecture, gelsolins have been shown to play roles in different cellular functions, including cell motility, apoptosis, and regulation of phagocytosis (169,170,171). Recent studies by Tomas et al. (172) have suggested novel roles for gelsolin in insulin secretion from MIN6 β-cell lines. A significantly higher abundance of gelsolin was detected in the B1 subline of MIN6 cells, which is highly responsive to glucose compared with the C3 subline of MIN6 cells, which is fairly refractory to glucose. Overexpression of gelsolin also potentiated GSIS in B1 cells. Compatible with its positive modulatory role(s) in GSIS are their observations indicating a marked reduction in GSIS in cells in which gelsolin was knocked down. It was concluded that gelsolin plays a novel regulatory role in GSIS, specifically at the level of F-actin organization. Follow-up studies from this laboratory by Yermen et al. (173) have suggested prosurvival roles for gelsolin in isolated β-cell preparations. Given these findings and based on roles for small G proteins in gelsolin-mediated effects on cell function, it remains to be determined whether such a cross-talk between G proteins and gelsolin underlies the sequence of events leading to F-actin organization, which is vital for GSIS to occur.
Together, based on the experimental evidence reviewed above, a model is proposed to suggest a potential cross-talk and interplay between farnesylated and geranylated G proteins in the signaling events leading to GSIS (Fig. 5). It is proposed that at least two G protein effector proteins (i.e., PAK1 and ERK) are involved in this signaling cascade, which could promote cytoskeletal rearrangement and vesicular transport through the intermediacy of IQGAPs and glesolins. Based on emerging evidence (as reviewed above in this section) in multiple cells including the islet β-cell, IQGAPs and gelsolins promote the cytoskeletal rearrangements and vesicular trafficking under the control of prenylated and geranylated small molecular mass GTPases (Fig. 5).
Figure 5.
A model for glucose-mediated activation of farnesylated and geranylgeranylated proteins leading to GSIS. Identification of ERK and PAK1 as target proteins. Glucose metabolism leads to the activation of islet endogenous FTases and GGTases culminating in the activation of farnesylated and geranylated proteins, respectively. Such conclusions were reached by pharmacological and molecular biological approaches. It appears that activation of a yet to be identified farnesylated protein(s) is necessary for glucose-mediated activation of ERK and subsequent effects on insulin gene transcription and insulin secretion. On the other hand, glucose-mediated activation of Cdc42 and Rac1 leads to regulation of PAK1 activity. Potential phosphoprotein substrates for PAK1 have not been identified in the β-cell up until now, but could include Rho-GDI. The activation of ERK and PAK (and other effector proteins) facilitate reorganization of the actin cytoskeletal architecture leading to translocation and fusion of insulin granules with the plasma membrane and release of insulin. An active involvement of IQGAPs and gelsolins in these signaling pathways in the organization of the cytoskeletal architecture is also proposed.
VIII. Regulation of the Metabolic Dysfunction of the Islet β-Cell by Small G Proteins
Although the data reviewed above support the overall hypothesis that specific G proteins (e.g., Cdc42, Rac1, and ARF-6) play positive modulatory roles in islet function and GSIS, a growing body of evidence appears to support the hypothesis that certain small GTPases could contribute to metabolic abnormalities, impaired insulin secretion, and eventual demise under the duress of various stimuli. For the sake of brevity, this section is divided into three subsections: 1) metabolic dysfunction induced due to increased generation of reactive oxygen species (ROS) and the associated increase in the oxidative stress in pancreatic β-cells under in vitro conditions in the presence of various stimuli and islets derived from animal models of diabetes; 2) metabolic dysfunction induced under the duress of cytokines (e.g., IL-1β); and 3) metabolic dysregulation and demise observed in β-cells after depletion of intracellular GTP.
A. The Rac1-NADPH oxidase-oxidative stress connection
Emerging evidence from in vitro and in vivo studies provides strong support to the hypothesis that chronic exposure of β-cells to elevated glucose (i.e., glucotoxicity), lipids (i.e., lipotoxicity), or glucose plus lipids (e.g., glucolipotoxicity) results in a significant metabolic dysregulation eventually leading to cell demise (174,175). Published evidence also suggests a marked increase in the generation of ROS, which manifests in increased oxidative stress in cells under the conditions of glucotoxicity (175). Several mechanisms have been put forth in this context, including depletion of intracellular redox state via the oxidation of reducing equivalents (e.g., reduced glutathione) and activation of superoxide-generating enzymatic machinery (176,177).
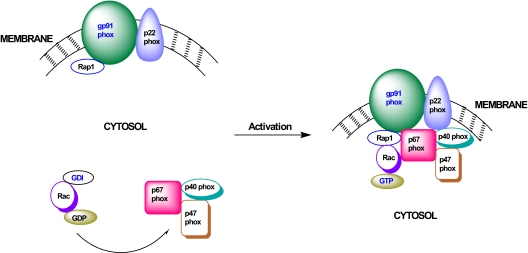
One of the enzymatic steps involved in the increased generation of ROS and associated induction of intracellular oxidative stress in the pancreatic β-cell includes activation of the phagocytic NADPH-oxidase (NOX) system (Fig. 6). NOX is a highly regulated membrane-associated protein complex that catalyzes the one electron reduction of oxygen to superoxide anion involving oxidation of cytosolic NADPH. The phagocytic NOX is a multicomponent system comprised of membrane as well as cytosolic components. The membrane-associated catalytic core is a complex comprising gp91phox, p22phox, and the small G protein Rap1. The cytosolic regulatory components include p47phox, p67phox, and the small G protein Rac (178,179,180). After stimulation, the cytosolic components of NOX translocate to the membrane fraction for association with the catalytic core for holoenzyme assembly (Fig. 6). Available evidence suggests that a protein kinase Cζ-sensitive phosphorylation of p47phox leads to its translocation to the membrane fraction (181). It has also been shown that functional activation of Rac (i.e., GTP-Rac) is vital for the holoenzyme assembly and activation of NOX.
Figure 6.
Positive modulatory roles for Rac1 (and Rap1?) in the activation of NOX and generation of ROS and subsequent oxidative stress in β-cells under the duress of various stimuli. Based on the current knowledge, it is evident that NOX plays a significant role(s) in the generation of ROS and associated increase in the oxidative stress in various in vitro and in vivo models of glucolipotoxicity and diabetes. Such conditions promote the activation of Rac1 (and Rap1) in the islet β-cell. After activation of Rac1, the cytosolic core complex of NOX (i.e., p67phox/p47phox/p40phox/ Rac1-GTP) translocates to the membrane to associate with the gp91phox, p22phox, and Rap1 for the holoenzyme assembly and functional activation of NOX.
Several recent studies have demonstrated localization and functional activation of the NOX in clonal β-cells, normal rat islets, and human islets under the duress of various stimuli known to cause metabolic dysregulation. Some of these stimuli include, but are not limited to, high glucose, saturated fatty acids, and proinflammatory cytokines (182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198). It has also been demonstrated that pharmacological inhibition of NOX by diphenylene iodinium chloride or antisense oligonucleotides for p47phox markedly attenuated glucose-induced ROS production and oxidative stress, suggesting a critical involvement of NOX in the metabolic dysfunction induced by glucose. Functional activation of this enzyme has also been demonstrated in islets from the diabetic animal models (db/db mice; Otsuka Long-Evans Tokushima Fatty rat, etc.) (184). Interestingly, treatment of these animals with angiotensin II type-1 receptor antagonists significantly reduced the expression of NOX subunits and the associated oxidative stress in islets derived from these animal models. These data implicate a significant contributory role for NOX in the metabolic dysfunction of the β-cell under conditions of oxidative stress (184).
Very little has been studied, however, in the islet to address potential contributory roles of small G proteins in the cascade of events leading to NOX-mediated metabolic dysfunction in β-cells exposed to various stimuli in vitro or in diabetic animal models. As depicted in Fig. 6, both Rac and Rap represent key components of the NOX holoenzyme and could serve as potential targets to suppress NOX activation. Along these lines, studies in cultured retinal pericytes have demonstrated (199) a role for NOX in palmitate-induced apoptosis. A significant increase in NOX activity, oxidative stress, and caspase-3 activity was demonstrable in cells exposed to palmitate. Overexpression of dominant-negative mutants of p67phox and Rac1 (N17Rac1) markedly inhibited the increase in caspase-3 activation. Furthermore, overexpression of an active mutant of Rac1 (V12Rac1) increased caspase-3 activity, suggesting that constitutive activation of Rac1 results in NOX activation culminating in the generation of oxidative stress and metabolic dysfunction. In this context, recent evidence from our laboratory (197,198) suggests that long-term exposure of INS 832/13 cells with glucose and/or palmitate significantly increased NOX-mediated induction of oxidative stress. Furthermore, pharmacological inhibition of Rac1 activation markedly reduced the oxidative stress and loss in metabolic cell viability under these conditions (197,198). To the best of the author’s knowledge, studies of potential involvement of Rap1 in this signaling cascade, specifically in the context of metabolic defects induced by glucose and/or palmitate, have not been conducted thus far.
In addition to inducing NOX-derived ROS accumulation and oxidative stress, hyperglycemic conditions have been shown to exert significant effects on G proteins and/or their regulatory accessory proteins within the islet β-cell. For example, Nagamatsu et al. (200) reported significant abnormalities in the expression of specific SNARE proteins, which have been implicated in insulin exocytosis via interaction with Cdc42. More recent studies by Abderrahmani et al. (92) have demonstrated glucotoxic effects on the expression of small G proteins (e.g., Rab3A and Rab27a) and their effector proteins (e.g., granuphilin/ Slp4 and Noc2) in INS-1 cells and rat islets. Further experiments by these investigators have suggested that hyperglycemia induces the activation of the transcriptional repressor, ICER, via a cAMP-dependent mechanism, which in turn inhibits the expression of Rab GTPases and their effector proteins. Together, these data suggest a significant defect in the expression of key signaling proteins involved in insulin secretion as a consequence of glucotoxicity. In this context, we recently reported a substantial reduction in the expression and the catalytic activity of NDPK in β-cells chronically exposed to glucose and/or palmitate (201). A cell-permeable analog of ceramide also mimicked the effects of palmitate by significantly reducing the expression of nm23-H1 and nm23-H2 and NDPK activity in these cells. These findings suggest that de novo generation of intracellular ceramide from palmitate might represent at least one of the signaling steps involved in lipid-induced effects on NDPK expression and function in β-cells. It is likely that such a reduction in these activities could translate into defective G protein activation and insulin secretion because we and others have demonstrated vital roles for NDPKs in G protein activation (for review, see Ref. 19).
