Abstract
Cellular actions of thyroid hormone may be initiated within the cell nucleus, at the plasma membrane, in cytoplasm, and at the mitochondrion. Thyroid hormone nuclear receptors (TRs) mediate the biological activities of T3 via transcriptional regulation. Two TR genes, α and β, encode four T3-binding receptor isoforms (α1, β1, β2, and β3). The transcriptional activity of TRs is regulated at multiple levels. Besides being regulated by T3, transcriptional activity is regulated by the type of thyroid hormone response elements located on the promoters of T3 target genes, by the developmental- and tissue-dependent expression of TR isoforms, and by a host of nuclear coregulatory proteins. These nuclear coregulatory proteins modulate the transcription activity of TRs in a T3-dependent manner. In the absence of T3, corepressors act to repress the basal transcriptional activity, whereas in the presence of T3, coactivators function to activate transcription. The critical role of TRs is evident in that mutations of the TRβ gene cause resistance to thyroid hormones to exhibit an array of symptoms due to decreasing the sensitivity of target tissues to T3. Genetically engineered knockin mouse models also reveal that mutations of the TRs could lead to other abnormalities beyond resistance to thyroid hormones, including thyroid cancer, pituitary tumors, dwarfism, and metabolic abnormalities. Thus, the deleterious effects of mutations of TRs are more severe than previously envisioned. These genetic-engineered mouse models provide valuable tools to ascertain further the molecular actions of unliganded TRs in vivo that could underlie the pathogenesis of hypothyroidism.
Actions of thyroid hormone that are not initiated by liganding of the hormone to intranuclear TR are termed nongenomic. They may begin at the plasma membrane or in cytoplasm. Plasma membrane-initiated actions begin at a receptor on integrin αvβ3 that activates ERK1/2 and culminate in local membrane actions on ion transport systems, such as the Na+/H+ exchanger, or complex cellular events such as cell proliferation. Concentration of the integrin on cells of the vasculature and on tumor cells explains recently described proangiogenic effects of iodothyronines and proliferative actions of thyroid hormone on certain cancer cells, including gliomas. Thus, hormonal events that begin nongenomically result in effects in DNA-dependent effects. l-T4 is an agonist at the plasma membrane without conversion to T3. Tetraiodothyroacetic acid is a T4 analog that inhibits the actions of T4 and T3 at the integrin, including angiogenesis and tumor cell proliferation. T3 can activate phosphatidylinositol 3-kinase by a mechanism that may be cytoplasmic in origin or may begin at integrin αvβ3. Downstream consequences of phosphatidylinositol 3-kinase activation by T3 include specific gene transcription and insertion of Na, K-ATPase in the plasma membrane and modulation of the activity of the ATPase.
Thyroid hormone, chiefly T3 and diiodothyronine, has important effects on mitochondrial energetics and on the cytoskeleton. Modulation by the hormone of the basal proton leak in mitochondria accounts for heat production caused by iodothyronines and a substantial component of cellular oxygen consumption. Thyroid hormone also acts on the mitochondrial genome via imported isoforms of nuclear TRs to affect several mitochondrial transcription factors. Regulation of actin polymerization by T4 and rT3, but not T3, is critical to cell migration. This effect has been prominently demonstrated in neurons and glial cells and is important to brain development. The actin-related effects in neurons include fostering neurite outgrowth. A truncated TRα1 isoform that resides in the extranuclear compartment mediates the action of thyroid hormone on the cytoskeleton.
Molecular mechanisms of genomic and nongenomic actions of thyroid hormone are described. Genomic actions are those that require a primary interaction of 3, 5, 3’-triiodo-L-thyronine (T3) with intranuclear isoforms of the nuclear thyroid hormone receptor (TR) and result in transcription of specific genes; mutations in these genes lead to thyroid hormone resistance syndromes or may in models promote thyroid cancer. Nongenomic actions of thyroid hormone include effects of L-thyroxine (T4) and T3 that are initiated at the plasma membrane receptor for thyroid hormone on integrin avb3 and transduced by extracellular signal regulated kinases 1/2 (ERK1/2) or phosphatidylinositol 3-kinase (PI3K) into nucleus-mediated events, including angiogenesis and tumor cell proliferation. Other nongemonic actions begin in cytoplasm at PI3K or ERK1/2, may involve extranuclear TR isoforms and result in expression of specific genes. Also discussed are nongenomic mechanisms of regulation of mitochondrial respiration by thyroid hormone and of control by T4 of the state of the actin cytoskeleton.
- I. Genomic Actions of Thyroid Hormone
- A. Multiple forms of thyroid hormone nuclear receptors
- B. Isoform-dependent functions of TRs
- C. Multilevel regulation of TR transcription activity
- D. TR mutations and disease
- II. Nongenomic Actions of Thyroid Hormone
- A. Initiation sites for nongenomic actions of thyroid hormone: plasma membrane and cytoplasm (Fig. 4)
- B. Examples of nongenomic actions of thyroid hormone
- III. Thyroid Hormone and Mitochondria
- A. Mitochondrial energetics and thyroid hormone
- B. Thyroid hormone and mitochondriogenesis
- C. Thyroid hormone-dependent induction of mitochondrial DNA
- D. Thyroid hormone-dependent nongenomic actions in mitochondria
- IV. Actions of Thyroid Hormone on the Cytoskeleton, Cell Migration
- A. Astrocytes
- B. Neurons
- C. The role of TRΔα1 gene in T4-dependent actin polymerization
- V. Concentrations of Thyroid Hormone at Which Molecular Actions of the Hormone Are Measured
- A. Deiodinases
- B. Thyroid hormone transporters
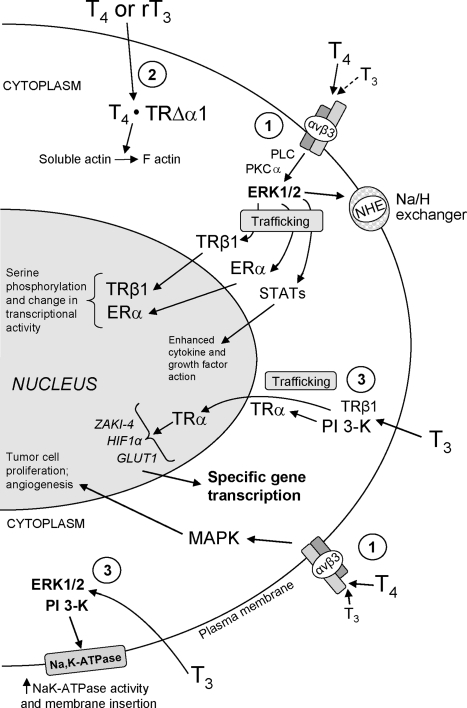
Figure 4.
Nongenomic actions of thyroid hormone that are initiated at the plasma membrane receptor on integrin αvβ3 or in cytoplasm. Via the integrin receptor, thyroid hormone from the cell surface stimulates MAPK (ERK1/2) through phospholipase C (PLC) and protein kinase C (PKC) (161). Hormone-activated ERK1/2 promotes trafficking of specific proteins resident in cytoplasm to the nuclear compartment and serine phosphorylation of nucleoproteins by activated ERK1/2 also translocated to the nucleus. Target proteins phosphorylated by hormone-directed ERK include estrogen receptor (ER)-α, TR-β1, signal transducing and activator of transcription (STAT)-1α, and CoA protein Trip230. Complex cellular events induced from the cell surface receptor include angiogenesis (endothelial and vascular smooth muscle cells) and tumor cell proliferation (160). In cytoplasm, T3 can nongenomically activate PI3K and initiate downstream transcription of specific genes. Activation of PI3K can involve TRβ1 or TRα resident in cytoplasm. A truncated form of TRα1 (TRΔα1) in cytoplasm mediates the action of T4 and rT3 on the actin cytoskeleton. T3 and T4 may also activate PI3K from the integrin αvβ3 hormone receptor site (148). GLUT1, Glucose transporter-1.
I. Genomic Actions of Thyroid Hormone
A. Multiple forms of thyroid hormone nuclear receptors
Using isolated rat nuclei, Tata and Widnell first showed that T3 stimulates DNA-dependent RNA-polymerase activity to increase synthesis of new RNAs (1). Subsequently, Oppenheimer et al. (2) and Samuels et al. (3) demonstrated high-affinity, low-capacity binding sites in the nuclei of rat tissues and cultured GH cells, respectively. These studies, whereas considered correlative at the time, strongly suggested that thyroid hormone nuclear receptors (TRs) mediated the transcriptional activities of T3. In the ensuing years, efforts were made to purify TRs from rat liver (4,5,6) but met with only limited success. It was not until the cloning of the TRs in 1986 that it became possible to characterize their molecular properties and to study directly how TRs regulate the transcriptional activities of T3 target genes (7,8).
TRs are members of the nuclear receptor superfamily and function as T3-inducible transcription factors. TRs are derived from two genes located on two different chromosomes (9) (Fig. 1A). The TRβ gene, located on chromosome 3, encodes three T3-binding TRβ isoforms (β1, β2, and β3) (10). These TRβ isoforms share high sequence homology in the DNA and T3-binding domains but differ in the length and amino acid sequences in the amino terminal A/B domain. Internal usage of ATG leads to the TRΔβ3 that lacks the amino A/B and DNA-binding domains but retains T3-binding activity (10). The TRα gene, located on chromosome 17, encodes one T3-binding TRα1 and two splicing variants (TRα2 and TRα3). These TRα1 variants differ from TRα1 in the length and amino acid sequences in the C-terminal region, beginning at amino acid 370. These TRα1 variants have no T3-binding activity (11). Truncated TRs, transcribed from an internal promoter located in intron 7, give rise to TRΔα1 and TRΔα2 that lack amino-terminal A/B and DNA domains but retain most of the T3-binding domain (12).
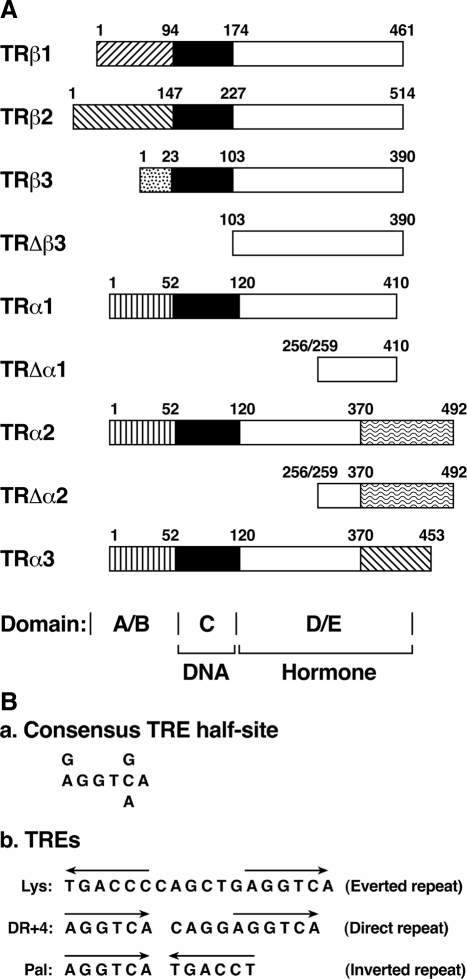
Figure 1.
A, Schematic representation of TR isoforms. TRs are encoded by the TRβ and TRα genes located on different chromosomes. Alternative splicing of primary transcripts gives rise to four thyroid hormone binding isoforms. TRs share high sequence homology in the DNA binding domain C (solid bar) and the hormone binding domain D/E (open bar). The amino-terminal A/B domains are variable in length and amino acid sequence as indicated by different symbols. The amino acids of the truncated TRs (TRΔβ3, TRΔα1, and TRΔα2) at the amino and carboxy termini are indicated by numbers. B, The DNA sequence (a) and the arrangement (b) of the TRE half-site binding motifs. The Lys TRE was identified in the promoter of lysozyme gene. DR+4 TRE represents the direct repeat separated by four nucleotides. Pal is TRE half-site in palindromic arrangement.