B. Small G proteins in the metabolic dysfunction induced by IL-1β
Type 1 diabetes develops as a consequence of the selective destruction of insulin-secreting β-cells, which is mediated principally by cytokines (e.g., IL-1β, TNFα, and IFNγ) secreted by the infiltrating immune cells. Several lines of evidence suggest that the demise of the pancreatic β-cells due to immune attack could be due to apoptotic and necrotic pathways (202,203,204,205,206). Earlier studies have demonstrated the involvement of small G proteins in metabolic dysfunction/apoptosis of multiple cell types under the duress of cytokines (207,208,209). Recent evidence also indicates involvement of small G proteins in IL-1β-mediated increase in inducible nitric oxide synthase (iNOS) expression and nitric oxide (NO) release in insulin-secreting cells. Using Clostridial toxins, which specifically monoglucosylate and inactivate specific G proteins, Tannous et al. (210) presented evidence for the involvement of small G proteins (e.g., H-Ras) in IL-1β-induced iNOS expression and NO release in insulin-secreting cells. These observations were further confirmed via pharmacological approaches. Two structurally dissimilar inhibitors of G protein farnesylation (e.g., manumycin and damnacanthal) (210) and more specific prodrug inhibitors of FTases (e.g., 3-allyl or vinyl farnesols) also inhibited IL-1β-mediated NO release from pancreatic β-cells (R.H. Amin and A. Kowluru, unpublished observations). Furthermore, these pro-drug inhibitors of FTases prevented IL-1β-induced loss of metabolic cell viability in isolated β-cells, implicating an obligatory role of a protein prenylation step in IL-1β-mediated effects on the β-cell. IL-1β-induced iNOS expression and NO release have also been shown to be sensitive to inhibition of protein palmitoylation. Preincubation of insulin-secreting cells with CLN, an inhibitor of protein palmitoylation, significantly reduced IL-1β-induced NO release from these cells (211). 2-Bromopalmitate, a structurally distinct inhibitor of protein palmitoylation (but not 2-hydroxymyristic acid, an inhibitor of protein myristoylation), also reduced IL-1β-induced NO release from these cells. One of the palmitoylated proteins was identified as H-Ras because CLN markedly reduced incorporation of [3H]palmitate into H-Ras and caused significant accumulation of Ras in the cytosolic fraction. Together, these data appear to indicate that one (or more) proteins that undergo prenylation and palmitoylation may be necessary for IL-1β-mediated effects on β-cells. On a mechanistic note, it appears that the integrity of lipid rafts and a potential interaction between H-Ras and Cav-1 may be necessary for IL-1β-mediated effects (212). It was observed that disruption of lipid rafts (e.g., with cyclodextrin) markedly reduced IL-1β-induced iNOS expression and NO release from β-cells. IL-1β transiently increased tyrosine phosphorylation of Cav-1, and overexpression of a tyrosine phosphorylation-deficient mutant (Y14F) or siRNA-mediated Cav-1 knock-down also resulted in marked attenuation of IL-1β-induced iNOS gene expression and NO release from these cells, further implicating Cav-1 in this signaling cascade. Lastly, IL-1β also promoted the translocation of H-Ras into lipid rafts. These findings led to the conclusion that tyrosine phosphorylation of Cav-1 and subsequent interaction among members of the Ras signaling pathway within the membrane lipid microdomains represent early signaling mechanisms of IL-1β in the pancreatic β-cells (212).
C. Small G proteins in the metabolic dysfunction induced by GTP depletion
Independent studies by two groups have suggested that exposure of isolated β-cells to MPA, a specific inhibitor of GTP biosynthesis, results in cell death via apoptosis. In the first study, Li et al. (213) observed that treatment of clonal β-cells with MPA resulted in inhibition of mitogenesis, cell number, DNA and protein contents, and metabolic cell viability. MPA-treated cells also displayed a strong and localized staining with propidium iodide, suggesting condensation and fragmentation of DNA. Electron microscopy of MPA-treated cells revealed typical signs of apoptosis, including condensed and marginated chromatin, apoptotic bodies, cytosolic vacuolization, and loss of microvilli. Based on these observations, it was concluded that prolonged depletion of GTP induces β-cell death, which may be mediated by one (or more) small G proteins. More recently, studies from Kim’s laboratory (214) have provided compelling evidence to suggest that MPA treatment of normal rat islets and clonal β-cells results in cell death. They demonstrated significant increases in c-jun N-terminal kinase (JNK), p38 MAPK, and caspase-3 cleavage in MPA-treated cells, which was reversed by coprovision of guanosine but not adenosine (214,215). These findings suggest that depletion of intracellular GTP results in cell death. MPA treatment led to a significant reduction in the expression of Rho-GDI, a small G protein regulator (Fig. 3) in these cells. Interestingly, overexpression of Rho-GDI markedly decreased JNK activation and improved loss in cell viability in MPA-treated cells. Moreover, siRNA-mediated knockdown of Rho-GDI potentiated MPA-induced JNK activation and cell demise (109). It was concluded that MPA induces apoptosis in insulin-secreting cells via down-regulation of Rho-GDI and increased activation of JNK. Together, these studies directly implicate role(s) for endogenous GTP and its binding proteins in the regulation of islet β-cell survival mechanisms.
IX. Insulin Secretory Abnormalities in Small G Protein Knockout Animal Models
In addition to the above reviewed observations from multiple laboratories that were accrued from in vitro experimental approaches, several lines of recent evidence are suggestive of significant abnormalities in various intracellular signaling mechanisms culminating in abnormal insulin secretion in G protein knockout models. They are reviewed briefly in this section.
Studies by Yaekura et al. (87) reported fasting hyperglycemia and glucose intolerance in Rab3A-null mice. Insulin secretion in response to arginine was similar in control and Rab3A knockout mice; these observations are indicative of a phenotype akin to insulin-secretory abnormalities demonstrable in type 2 diabetes. Despite no apparent differences in β-cell mass and insulin production between the control and the Rab3A-null groups, secretagogue-induced insulin secretion was impaired significantly in islets derived from Rab3A-null mice. Glucose oxidation and glucose-induced augmentation in intracellular calcium concentrations, however, were comparable in islets from the control and Rab3A-null animals. It was concluded that Rab3A plays a major role in GSIS by replenishing the readily releasable pool of insulin-laden secretory granules. Isolated islets from the Rab27a-null Ashen mice have been shown to exhibit defective glucose-, but not KCl-, phorbol ester or forskolin-induced insulin secretion (91). No significant changes in the intracellular calcium concentrations or dynamics of fusion pore openings after glucose stimulation were noticed. A marked attenuation in the exocytosis of predocked granules and replenishment of the releasable pool of granules after glucose stimulation was also demonstrated in this animal model (71). Furthermore, islets from the Rab27a-null mice exhibited significant defects in the replenishment of the readily releasable and immediately releasable pools of secretory granules in response to depolarization-sensitive calcium influx. High glucose promoted the immediately releasable pools in these islets, albeit a significant defect in the readily releasable pool is noticed. Islets from Rab3a-null mice exhibited no defects in the replenishment of either of the pools, implying distinct roles of Rab3a and Rab27a in the secretory granule pathway (95).
Recently, Asahara et al. (74) have reported significant abnormalities in islet function and insulin secretory defects in a Rac1-null mouse model. An impairment in glucose tolerance and hypoinsulinemia in Rac1-null mice (βRac1−/− mice) were noticed. Similar to what was observed in the in vitro studies in cultured β-cells, glucose-induced, but not KCl-induced, insulin secretion was attenuated in islets derived from the βRac1−/− mice. Further in vitro data from studies in INS-1 cells not only confirmed the vital roles for Rac1 but also provided additional support to previously proposed roles for this protein in modulation of insulin secretion by Rac1 through modulation of cytoskeletal proteins within the β-cell.
Studies by Font de Mora et al. (121) have suggested roles for the Ras-G protein regulatory factor (GRF) 1 signaling pathway in normal β-cell growth. The GRF1-null mice exhibited a significant reduction in body weight, hypoinsulinemia, and impaired glucose tolerance. Defects in β-cell proliferation and neogenesis were also noted in these animals. Lastly, islets from the GRF1 knockout models failed to respond to IGF in eliciting activation of Akt and ERK signaling steps. Together, these findings implicate a vital role for Ras-GRF1 signaling cascade in the development and functional aspects of β-cells. Interestingly, studies by Efrat et al. (216) demonstrated that male transgenic mice overexpressing human H-Ras developed β-cell degeneration and the onset of diabetes. These animals exhibited hyperglycemia, glycosuria, and hypoinsulinemia. A significant abnormality in islet ultrastructure has also been noted in these animals. Interestingly, overexpression of the Ras oncogene in female mice resulted in no phenotypical changes suggesting a clear role for this protein in gender-specific effects in the development of islet lesion and the onset of diabetes. Together, findings from the GRF1 knockout model and the H-Ras transgenic mouse model provide interesting clues with regard to the relevant roles of these two proteins in islet β-cell proliferation, survival, and demise.
Studies by Srivastava et al. (217) in the Anx7 (a gene that encodes for a calcium-activated G protein) knockout mouse have provided evidence into calcium signaling through inositol triphosphate stores in GSIS. A significant reduction in GSIS from the islets derived from the knockout mouse was reported, despite an 8- to 10-fold higher insulin content in mutants compared with their control counterparts. Although the glucose-induced increases in intracellular calcium concentrations were comparable between control and knockout mice, the ability of inositol triphosphate-generating agonists to mobilize intracellular calcium was significantly attenuated in Anx 7(+/−) knockout mouse islets. These studies thus established a link between putative contributory roles for inositol triphosphate-mobilizable calcium stores in GSIS and its regulation by a calcium-activated G protein in the islet β-cell.
In the final section of this review, I will review data from two laboratories suggesting potential defects in G protein-mediated signaling pathways in islets derived from animal models of diabetes. Potential reversal of the insulin secretory defects by G protein agonists is also reviewed.
X. Insulin Secretory Defects in Diabetic Animals: Reversal by G Protein Agonists
Very little is known with regard to the potential abnormalities in G protein function and activation in islets derived from animal models of diabetes. Along these lines, published evidence from two laboratories appears to suggest reversal of insulin secretory defects in the Goto-Kakizaki rat (GK rat), a model for type 2 diabetes, by Mas analogs, which are known to directly activate G proteins largely by GTP/GDP exchange mechanisms.
A. Effects of Mas on G protein activation and insulin secretion from isolated β-cells
Mas, a tetradecapeptide from wasp venom, activates a wide variety of G proteins, presumably via GTP/GDP exchange (218,219). Numerous previous studies have demonstrated stimulation of insulin secretion by Mas in normal rat islets, human islets, and clonal β-cells (48,218,219,220,221,222,223,224,225). However, the precise mechanisms underlying Mas-stimulated insulin secretion (MSIS) remain partially understood. In the context of the potential involvement of G proteins in MSIS, it was reported that MSIS and GSIS from the β-cell involve the intermediacy of a Rac1-mediated signaling step (222). Both glucose and Mas increased translocation and membrane association of Rac1 in insulin-secreting cells. Interestingly, unlike GSIS, MSIS was resistant to inhibitors of protein prenylation, suggesting that prenylation of Rac1 is not critical for MSIS. These data appear to suggest that Mas activates Rac1, presumably via GTP/GDP exchange. Moreover, transfection of a dominant-negative Rac1 (N17Rac1) mutant markedly attenuated MSIS and GSIS (222). These data further implicate that the GTP-hydrolytic cycle is essential for Rac1-mediated secretion. In addition, findings from Sharp’s laboratory (220) have identified Cdc42 as one of the target proteins for Mas action in β-cells. Together, these findings implicate specific roles for Cdc42 and Rac1 in GSIS and MSIS.
B. Reversal of insulin secretory defects in diabetic animals by analogs of Mas
Recently published evidence suggests reversal of insulin secretory defects seen in islets derived from diabetic animal models (e.g., the GK rat) by Mas analogs. The GK rat, which is a well-accepted model for type 2 diabetes, is developed by repeated breeding of glucose-intolerant Wistar rats (226,227). Using this animal model, Metz et al. (228) first reported that despite intact contents of insulin, GK rat islets had markedly deficient insulin release in response to glucose. In contrast, Mas completely circumvented any secretory defect. Effects of Mas were structure-specific because no reversal of secretory defects was demonstrable by Mas-17, which is an inactive analog of Mas. Therefore, it appears that a defect late in the signal transduction in the GK rat islet, possibly taking place at the activation of a Mas-sensitive G protein step in exocytosis, may be responsible for defective insulin secretion in this animal model (228). Compatible with the above observations are the findings of Ostenson et al. (229) indicating that galparan, a 27-amino acid chimeric peptide (consisting of galanin linked to Mas), potently stimulated insulin secretion from islets derived from control and GK rats equipotently. It appears that galparan stimulates insulin secretion at a distal site in the stimulus-secretion coupling of the islet β-cell (229). Future research will identify putative G proteins, the functional activation of which might be impaired in the GK islet, which are reversed by direct activation by Mas. It is likely that such defects might lie at the level of GDP/GTP exchange, although this remains to be verified experimentally. It is also likely that such defects might underlie defects in NDPK-mediated activation of Mas-sensitive G proteins. Indeed, published evidence appears to support such a viewpoint. First, it has been shown that the NDPK is activated by Mas in a variety of insulin-secreting β-cells (230). Second, the expression and the catalytic activity of NDPK are significantly reduced in islets from the GK rat compared with the control Wistar rats (231). On the basis of these findings, it is likely that a defect in the late signaling steps in these islets, possibly occurring at the site of activation by NDPK of a Mas-sensitive G protein-dependent step, might contribute toward impaired insulin secretion in this animal model. These aspects are currently being addressed in our laboratory.
XI. Conclusions and Future Directions
To conclude, the field of research to understand the regulatory roles of G proteins in general and small G proteins in particular has seen tremendous progress since the early 1990s. A review of published evidence from multiple laboratories indicates that at least three major classes of small G proteins are localized in the pancreatic β-cell. Although a wide variety of insulin-secreting cells including clonal β-cells, normal rat islets, mouse islets and human islets are used to study the localization, identification, and characterization of small G proteins, the majority of these observations clearly implicate regulatory roles for this class of signaling proteins (e.g., Cdc42, Rac1, and ARF-6) in insulin secretion. Several regulatory proteins/factors for these G proteins have also been identified and characterized in these cellular preparations. It is also evident from the above discussion that these factors regulate the functions of their respective G protein partners in a tightly controlled fashion. Although not reviewed herein, a considerable amount of research has already been conducted and is ongoing toward the understanding of protein-protein interactions between the secretory granule and plasma membrane-associated small G proteins and their effector proteins to facilitate optimal priming and docking of the granules for fusion and the release of granular contents into the circulation. The reader is referred to a recent review by Wang and Thurmond (26) for experimental data and discussion along these lines. Also, data are emerging in other endocrine cells to suggest activation of G protein-sensitive PLases in the generation of fusogenic lipids for fusion of the secretory vesicles/granules with the plasma membrane leading to exocytotic secretion of respective hormones. In addition to the aforementioned regulatory proteins/factors, scaffolding proteins are also emerging as novel regulators of G protein function in isolated β-cells. Certainly this area is open for extensive research in the future, given the ability of these proteins to interact with a wide variety of effectors in multiple cell types, including the pancreatic β-cell.
This review also suggests not so friendly roles for small G proteins in the induction of cellular signaling pathways/events leading to the generation of ROS and associated oxidative stress in the islet β-cell under the duress of various stimuli (e.g., high glucose, high lipids, and cytokines). These observations from several laboratories suggest that the phagocytic NOX could represent one of the potential drug targets to prevent oxidative stress-mediated cellular dysfunction and demise. Certainly, data from studies using the angiotensin receptor blockers both in vitro and in vivo have provided fresh insights/clues and should be pursued further. In addition, very little has been done in the context of inhibiting G protein (e.g., Rap1 and Rac1) function to relieve the damaging effects of NOX activation. One potential caveat here is that both Rac1 and Rap1 are critical for islet survival mechanisms as well.
Data from small G protein knockout models are encouraging, and they further support a role for this class of signaling proteins in insulin secretory function and maintenance of optimal β-cell mass. Future studies on the quantitation of functional status of various effector proteins for the respective small G proteins in the knockout models will provide valuable information with regard to each of these signaling pathways in islet function, survival, and demise. In addition to potential defects in the expression and function of specific G proteins in models of impaired insulin secretion and diabetes, it should be kept in mind that potential alterations in the expression and function of enzymes involved in the posttranslational modification of G proteins could lead to mislocalization of these G proteins culminating in dysregulation of the islet. Indeed, recent studies by Roberts et al. (232) have suggested that the membrane targeting, association, and functional activation of Rho family of small G proteins are dictated precisely by the concerted actions of the endopeptidase (which cleaves the AAX residues of CAAX motif of these G proteins) and the methyltransferase (which then facilitates the methylation of the carboxylated anion of the prenylated Rho G proteins). Future studies will need to examine the functional status and activation of these enzymes in models of impaired insulin secretion and diabetes.
Acknowledgments
I dedicate this review to my colleague, collaborator, and above all my very good friend, Dr. Stewart Metz. Dr. Metz’s enthusiasm, quest for scientific knowledge, and insight have guided me throughout the years. His vision in the area of GTP and G proteins in islet function has allowed me to contribute to this fertile area of islet β-cell research for more than 15 yr. I also sincerely thank all of my colleagues and collaborators at the William S. Middleton VA Medical Center and the University of Wisconsin (Madison) and the John D. Dingell VA Medical Center and Wayne State University (Detroit) for their continued support and assistance in the accrual of experimental data, which are cited in this review.
Footnotes
This work is supported by the National Institutes of Health (DK 94201), the Department of VA Medical Research Service MERIT award, and a Senior Research Career Scientist Award to the author.
Disclosure Summary: The author has nothing to disclose.
First Published Online November 4, 2009
Abbreviations: AA, Arachidonic acid; AFC, acetyl farnesyl cysteine; ARF-6, ADP-ribosylation factor-6; ARNO, cytohesin/Arf nucleotide-binding site opener; Cav-1, caveolin-1; CLN, cerulenin; CML, carboxylmethylation; CoA, coenzyme A; Epac, exchange proteins directly activated by cAMP; FTase, farnesyltransferase; FTI, farnesyltransferase inhibitor; GAP, GTPase-activating protein; GDI, GDP-dissociation inhibitor; GEF, GDP/GTP-exchange factor; GGTase, geranylgeranyltransferase; GGTI, geranylgeranyltransferase inhibitor; GK rat, Goto-Kakizaki rat; GLP-1, glucagon-like peptide-1; G protein, GTP-binding protein; GRF, G protein regulatory factor; GSIS, glucose-stimulated insulin secretion; GTPase, guanosine triphosphatase; HMG-CoA, hydroxymethylglutaryl CoA; iNOS, inducible NO synthase; IQGAP, IQ motif-containing GTPase-activating protein 1; JNK, c-Jun N-terminal kinase; LOVA, lovastatin; LPC, lysophosphatidylcholine; Mas, mastoparan; MPA, mycophenolic acid; MSIS, mastoparan-stimulated insulin secretion; MVA, mevalonic acid; NDPK, nucleoside diphosphate kinase; NO, nitric oxide; NOX, NADPH-oxidase; PA, phosphatidic acid; PI (4,5) P2, phosphatidylinositol 4,5 bisphosphate; PLases, phospholipases; PLD, phospholipase-D; PTX, pertussis toxin; ROS, reactive oxygen species; siRNA, small interfering RNA; SNARE protein, soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein; Tiam1, T-lymphoma invasion and metastasis1; VAMP2, vesicle-associated membrane protein 2.