Similar to other members of the receptor superfamily, TRs consist of single polypeptide chains with modular functional domains (13,14) (Fig. 1A). The amino-terminal A/B domains vary among TR isoforms that are involved in transcription regulation. Located centrally in the molecules is the highly conserved DNA-binding domain that interacts with thyroid hormone response elements (TREs) of T3 target genes. The carboxyl-terminal ligand-binding domain (LBD) shares high sequence homology among TR isoforms that assume multifunctions. LBD not only binds thyroid hormones but also interacts with a host of corepressors (CoRs) and coactivators (CoAs), collectively known as receptor coregulators (15). The LBD is also involved in homodimerization of DNA-bound TRs and their heterodimerization with other members of the receptor superfamily, notably with the retinoid X receptors (RXRs). Analysis of the structure of LBD indicates that ligand binding induces dramatic structural changes that facilitate dissociation of repressors and association of activators (16,17).
The expression of TR isoforms is tissue-dependent and developmentally regulated (9). TRα1 is constitutively expressed at embryonic development, and TRβ is expressed toward the later stage of development (18). TRβ1 is expressed predominantly in the kidneys, liver, brain, heart, and thyroid; at lower levels in the skeletal muscle, lungs, and spleen; but not at all in the testes (9,10,19). TRβ2 is mainly expressed in the brain, retina, and inner ears; at low levels in the lungs and heart; but not in other tissues (10,20). TRβ3 is predominantly expressed in the kidneys, liver, and lungs; at low levels in the skeletal muscle, spleen, brain, and heart; but not at all in the testis (10). TRα1 and TRα2 are expressed at the highest levels in the brain; at lower levels in the kidneys, skeletal muscle, lungs, heart, testes, and liver; but not in the testes (10).
B. Isoform-dependent functions of TRs
The molecular diversity of TRs raises the question as to whether the TR isoforms have distinct functions or simply serve a redundant role for each other. The tissue-dependent and developmentally regulated differential expression of TR isoforms suggests that TRs may mediate subtype-specific functions. This possibility is supported by distinct patterns of spatiotemporal TR isoform expression in the embryonic and postnatal nervous system (18). TRα1- and TRα2 mRNAs are similarly and widely expressed in the fetal neocortical plate, site of cortical neuronal differentiation. In contrast, TRβ1 transcripts are restricted in distribution, with prominent expression in zones of neuroblast proliferation such as the germinal trigone and the cortical ventricular layer. TRβ2 transcripts are expressed in the developing hippocampus and striatum (20). These differential spatiotemporal expression patterns suggest that TR isoforms could play distinct functional roles during development. Gene inactivation studies, however, provide in vivo evidence to indicate that the TR isoforms can have both subtype-specific and overlapping functional roles (21,22,23,24,25). Mice lacking TRα1 have a lower heart rate, abnormal heart contractility, and decreased body temperature (23). In contrast, mice in which the TRβ gene is selectively inactivated have a mild dysfunction of the pituitary-thyroid axis and a deficit in auditory function and eye development (20,21,22). Moreover, mice in which both TRα1 and TRα2 are deleted have impaired postnatal development and decreased postnatal survival (24). These distinct phenotypes exhibited by mice in which individual TR genes are selectively deleted indicate that TR isoforms mediate specific functions. When both TRα and TRβ genes are inactivated, an array of phenotypes are detected, including severe dysfunction of the pituitary-thyroid axis, retarded growth, and delayed bone maturation, which are not found in the single receptor-deficient mice (25). These findings indicate that TRα1 and TRβ can substitute for each other to mediate some actions of T3 and also mediate isoform-specific functions.
The TR isoform-dependent phenotypes exhibited by mice deficient in TR genes prompted the question as to whether TR isoforms specifically regulate certain target genes that mediate the TR isoform-dependent phenotypic expression. The question was addressed by using the cDNA microarray approach to compare the gene expression profiles in the livers of TRα knockout, TRβ knockout, and wild-type mice (26). Yet, hierarchical clustering analyses indicate that no clusters of target genes that are selectively activated by either TR isoform were identified (26), suggesting that TR isoform-specific regulation of target genes is rare in the liver. However, using a similar approach, Flores-Morales et al. (27) found 155 hepatic genes that are regulated after treatment with T3 for 2 h in wild-type mice, whereas only 84 hepatic genes are regulated in TRβ knockout mice under the same conditions. These findings raised the possibility that some T3 target genes in the liver could be specifically regulated by TRβ1. It is currently unclear what underlies the differences between these two studies. It is possible that the mouse strains and the different experimental conditions could account for the discrepancy. Moreover, it has been shown that TRα1 and TRα2 isoforms are zonally expressed around the central vein in rat liver and that the portal to central gradient of TRα1 is broader than that of TRβ1. Moreover, the expression of the TRα2 protein showed a diurnal variation with a peak in the afternoon when the animals are least active, whereas no such variation was found for the TRα1 protein (28). Although it is not clear whether such zonal distribution and diurnal changes of TR isoforms in rats also occur in mice, the possibility exists that sampling of livers for array analyses could also contribute to the discrepancy observed by these two studies.
However, it is important to understand the underlying mechanisms resulting in distinct phenotypic expression in mice deficient in one TR isoform or the other, or both. The availability of various genetically engineered mice would allow the use of high throughput array approach to ascertain whether there are clusters of genes that are preferentially regulated by one TR isoform that could impact the biological functions of other target tissues, such as brain, heart, bone, kidney, and thyroid. The major TR isoform in the brain, heart, and bone is TRα, and that in the kidney and thyroid is TRβ. Consistent results obtained under defined conditions could reveal whether tissue-dependent abundance of TR isoform proteins might be one of the mechanisms underlying the phenotypic expression of mice deficient in one TR isoform or the other. This question warrants additional studies in the future.
C. Multilevel regulation of TR transcription activity
1. Diversity of TREs
TRs are DNA-binding transcription factors that recognize specific DNA sequences on the promoters of T3 target genes. In the years after the cloning of TR cDNAs, flurries of activity to map and characterize TREs on T3 target genes led to identification of TREs with a core consensus sequence of the hexanucleotide “half-site” (A/G)GGT(C/A/G)A (Fig. 1B). Analyses of the TREs in the promoters of many T3 target genes have shown that the TRE half-sites exist in pairs (Fig. 1B). The half-site binding motif can be arranged as an everted repeat (as in the chicken lysozyme gene, Lys; Fig. 1B), as a direct repeat (as in the malic enzyme gene, DR+4; Fig. 1B), or as an inverted repeat (palindrome, Pal, as in the GH gene; Fig. 1B). The spacing between the two half-sites also varies, depending on the orientation of the half-sites. For the everted repeat, there is a spacing of six nucleotides. For the direct repeat, the spacing is four nucleotides. For the palindromic arrangement, no spacing separates the two half-sites. Analyses of T3 target genes showed that TRE direct repeats are more common than TRE inverted repeats (29). Furthermore, in the promoters of some genes, clusters of mixed types of TREs are present to interact with TR for maximal T3 responses. The GH gene is an example in that one hexamer is common for a direct repeat TRE, and a palindromic TRE is common for cooperative binding of the TR dimer-T3 complex (30).
2. Crosstalk with other signaling pathways via heterodimeric partners of TR
TRs bind to TREs not only as homodimers but also as heterodimers with other members of the receptor superfamily, such as RXRs, vitamin D receptor, and all subtypes of the retinoic acid receptors. Heterodimerization with RXR dramatically increases the binding of TRs to TREs, the responsiveness of TR to T3, and the transcriptional activation (31). Thus, heterodimerization provides an important means to modulate the functions of TR. Due to ubiquitous distribution of RXR and its promiscuity in heterodimerization with many members of the receptor superfamily, heterodimerization with RXR provides a means for TR to crosstalk with other receptors. Crosstalk with peroxisome proliferator-activated receptor (PPAR) signaling via heterodimerization with RXR by TR is a prominent example. PPARγ regulates the expression of its target genes by binding to the PPAR response element (direct repeat+1; DR1) as a heterodimer with RXR. Recently, it was shown that TRβ competes with PPARγ for binding to DR1 as a heterodimer with RXR in vitro and in vivo to repress the transcriptional activity of PPARγ (32). Because PPARγ plays a key role in lipid metabolism, carcinogenesis, and cardiovascular diseases (33,34,35), this mode of TR action via crosstalk with other receptors expands TR effects via direct binding to TREs on the promoter of target genes.
The gene regulatory activity of TR could also be affected by other receptors that heterodimerize with RXR. This is shown by the regulation of 7α-hydroxylase (CYP7A1) that is the rate-limiting enzyme in cholesterol metabolism. TRβ/RXR binds to the TRE (DR+4) on the promoter of the CYP7A1 gene to positively regulate its expression. However, the liver X receptor (LXR)/RXR heterodimer also binds to the same TRE site to activate the expression of the CYP7A1 gene. Cell-based studies indicate that cotransfection of TRβ with LXR-α inhibits the activity of LXR-α transcription activity of the CYP7A1 promoter (36). This inhibition is due to competition of LXR with TRβ for heterodimerizing with RXR in binding to DR+4 (36). These findings show that LXR crosstalks with TRβ via heterodimerization with RXR, and via this network the activity of TRβ is modulated by other receptors.
3. Modulation of TR functions by other cellular proteins
Central to the understanding of TR action are the mechanisms by which the diverse effects of T3 are achieved. Recent studies suggest that the diverse effect of TR could be mediated by interaction with a host of cellular proteins. These cellular proteins could be expressed in a tissue-dependent and developmentally regulated manner. This, together with the known differential expression of TR isoforms, diversity of TREs, and various heterodimeric partners, could further fine-tune the transcriptional activity of TR in different tissues. Thus, the diverse effects of TR could be achieved via combinatorial complexes of TR with various cellular proteins. Many TR-interacting proteins have recently been identified and are briefly reviewed in Section I.C.3.a.
a. Nuclear receptor coregulators.
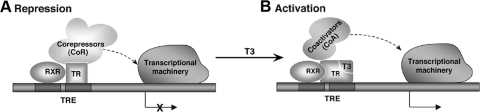
The search for bridging factors that could communicate the signals of TRE-bound TR with the basal transcription machinery for efficient transcription led to the identification of the first transcriptional mediator that interacts with both TRβ1 and RXR in a ligand-dependent fashion. This protein, Trip1 (for TR-interacting protein), shares striking sequence conservation with the yeast transcriptional mediator Sug1 (37). Soon after the discovery of Trip1, many coregulatory proteins (CoR and CoA) for TRs have also been discovered via various screening strategies. Structures and functions of these coregulators have been intensively studied in the past decade, and much has been learned about how TRs interact with the coregulators. A simplified molecular model for the regulation of T3-dependent positively regulated genes is shown in Fig. 2. In the absence of T3, the unliganded TR binds to TRE as a heterodimer with RXR. The association of unliganded TR/RXR with CoA results in the repression of basal transcriptional activity (Fig. 2A). Binding of T3 to TR induces structural changes (16), allowing the liganded TR to recruit CoA and other associated proteins to modify chromatin structures to facilitate transcriptional activation (Fig. 2B).
Figure 2.
A simplified molecular model for transcriptional repression by unliganded TR (A) and activation by liganded TR (B). Interaction of the unliganded TR with the complex of CoRs and its associated proteins leads to repression of transcription (A). Interaction of the liganded TR with CoAs (e.g., SRC/p160 or TRAP/DRIP complex) leads to transcriptional activation (B).
Coactivators (CoAs).