References
- Malaisse WJ 1972 Hormonal and environmental modification of islet activity. In: Steiner DF, Frankel N, eds. Handbook of physiology. New York: American Physiological Society; 237–260 [Google Scholar]
- Prentki M, Matschinsky FM 1987 Calcium, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev 67:1185–1248 [DOI] [PubMed] [Google Scholar]
- MacDonald MJ 1990 Elusive proximal signals of β-cells for insulin secretion. Diabetes 39:1461–1466 [DOI] [PubMed] [Google Scholar]
- Laychock SG 1990 Glucose metabolism, second messengers and insulin secretion. Life Sci 47:2307–2316 [DOI] [PubMed] [Google Scholar]
- Newgard CB, McGarry JD 1995 Metabolic coupling factors in pancreatic β-cell signal transduction. Annu Rev Biochem 64:689–719 [DOI] [PubMed] [Google Scholar]
- Deeney JT, Prentki M, Corkey BE 2000 Metabolic control of β-cell function. Semin Cell Dev Biol 11:267–275 [DOI] [PubMed] [Google Scholar]
- Newgard CB, Lu D, Jensen MV, Schissler J, Boucher A, Burgess S, Sherry AD 2002 Stimulus/secretion coupling factors in glucose-stimulated insulin secretion: insights gained from a multidisciplinary approach. Diabetes 51(Suppl 3): S389–S393 [DOI] [PubMed] [Google Scholar]
- Berggren PO, Leibiger IB 2006 Novel aspects on signal transduction in the pancreatic β cell. Nutr Metab Cardiovasc Dis 16(Suppl 1):S7–S10 [DOI] [PubMed] [Google Scholar]
- Metz SA 1988 Membrane phospholipid turnover as an intermediary step in insulin secretion. Putative roles of phospholipases in cell signaling. Am J Med 85:9–21 [DOI] [PubMed] [Google Scholar]
- Metz SA 1991 The pancreatic islet as a Rubik’s cube. Is phospholipid hydrolysis a piece of the puzzle?. Diabetes 40:1565–1573 [DOI] [PubMed] [Google Scholar]
- Lawrence M, Shao C, Duan L, McGlynn K, Cobb MH 2008 The protein kinases ERK1/2 and their roles in pancreatic β cells. Acta Physiol (Oxf) 192:11–17 [DOI] [PubMed] [Google Scholar]
- Lang J 1999 Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem 259:3–17 [DOI] [PubMed] [Google Scholar]
- Poitout V 2008 Phospholipid hydrolysis and insulin secretion: a step toward solving the Rubik’s cube. Am J Physiol Endocrinol Metab 294:E214–E216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PM, Persaud SJ 1998 Protein kinases, protein phosphorylation, and the regulation of insulin secretion from pancreatic β-cells. Endocr Rev 19:429–461 [DOI] [PubMed] [Google Scholar]
- Easom RA 1999 CaM kinase II: a protein kinase with extraordinary talents germane to insulin exocytosis. Diabetes 48:675–684 [DOI] [PubMed] [Google Scholar]
- Nesher R, Anteby E, Yedovizky M, Warwar N, Kaiser N, Cerasi E 2002 β-cell protein kinases and the dynamics of the insulin response to glucose. Diabetes 51(Suppl 1):S68–S73 [DOI] [PubMed] [Google Scholar]
- Khoo S, Gibson TB, Arnette D, Lawrence M, January B, McGlynn K, Vanderbilt CA, Griffen SC, German MS, Cobb MH 2004 MAP kinases and their roles in pancreatic β-cells. Cell Biochem Biophys 40(Suppl 3):191–200 [DOI] [PubMed] [Google Scholar]
- Kowluru A 2002 Identification and characterization of a novel protein histidine kinase in the β cell: evidence for its regulation by mastoparan, an activator of G-proteins and insulin secretion. Biochem Pharmacol 63:2091–2100 [DOI] [PubMed] [Google Scholar]
- Kowluru A 2008 Emerging roles for protein histidine phosphorylation in cellular signal transduction: lessons from the islet β-cell. J Cell Mol Med 12:1885–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz SA, Rabaglia ME, Pintar TJ 1992 Selective inhibitors of GTP synthesis impede exocytotic insulin release from intact rat islets. J Biol Chem 267:12517–12527 [PubMed] [Google Scholar]
- Metz SA, Meredith M, Rabaglia ME, Kowluru A 1993 Small elevations of glucose concentration redirect and amplify the synthesis of guanosine 5′-triphosphate in rat islets. J Clin Invest 92:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Noda M, Sharp GW 1998 Nutrient augmentation of Ca2+-dependent and Ca2+-independent pathways in stimulus-coupling to insulin secretion can be distinguished by their guanosine triphosphate requirements: studies on rat pancreatic islets. Endocrinology 139:1172–1183 [DOI] [PubMed] [Google Scholar]
- Straub SG, James RF, Dunne MJ, Sharp GW 1998 Glucose augmentation of mastoparan stimulated insulin secretion in rat and human pancreatic islets. Diabetes 47:1053–1057 [DOI] [PubMed] [Google Scholar]
- Kowluru A, Seavey SE, Li G, Sorenson RL, Weinhaus AJ, Nesher R, Rabaglia ME, Vadakekalam J, Metz SA 1996 Glucose- and GTP-dependent stimulation of the carboxylmethylation of Cdc42 in rodent and human pancreatic islets and pure β cells: evidence for an essential role for GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest 98:540–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A 2003 Regulatory roles for small G-proteins in the pancreatic β cell: lessons from models of impaired insulin secretion. Am J Physiol Endocrinol Metab 285:E669–E684 [DOI] [PubMed] [Google Scholar]
- Wang Z, Thurmond DC 2009 Mechanisms of biphasic insulin-granule exocytosis- roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 122:893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RP, Seaquist ER, Walseth TF 1991 G proteins and modulation of insulin secretion. Diabetes 40:1–6 [DOI] [PubMed] [Google Scholar]
- Seaquist ER, Walseth TF, Redmon JB, Robertson RP 1994 G-protein regulation of insulin secretion. J Lab Clin Med 123:338–345 [PubMed] [Google Scholar]
- Gilman AG 1987 G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56:615–649 [DOI] [PubMed] [Google Scholar]
- Birnbaumer L 1992 Receptor-to-effector signaling through G proteins: roles for γ dimers as well as α subunits. Cell 71:1069–1072 [DOI] [PubMed] [Google Scholar]
- Clapham DE, Neer EJ 1993 New roles for G-protein β γ-dimers in transmembrane signaling. Nature 365:403–406 [DOI] [PubMed] [Google Scholar]
- Katada T, Ui M 1979 Islet activating protein. Enhanced secretion and cyclic AMP accumulation in pancreatic islets due to activation of native calcium ionophores. J Biol Chem 254:469–479 [PubMed] [Google Scholar]
- Nakaki T, Nakadate T, Ishii K, Kato R 1981 Postsynaptic α2 adrenergic receptors in isolated rat islets of Langerhans: inhibition of insulin release and cyclic 3′5′ adenosine monophosphate accumulation. J Pharmacol Exp Ther 216:607–612 [PubMed] [Google Scholar]
- Hillaire-Buys D, Gross R, Roye M, Ribes G, Loubatières-Mariani MM 1992 Adrenergic inhibition of insulin secretion involves pertussis toxin-sensitive and -insensitive mechanisms. Eur J Pharmacol 218:359–362 [DOI] [PubMed] [Google Scholar]
- El-Mansoury AM, Morgan NG 1998 Activation of protein kinase C modulates α2-adrenergic signalling in rat pancreatic islets. Cell Signal 10:637–643 [DOI] [PubMed] [Google Scholar]
- Seaquist ER, Neal AR, Shoger KD, Walseth TF, Robertson RP 1992 G-proteins and hormonal inhibition of insulin secretion from HIT-T15 cells and isolated rat islets. Diabetes 41:1390–1399 [DOI] [PubMed] [Google Scholar]
- Kowluru A, Metz SA 1994 GTP and its binding proteins in the regulation of insulin exocytosis. In: Draznin B, LeRoith D, eds. Molecular biology of diabetes. Totowa, NJ: Humana; 249–283 [Google Scholar]
- Kowluru A, Robertson RP, Metz SA 2000 GTP-binding proteins in the regulation of pancreatic β cell function. In: LeRoith D, Olefsky JM, Taylor S, eds. Diabetes mellitus—fundamental and clinical text. Philadelphia: J. B. Lippincott Co.; 78–94 [Google Scholar]
- Kowluru A, Metz SA 1994 Stimulation by PGE2 of a high-affinity GTPase in the secretory granules of normal rat and human pancreatic islets. Biochem J 297:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A, Seavey SE, Rhodes CJ, Metz SA 1996 A novel regulatory mechanism for trimeric GTP-binding proteins in the membrane and secretory granule fractions of human and rodent β cells. Biochem J 313:97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad RJ, Young RA, Record RD, Smith RM, Butkerait P, Manning D, Jarett L, Wolf BA 1995 The heterotrimeric G-protein Gi is localized to the insulin secretory granules of β-cells and is involved in insulin exocytosis. J Biol Chem 270:12869–12876 [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T 2001 Small GTP-binding proteins. Physiol Rev 81:153–208 [DOI] [PubMed] [Google Scholar]
- Casey PJ, Seabra MC 1996 Protein prenyltransferases. J Biol Chem 271:5289–5292 [DOI] [PubMed] [Google Scholar]
- Maurer-Stroh S, Washietl S, Eisenhaber F 2003 Protein prenyltransferases. Genome Biol 4:212.1–212.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra MC, Reiss Y, Casey PJ, Brown MS, Goldstein JL 1991 Protein farnesyltransferase and geranylgeranyl transferase share a common α subunit. Cell 65:429–434 [DOI] [PubMed] [Google Scholar]
- Fu HW, Casey PJ 1999 Enzymology and biology of CaaX protein prenylation. Rec Prog Horm Res 54:315–342, discussion 342–343 [PubMed] [Google Scholar]
- Kowluru A 2008 Protein prenylation in glucose-induced insulin secretion from the pancreatic islet β-cell: a perspective. J Cell Mol Med 12:164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz SA, Rabaglia ME, Stock JB, Kowluru A 1993 Modulation of insulin secretion from normal rat islets by inhibitors of the post-translational modifications of GBPs. Biochem J 295:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthakal R, Kaur H, Goalstone M, Kowluru A 2007 Dominant negative α-subunit of farnesyl-and geranyl geranyltransferase inhibits glucose-stimulated, but not KCl-stimulated, insulin secretion in INS 832/13 cells. Diabetes 56:204–210 [DOI] [PubMed] [Google Scholar]
- Li G, Regazzi R, Roche E, Wollheim CB 1993 Blockade of mevalonate production by lovastatin attenuates bombesin and vasopressin potentiation of nutrient-induced insulin secretion in HIT-T15 cells. Probable involvement of small GTP-binding proteins. Biochem J 289:379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin R, Chen HQ, Tannous M, Gibbs R, Kowluru A 2002 Inhibition of glucose- and calcium-induced insulin secretion from βTC3 cells by novel inhibitors of protein isoprenylation. J Pharmacol Exp Ther 303:82–88 [DOI] [PubMed] [Google Scholar]