The steroid hormone receptor CoA (SRC-1) is the first CoA to be cloned that binds to the liganded TR in addition to other members of the receptor superfamily (38,39). Subsequently identified were other related CoAs, known as SRC-2/TIF2 (transcription intermediary factor 2)/GRIP1 (GR-interacting protein 1) and SRC-3/TRAM-1 (thyroid hormone receptor activator molecule 1)/RAC3 (receptor-associated CoA 3)/ACTR (activator of thyroid receptor)/pCIP (p300/CBP cointegrator-associated protein) (40). These CoAs share considerable sequence homology (∼40%), have functional properties similar to SRC-1, and are now designated as the SRC/p160 family members (40). These SRCs share a highly conserved basic helix loop helix (bHLH) and Per-ARNT-Sim (PAS) A/B domain (bHLH-PAS; Fig. 3) in the amino-terminal region that functions as a DNA-binding domain or dimerization interaction region for other transcription factors (41). In the center of the molecule, three copies of signature motifs LXXLL (in the one letter code of amino acids, X indicates any amino acid) termed the NR box are located (41,42,43,44). Mutational analysis indicates that distinct NR boxes interact differentially with different nuclear receptors (receptor-specific codes), and the selectivity may arise from the amino acids located adjacent to the different LXXLL motifs (42). X-ray crystallographic analysis of TRβ-LBD complexed with T3, and a 13-amino acid peptide NR box of GRIP1 revealed the details in the molecular interaction of receptor and CoA (45). The leucines of the α-helical NR box make contacts with a hydrophobic groove consisting of residues from helices 3, 4, 5, and 12 of TRβ. A single LXXLL peptide interacts with each monomer of TRβ dimer (42).
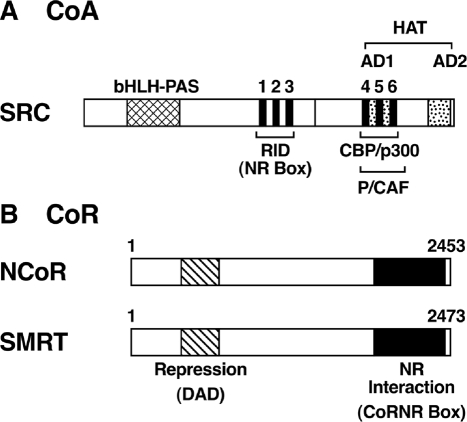
Figure 3.
Schematic representation of SRC/p160 CoA family (A) and NCoR/SMRT CoRs (B). A, The location of the receptor interaction domain (RID) in the SRC is indicated. RID and activation domain 1 (AD1) each contains three LXXLL motifs. The specific domains for interaction with P/CAF, CBP/p300, as well as the histone acetyltransferase domain, are indicated. Located near the amino-terminal region is the highly conserved bHLH–PAS domain that functions as a DNA-binding or dimerization surface in many transcription factors. B, The nuclear receptor (NR) interaction region that contains the “CoRNR box” motifs near the C-terminal region is indicated. Near the amino-terminal region is the deacetylase activation domain (DAD) that interacts with and activates HDAC3, required for repression by TR.
Near the C-terminal region, SRC-1 contains two activation domains, AD1 and AD2, responsible for its coactivation function. Both AD domains are required for optimal coactivation although they act independently. AD also contains three additional LXXLL motifs that interact with general transcriptional activators CBP/p300 and P/CAF (41,44). Like CBP/p300, SRC-1 also functions as a histone acetyltransferase, and this activity is localized in the C-terminal region of the protein (Fig. 3). The histone acetyltransferase functions to modify chromatin structures to facilitate transcriptional activity of nuclear receptors.
In addition to SRC/p300 complexes, the transcriptional activity of TRs is also regulated by other large complexes. Using epitope-tagged TR, TR-associated proteins (TRAP) were affinity-purified from a cell line stably expressing TR (46). The TRAP complex, which does not exhibit intrinsic histone acetyltransferase activity, was shown to enhance the transcription activity of TR in a chromatin-free system (47). This complex contains at least nine proteins, with molecular weight ranging from approximately 70 to approximately 230 kDa. One of these proteins, TRAP220, was found to interact with TR in response to T3 (47,48). A complex containing similar components was found to interact with vitamin D receptor (vitamin D receptor interacting proteins; DRIPs) and to enhance its transcriptional activity (49). Thus, different receptors could be mediated by the TRAP/DRIP complex for the regulation of transcriptional activity.
That TR is associated with multiple complexes prompted the question as to how different complexes collaborate to activate ligand-dependent transcription activity of TR. Several interesting models have been proposed. These different complexes could perform different tasks in a sequential order, in a combinatorial overlapping utilization of the complexes, or in a target gene-specific utilization of complexes in responding to different signals (50). It is possible that the mode of actions could depend on the target genes and the cellular context. The elucidation of these possibilities will require additional studies.
Corepressors (CoRs).
One of the functional characteristics of TR is its ability to repress, or silence, the basal transcription in the absence of ligand. Intensive studies in the past decade have indicated that this repression occurs via interaction of TR with CoR proteins. The first CoRs cloned were NCoR (nuclear receptor CoR) (51) and its homolog, SMRT (silencing mediator of retinoid and thyroid hormone receptor) (52). Subsequently, other nuclear proteins such as Hairless (53), Alien (54), RIP-140 (55,56), and SUN-CoR (57) were reported to function as CoRs for TRs. Our current understanding of how CoRs mediate the basal repression of TRs is mostly learned from the studies using NCoR and SMRT because they are the best characterized.
NCoR or SMRT binds to the surface of the TR molecule consisting of helices 3, 4, and 5 via its “CoRNR” box with repeated motifs of (I/L)XX(I/V)I in the C-terminal region (58,59) (Fig. 3B). NCoR and SMRT are associated with other proteins such as transducin-like protein (TBL1, or TBL1R) and histone deacetylase 3 (HDAC3) to form large repression complexes (60). NCoR and SMRT interact with HDAC3 via a region denoted the deactylase activation domain that contains the SANT1 motif (59,60) (Fig. 3B). HDAC3 is required for repression by TR (59). However, the repression by TR could also be mediated by HDAC3-independent mechanisms via TBL1, which interacts with histones (60,61).
Although NCoR and SMRT are highly homologous and the mode of action appears to be similar, the gene inactivation in mice suggests that these two CoRs have nonredundant biological functions (62). Mice with NCoR knockout are embryonic lethal, suggesting the SMRT cannot compensate for the functions of NCoR involved in development and survival (63). Although NCoR has been implicated in human diseases such as acute promyelocytic leukemia (retinoic acid receptor translocation) (64), acute myeloid leukemia (AML1-ETO translocation) (65), and thyroid hormone resistance (66), and more recently in the regulation of oncogenic functions of a mutated TRβ (denoted TRβPV) in thyroid carcinogenesis (67) (see Section I.D.1.b.), it is not yet known whether SMRT is involved in human diseases. Thus, these observations suggest that these two CoRs could mediate distinct biological functions. With the increasing numbers of CoRs that are being discovered and being characterized, novel mechanisms in the regulation of TR functions should soon be forthcoming.
b. Other TR interacting proteins.
In addition to nuclear receptor coregulators that modulate the transcriptional activity of TR in a hormone-dependent manner, the activity of TRs can also be regulated by other cellular proteins. These TR-interacting proteins are functionally diverse, ranging from transcription regulators, to cytoskeletal architecture modulators, to tumor suppressors and promoters. In their own right, these TR-interacting proteins occupy a critical position in regulating cellular functions. The mode of interaction of TRs with each protein varies, and the mechanisms by which these proteins alter the functions of TR also differ. The interaction with TR also alters the functions of these proteins.
Cyclin D1.
Cyclin D1 regulates cell cycle progression, and its overexpression is associated with tumorigenesis (68). Lin et al. (69) found that cyclin D1 physically interacts with the C-terminal region of the LBD of TRβ1 in a T3-independent manner. Cyclin D1 acts to repress both the silencing activity of the unliganded TRβ1 and the transcriptional activity of the liganded TRs. The binding of TR to TRE is not affected by its association with cyclin D1. Interestingly, cyclin D1 acts as a bridging factor to recruit HDAC3 to augment the silencing activity of the unliganded TRβ1 and to mediate the repression of the T3-dependent transcriptional activity. Thus, the interaction of TR with cyclin D1 raises the possibility that TR could mediate its functions via the cyclin D1 regulatory network (70).
EAR-2.
EAR-2, an orphan nuclear receptor, was identified as an interacting protein for TRβ in human colon carcinoma RKO cells (71). EAR-2 is a distant member of the chick ovalbumin upstream promoter-transcription factor of the orphan nuclear receptor family. TRβ1 physically interacts with EAR-2 in vitro and in cells independent of T3. The binding site was mapped to the C-terminal region of TRβ. Binding of TRβ1 to TREs is competitively inhibited by EAR-2. In several cultured cell lines, both the T3-dependent and T3-independent TRβ1 transcriptional activities are repressed by EAR-2. However, the sensitivity of repression depends on the cell type, thereby suggesting that the cellular context plays an important role in the repression effect of EAR-2 (71). One of the possible cellular factors that could affect the cell-type dependent repression effect of EAR-2 is SRC-1 because the repression effect of EAR-2 on the T3-dependent transcriptional activity is reversed by the transfected SRC-1 in cells (71). Because the expression of SRC-1 is cell-type dependent (38), the extent of negative regulation most likely will depend on the expression levels of EAR-2, TR, and CoAs in cells. This dependence suggests that the diverse functions of TR are likely mediated by a large network of regulatory proteins.
Tumor suppressor p53.
The tumor suppressor p53 is a critical transcription factor in the regulation of the cell cycle and in tumorigenesis (72). Yap et al. (73) found that TRβ1 physically interacts with p53 through its DNA-binding domain. The regions of p53 responsible for its interaction with TRβ1 are located in the DNA-binding domain and at the carboxyl-terminal polymerization domain (74). The TR DNA-binding domain is the interaction site with p53 (74,75). This physical interaction leads to the inhibition of the binding of TRβ1 to TREs. In transfected cells, p53 represses the T3-dependent transcription of TRβ1 (73). In rat pituitary GH-producing GC cells that endogenously express TR, expression of p53 leads to a repression of a TR-target gene, the GH gene (76). Thus, p53 is a negative regulator of the transcriptional activity of TRβ1.
That the DNA-binding and the carboxyl-terminal polymerization domains of p53 are the binding sites with TRβ1 raised the possibility that the activity of p53 could be affected by binding to TRβ1. Indeed, the association of p53 with TRβ1 increases the binding of p53 to p53 DNA-binding elements (74). This increase in DNA-binding, however, results in repression of p53-dependent transcription activation in transfected cells. Furthermore, this association leads to an inhibition of the p53-mediated induction of bax and gadd45 expression (74). Because the expression of bax and gadd45 is directly regulated by p53, these results indicate that TRβ1 can modulate p53-regulated gene expression. Taken together, the crosstalk between these two important transcription factors could play an important role in the biology of normal and cancer cells.
Gelsolin.
Gelsolin is an actin-binding protein, and in the presence of calcium it is able to sever actin and cap the growing end of the released filament (77). It is involved in controlling cell morphology, motility, growth, and apoptosis (78). Phosphoinositides can bind to gelsolin and regulate its interaction with actin (79). Its important role in phospholipid signaling pathways was recently demonstrated by the observation that gelsolin-induced epithelial cell invasion is dependent on phosphatidylinositol 3-kinase (PI3K)-Rac pathways (79). During thyroid carcinogenesis, the expression of gelsolin was found down-regulated (80). Additional biochemical analyses demonstrated that gelsolin physically interacts with TRβ and its mutant TRβPV in vivo and in vitro. The interaction regions were mapped to the C terminus of gelsolin and the DNA-binding domain of TR. Interestingly, the interaction of gelsolin is weakened by the mutation of TR, resulting in perturbation of cytoskeletal architectures. These results revealed a novel function of TR in maintaining the integrity of cellular cytoskeletal structure and functions via physical interaction with gelsolin (80).
Pituitary tumor-transforming gene (PTTG).
PTTG, also known as securin, is a critical mitotic checkpoint protein that helps hold sister chromatids together before entering anaphase (81). It was originally isolated from GH4 pituitary cells and shown to cause in vitro cell transformation and to induce tumor formation in vivo (82). Overexpression of PTTG has been detected in human thyroid carcinomas (83,84), colorectal carcinoma (85), pituitary adenomas (86), and hematopoietic neoplasms (87). Importantly, PTTG was found to induce genetic instability in a variety of cells including thyroid cells (88,89). The overexpression of PTTG in thyroid tumors of a mouse model of thyroid cancer that harbors a TRβ mutant (TRβPV/PV mice), TRβPV, led to the discovery that PTTG is a TR-interacting protein (89). In vitro and cell-based studies showed that the PTTG protein is physically associated with TRβ as well as the mutated TRβPV. The DNA-binding domain of TR is the site that interacts with the amino-terminal region of PTTG. Concomitant with T3-induced degradation of TRβ (90), PTTG proteins are degraded by the proteasome machinery, but no such degradation occurs when PTTG is associated with the mutant TRβPV (91). A recent study has demonstrated the interaction of SRC-3 with proteasome activator 28γ (PA28γ) and that the degradation of SRC-3 is mediated by the PA28γ proteasome (92). The direct interaction of TRβ with SRC-3 upon T3-binding activates the PA28γ proteasome-mediated degradation of PTTG. In contrast, TRβPV, which does not bind T3, could not form active complexes via direct interaction with SRC-3/PA28γ. The discovery that the liganded TRβ, via physical interaction with PTTG, regulates cellular levels of PTTG protein has important implications in cancer biology in that aberrant accumulation of PTTG is known to cause genetic instability that could underlie cancer development.
β-Catenin.
β-Catenin, a structural component of cell adhesion complexes, interacts with the transmembrane E-cadherin to regulate actin filament assembly to regulate cellular functions (93). In addition, β-catenin also functions as a CoA for a family of transcription factors known as the T-cell factor/lymphoid enhancer factor (TCF/LEF). Upon increased cellular levels and nuclear accumulation, β-catenin/TCF complexes bind to the promoters of downstream target genes involved in cell proliferation, survival, and migration (94). The cellular abundance of β-catenin was found highly elevated in the thyroid tumors of TRβPV/PV mice (95). Studies to understand how TRβ and its mutants regulate the cellular levels of β-catenin in vivo led to the discovery that TRβ interacts with β-catenin in vitro as well as in vivo (95). The interaction region of TR was mapped to the DNA-binding domain, and the interaction of β-catenin with TR favors the unliganded state of TRβ. Via physical interaction in a T3-dependent manner, TRβ could regulate the β-catenin protein levels through Adenomatous Polyposis Coli-independent proteasome pathways. The functional significance of the regulatory mechanism is evident in that TRβPV/PV mice, which harbor a mutated TR (TRβPV) that has lost T3-binding activity, have elevated β-catenin protein levels. This aberrantly increased β-catenin protein level could lead to activation of β-catenin-regulated downstream target genes to contribute to thyroid carcinogenesis in these TRβPV/PV mice.
D. TR mutations and disease
Given the critical role of TRs in cellular functions, it is reasonable to expect that mutations of TRs could have deleterious effects. Indeed, shortly after the cloning of the TR genes (7,8), a tight linkage was discovered between the affected family members with resistance to thyroid hormone (RTH) and the TRβ gene (96). The identification of a Pro453 His mutation in the TRβ gene of one kindred established that RTH is caused by mutations of the TRβ gene (97). To date, about 124 different mutations in the TRβ gene have been reported in more than 374 families and 532 affected individuals (98). In addition to RTH, other abnormalities associated with mutations of the TRβ gene have been uncovered from the mouse models harboring TRβ mutations (99). So far, mutations of the TRα gene have not been reported in humans. However, abnormalities from mice harboring mutations of the TRα gene have been described (99). Significant advances have been made in understanding the in vivo actions of TR mutants by using the genetically engineered mice that will be highlighted in Section I.D.1.
1. Mutations of the TRβ gene
a. RTH.
RTH is a syndrome characterized by reduced sensitivity of tissues to the actions of thyroid hormone (100,101). The hallmark of RTH is elevated thyroid hormone associated with nonsuppressible TSH. Other clinical signs are goiter, short stature, decreased weight, tachycardia, hearing loss, attention deficit/hyperactivity disorder, decreased IQ, and dyslexia (100,101). The clinical manifestations vary between families with different mutations, between families with the same mutation, and also between members of the same family with identical mutations. Most patients are heterozygous, with only one mutated TRβ gene, and their clinical symptoms are mild (100,101). Only one patient homozygous for a mutant TRβ has been reported (102). This patient, who died young, displayed an extraordinary and complex phenotype of extreme RTH with very high levels of thyroid hormones and TSH (102).
Two knockin mutant mice harboring mutations of the TRβ gene have been created to understand the molecular basis of RTH, one harboring a carboxyl-terminal 14-amino acid frame-shift mutation (TRβPV mouse) (103) and the other a Δ337T mutation (TRβΔ337T mouse) (104). These two knockin mice exhibit RTH phenotypes including dysregulation in the pituitary-thyroid axis, abnormal regulation of serum cholesterol, and neurological dysfunction (36,104,105,106). Consistent with phenotypes of RTH patients, TRβPV mice also exhibit growth retardation (103), hearing defects (107), and thyrotoxic skeletal phenotype (108).
Using TRβPV mice, Zhang et al. (109) elucidated the molecular mechanisms of the dominant activity of TRβ mutants in vivo. In the liver nuclear extracts of TRβPV/+ mice, PV forms not only TRE-bound homodimers, but also TRE-bound heterodimers with TRβ1, TRα1, or RXR. In TRβPV/PV mice, in addition to PV/PV homodimers, the lack of wild-type TRβ1 facilitates the formation of TRE-bound PV/TRα1 and PV/RXR heterodimers. Therefore, in vivo, PV competes with TRβ or wild-type TRα1 for binding to TRE and for heterodimerization with RXRs (109). Such competition leads to repression of the T3-positively regulated target genes—S14, malic enzyme, and type 1 deiodinase—in the liver of TRβPV mice. These studies demonstrate that one of the molecular mechanisms by which TRβ mutants exert their dominant-negative activity in vivo is through competition of inactive PV dimers with TRs for binding to TRE and of the mutant PV with RXR for binding to TRE of T3-target genes.
Although most heterozygous RTH patients are clinically euthyroid, some are hypothyroid, and some may appear thyrotoxic. Intriguingly, the same individual may present evidence of hypothyroidism in one tissue, whereas showing signs of thyrotoxicosis in other tissues (100,101). Using TRβPV mice, Zhang et al. (109) showed that differential expression of TR isoforms in tissues contributes to variable clinical manifestations in RTH. Using mice from the cross of TRβPV mice and SRC-1-deficient mice, Kamiya et al. (105) showed that lack of SRC-1 modulates the degree of resistance to thyroid hormone in a target tissue-dependent manner and alters abnormal expression patterns of several T3 target genes in tissues. Thus, differential expression of CoAs such as SRC-1 also contributes to the variable clinical manifestations in RTH.
To test whether TRα1 plays a compensatory role in maintaining the normal physiological functions of T3 in RTH patients who are clinically euthyroid, Suzuki and Cheng crossed TRβPV mice with mice deficient in TRα1 (110) and compared the phenotypes of TRβPV mice with or without TRα1 (110). Lack of TRα1 worsens the RTH symptoms and suggests that TRα1 plays an important and previously unrecognized compensatory role in maintaining the physiological functions of T3 in heterozygous patients with RTH. It is clear from these studies that complex regulation of actions of TRβ mutants and cellular context lead to varied manifestations of the RTH phenotype.
b. Thyroid cancer.
Despite reports to indicate a close association of TRβ mutants in human cancers (111,112), direct evidence to support their oncogenic actions is lacking. The remarkable discovery that TRβPV/PV mice spontaneously develop follicular thyroid carcinoma has provided an unprecedented opportunity to elucidate the oncogenic actions of TRβ mutants in vivo (113). The pathological progression from hyperplasia to capsular invasion, vascular invasion, anaplasia, and metastasis to the lung is similar to human thyroid cancer (113). The mutation of TRβPV is highly penetrated, as evidenced by the fact that by 1 yr of age, all TRβPV/PV mice have developed thyroid cancer.
cDNA microarray analysis identified altered expression of 280 genes during thyroid carcinogenesis (114), indicating that complex alterations of multiple signaling pathways induced by TRβPV could contribute to thyroid carcinogenesis. Among the altered signaling pathways, the repression of the PPARγ (19) is particularly relevant in view of findings that altered PPARγ expression and function could be an important risk factor in the development of human thyroid carcinomas (115,116,117,118). Further biochemical and molecular analysis indicates that TRβPV acts not only to repress the expression of PPARγ, but also to inhibit its transcriptional activity. Such repression of PPARγ activity leads to the activation of the nuclear factor κB downstream signaling, thereby promoting tumor cell proliferation and inhibiting apoptosis. Via such actions, TRβPV functions as an oncogene to mediate thyroid carcinogenesis (119).
The oncogenic actions of TRβPV are not limited via transcription regulation initiated at the nucleus site. Molecular analyses showed that TRβPV could also act via nongenomic actions to activate other oncogenes and key effectors of critical cellular signaling pathways to promote carcinogenesis. Several interacting oncogenes and key regulators have been identified. They are the regulatory subunit p85α of PI3K (67,120), PTTG (91), and β-catenin (95). The physical interaction of TRβPV with the regulatory subunit p85α of PI3K leads to the activation of PI3K-AKT-mammalian target of rapamycin-p70S6K pathway to promote tumor cell proliferation and organ growth (67,120). This interaction also leads to activation of the PI3K-integrin-linked kinase-matrix metalloproteinase-2 pathway to increase cell invasion and metastasis (120). The physical association of TRβPV with PTTG blocks the degradation of PTTG via proteasome machinery, resulting in an aberrant accumulation of cellular PTTG to disrupt mitotic progression and chromosomal abnormalities (91). The physical interaction of TRβPV with β-catenin prevents the degradation of β-catenin, leading to sustained activation of β-catenin-mediated downstream target gene expression to contribute to thyroid carcinogenesis (93). Thus, TRβPV via nucleus-initiated transcription as well as nongenomic signaling pathways functions as an oncogene in thyroid carcinogenesis.
c. Pituitary tumors.
In addition to RTH and thyroid carcinoma, TRβPV/PV mice also spontaneously develop TSH-secreting pituitary adenomas (TSH-omas) (121). TSH-omas are tumors that constitutively secrete TSH. The molecular genetics underlying this abnormality are not well understood. Clues about genetic alterations leading to TSH-omas began to emerge when mutated TRβ was identified in several patients with TSH-omas (122,123). The TRβPV/PV mouse has provided an opportunity to address the role of TRβ mutants in the pathogenesis of TSH-omas (121). Extensive biochemical and cell-based studies indicated that TRβPV increases expression of cyclin D1 at both the mRNA and protein levels, leading to the activation of the CDK/retinoblastoma (Rb)/E2F pathway that mediates, at least in part, the aberrant proliferation of thyrotrophs in TRβPV/PV mice. Thus, these findings provide mechanistic insights into the pathogenesis of TSH-omas in patients and raise the possibility that the mutated TRβ could serve as a molecular marker for diagnosis.
2. Mutations of the TRα gene
Dwarfism and metabolic disorders.
The intriguing fact that no mutations of the TRα gene have ever been identified in RTH patients has perplexed investigators for years and raised the fundamental issue of whether mutations of the TRα gene are lethal or can cause other human diseases. This perplexing question led to the creation of knockin mice harboring the same PV mutation in the TRα gene locus (TRα1PV mice) (124) to unequivocally resolve this fundamental issue. The mutation of both alleles of the TRα gene is not lethal to the embryos, although neonates die shortly after birth. The mutation of one TRα allele results in dwarfism and other abnormalities that are clearly distinct from those caused by mutations of the TRβ gene. Notably, TRαPV/+ mice exhibit no apparent abnormalities in thyroid function tests. These differences in the pituitary-thyroid axis are consistent with the fact that no TRα mutations have been identified in RTH patients. These results indicate that mutated TRα1 and TRβ have distinct biological functions in vivo and thus lead to different diseases.