- Leiser M, Efrat S, Fleischer N 1995 Evidence that Rap1 carboxylmethylation is involved in regulated insulin secretion. Endocrinology 136:2521–2530 [DOI] [PubMed] [Google Scholar]
- Li G, Kowluru A, Metz SA 1996 Characterization of prenylcysteine methyltransferase in insulin-secreting cells. Biochem J 316:345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett S, Brown DA, Linder ME 2000 Lipid-dependent targeting of G proteins into rafts. J Biol Chem 275:2191–2198 [DOI] [PubMed] [Google Scholar]
- Bourne HR 1988 Do GTPases direct membrane traffic in secretion? Cell 53:669–671 [DOI] [PubMed] [Google Scholar]
- Yajima H, Komatsu M, Yamada S, Straub SG, Kaneko T, Sato Y, Yamauchi K, Hashizume K, Sharp GW, Aizawa T 2000 Cerulenin, an inhibitor of protein acylation, selectively attenuates nutrient stimulation of insulin release: a study in rat pancreatic islets. Diabetes 49:712–717 [DOI] [PubMed] [Google Scholar]
- Straub SG, Sharp GW 2007 Inhibition of insulin secretion by cerulenin might be due to impaired glucose metabolism. Diabetes Metab Res Rev 23:146–151 [DOI] [PubMed] [Google Scholar]
- Deeney JT, Gromada J, Høy M, Olsen HL, Rhodes CJ, Prentki M, Berggren PO, Corkey BE 2000 Acute stimulation with long chain acyl-CoA enhances exocytosis in insulin-secreting (HIT T-15 and NMRI β-cells). J Biol Chem 275:9363–9368 [DOI] [PubMed] [Google Scholar]
- Cheng H, Straub SG, Sharp GW 2003 Protein acylation in the inhibition of insulin secretion by norepinephrine, somatostatin, galanin, and PGE2. Am J Physiol Endocrinol Metab 285:E287–E294 [DOI] [PubMed] [Google Scholar]
- De Vos ML, Lawrence DS, Smith CD 2001 Cellular pharmacology of cerulenin analogs that inhibit protein palmitoylation. Biochem Pharmacol 62:985–995 [DOI] [PubMed] [Google Scholar]
- Kowluru A 2005 Novel regulatory roles for protein phosphatase-2A in the islet β cell. Biochem Pharmacol 69:1681–1691 [DOI] [PubMed] [Google Scholar]
- Kowluru A, Li G, Metz SA 1997 Glucose activates the carboxyl methylation of γ subunits of trimeric GTP-binding proteins in pancreatic β cell. Modulation in vivo by calcium, GTP, and pertussis toxin. J Clin Invest 100:1596–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A, Li G, Rabaglia ME, Segu VB, Hofmann F, Aktories K, Metz SA 1997 Evidence for differential roles of the Rho subfamily of GTP-binding proteins in glucose- and calcium-induced insulin secretion from pancreatic β cells. Biochem Pharmacol 54:1097–1108 [DOI] [PubMed] [Google Scholar]
- Regazzi R, Kikuchi A, Takai Y, Wollheim CB 1992 The small GTP-binding proteins in the cytosol of insulin-secreting cells are complexed to GDP dissociation inhibitor proteins. J Biol Chem 267:17512–17519 [PubMed] [Google Scholar]
- Kowluru A, Rabaglia ME, Muse KE, Metz SA 1994 Subcellular localization and kinetic characterization of guanine nucleotide binding proteins in normal rat and human pancreatic islets and transformed β cells. Biochim Biophys Acta 1222:348–359 [DOI] [PubMed] [Google Scholar]
- Daniel S, Noda M, Cerione RA, Sharp GW 2002 A link between Cdc42 and syntaxin is involved in mastoparan-stimulated insulin release. Biochemistry 41:9663–9671 [DOI] [PubMed] [Google Scholar]
- Kowluru A, Chen HQ, Tannous M 2003 Novel roles for the Rho subfamily of GTP-binding proteins in succinate-induced insulin secretion from βTC3 cells: further evidence in support of succinate mechanism of insulin release. Endocr Res 29:363–376 [DOI] [PubMed] [Google Scholar]
- Nevins AK, Thurmond DC 2003 Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am J Physiol Cell Physiol 285:C698–C710 [DOI] [PubMed] [Google Scholar]
- Nevins AK, Thurmond DC 2005 A direct interaction between Cdc42 and vesicle-associated membrane protein 2 regulates SNARE-dependent insulin exocytosis. J Biol Chem 280:1944–1952 [DOI] [PubMed] [Google Scholar]
- Nevins AK, Thurmond DC 2006 Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic β-cells. J Biol Chem 281:18961–18972 [DOI] [PubMed] [Google Scholar]
- Wang Z, Oh E, Thurmond DC 2007 Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem 282:9536–9546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Luo R, Kowluru A, Li G 2004 Novel regulation by Rac1 of glucose-and forskolin-induced insulin secretion in INS-1 β cell. Am J Physiol Endocrinol Metab 286:E818–E827 [DOI] [PubMed] [Google Scholar]
- McDonald P, Veluthakal R, Kaur H, Kowluru A 2007 Biologically active lipids promote trafficking and membrane association of Rac1 in insulin-secreting INS832/13 cells. Am J Physiol Cell Physiol 292:C1216–C1220 [DOI] [PubMed] [Google Scholar]
- Asahara A, Kido Y, Shigeyama Y, Matsuda T, Takeda A, Inoue T, Shibutani Y, Koyanagi M, Uchida T, Kasuga M 2008 Rac1 regulates glucose induced insulin secretion through modulation of cytoskeletal organization in β cells. Diabetes 57:A55 (supplement 1) [Google Scholar]
- Greiner TU, Kesavan G, Ståhlberg A, Semb H 2009 Rac1 regulates pancreatic islet morpholgenesis. BMC Dev Biol 9:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A, Amin R 2002 Inhibitors of post-translational modifications of G-proteins as probes to study the pancreatic beta cell function: potential therapeutic implications. Curr Drug Targets Immune Endocr Metabol Disord 2:129–139 [PubMed] [Google Scholar]
- Lawrence JT, Birnbaum MJ 2003 ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA 100:13320–13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodnitzky JA, Syed N, Kimber MJ, Day TA, Donaldson JG, Hsu WH 2007 Somatostatin receptors signal through EFA6A-ARF6 to activate phospholipase D in clonal β-cells. J Biol Chem 282:13410–13418 [DOI] [PubMed] [Google Scholar]
- Hammar E, Tomas A, Bosco D, Halban PA 2009 Role of the Rho-ROCK (Rho-associated kinase) signaling pathway in the regulation of pancreatic β-cell function. Endocrinology 150:2072–2079 [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, Seino S 2007 Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA 104:19333–19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzi R, Ravazzola M, Iezzi M, Lang J, Zahraoui A, Andereggen E, Morel P, Takai Y, Wollheim CB 1996 Expression, localization and functional role of small GTPases of the Rab3 family in insulin-secreting cells. J Cell Sci 109:2265–2273 [DOI] [PubMed] [Google Scholar]
- Li G, Regazzi R, Balch WE, Wollheim CB 1993 Stimulation of insulin release from permeabilized HIT-T15 cells by a synthetic peptide corresponding to the effector domain of the small GTP-binding protein rab3. FEBS Lett 327:145–149 [DOI] [PubMed] [Google Scholar]
- Olszewski S, Deeney JT, Schuppin GT, Williams KP, Corkey BE, Rhodes CJ 1994 Rab3A effector domain peptides induce insulin exocytosis via specific interaction with cytosolic protein doublet. J Biol Chem 269:27987–27991 [PubMed] [Google Scholar]
- Iezzi M, Escher G, Meda P, Charollais A, Baldini G, Darchen F, Wollheim CB, Regazzi R 1999 Subcellular distribution and function of Rab3A, B, C and D isoforms in insulin-secreting cells. Mol Endocrinol 13:202–212 [DOI] [PubMed] [Google Scholar]
- Iezzi M, Regazzi R, Wollheim CB 2000 The Rab3-interacting molecular RIM is expressed in pancreatic β-cells and is implicated in insulin exocytosis. FEBS Lett 474:66–70 [DOI] [PubMed] [Google Scholar]
- Kajio H, Olszewski S, Rosner PJ, Donelan MJ, Geoghegan KF, Rhodes CJ 2001 A low-affinity Ca2+-dependent association of calmodulin with Rab3A effector domain inversely correlates with insulin exocytosis. Diabetes 50: 2029–2039 [DOI] [PubMed] [Google Scholar]
- Yaekura K, Julyan R, Wicksteed BL, Hays LB, Alarcon C, Sommers S, Poitout V, Baskin DG, Wang Y, Philipson LH, Rhodes CJ 2003 Insulin secretory deficiency and glucose intolerance in Rab3A null mice. J Biol Chem 278:9715–9721 [DOI] [PubMed] [Google Scholar]
- Waselle L, Coppola T, Fukuda M, Iezzi M, El-Amraoui A, Petit C, Regazzi R 2003 Involvement of the Rab27 binding protein Sla2c/MyRIP in insulin exocytosis. Mol Biol Cell 14:4103–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviet S, Coppola T, Haynes LP, Burgoyne RD, Regazzi R 2004 The Rab-binding protein Noc2 is associated with insulin-containing secretory granules and is essential for pancreatic β-cell exocytosis. Mol Endocrinol 18:117–126 [DOI] [PubMed] [Google Scholar]
- Torii S, Takeuchi T, Nagamatsu S, Izumi T 2004 Rab27 effector granuphilin promotes the plasma membrane targeting of insulin granules via interaction with syntaxin1a. J Biol Chem 279:22532–22538 [DOI] [PubMed] [Google Scholar]
- Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, Kasai H, Nagamatsu S, Gomi H, Izumi T 2005 Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J Clin Invest 115:388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abderrahmani A, Cheviet S, Ferdaoussi M, Coppola T, Waeber G, Regazzi R 2006 ICER induced by hyperglycemia represses the expression of genes essential for insulin exocytosis. EMBO J 25:977–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Fujita T, Gomi H, Izumi T 2008 Docking is not a prerequisite but a temporal constraint for fusion of secretory granules. Traffic 9:1191–1203 [DOI] [PubMed] [Google Scholar]
- Kimura T, Kaneko Y, Yamada S, Ishihara H, Senda T, Iwamatsu A, Niki I 2008 The GDP-dependent Rab27a effector coronin 3 controls endocytosis of secretory membrane in insulin-secreting cell lines. J Cell Sci 121:3092–3098 [DOI] [PubMed] [Google Scholar]
- Merrins MJ, Stuenkel EL 2008 Kinetics of Rab27a-dependent actions on vesicle docking and priming in pancreatic β-cells. J Physiol 586:5367–5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffa L, Fuchs E, Pietropaolo M, Barr F, Solimena M 2008 ICA69 is a novel Rab2 effector regulating ER-Golgi trafficking in insulinoma cells. Eur J Cell Biol 87:197–209 [DOI] [PubMed] [Google Scholar]
- Leech CA, Holz GG, Chepurny O, Habener JF 2000 Expression of cAMP-regulated guanine nucleotide exchange factors in pancreatic β-cells. Biochem Biophys Res Commun 278:44–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan EP, Gao X, Leung YM, Gaisano HY 2007 Activation of exchange protein directly activated by cyclic adenosine monophosphate and protein kinase A regulate common and distinct steps in promoting plasma membrane exocytic and granule-to granule fusions in rat islet β cells. Pancreas 35:e45–e54 [DOI] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, Harbeck M, Roe MW, Holz GG 2005 A cAMP and Ca2+ coincidence detector in support of Ca2+ release in mouse pancreatic β cells. J Physiol 566:173–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Holz G 2004 New insights concerning the glucose-dependent insulin secretagogue action of glucagon-like peptide-1 in pancreatic β-cells. Horm Metab Res 36:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG 2004 Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic β-cell. Diabetes 53:5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SL, Monks LK, Gao H, Deaville P, Morgan NG 2002 Identification of the monomeric G-protein, Rhes, as an efaroxan-regulated protein in the pancreatic β-cell. Br J Pharmacol 136:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharoyko VV, Zaitseva II, Varsanyi M, Portwood N, Leibiger B, Leibiger I, Berggren PO, Efendiæ S, Zaitsev SV 2005 Monomeric G-protein, Rhes, is not an imidazoline-regulated protein in pancreatic β-cells. Biochem Biophys Res Commun 338:1455–1459 [DOI] [PubMed] [Google Scholar]
- Taylor JP, Jackson DA, Morgan NG, Chan SL 2006 Rhes expression in pancreatic β-cells is regulated by efaroxan in a calcium-dependent process. Biochem Biophys Res Commun 349:809–815 [DOI] [PubMed] [Google Scholar]
- Finlin BS, Mosley AL, Crump SM, Correll RN, Ozcan S, Satin J, Andres DA 2005 Regulation of L-type Ca2+ channel activity and insulin secretion by the Rem2 GTPase. J Biol Chem 280:41864–41871 [DOI] [PubMed] [Google Scholar]
- Lopez JA, Kwan EP, Xie L, He Y, James DE, Gaisano HY 2008 The RalA GTPase is a central regulator of insulin exocytosis from pancreatic islet β cells. J Biol Chem 283:17939–17945 [DOI] [PubMed] [Google Scholar]
- Adra CN, Iyengar AR, Syed FA, Kanaan IN, Rilo HL, Yu W, Kheraj R, Lin SR, Horiuchi T, Khan S, Weremowicz S, Lim B, Morton CC, Higgs DR 1998 Human ARHGDIG, a GDP-dissociation inhibitor for Rho proteins: genomic structure, sequence, expression analysis, and mapping to chromosome 16p13.3. Genomics 53:104–109 [DOI] [PubMed] [Google Scholar]
- Kowluru A, Veluthakal R 2005 Rho guanosine diphosphate-dissociation inhibitor plays a negative modulatory role in glucose stimulated insulin secretion. Diabetes 54:3523–3529 [DOI] [PubMed] [Google Scholar]
- Park YJ, Ahn HJ, Chang HK, Kim JY, Huh KH, Kim MS, Kim YS 2009 The RhoGDI-α/JNK signaling pathway plays a significant role in mycophenolic acid-induced apoptosis in an insulin-secreting cell line 2009. Cell Signal 21:356–364 [DOI] [PubMed] [Google Scholar]
- Scheffzek K, Stephan I, Jensen ON, Illenberger D, Gierschik P 2000 The Rac-RhoGDI complex and the structural basis for the regulation of Rho proteins by RhoGDI. Nat Struct Biol 7:122–126 [DOI] [PubMed] [Google Scholar]
- DerMardirossian C, Bokoch GM 2005 GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol 15:356–363 [DOI] [PubMed] [Google Scholar]
- Olofsson B 1999 Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal 11:545–554 [DOI] [PubMed] [Google Scholar]
- DerMardirossian CM, Bokoch GM 2006 Phosphorylation of RhoGDI by p21-activated kinase 1. Methods Enzymol 406:80–90 [DOI] [PubMed] [Google Scholar]
- Kowluru A, Metz SA 1994 Regulation of guanine-nucleotide binding proteins in islet subcellular fractions by phospholipase-derived lipid mediators of insulin secretion. Biochim Biophys Acta 1222:360–368 [DOI] [PubMed] [Google Scholar]
- Gilligan M, Welsh GI, Flynn A, Bujalska I, Diggle TA, Denton RM, Proud CG, Docherty K 1996 Glucose stimulates the activity of the guanine nucleotide-exchange factor elF-2B in isolated rat islets of Langerhans. J Biol Chem 271:2121–2125 [DOI] [PubMed] [Google Scholar]
- Arava Y, Seger R, Walker MD 1999 GRFβ, a novel regulator of calcium signaling, is expressed in pancreatic β cells and brain. J Biol Chem 274:24449–24452 [DOI] [PubMed] [Google Scholar]
- Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S 2001 Critical role of cAMP-GEFII-Rim 2 complex in incretin-potentiated insulin secretion. J Biol Chem 276:46046–46053 [DOI] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Holz GG 2001 cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic β-cells. J Physiol 536:375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous M, Veluthakal R, Amin R, Kowluru A 2002 IL-1 β-induced nitric oxide release from insulin-secreting β-cells: further evidence for the involvement of GTP-binding proteins. Diabetes Metab 28:3S78–3S84; discussion 3S108–3S112 [PubMed] [Google Scholar]
- Coppola T, Perret-Menoud V, Gattesco S, Magnin S, Pombo I, Blank U, Regazzi R 2002 The death domain of Rab3 guanine nucleotide exchange protein in GDP/GTP exchange activity in living cells. Biochem J 362:273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font de Mora J, Esteban LM, Burks DJ, Núñez A, Garcés C, García-Barrado MJ, Iglesias-Osma MC, Moratinos J, Ward JM, Santos E 2003 Ras-GRF1 signaling is required for normal β-cell development and glucose homeostasis. EMBO J 22:3039–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson L, Ma X, Renström E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, Sewing S, Rorsman P 2003 SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol 121:181–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon G, Pappan KL, Marshall CA, Schaffer JE, McDaniel ML 2004 cAMP dose-dependently prevents palmitate-induced apoptosis by both protein kinase A- and cAMP-guanine nucleotide exchange factor-dependent pathways in β-cells. J Biol Chem 279:8938–8945 [DOI] [PubMed] [Google Scholar]
- Hashiguchi H, Nakazaki M, Koriyama N, Fukudome M, Aso K, Tei C 2006 Cyclic AMP/cAMP-GEEF pathway amplifies insulin exocytosis induced by Ca2+ and ATP in rat islet β-cells. Diabetes Metab Res Rev 22:64–71 [DOI] [PubMed] [Google Scholar]
- Veluthakal R, Madathilparambil SV, McDonald P, Olson LK, Kowluru A 2009 Regulatory roles of Tiam1, a guanine nucleotide exchange factor for Rac1, in glucose-stimulated insulin secretion in pancreatic β-cells. Biochem Pharmacol 77:101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caumont AS, Galas MC, Vitale N, Aunis D, Bader MF 1998 Regulated exocytosis in chromaffin cells. Translocation of ARF6 stimulates plasma membrane-associated phospholipase D. J Biol Chem 273:1373–1379 [DOI] [PubMed] [Google Scholar]
- Kahn RA, Volpicelli-Daley L, Bowzard B, Shrivastava-Ranjan P, Li Y, Zhou C, Cunningham L 2005 Arf family GTPases: roles in membrane traffic and microtubule dynamics. Biochem Soc Trans 33:1269–1272 [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Honda A 2005 Localization and function of Arf family GTPases. Biochem Soc Trans 33:639–642 [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C, Chavrier P 2006 ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 7:347–358 [DOI] [PubMed] [Google Scholar]
- Gillingham AK, Munro S 2007 The small G proteins of the ARF family and their regulators. Annu Rev Cell Dev Biol 23:579–611 [DOI] [PubMed] [Google Scholar]
- Dunlop M, Metz SA 1989 A phospholipase D-like mechanism in pancreatic islet cells: stimulation by calcium, phorbol ester and sodium fluoride. Biochem Biophys Res Commun 163:922–928 [DOI] [PubMed] [Google Scholar]
- Metz SA, Dunlop M 1990 Stimulation of insulin release by phospholipase D. A potential role for endogenous phosphatidic acid in pancreatic islet function. Biochem J 270:427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad RJ, Jolly YC, Wolf BA 1991 Glucose and carbachol synergistically stimulate phosphatidic acid accumulation in pancreatic islets. Biochem Biophys Res Commun 180:960–966 [DOI] [PubMed] [Google Scholar]
- Hughes WE, Elgundi Z, Huang P, Frohman MA, Biden TJ 2004 Phospholipase D1 regulates secretagogue-stimulated insulin release in pancreatic β-cells. J Biol Chem 279: 27534–27541 [DOI] [PubMed] [Google Scholar]
- Vitale N, Chasserot-Golaz S, Bader MF 2002 Regulated secretion in chromaffin cells: an essential role for ARF6-regulated phospholipase D in the late stages of exocytosis. Ann NY Acad Sci 971:193–200 [DOI] [PubMed] [Google Scholar]
- Caumont AS, Vitale N, Gensse M, Galas MC, Casanova JE, Bader MF 2000 Identification of a plasma membrane-associated guanine nucleotide exchange factor for ARF6 in chromaffin cells. Possible role in regulated exocytotic pathway. J Biol Chem 275:15637–15644 [DOI] [PubMed] [Google Scholar]
- Cohen LA, Honda A, Varnai P, Brown FD, Balla T, Donaldson JG 2007 Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol Biol Cell 18:2244–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzakri K, Ribaux P, Tomas A, Parnaud G, Rickenbach K, Halban PA 2008 Rab GTPase activating protein AS160 is a major downstream effector of protein kinase B/Akt signaling in pancreatic β-cells. Diabetes 57:1195–1204 [DOI] [PubMed] [Google Scholar]
- Otsuki Y, Tanaka M, Yoshii S, Kawazoe N, Nakaya K, Sugimura H 2001 Tumor metastasis suppressor nm23H1 regulates Rac1 GTPase by interaction with Tiam1. Proc Natl Acad Sci USA 98:4385–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Tseng YH, Kantor JD, Rhodes CJ, Zetter BR, Moyers JS, Kahn CR 1999 Interaction of the Ras-related protein associated with diabetes rad and the putative tumor metastasis suppressor NM23 provides a novel mechanism of GTPase regulation. Proc Natl Acad Sci USA 96:14911–14918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A, Veluthakal R, Kaetzel DM 2006 Regulatory roles for nm23/nucleoside diphosphate kinase-like enzymes in insulin secretion from the pancreatic islet β-cell. J Bioenerg Biomembr 38:227–232 [DOI] [PubMed] [Google Scholar]