That mutations of the TRα gene lead to phenotypes differing from the mutations of the TRβ gene was also confirmed in two other knockin mutant mice harboring different mutations, TRα1R384C (125) and TRα1P398H (126). Interestingly, among the three knockin mutant mice, there are differences in phenotypes. TRαR384C/+ mice exhibit juvenile growth retardation that is overcome in adult mice. The milder impairment in growth was attributed to a mutant (TRα1R384C) with a weaker dominant-negative activity (125). In contrast to the TRα1PV mutation, with no T3-binding and transactivation capacity, TRα1R384C only partially lost T3-binding activity, and its transactivation activity could be restored by increased T3 concentration. In vivo, the growth impairment in TRαR384C/+ mice could be rescued by elevated thyroid hormone via an independent mutation in the TRβ gene. In contrast to TRα1PV and TRα1R384C knockin mice that exhibit dwarfism and reduction in fat mass, TRα1P398H mutant mice have increased body fat accumulation and elevated serum levels of leptin, glucose, and insulin. In addition, there is a marked impairment in sympathetic-mediated lipolysis in white adipose tissue (126).
Recent studies indicate that the lean phenotype exhibited by TRα1PV and TRα1R384C knockin mice is partly due to the reduction in fat mass (127,128). However, the underlying mechanisms that lead to abnormal lipid metabolism differ in these two mutant mice. In TRαPV/+ mice, the reduced white adipose mass is due to the repression by TRα1PV of the expression of PPARγ, the key regulator of adipogenesis at both the mRNA and protein levels and the inhibition of the transcription activity of PPARγ. By contrast, TRαR384C/+ mice are hypermetabolic, showing increased lipid mobilization and β-oxidation in adipose tissues. The blockade of sympathetic signaling to brown adipose tissue normalized the metabolic phenotype despite a continued perturbed hormone signaling in this cell type (128). In contrast, the brown adipose tissue of TRαPV/+ mice is not affected by PV mutation (127).
The manifestation of lean phenotype in TRα1PV (124,127) and TRα1R384C type (128), but of increased fat accumulation in TRα1P398H mutant mice (126) suggests that the phenotypic expression of a knockin mutant is sensitive to the location and type of mutation in TRα1. These three mutations—TRα1PV, TRα1R384C, and TRα1P398H—are all located in helix 12, which in wild-type TR undergoes dramatic structural reorganization in response to T3 binding (16), suggesting that the movement and reorganization of helix 12 in relation to the remainder of the TRα1 molecule could be sensitive to mutational alteration. Previously, it has been shown that in vitro different RTH TRβ mutants interact differently with CoRs (66,129,130). Although it is currently unknown how these TRα1 mutants interact with CoRs in vivo, it is conceivable that a different mode of interaction of TRα1 mutants with various CoRs could lead to differential transcriptional repression of different target genes, resulting in the manifestation of different phenotypes. Verification of this possibility in future studies would certainly further advance our understanding of the actions of TRα1 mutants in vivo and the molecular basis of diseases due to mutation of the TRα gene.
II. Nongenomic Actions of Thyroid Hormone
A. Initiation sites for nongenomic actions of thyroid hormone: plasma membrane and cytoplasm (Fig. 4)
1. Sites on the plasma membrane at which thyroid hormone actions may be initiated
High-affinity binding sites for thyroid hormone analogs have for many years been recognized on the plasma membrane of erythrocytes (131,132) and other cells (133,134) and sometimes have been linked to local membrane functions, such as the calcium pump (132,135); see Section II.B.1.a). There was a reluctance to describe these sites as receptors because: 1) complex cellular functions ascribed to thyroid hormone did not follow hormone-binding to the sites; 2) specific identities of membrane binding sites for iodothyronines were not established; and 3) nuclear receptors for thyroid hormone appeared to account for most of the acknowledged actions of thyroid hormone. Rapid-onset membrane effects, such as stimulation of cellular glucose uptake (136) and changes in sodium current (137), were attributed to thyroid hormone in intact cells but implicated an initiation site at or near the plasma membrane.
Recently, a structural protein of the plasma membrane, integrin αvβ3, has been shown to contain a binding domain for iodothyronines that is an initiation site for hormone-directed complex cellular events, such as cell division (138) and angiogenesis (139). This qualifies the binding site for characterization as a receptor. The receptor has been localized to the Arg-Gly-Asp (RGD) recognition site on the integrin that is important to the binding of a number of extracellular matrix proteins (140) and growth factors (141,142,143). From this site, the thyroid hormone signal is transduced by MAPK (ERK1/2) into angiogenesis in endothelial cells (139) and cell proliferation of tumor cell lines (138,144,145) and tumor xenografts (146,147). T4 in concentrations that approximate physiological (10−10 m free T4) and T3 in supraphysiological concentrations cause ERK-dependent cell proliferation. It is now clear that the hormone receptor domain on the integrin is more complex than initially thought. That is, there is a T3-specific site in the domain, as well as a site at which both T4 and T3 may act (148). The T3-specific site activates PI3K and is linked not to cell proliferation, but to trafficking of certain intracellular proteins such as shuttling of TRα from cytoplasm to nucleus, and to the transcription of specific genes, such as hypoxia-inducible factor-1α (HIF-1α) (148). T4 is unable to activate PI3K. A deaminated derivative of T4, tetraiodothyroacetic acid (tetrac), is an antagonist at the integrin receptor domain and blocks actions of agonist hormone analogs at both the T4/T3 site and the T3-specific site. Interestingly, RGD peptides block the T3 site, but at the T4/T3 site do not inhibit cell proliferation; they selectively inhibit thyroid hormone-enhanced shuttling of TRβ1 to the nucleus from cytoplasm. The effects of these hormone analogs at the integrin receptor domain are reproduced by their reformulation into nanoparticles that prohibit access of the agents to the cell interior.
Other laboratories have in the past 5 yr clearly shown that T3 can activate the PI3K/protein kinase B/Akt signal transduction pathway (149,150,151), but have reported that the activation process begins in cytoplasm. The consequences of this action of T3 include transcription of the HIF1-α gene and activation of plasma membrane Na,K-ATPase and its insertion in the plasma membrane (see Section II.A.2).
Binding sites for thyroid hormone have also been described in synaptosomes of chick embryo brain (152). These sites in cerebral cortex have a higher affinity for T3 than for T4. Interestingly, the binding capacity of one species of binding sites declines importantly after hatching. A synaptosomal binding site for iodothyronines appears to be associated with one or more G proteins (153). The application of the term “receptor” to these moieties awaits further definition of their function(s).
2. Sites in cytoplasm at which thyroid hormone actions may be initiated
Proteins in cytoplasm that bind iodothyronines are either nuclear receptors that reside in cytoplasm, apparently transiently, or native cytoplasmic proteins. Nuclear thyroid hormone receptors at one time were seen to be functional only within the nuclear compartment, despite their identification in cytoplasm (154,155,156). However, nuclear TRβ1 detected in cytoplasm and complexed with T3 has been shown by Cao et al. (157) to interact with the p85 regulatory subunit of PI3K, leading downstream to specific gene transcription (157,158). Among the genes transcribed by this mechanism are ZAKI-4, a calcineurin-like protein, and HIF-1α (149,158). HIF-1α protein is involved in expression of a series of genes important to carbohydrate metabolism, including glucose transporter-4. TRβ1 was also shown to direct via PI3K the slowing of deactivation of KCNH2 channels in the plasma membrane of pituicytes (151). Lei et al. (159) have implicated cytoplasmic TRβ1 and PI3K in modulation of activity of Na, K-ATPase, in insertion of the sodium pump protein in the plasma membrane, and in transcription of the Na, K-ATPase gene. TRα1 may also reside in endothelial cell cytoplasm. Hiroi et al. (160) have reported that this receptor, when extranuclear, may activate endothelial cell nitric oxide synthase and is thought to contribute to vasodilatation induced by thyroid hormone. Finally, TRΔα1 is a truncated form of a nuclear receptor shown to contribute to regulation by thyroid hormone of the state of the actin cytoskeleton (161) (see Section IV).
The above effects that include nuclear thyroid hormone receptors that reside in cytoplasm—and, in certain cases, PI3K—are felt to be initiated in cytoplasm and not at the plasma membrane. It should be noted, however, that MAPK (ERK1/2) activation has been shown by Lei et al. (162) to precede the stimulation of PI3K by T3 that leads to changes in plasma membrane Na, K-ATPase activity. This raises the possibility that the cell surface integrin receptor for iodothyronines that is capable of activating both ERK1/2 and PI3K (148), as described in Section II.A.1, may be implicated in certain actions of T3 on Na, K-ATPase. It will be useful to investigate whether tetrac or RGD peptides or antibody to αvβ3 affect the cytoplasmic nuclear receptor/PI3K-based mechanisms that are involved in gene transcription and modulation of plasma membrane Na, K-ATPase activity.
Several proteins that reside largely, if not exclusively, in cytoplasm are known to bind iodothyronines and are not nuclear thyroid hormone receptors or hormone receptor-derived (163,164,165,166,167). Hashizume and co-workers (163,168) described a dimeric 76 kDa rat liver cytosol binding protein for T3 whose binding capacity for the hormone was enhanced by NADPH. Affinity of the protein for d-T3 was higher than for l-T3. A 38-kDa human kidney cytosol protein similar to that described by Hashizume et al. (163) was identified by Vie et al. (166) that bound T3 with an affinity comparable to that of nuclear receptors for the hormone. T3 binding was regulated by NADPH/NADP+. An insight into function of these cytosol thyronine-binding proteins (CTBPs) was provided when NADP was shown to transform the NADPH-activated liver cytosol protein into a form capable of transferring T3 to the nuclear compartment (164). Cytosolic pyruvate kinase monomers M1 (PKM1) and PKM2 are both capable of binding T3 (167,169). The chemistry of the interaction of hormone and kinase is interesting. T3 inhibits kinase activity of both p58 PKM1 and p58 PKM2. At least in the case of PKM1, enzyme activity is restored by the addition of fructose 1, 6-diphosphate (167).
B. Examples of nongenomic actions of thyroid hormone
1. Actions of thyroid hormone that are expressed at the plasma membrane
a. Ca2+-ATPase activity.
The existence of nongenomic actions of thyroid hormone at the plasma membrane was first demonstrated in membranes harvested from mature, i.e., enucleate, human and rat erythrocytes. In studies of such membranes conducted more than 20 yr ago by Galo et al. (135), calcium pump (Ca2+-ATPase) activity was shown to be modulated by thyroid hormone. The hormone concentrations used in these studies were near-physiological. Galo et al. (135) also showed that whether iodothyronines increased or decreased, the level of activity of the pump depended upon the lipid content of the rodent diet. That is, increased saturated fat intake permitted the stimulation of the calcium pump by T4 and T3. This inferred that the lipid microenvironment of the Ca2+-ATPase in rodent red blood cell membranes was a modulator of pump activity.
Other investigators subsequently confirmed this effect of thyroid hormone in vitro on calcium pump activity (170,171), established that the calmodulin-Ca2+ complex was essential to thyroid hormone action (170), and showed that transduction of the hormone signal into Ca2+-ATPase activity required specific kinases (172). Functional significance of the action of the hormone on Ca2+-ATPase activity was revealed when Ca2+ efflux from inside-out vesicles of human erythrocytes was shown in vitro to increase in response to physiological concentrations of thyroid hormone (173). The mechanism by which thyroid hormone rapidly activates the enzyme in vitro appears to involve activation of phospholipase C, release of inositol 3,4,5-trisphosphate, and consequent activation of protein kinase C (172).
The paradigm in these studies of isolated membranes was acute exposure of the preparations in vitro to physiological concentrations of T4 or supraphysiological levels of T3. Clinical studies revealed that red blood cells obtained from hyper- and hypothyroid patients (174) had membrane Ca2+-ATPase activities that were, respectively, increased and decreased. Another clinical report described hormone effects on Ca2+-ATPase activity and on intracellular free Ca2+ concentration in polymorphonuclear leukocytes (175). In vitro studies included in the latter report presented evidence for a direct action of the hormone on membrane calcium pump activity.
Several laboratories have explored action of thyroid hormone on Ca2+-ATPase activities in excitable cells of animals. Zinman et al. (176) recently showed that T3 and T4 acutely reversed calcium overload induced in neonatal rat cardiomyoctes. That this reflected increased pumping of Ca2+ from sarcoplasm into sarcoplasmic reticulum (SR) was shown when pharmacological inhibition of SR Ca2+-ATPase blocked reduction of sarcoplasmic [Ca2+] by thyroid hormone. Intracellular calcium overload was induced in these cells by increasing extracellular [Ca2+] or decreasing extracellular [Na+], where the latter caused reverse-mode Na+/Ca2+ exchange.