- Béglé A, Tryoen-Tóth P, de Barry J, Bader MF, Vitale N 2009 ARF6 regulates the synthesis of fusogenic lipids for calcium-regulated exocytosis in neuroendocrine cells. J Biol Chem 284:4836–4845 [DOI] [PubMed] [Google Scholar]
- Bader MF, Vitale N 2009 Phospholipase D in calcium-regulated exocytosis: lessons from chromaffin cells. Biochim Biophys Acta 1791:936–941 [DOI] [PubMed] [Google Scholar]
- Khoo S, Griffen SC, Xia Y, Baer RJ, German MS, Cobb MH 2003 Regulation of insulin gene transcription by ERK1 and ERK2 in pancreatic β cells. J Biol Chem 278:32969–32977 [DOI] [PubMed] [Google Scholar]
- Arnette D, Gibson TB, Lawrence MC, January B, Khoo S, McGlynn K, Vanderbilt CA, Cobb MH 2003 Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic β cells. J Biol Chem 278:32517–32525 [DOI] [PubMed] [Google Scholar]
- Gibson TB, Lawrence MC, Gibson CJ, Vanderbilt CA, McGlynn K, Arnette D, Chen W, Collins J, Naziruddin B, Levy MF, Ehrlich BE, Cobb MH 2006 Inhibition of glucose-stimulated activation of extracellular signal-regulated protein kinase 1 and 2 by epinephrine in pancreatic β-cells. Diabetes 55:1066–1073 [DOI] [PubMed] [Google Scholar]
- Lawrence M, Shao C, Duan L, McGlynn K, Cobb MH 2008 The protein kinases ERK1/2 and their roles in pancreatic β cells. Acta Physiol (Oxf) 192:11–17 [DOI] [PubMed] [Google Scholar]
- Khoo S, Gibson TB, Arnette D, Lawrence M, January B, McGlynn K, Vanderbilt CA, Griffen SC, German MS, Cobb MH 2004 MAP kinase and their roles in pancreatic β-cells. Cell Biochem Biophys 40:191–200 [DOI] [PubMed] [Google Scholar]
- Frödin M, Sekine N, Roche E, Filloux C, Prentki M, Wollheim CB, Van Obberghen E 1995 Glucose, other secretagogues, and nerve growth factor stimulates mitogen-activated protein kinase in the insulin-secreting β-cell line INS-1. J Biol Chem 270:7882–7889 [DOI] [PubMed] [Google Scholar]
- Ehses JA, Pelech SL, Pederson RA, McIntosh CHS 2002 Glucose-dependent insulinotropic polypeptide activates the Raf-Mek1/2-ERK1/2 module via cyclic AMP/cAMP-dependent protein kinase/Rap1-mediated pathway. J Biol Chem 277:37088–37097 [DOI] [PubMed] [Google Scholar]
- Briaud I, Lingohr MK, Dickson LM, Wrede CE, Rhodes CJ 2003 Differential activation mechanisms of Erk-1/2 and p70S6K by glucose in pancreatic β cells. Diabetes 52:974–983 [DOI] [PubMed] [Google Scholar]
- Trümper J, Ross D, Jahr H, Brendel MD, Göke R, Hörsch D 2005 The Rap-B-Raf signaling pathway is activated by glucose and glucagon-like peptide-1 in human islet cells. Diabetologia 48:1534–1540 [DOI] [PubMed] [Google Scholar]
- Gomez E, Pritchard C, Herbert TP 2002 cAMP-dependent protein kinase and Ca2+ influx through L-type voltage-gated calcium channels mediate Raf-independent activation of extracellular regulated kinase in response to glucagon-like peptide-1 in pancreatic β-cells. J Biol Chem 277:48146–48151 [DOI] [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH 2007 Differential regulation and properties of MAPKs. Oncogene 26:3100–3112 [DOI] [PubMed] [Google Scholar]
- Veluthakal R, Rhodes CJ, Kowluru A, Protein prenylation is necessary for glucose-induced activation of ERK1/2 and insulin secretion in pancreatic β-cells. Proc 68th Annual Meeting of the American Diabetes Association, San Francisco, CA, 2008 (Abstract LB-4888) [Google Scholar]
- Kolch W 2005 Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol 6:827–837 [DOI] [PubMed] [Google Scholar]
- Roy M, Li Z, Sacks DB 2004 IQGAP1 binds to ERK2 and modulates its activity. J Biol Chem 279:17329–17337 [DOI] [PubMed] [Google Scholar]
- Briggs MW, Sacks DB 2003 IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep 4: 571–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateer SC, Wang N, Bloom GS 2003 IQGAPs: integrators of the cytoskeleton, cell adhesion machinery, and signaling networks. Cell Motil Cytoskeleton 55:147–155 [DOI] [PubMed] [Google Scholar]
- Brandt DT, Grosse R 2007 Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep 8:1019–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Kaibuchi K 1999 Cdc42, Rac1, and their effector IQGAP1 as molecular switches for cadherin-mediated cell-cell adhesion. Biochem Biophys Res Commun 262:1–6 [DOI] [PubMed] [Google Scholar]
- Kurella VB, Richard JM, Parke CL, Lecour Jr LF, Bellamy HD, Worthylake DK 2009 Crystal structure of the GAP-related domain from IQGAP1. J Biol Chem 284:14857–14865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K 1996 Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem 271:23363–23367 [DOI] [PubMed] [Google Scholar]
- Nauert JB, Rigas JD, Lester LB 2003 Identification of IQGAP1/AKA79 complex in β-cells. J Cell Biochem 90:97–108 [DOI] [PubMed] [Google Scholar]
- Rittmeyer EN, Daniel S, Hsu SC, Osman MA 2008 A dual role for IQGAP1 in regulating exocytosis. J Cell Sci 121:391–403 [DOI] [PubMed] [Google Scholar]
- Yan J, Yang Y, Zhang H, King C, Kan HM, Cai Y, Yuan CX, Bloom GS, Hua X 2009 Menin interacts with IQGAP1 to enhance intercellular adhesion of β-cells. Oncogene 28:973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H, Adachi M, Matsui T, Okawa K, Tsukita S, Tsukita S 2008 IQGAP3 regulates cell proliferation through the Ras/ERK signalling cascade. Nat Cell Biol 10:971–978 [DOI] [PubMed] [Google Scholar]
- Wang S, Watanabe T, Noritake J, Fukata M, Yoshimura T, Itoh N, Harada T, Nakagawa M, Matsuura Y, Arimura N, Kaibuchi K 2007 IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite growth. J Cell Sci 120:567–577 [DOI] [PubMed] [Google Scholar]
- Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D 2004 Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci 61:2614–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro A 1998 The small GTP-binding protein Rac promotes the dissociation of gelsolin from actin filaments in neutrophils. J Biol Chem 273:805–813 [DOI] [PubMed] [Google Scholar]
- De Corte V, Bruyneel E, Boucherie C, Mareel M, Vandekerckhove J, Gettemans J 2002 Gelsolin-induced epithelial cell invasion is dependent on Ras-Rac signaling. EMBO J 21:6781–6790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas A, Yermen B, Min L, Pessin JE, Halban PA 2006 Regulation of pancreatic β-cell insulin secretion by actin cytoskeleton remodelling: role of gelsolin and cooperation with the MAPK signaling pathway. J Cell Sci 119:2156–2167 [DOI] [PubMed] [Google Scholar]
- Yermen B, Tomas A, Halban PA 2007 Pro-survival role of gelsolin in mouse β-cells. Diabetes 56:80–87 [DOI] [PubMed] [Google Scholar]
- Poitout V, Robertson RP 2008 Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocr Rev 29:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM 2003 Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 52:1–8 [DOI] [PubMed] [Google Scholar]
- Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H 2003 Glucose toxicity in β cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 52:581–587 [DOI] [PubMed] [Google Scholar]
- Robertson RP, Tanaka Y, Takahashi H, Tran PO, Harmon JS 2005 Prevention of oxidative stress by adenoviral overexpression of glutathione-related enzymes in pancreatic islets. Ann NY Acad Sci 1043:513–520 [DOI] [PubMed] [Google Scholar]
- Guichard C, Moreau R, Pessayre D, Epperson TK, Krause KH 2008 NOX family of NADPH oxidases in liver and in pancreatic islets: a role in metabolic syndrome and diabetes? Biochem Soc Trans 36:920–929 [DOI] [PubMed] [Google Scholar]
- Hordijk PL 2006 Regulation of NADPH oxidases: the role of Rac proteins. Circ Res 98:453–462 [DOI] [PubMed] [Google Scholar]
- Sumimoto H 2008 Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J 275:3249–3277 [DOI] [PubMed] [Google Scholar]
- Dang PM, Fontayne A, Hakim J, El Benna J, Périanin A 2001 Protein kinase ζ phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol 166:1206–1213 [DOI] [PubMed] [Google Scholar]
- Janciauskiene S, Ahrén B 2000 Fibrillar islet amyloid peptide differentially affects oxidative mechanisms and lipoprotein uptake in correlation with cytotoxicity in two insulin producing cell lines. Biochem Biophys Res Commun 267:619–625 [DOI] [PubMed] [Google Scholar]
- Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR 2003 Pancreatic β-cells express phagocyte-like NAD(P)H oxidase. Diabetes 52:1457–1463 [DOI] [PubMed] [Google Scholar]
- Nakayama M, Inoguchi T, Sonta T, Maeda Y, Sasaki S, Sawada F, Tsubouchi H, Sonoda N, Kobayashi K, Sumimoto H, Nawata H 2005 Increased expression of NAD(P)H oxidase in islets of animal models of type 2 diabetes and its improvement by an AT1 receptor antagonist. Biochem Biophys Res Commun 332:927–933 [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Nawata H 2005 NAD(P)H oxidase activation: a potential target mechanism for diabetes vascular complications, progressive β-cell dysfunction and metabolic syndrome. Curr Drug Targets 6:495–501 [DOI] [PubMed] [Google Scholar]
- Cunningham GA, McClenaghan NH, Flatt PR, Newsholme P 2005 L-Alanine induces changes in metabolic and signal transduction gene expression in a clonal rat pancreatic β-cell line and protects from pro-inflammatory cytokine-induced apoptosis. Clin Sci (Lond) 109:447–455 [DOI] [PubMed] [Google Scholar]
- Lupi R, Del Guerra S, Bugliani M, Boggi U, Mosca F, Torri S, Del Prato S, Marchetti P 2006 The direct effects of the angiotensin-converting enzyme inhibitors, zofenoprilat and enalaprilat, on isolated human pancreatic islets. Eur J Endocrinol 154:355–361 [DOI] [PubMed] [Google Scholar]
- Shao J, Iwashita N, Ikeda F, Ogihara T, Uchida T, Shimizu T, Uchino H, Hirose T, Kawamori R, Watada H 2006 Beneficial effects of candestartan, and angiotensin II type 1 receptor blocker, on β-cell function and morphology in db/db mice. Biochem Biophys Res Commun 344:1224–1233 [DOI] [PubMed] [Google Scholar]
- Uchizono Y, Takeya R, Iwase M, Sasaki N, Oku M, Imoto H, Iida M, Sumimoto H 2006 Expression of isoforms of NADPH oxidase components in rat pancreatic islets. Life Sci 80:133–139 [DOI] [PubMed] [Google Scholar]