This set of observations on Ca2+-ATPase function provides in part an explanation for the inotropic action of thyroid hormone. However, T3 has also been shown to increase transcription of SR Ca2+-ATPase gene (SERCA2) (177). This genomic action of the hormone obviously contributes to inotropism and to normal diastolic relaxation (178). TRα mediates this genomic effect of the hormone (179).
Ca2+-ATPase activity of myocardiocyte sarcolemma is also subject to modulation by thyroid hormone (180). Although this calmodulin-requiring function of thyroid hormone serves to reduce cardiac sarcoplasmic [Ca2+], its quantitative contribution to regulation of sarcoplasmic [Ca2+] is small. More likely, this action is relevant to local, subsarcolemmal regulation of the Na+/Ca2+ exchanger (181).
The Ca2+-ATPase activity of adult rat cerebrocortical synaptosomes can rapidly increase upon in vitro exposure to T3 (182). Only seconds are required to obtain this effect with concentrations of T3 that are within the physiological range (Table 1), but the maximum effect is achieved at supraphysiological levels of the hormone. A correlate of the change in enzyme activity is a rise in intrasynaptosomal free ionized [Ca2+] with T3 treatment (183) and subsequent activation of synaptosomal nitric acid synthase (184). It was felt that T3 interacted directly with the endofacial aspect of the ATPase. Our knowledge of the thyroid hormone-Ca2+-ATPase relationship in other tissues implicates an intermediate transduction mechanism for the hormone signal, as noted above, but the activation of synaptosomal Ca2+-ATPase occurs sufficiently rapidly that a second and rapid mechanism for stimulation of the ATPase by the hormone may exist (184). Increases in Ca2+-ATPase activity were also obtained in vivo when T3 was administered to intact animals and synaptosomes prepared from brain.
Table 1.
Threshold concentrations of iodothyronines at which selected nongenomic actions of thyroid hormone are detected
| Action of thyroid hormone | Thyroid hormone analog | Hormone concentration | Ref. |
|---|---|---|---|
| Membrane Ca2+-ATPase activity | T4 | 10−10m | 132 |
| 2-Deoxyglucose transport | T3 | 10−9m | 191 |
| Na, K-ATPase activity | T3 | 10−9m | 159 |
| Na+ current: myocardiocytes | T4, T3 | 5 × 10−9m | 137 |
| Na+ current: sensory neurons | T4 | 3 × 10−8m | 205 |
| Na+/H+ exchanger | T3 | 10−10m | 197 |
| Cancer cell proliferation | T4 | 10−10m free | 138 |
| Angiogenesis | T4 | 10−10m free | 139 |
| TRa shuttling to nucleus | T4 | 10−10m free | 214 |
| Initiation of transcription of HIF1α gene | T3 | 10−9m | 148 |
Nuclear thyroid hormone receptor resident in cytoplasm.
Calmodulin is a cytoplasmic intracellular Ca2+-binding protein that is important to the modulation of plasma membrane Ca2+-ATPase activity and is essential to the ability of thyroid hormone to increase activity of this ATPase (170). Calmodulin is also found in synaptosomes (185). In the case of SR Ca2+-ATPase, pump activity is regulated primarily by phospholamban, a single-pass membrane protein that, unphosphorylated, inhibits calcium uptake by SR. Inhibition is reversed by dual-site phosphorylation at a specific serine and threonine (186). But, as noted above, pharmacological inhibition of calmodulin can block acute stimulation by thyroid hormone of SR Ca2+-ATPase activity in intact cardiac myocytes (176). The basis for this is the observation that calmodulin participates in the regulation of phospholamban activity via phosphorylation of phospholamban that is calmodulin kinase II-dependent at one residue (Thr-17) or is protein kinase A-mediated at Ser-16 (187,188,189). Because thyroid hormone is capable of nongenomically activating protein kinase A activity (142), as well as calmodulin-dependent action, both phosphorylation steps may be influenced by the hormone. Specific phosphorylation of another serine of phospholamban has also been implicated in the enhancement of SR Ca2+-ATPase activity by phospholamban (187). Actions of thyroid hormone on SR Ca2+-ATPase have the ability to increase the velocity of cardiac muscle relaxation and, by increasing SR [Ca2+] during diastole, enhancing contractility.
b. 2-Deoxyglucose uptake.
In a series of papers beginning in 1979, Segal and Ingbar (190,191,192) showed that thyroid hormone, specifically T3, rapidly increased the rate of 2-deoxyglucose uptake by thymocytes. The hormone concentration required was near-physiological (Table 1). The molecular basis of this action of the hormone was not fully defined, but an increase in [Ca2+]i preceded glucose uptake induced by T3 (191). There was also a component to the regulation of this hormonal effect that was contributed by cAMP (192). This raised the possibility that nongenomic transduction of the thyroid hormone signal relevant to other cell processes might involve cyclic nucleotides. This has not been shown widely to be the case.
The functional significance of the action of thyroid hormone on cellular uptake of 2-deoxyglucose is speculative because the action has not been compared quantitatively with that of insulin. However, in addition to its action on thymocytes, T3 was shown to enhance glucose uptake in heart cells, diaphragm, and fat cells (193). At what cellular site the thyroid hormone effect on glucose uptake is initiated is not known.
c. Na, K-ATPase activity.
Lei, Ingbar, Mariash, and co-workers (159,194) have shown that T3 at 10−9 m can by a nongenomic mechanism increase activity of the plasma membrane Na, K-ATPase (sodium pump) in lung alveolar cells. Transduction of the thyroid hormone signal into sodium pump activity is via both MAPK (162) and PI3K (150,162). T3 is also a part of the control process for insertion of Na, K-ATPase units into the cell membrane (159). In the euthyroid intact organism, Na, K-ATPase is tonically exposed to iodothyronines, and thus the basal activity of the pump is in part regulated by ambient levels of T3.
In contrast to these observations in intact cells, the activity of Na, K-ATPase present in synaptosomes has been shown to decrease in response to T3 and, to a lesser extent, other thyroid hormone analogs (195). It is possible that this observation and those made in pulmonary alveolar epithelial cells reflect specialized tissue functions that differentially recognize thyroid hormone and that there are different proportions of certain ion transport mechanisms in the synaptosome and in intact cells. Regulation of [Na+]i or intrasynaptosomal [Na+] is complex and subject to contributions from the Na+/Ca2+ exchanger and Na+/H+ antiporter, as well as sodium current and Na, K-ATPase (see Section II.B.3.a). It thus can be difficult to distinguish primary from secondary effects of the hormone on a given ion transport mechanism.
Gick, Ismail-Beigi, and Edelman (196) showed that transcriptional regulation of the Na, K-ATPase gene in rat liver and kidney was a function of T3 by a genomic mechanism. However, because of the substantial differences that were observed in gene transcription rates and mRNA abundance, these authors proposed that both genomic and nongenomic mechanisms were invoked by T3. That is, the nongenomic contribution might be via stabilization of mRNA (see Section II.B.3.a). By nongenomic and genomic mechanisms, then, thyroid hormone participates in the setting/maintenance of intracellular concentrations of Na+ and K+.
d. Na+/H+ antiporter.
Incerpi et al. (197) established that the sodium-proton exchanger (Na+/H+ antiporter) was subject to regulation by T3. The model tissue was rat skeletal muscle (L6 myoblasts). The rapid onset of this action supported a nongenomic mechanism for the effect of the iodothyronine, and subsequent studies by this group showed that the hormonal effect was mediated by MAPK (ERK1/2) (198). Approximately physiological concentrations of T3 were effective in this system (Table 1) (197). An implication of this set of observations is that ambient thyroid hormone, by contributing to the setpoint of the antiporter, in part defines the capacity of cells to recover from acid loads. In the L6 myoblast, for example, the presence of T3 accelerated return to normal intracellular pH after an NH4Cl load (198). This observation is likely to be of relevance to hyopoxic/ischemic stress in cardiac and striated muscle. The exchange of Na+ for protons by the antiporter serves to acidify the immediate environment of the cell and to increase [Na+]i. The latter result may be an indirect mechanism by which plasma membrane Na, K-ATPase activity is enhanced in cells exposed to thyroid hormone.
The activity of multidrug resistance (P-glycoprotein) pumps (199) in the plasma membranes of cancer cells reduces the intracellular residence times of certain cancer chemotherapeutic agents. Tetrac is a deaminated analog of T4 that inhibits binding of agonist thyroid hormone analogs, such as T4 and T3, to the cell surface receptor for thyroid hormone on integrin αvβ3 (161,200,201). Tetrac, itself, is not an agonist at the integrin receptor. It has been shown that tetrac increases the residence time of doxorubicin in doxorubicin-resistant human breast cancer (MCF-7) cells (146) in vitro and thus is capable of reversing chemoresistance in such cells. It may be postulated that, at least in part, this action of tetrac is due to its inhibition of the contribution of thyroid hormone to basal activity of the Na+/H+ exchanger and fostering of a decrease in intracellular pH (202).
The increase in [Na+]i that is the result of stimulation of Na+ current by thyroid hormone or of Na+/H+ exchange by iodothyronines may secondarily increase [Ca2+]i via stimulation of reverse-mode Na+/Ca2+ exchange (176). It was noted above that reverse-mode sodium/calcium exchange may cause intracellular calcium overload when extracellular [Na+] is reduced. Without acting directly on Na+/Ca2+ exchange, then, the hormone may influence this cellular mechanism.
e. Na+ current.
Craelius, Green, and Harris (137,203) have described a rapid onset effect of thyroid hormone analogs on slowing of the inactivation of the Na+ current. The model cell was neonatal rat myocardiocytes. A hormonal effect was apparent within 1 min of exposure of the cells to T3. Concentrations of T4 and T3 were effective in this model (Table 1) (137). The increased inward flux of Na+ that results from this hormonal action amplifies cell depolarization and may also contribute to activation of membrane Na, K-ATPase or Na+/Ca2+ exchange. This action of the hormone on the sodium current is a mechanism by which thyroid hormone in excess may increase cell excitability. In the case of the heart, this action of iodothyronines is antagonized by lidocaine (203,204) and may be postulated to contribute to abnormal cardiac rhythms.
Huang et al. (204) also carried out a structure-activity analysis of iodothyronines in this model of hormone action. T3 and T4 were equally active at 10 nm. Deaminated hormone analogs such as tetrac and triiodothyroacetic acid did not affect Na+ current, but pretreatment of cells with rT3 inhibited the effects of T4 and T3.
Recent voltage clamp study by Yonkers and Ribera (205,206) documented chronic and acute effects of T4 (10−8 m) (Table 1) on sodium current (INa) in developing sensory neurons in the zebrafish. T3 was not active in this model system. Hormonal action on INa in the neuron was inhibited by tetrac and by function-blocking antibody to integrin αvβ3, indicating that the effect of T4 was initiated at the cell surface integrin receptor for the hormone. As is the case in regulation of tumor or endothelial cell proliferation and of the state of the actin cytoskeleton discussed in Section IV, T4 may act in this neuronal sodium current paradigm as a hormone, rather than a prohormone precursor to T3.
f. Endocytosis.
Endocytosis is an inherent property of the lipid-enriched plasma membrane and is requisite to the metabolism/degradation of cell membrane components and to trafficking of specific membrane component proteins within the cell. For example, thyroid hormone promotes the endocytosis of type 2 (207) and type 3 iodothyronine 5′-monodeiodinases (208), enzyme family members that are responsible for the conversion of T4 to T3. The molecular mechanisms involved in this action of thyroid hormone are incompletely understood. However, the state of actin is a component of endocytosis (209), and regulation of the actin cytoskeleton is a role of iodothyronines (see Section IV).
g. Epidermal growth factor (EGF) receptor (EGFR) activity.
The EGFR in the plasma membrane transduces the EGF signal at the target cells of the growth factor. This receptor has been of special interest to oncology research because of the EGF dependence of proliferation that has been defined in a variety of tumor cells (210,211). Pharmacological and antibody inhibitors of EGFR tyrosine kinase activity have recently been developed as cancer chemotherapeutic agents (212,213).
It has been shown that thyroid hormone is capable of refining the function of the EGFR. For example, the presence of thyroid hormone in in vitro experiments permits the EGFR in tumor cells to distinguish between EGF and TGF-α, two natural ligands of the receptor (142). In these HeLa cell studies, the downstream consequences of TGF-α-binding and EGF-binding at the receptor are identical in the absence of thyroid hormone, but different when physiological levels of thyroid hormone are present, e.g., on c-fos expression. This action of thyroid hormone on EGFR may be relevant to the proliferative effect of the hormone on tumor cells.
2. Complex cellular actions initiated at or modulated by the integrin receptor for thyroid hormone
a. Protein trafficking.
It was mentioned above that iodothyronines can nongenomically influence the internalization of plasma membrane proteins, such as the 5′-iodothyronine monodeiodinase (207). However, these observations provide only a limited insight into the effects of the hormone on movement of specific proteins throughout the cell. Thus, Baumann et al. (154) and Zhu et al. (155) detected nuclear TRβ1 in cytoplasm and showed a decade ago that T3 caused movement of a readily detectable TR-green fluorescent protein (GFP) chimera into the nucleus. Physiological concentrations of thyroid hormone may support cytoplasm-to-nucleus shuttling of TR (214) (Table 1). The finding of the nuclear receptor in cytoplasm was somewhat surprising, given the canonical view that the receptor was exclusively contained within the nucleus. However, nascent receptor must move through the cytoplasm from endoplasmic reticulum, where it is synthesized, to the nucleus, thus providing one explanation for the presence of TR in cytoplasm. Subsequently, Moeller et al. (149) showed that TRβ1 in cytoplasm was functional and capable of binding in T3-treated cells to p85α, the regulatory subunit of the signal transducing protein, PI3K, a step premonitory to downstream transcription of genes important to glucose metabolism in cells (Fig. 4).
TRα1 in cytoplasm may also interact with the regulatory subunit of PI3K in endothelial cells (160). Translocation has also been described of cytoplasmic TRα1 to the nucleus in thyroid hormone-treated cells (148), and a truncated isoform of the TRα1 is also found in cytoplasm and mediates the action of T4 and rT3 on the actin cytoskeleton (see Section IV).
From the standpoint of a mechanism by which thyroid hormone may affect trafficking of TR, Cao et al. (214) have disclosed that this process is modulated from the plasma membrane by the thyroid hormone receptor on integrin αvβ3. T4 and T3 both promote translocation of cytoplasmic TRβ1-GFP to the cell nucleus by a mechanism that is inhibited by tetrac, by RGD peptide, and by antibody to integrin αvβ3. The thyroid hormone receptor is located at the RGD recognition site on the integrin that is critical to integrin-extracellular matrix protein interactions (140). Interestingly, exposure to thyroid hormone of normal cells engineered to contain TRβ1-GFP and fluorescently-labeled MAPK (ERK1/2) shows that complexes of cytoplasmic MAPK-TR and nuclear MAPK-TR develop rapidly (214). It has been proposed that the interaction of TR and MAPK in cytoplasm is a prerequisite to transfer of TR to the nucleus (214). An ERK1/2 docking site on TRβ1 has been identified (215), and thyroid hormone-activated ERK1/2 is known to phosphorylate TRβ1 at Ser-142 (156). Studied in the cell nucleus, this phosphorylation has functional consequences, including the recruitment of CoA proteins (Fig. 4). The observation that TRβ1 and ERK1/2 form a complex in cytoplasm raises the possibility that phosphorylation may occur outside the nucleus.
Several other proteins move between cellular compartments in response to thyroid hormone. Trip230 is an activator protein whose transport from the Golgi apparatus to the cell nucleus is facilitated by T3 (216). The translocation of signal transducer and activator of transcription-1α (STAT-1α) from cytoplasm to nucleus is also enhanced by treatment of cells with thyroid hormone (217). The STAT family is important to the transduction of a number of polypeptide or protein factor signals at the cell surface into cell responses. These factors include EGF (218) and interferon-γ (IFN-γ). In the case of IFN-γ, exposure of HeLa cells to thyroid hormone leads to activation of MAPK (ERK1/2), formation of STAT1α-MAPK nuclear complexes, specific serine phosphorylation (residue 727) of the already tyrosine-phosphorylated STAT1α, and material potentiation of the transduction of the IFN signal. That is, the tyrosine phosphorylation of STAT1α is required for signal transduction, but concomitant serine phosphorylation amplifies the transduction of signals by the protein. By this mechanism, thyroid hormone is capable of enhancing the antiviral activity of IFN-γ by up to 100-fold (217).
ERα may also translocate to the nucleus in thyroid hormone-treated breast cancer cells (144), but no other members of the nuclear hormone receptor superfamily have as yet been reported to move into the nucleus in response to cell exposure to thyroid hormone. However, MAPK-docking sites exit on several other superfamily members (145). These receptors thus are subject to serine (or threonine) phosphorylation by MAPK (ERK1/2) in thyroid hormone-treated cells.
Another nongenomic protein trafficking observation in thyroid hormone-treated cells is the transfer of the oncogene suppressor protein, p53, from cytoplasm to the nucleus (219). p53 is another protein that is subject to serine phosphorylation by iodothyronine-activated MAPK (ERK1/2). Once in the nucleus, p53 can be recovered in protein complexes that include MAPK and TR (219). Activated p53 is transcriptionally active, and it is reasonable to assume that this action of thyroid hormone on p53 is protective, that is, it contributes to the cellular defense against oncogenesis. It has not been shown, however, that this action of the hormone is a cellular defense mechanism. In fact, this p53-related observation needs to be reconciled with the fact that thyroid hormone is antiapoptotic (144) and is a proliferation factor for certain tumor cells. The latter include human breast cancer (145), glioma/glioblastoma (138,220), thyroid cancer (144), and head-and-neck cancer (H. Y. Lin, F. B. Davis, and P. J. Davis, unpublished observations). A distinction must be drawn here between oncogenesis and proliferation. There is little evidence that the hormone is oncogenic; rather, the proliferation of already-established cancer cells is promoted by T4 and T3.
MAPK (ERK1/2) is activated (tyrosine-phosphorylated) by exposure of cells to thyroid hormone, as noted above. Activated MAPK rapidly translocates to the nucleus, regardless of the activating factor. The ability to promote this translocation thus is not a specific attribute of thyroid hormone.
b. Cell migration.
Migration of neuronal and glial cells has been shown by Farwell et al. to be regulated by thyroid hormone (221). T4 and rT3 are important here, whereas T3 does not influence motility of these cells. Migration is dependent upon the presence of sufficient cytoskeletal F-actin in cells to support cell motility. Leonard and co-workers (222) have also described a role of thyroid hormone in the conversion of soluble actin into F-actin (see Section IV).
c. Platelet aggregation.
Platelets bear integrin αvβ3 and have been studied by Mousa et al. (223) to determine whether thyroid hormone, acting via this integrin, is capable of modifying platelet function. Human platelets agglutinate in response to physiological concentrations of free T4 and, as a biological indicator of agglutination, secrete ATP in response to the hormone. To support the concept that this hormonal action occurs via a cell surface receptor, the investigators reproduced with agarose-T4 the effect of unmodified T4. RGD peptide blocked this action of T4 and agarose-T4, indicating that the action of the hormone was initiated at the RGD recognition site on the heterodimeric integrin, where the thyroid hormone receptor is located. T3, however, did not cause platelet agglutination or ATP secretion, nor did diiodothyropropionic acid, another iodothyronine analog with thyroid hormone agonist properties. Where angiogenesis is desired in the setting of tissue, e.g., mammalian limb, ischemia (224), a proangiogenic thyroid hormone analog would be desirable that lacks the ability to aggregate platelets (see Section II.B.2.d).
d. Cell proliferation: angiogenesis.
Several laboratories have shown that thyroid hormone fosters new blood vessel formation. Tomanek et al. (225) studied angiogenesis in the setting of experimental myocardial infarction and found that administration of thyroid hormone in above-physiological concentrations in the rat resulted in an increase in abundance of new blood vessels, compared with untreated controls. The mechanism involved accumulation of basic fibroblast growth factor (bFGF). The desirability of new blood vessel formation in the setting of tissue damage is apparent in terms of limiting future, recurrent ischemia in the areas of tissue vulnerability. Gerdes and co-workers have shown that iodothyronines increase new blood vessel growth in brain (226), as well as myocardium (227).
Davis and co-workers have defined the proangiogenic activity of thyroid hormone in two standard experimental models, the chick chorioallantoic membrane (CAM) system (139,228) and the human dermal microvascular endothelial cell (HDMEC) microtubule assay (229). In these models, T4 at 10−10 m (Table 1) and T3 at higher concentrations are both active. These studies of the action of T4 have included propylthiouracil to prevent conversion of T4 to T3 by cellular 5′-iodothyronine monodeiodinase. The importance of this manipulation is that: 1) it supports intrinsic proangiogenic activity of T4; and 2) it opposes the impression that T4 is solely a prohormone for T3. Both the CAM and HDMEC microtubule models reveal that RGD peptide (but not the control RGE moiety), tetrac, and monoclonal antibody to integrin αvβ3 serve to block thyroid hormone-induced angiogenesis. The results indicate that the proangiogenic action of thyroid hormone is initiated at its plasma membrane receptor. The angiogenic response is dependent upon activation of MAPK (ERK1/2) by the hormone-integrin complex. In addition, exposure of the CAM to thyroid hormone results in the release about the treated cells of bFGF (FGF2) (228) that is seen, in an autocrine manner, to cause endothelial cell proliferation. Addition of bFGF antibody to the model blocks the angiogenic response to the hormone. At least in part, then, the angiogenic response to thyroid hormone is mediated by release of one or more vascular growth factors.
Tetrac inhibits iodothyronine-induced angiogenesis by blocking binding of agonist thyroid hormones to the integrin receptor. However, tetrac will block the angiogenic properties of several polypeptide or protein vascular growth factors in the absence of thyroid hormone. These factors include, as noted above, vascular endothelial growth factor and bFGF. It has also been shown that angiopoietin-2 (Ang-2) gene expression (229) is increased by thyroid hormone, but not the expression of Ang-1. The significance of the Ang-1 vs. Ang-2 observations is that Ang-2 protein destabilizes the vascular microtubules, a step premonitory to vascular endothelial growth factor action, whereas Ang-1 stabilizes microtubles.
It is assumed that the antiangiogenic activity of tetrac is expressed via the RGD recognition site on integrin αvβ3 at which the thyroid hormone receptor is located. The vascular growth factors whose actions are inhibited by tetrac contain an RGD sequence; the sequence must be appreciated by the integrin before the factors become capable of acting at their specific receptors that are anatomically close to the integrin. That is, tetrac may interfere with crosstalk between the integrin and specific vascular growth factor receptors geographically clustered on the endothelial cell surface.
e. Cell proliferation: cancer cells.
The possibility that thyroid hormone in physiological concentrations may support tumor cell proliferation is evident from several experimental studies (138,144,145) and from limited clinical evidence (220,230). In patients with advanced glioblastoma multiforme, reduction by pharmacological means of circulating thyroid hormone levels had a significant therapeutic benefit (220), and a retrospective analysis of experience at the M.D. Anderson Cancer Center with spontaneous hypothyroidism and breast cancer suggested that hypothyroidism delayed tumor appearance and decreased aggressiveness (230). On the other hand, several authors and the American Thyroid Association concluded more than two decades ago that thyroid hormone replacement therapy did not affect breast cancer clinical behavior (231).
The experimental studies that have supported the existence of a proliferative effect of thyroid hormone in vitro include those on glioma cells (138), on human breast cancer cells (145), and on human thyroid cancer cell lines (144). In all such studies thyroid hormone was tested in physiological concentrations. The fact that tetrac opposed the trophic effect of agonist thyroid hormone analogs indicated that the cell surface receptor for the hormone might mediate the proliferative effect. This conclusion was supported by additional experiments in which agarose-T4, which does not gain access to the cell interior, mimicked unmodified T4. The proliferative effect of iodothyronines has been blocked by RGD peptide and antibody to integrin αvβ3. Inhibition of the MAPK signal transduction pathway with PD 98059–which inhibits the cascade at MAPK kinase—also blocks the action of thyroid hormone on tumor cell division (144,145).