- Morgan D, Oliveira-Emilio HR, Keane D, Hirata AE, Santos da Rocha M, Bordin S, Curi R, Newsholme P, Carpinelli AR 2007 Glucose, palmitic and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal cell line. Diabetologia 50:359–369 [DOI] [PubMed] [Google Scholar]
- Lastra G, Manrique C 2007 The expanding role of oxidative stress, rennin angiotensin system, and β-cell dysfunction in the cardiometabolic syndrome and type 2 diabetes mellitus. Antioxid Redox Signal 9:943–954 [DOI] [PubMed] [Google Scholar]
- Chu KY, Leung PS 2007 Angiotensin II type 1 receptor antagonism mediates uncoupling protein 2-driven oxidative stress and ameliorates pancreatic islet β-cell function in young type 2 diabetic mice. Antioxid Redox Signal 9:869–878 [DOI] [PubMed] [Google Scholar]
- Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R 2007 Diabetes associated cell stress and dysfunction: role of mitochondrial and non mitochondrial ROS production and activity. J Physiol 583:9–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KK, Leung PS 2008 Effects of hyperglycemia on angiotensin II receptor type1 expression and insulin secretion in an INS-1E pancreatic β-cell line. JOP 9:290–299 [PubMed] [Google Scholar]
- Imoto H, Sasaki N, Iwase M, Nakamura U, Oku M, Sonoki K, Uchizono Y, Iida M 2008 Impaired insulin secretion by diphenyleneiodium associated with perturbation of cytosolic Ca2+ dynamics in pancreatic β-cells. Endocrinology 149:5391–5400 [DOI] [PubMed] [Google Scholar]
- Janciauskiene S, Ahren B 2000 Fibrillar islet amyloid polypeptide differentially affects oxidative mechanisms and lipoprotein uptake in correlation with cytotoxicity in two insulin-producing cell lines. Biochem Biophys Res Commun 267:619–625 [DOI] [PubMed] [Google Scholar]
- Syed I, Veluthakal R, Kowluru A 2009 Lipotoxicity of the β cell involves ceramide-mediated activation of phagocytic NADPH oxidase. Diabetes 58(Suppl 1):A424 [Google Scholar]
- Veluthakal R, Syed I, Jayaram B, Kowluru A 2009 Rac1 regulates glucolipotoxicity of the islet β-cell. Diabetes 58(Suppl 1):A428 [Google Scholar]
- Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y 2005 Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes 54:1838–1845 [DOI] [PubMed] [Google Scholar]
- Nagamatsu S, Nakamichi Y, Yamamura C, Matsushima S, Watanabe T, Ozawa S, Furukawa H, Ishida H 1999 Decreased expression of t-SNARE, syntaxin 1, and SNAP-25 in pancreatic β-cells is involved in impaired insulin secretion from diabetic GK rat islets: restoration of decreased t-SNARE proteins improves impaired insulin secretion. Diabetes 48:2367–2373 [DOI] [PubMed] [Google Scholar]
- Veluthakal R, Suresh MV, Kowluru A 2009 Down-regulation of expression and function of nucleoside diphosphate kinase in insulin-secreting β-cells under in vitro conditions of glucolipotoxicity. Mol Cell Biochem 329:121–129 [DOI] [PubMed] [Google Scholar]
- Thomas HE, Darwiche R, Corbett JA, Kay TW 2002 Interleukin-1 plus γ-interferon-induced pancreatic β cell dysfunction is mediated by β-cell nitric oxide production. Diabetes 51:311–316 [DOI] [PubMed] [Google Scholar]
- Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL 2005 Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54(Suppl 2):S97–S107 [DOI] [PubMed] [Google Scholar]
- Steer SA, Scarim AL, Chambers KT, Corbett JA 2006 Interleukin-1 stimulates β-cell necrosis and release of the immunological adjuvant HMGB1. Plos Med 3:e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Lee MS 2009 Recent progress in research on β-cell apoptosis by cytokines. Front Biosci 14:657–664 [DOI] [PubMed] [Google Scholar]
- Hohmeier HE, Tran VV, Chen G, Gasa R, Newgard CB 2003 Inflammatory mechanisms in diabetes: lessons from the β-cell. Int J Obes Relat Metab Disord 27(Suppl 3): S12–S16 [DOI] [PubMed] [Google Scholar]
- Pahan K, Sheikh FG, Namboodiri AM, Singh I 1997 Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest 100:2671–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahan K, Liu X, McKinney MJ, Wood C, Sheikh FG, Raymond JR 2000 Expression of a dominant-negative mutant of p21(ras) inhibits induction of nitric oxide synthase and activation of nuclear factor-κB in primary astrocytes. J Neurochem 74:2288–2295 [DOI] [PubMed] [Google Scholar]
- Kowluru A, Morgan NG 2002 GTP-binding proteins in cell survival and demise: the emerging picture in the pancreatic β-cell. Biochem Pharmacol 63:1027–1035 [DOI] [PubMed] [Google Scholar]
- Tannous M, Amin R, Popoff MR, Fiorentini C, Kowluru A 2001 Positive modulation by Ras of interleukin-1β-mediated nitric oxide generation in insulin-secreting clonal β (HIT-T15) cells. Biochem Pharmacol 62:1459–1468 [DOI] [PubMed] [Google Scholar]
- Chen HQ, Tannous M, Veluthakal R, Amin R, Kowluru A 2003 Novel roles for palmitoy-lation of Ras in IL-1 β-induced nitric oxide release and caspase 3 activation in insulin-secreting β cells. Biochem Pharmacol 66:1681–1694 [DOI] [PubMed] [Google Scholar]
- Veluthakal R, Chvyrkova I, Tannous M, McDonald P, Amin R, Hadden T, Thurmond DC, Quon MJ, Kowluru A 2005 Essential role for membrane lipid rafts in interleukin-1β-induced nitric oxide release from insulin-secreting cells: potential regulation by caveolin-1. Diabetes 54: 2576–2585 [DOI] [PubMed] [Google Scholar]
- Li G, Segu VB, Rabaglia ME, Luo RH, Kowluru A, Metz SA 1998 Prolonged depletion of guanosine triphosphate induces death of insulin-secreting cells by apoptosis. Endocrinology 139:3752–3762 [DOI] [PubMed] [Google Scholar]
- Kim JY, Yoon SY, Park J, Kim YS 2006 Mycophenolic acid induces islet apoptosis by regulating mitogen-activated protein kinase activation. Transplant Proc 38:3277–3279 [DOI] [PubMed] [Google Scholar]
- Lee CC, Jeng LB, Li PC, Yang HR, Lu CW, Chen TH, Cheng HT, Chang SC, Lin MS, Poon KS, Chen YF, Ho YJ 2008 Molecular mechanisms of cell death of mycophenolic acid-treated primary isolated rat islets: implication of mitogen-activated protein kinase activation. Transplant Proc 40:2525–2526 [DOI] [PubMed] [Google Scholar]
- Efrat S, Fleischer N, Hanahan D 1990 Diabetes induced in male transgenic mice by expression of human H-ras oncoprotein in pancreatic β cells. Mol Cell Biol 10:1779–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava M, Eidelman O, Leighton X, Glasman M, Goping G, Pollard HB 2002 Anx7 is required for nutritional control of gene expression in mouse pancreatic islets of Langerhans. Mol Med 8:781–797 [PMC free article] [PubMed] [Google Scholar]
- Higashijima T, Burnier J, Ross EM 1990 Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. J Biol Chem 265:14176–14186 [PubMed] [Google Scholar]
- Higashijima T, Uzu S, Nakajima T, Ross EM 1988 Mastoparan, a peptide toxin from Wasp venom, mimics receptors by activating GTP-binding proteins. J Biol Chem 263:6491–6494 [PubMed] [Google Scholar]
- Yajima H, Komatsu M, Yamada S, Straub SG, Kaneko T, Sato Y, Yamauchi K, Hashizume K, Sharp GW, Aizawa T 2000 Cerulenin, an inhibitor of protein acylation, selectively attenuates nutrient stimulation of insulin release: a study in rat pancreatic islets. Diabetes 49:712–717 [DOI] [PubMed] [Google Scholar]
- Hällbrink M, Saar K, Ostenson CG, Soomets U, Efendic S, Howl J, Wheatley M, Zorko M, Langel U 1999 Effects of vasopressin-mastoparan chimeric peptides on insulin release and G-protein activity. Regul Pept 82:45–51 [DOI] [PubMed] [Google Scholar]
- Amin RH, Chen HQ, Veluthakal R, Silver RB, Li J, Li G, Kowluru A 2003 Mastoparan-induced insulin secretion from insulin-secreting βTC3 and INS-1 cells: evidence for its regulation by Rho subfamily of G-proteins. Endocrinology 144:4508–4518 [DOI] [PubMed] [Google Scholar]
- Gao Z, Reavey-Cantwell J, Young RA, Jegier P, Wolf BA 2000 Synaptotagmin III/VII isoforms mediate Ca2+-induced insulin secretion in pancreatic islet beta cells. J Biol Chem 275:36079–36085 [DOI] [PubMed] [Google Scholar]
- Komatsu M, McDermott AM, Gillison SL, Sharp GWG 1993, Mastoparan stimulates exocytosis at a Ca2+-independent late site in stimulus–secretion coupling. Studies with RINm5F β-cell line. J Biol Chem 268:23297–23306 [PubMed] [Google Scholar]
- Jones PM, Mann FM, Persaud SJ, Wheeler-Jones CPD 1993 Mastoparan stimulates insulin secretion from pancreatic β-cells by effects at a late stage in the secretory pathway. Mol Cell Endocrinol 94:97–103 [DOI] [PubMed] [Google Scholar]
- Goto Y, Kakizaki M, Masaki N 1975 Spontaneous diabetes produced by selective breeding of normal Wistar rats. Proc Jpn Acad 51:80–85 [Google Scholar]
- Portha B 2005 Programmed disorders of β-cell development and function as one cause for type 2 diabetes? The GK rat paradigm. Diabetes Metab Res Rev 21:495–504 [DOI] [PubMed] [Google Scholar]
- Metz SA, Meredith M, Vadakekalam J, Rabaglia ME, Kowluru A 1999 A defect late in stimulus-secretion coupling impairs insulin secretion in Goto Kakizaki diabetic rats. Diabetes 48:1754–1762 [DOI] [PubMed] [Google Scholar]
- Ostenson CG, Zaitsev S, Berggren PO, Efendic S, Langel U, Bartfai T 1997 Galparan: a powerful insulin-releasing chimeric peptide acting at a novel site. Endocrinology 138:3308–3313 [DOI] [PubMed] [Google Scholar]
- Kowluru A, Metz SA 1994 Characterization of nucleoside diphosphokinase activity in human and rodent pancreatic β cells: evidence for its role in the formation of guanosine triphosphate, a permissive factor for nutrient-induced insulin secretion. Biochemistry 33:12495–12503 [DOI] [PubMed] [Google Scholar]
- Kowluru A 2003 Defective protein histidine phosphorylation in islets from the Goto-Kakizaki diabetic rat. Am J Physiol Endocrinol Metab 285:E498–E503 [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Mitin N, Keller PJ, Chenette EJ, Madigan JP, Currin RO, Cox AD, Wilson O, Kirschmeier P, Der CJ 2008 Rho family GTPase modification and dependence on CAAX motif-signaled posttranslational modification. J Biol Chem 283:25150–25163 [DOI] [PMC free article] [PubMed] [Google Scholar]