Exactly what the events downstream of MAPK (ERK1/2) are in tumor cell proliferation caused by integrin-initiated thyroid hormone action is not clear in the various types of tumor cells mentioned above. Activated MAPK translocates to the cell nucleus, as noted earlier, and it is likely that nuclear MAPK serine phosphorylates transcriptionally-active nucleoproteins, as has been shown for STAT1α and TRβ1 (156,217). In the case of human breast cancer (MCF-7) cells, it has been shown that thyroid hormone-activated MAPK phosphorylates nuclear ERα at Ser-118 (145), and this is an antecedent of hormone-induced cell division. This mimics precisely the action of estradiol on MCF-7 cells.
Another mechanism by which thyroid hormone may stimulate cancer cell proliferation is by potentiation of the action of EGF on tumor cells. EGF is widely understood to be a proliferative factor (212). As noted above, the hormone can modify the actions of EGF at its plasma membrane receptor (219).
Thyroid hormone has been shown to be antiapoptotic (144). This action of the hormone supports cancer cell proliferation in the setting of proapoptotic agents, such as the stilbene, resveratrol. Such observations suggest that circulating levels of thyroid hormone in patients enrolled in clinical trials could blunt the activity of proapoptotic drugs. This conjecture has not yet been investigated in animal models of cancer. The mechanism of the antiapoptotic effect at least in part involves inhibition of p53 activation in tumor cell nuclei (H. Y. Lin, F. B. Davis, and P. J. Davis, unpublished observations). On the other hand, tetrac is proapoptotic and blocks the antiapoptotic effects of T4 (144,146,232).
3. Nongenomic cellular actions of thyroid hormone whose site(s) of initiation are not yet known
a. Stabilization of mRNA by thyroid hormone.
It has been known for more than a decade that the genomic activity of thyroid hormone was complimented by a nongenomic effect of the hormone on the half-lives of mRNAs transcribed from thyroid hormone-responsive genes. Among the transcripts so affected by the hormone are acetylcholinesterase mRNA (233), apolipoprotein AI (234), and uncoupling protein (UCP) mRNA (235), as well as Na, K-ATPase mRNA (196), as mentioned above. The mechanism is not yet clear for stabilization of mRNAs by thyroid hormone treatment of cells, but a serine-threonine kinase pathway has been implicated in the process (233). This prolongation of mRNA half-life is in contrast to the effects of the hormone on gene products, where iodothyronines generally increase protein turnover.
b. Actions of thyroid hormone in cytoplasm.
Viewed in the context of genomic actions of thyroid hormone, the existence of the hormone in cytoplasm may be conceived as a transient phenomenon, reflecting transport from the cell surface and cytoplasm into the nucleus. However, several cytoplasmic proteins are known to bear binding sites for iodothyronines, and these were identified above in Section II.A.2, which describes putative receptor sites in cytoplasm for thyroid hormone. Pyruvate kinase monomers M1 and M2 have their enzyme activities modulated when they bind T3 (167,236,237), and a dimeric CTBP may facilitate nuclear uptake of T3 (163,164). The regulation by the intracellular ratio of NADP/NADPH of the activity of CTBP has been described (163,166). Somewhat surprising in studies of CTBP, however, was that the protein had a higher affinity for d-T3 than for l-T3 (166,168).
III. Thyroid Hormone and Mitochondria
The ability of thyroid hormone to regulate energy utilization was first recognized more than 100 yr ago and is still a contemporary topic in thyroid research (238,239). To date, we still do not understand the molecular events by which thyroid hormone controls this essential function. Energy, in the form of ATP, is the currency required for life, and the mitochondrion is its principal source. This fact has made this organelle an obvious target for thyroid hormone action. In addition to providing about 90% of the energy currency of the cell, these acquired organelles also play essential roles in cell signaling and cell survival, two other cell regulatory cascades that are possible targets for thyroid hormone action. In this section we will focus on the effects of thyroid hormone on mitochondrial energetics and mitochondriogenesis.
Several provisos should be kept in mind when evaluating the influence of thyroid hormone on metabolism. First, most studies have focused exclusively on T3—at pharmacological levels—and have ignored the other iodothyronine metabolites found within cells, specifically, T4, rT3, and 3,3′-diiodothyronine. Secondly, the conventional view that thyroid hormone action is mediated by altered gene expression has often been used to dismiss actions that very likely are mediated by direct/nongenomic actions that occur within cellular compartments other than the nucleus. Finally, the impression that the mitochondrion is a ubiquitous organelle of bacterial origin often confounds the real differences that exist between these organelles in different tissues. For example, functional analysis of liver mitochondria is very likely to differ from that of mitochondria isolated from skeletal muscle or fat because mitochondria share only about 60% of their proteomes; up to 40% is derived in a tissue-specific fashion from the cells in which they reside. Such tissue-specific specialization generates mitochondria that are unique to their tissue of origin (240,241). Too often, reductionism obscures the biology of hormone action by extending tissue-specific observations to the whole organism, especially for mitochondrial functions.
A. Mitochondrial energetics and thyroid hormone
1. ATP generation
The mitochondrion consumes metabolic fuels and generates ATP by oxidative phosphorylation. Oxidation of energy substrates at the inner mitochondrial membrane generates a proton gradient that is used by ATP synthase to phosphorylate ADP, and the newly generated ATP is then exported across the inner membrane by the ADP/ATP translocator. The coupling of fuel consumption to ATP generation is tightly controlled but is not absolute, and uncoupling of substrate oxidation to ATP generation produces heat. In large part, it is the heat generated by uncoupled oxidative phosphorylation in mitochondria that constitutes the basal metabolic rate, the physiological process influenced by thyroid hormone. Because ATP generation depends upon the proton gradient across the inner mitochondrial membrane, disturbing this gradient by “proton leak” is one biochemical mechanism that uncouples fuel oxidation from ATP generation leading to the production of heat. Proton leaks can be generated by altering proton trafficking across the inner membrane or by covalent modification of the ADP/ATP translocator (242); both factors uncouple fuel oxidation from ATP generation and lead to heat generation.
2. Heat generation
There are two components to mitochondrial heat generation: 1) basal proton leak, which accounts for up to 30% of oxygen consumption and is thought to affect whole-body energy utilization; and 2) inducible proton leaks—proton leaks mediated by dynamically regulated UCPs that belong to the superfamily of mitochondrial anion carriers (reviewed in Refs. 243 and 244). Although it is the latter family of UCPs that have received attention because of their role in brown adipose tissue, it appears that the “basal proton leak” component of mitochondrial heat generation is the component that is influenced by thyroid hormone (245). In rodents, the catecholamine and thyroid hormone-dependent expression of UCP1 is a major player for facultative thermogenesis by brown fat mitochondria, but UCP1 is unique to this tissue and its sisters; principally UCP2 and UCP3 found in skeletal muscle do not appear to serve the same heat-generating function (246,247,248). Thus, the effects of thyroid hormone on basal proton conductance in mitochondria, outside of brown fat, remain an unknown in need of further study.
B. Thyroid hormone and mitochondriogenesis
The ability of thyroid hormone to increase the number of mitochondria in a cell is well known and, like its effects on energetics, remains poorly understood. Clearly, an increase in the number of mitochondria in a cell provides the machinery required to enhance ATP generation, consume more oxygen, and generate more heat. The direct effects of thyroid hormone on mitochondriogenesis are complicated by the fact that much of the mitochondrial proteome is imported from genes located in the cell nucleus. At least two general mitochondrial transcription factors encoded by the cell genome, the cold-induced CoA of nuclear receptors, PPAR gamma coactivator-1 (PGC-1) (249), and the mitochondrial transcription factor A (250,251), appear to be thyroid hormone dependent (252). Direct actions of thyroid hormone on the mitochondrial genome have recently received a boost by the identification of validated thyroid hormone “receptors” in the mitochondrial matrix. The recent demonstration that truncated forms of the TRα1 (253,254) and TRβ1 (255) are specifically imported into the mitochondrion, show high-affinity T3 binding, and stimulate generalized transcription of the mitochondrial genome provides an attractive mechanism by which thyroid hormone can directly affect mitochondrial replication. The fact that both nuclear and mitochondrial genomes are targets for thyroid hormone activation and can ultimately lead to the propagation of mitochondria is not unsurprising and is just another example of the communication network that exists between the mitochondrion and nuclear genomes that cooperate to reproduce new, fully functional mitochondria.
C. Thyroid hormone-dependent induction of mitochondrial DNA
Work from Wrutniak and co-workers (253) and Lechleiter and colleagues (256,257) has demonstrated definitively that TRα1 lacking the A/B domain is imported into the mitochondrion and: 1) impacts global gene expression by the mitochondrial genome (253,256); 2) participates in the changes in proton gradient; and 3) enhances inositol-1,4,5-trisphosphate-mediated calcium signaling (257). This work is consistent with that of others that indicate that the A/B domain is required for nuclear targeting of the TR gene products (255). Once inside the mitochondria, TRα1 interacts with a common transcriptional machinery and initiates global increases in mitochondrial gene expression by interacting with two authentic TREs located in the D-loop, an element of the mitochondrial genome that also contains the promoters for the general mitochondrial transcription factor A. In addition to the TREs found in the D-loop, authentic TREs are also located in the mitochondrial genes encoding the 12S and 16S rRNA in mitochondria (253). Thus, the N-terminal truncated TRα1 can serve as a T3-dependent transcription factor that initiates global mitochondrial transcription.
D. Thyroid hormone-dependent nongenomic actions in mitochondria
An intriguing recent finding is that the N terminus of Xenopus xTRβA1 is nearly identical to that of the mitochondrial targeted, A/B domain-deficient TRα1 encoded by transcripts originating from an internal AUG start site in exon 3 of the mouse TRα gene. Expression of xTRβA1 in frog oocytes or in mammalian cells leads to its appearance in the mitochondria matrix where it: 1) increases the proton gradient; and 2) inhibits cytochrome c release in thyroid hormone-dependent fashion. Not surprisingly, ligand-dependent suppression of cytochrome c release mediated by xTRβA1 leads to a fall in apoptotic activity in vitro and in CV1 cells (256). The ability of the T3-liganded xTRβA1 to increase mitochondrial membrane potential is counterintuitive, especially when an elevated proton leak is thought to mediate the thyroid hormone-dependent heat generation. However, Lechleiter and colleagues (257) argue that these T3-dependent effects observed in isolated mitochondria eliminate mitigating factors present in other cellular compartments and restrict those actions of T3 to mitochondria alone, possibly through interactions with electron chain components. These findings, together with the tissue-specific diversity of mitochondrial proteome, suggest that there is much more to be learned before the role of thyroid hormone on mitochondrial function is understood.
IV. Actions of Thyroid Hormone on the Cytoskeleton, Cell Migration
The ability of T4 to influence actin polymerization and, thereby, the physical state of a key component of the cytoarchitecture of cells is another novel, nonenomic action of thyroid hormone (222,258,259). The microfilament network is one of the major structural components of the cell and is composed of a dynamic, continuously remodeled fiber network composed of filamentous actin bundles, F-actin. In hypothyroid rodents, both neurons and astrocytes have poorly developed actin cytoskeletons that cannot be restored to normal by T3 replacement. By contrast, both T4 and rT3 initiate the reappearance of filamentous actin bundles in both cell types within minutes and without any change in total actin mRNA or protein content (222,258).
A. Astrocytes
The loss of the actin cytoskeleton has significant consequences on cerebellar maturation, rendering the astrocyte incapable of depositing neuronal guidance protein(s), such as laminin, onto their cell surface. This, in turn, impairs the recognition of guidance cues by the actin-anchored, transmembrane recognition apparatus in the neuronal growth cone (259). Laminin is an astrocyte-derived extracellular matrix protein that is secreted by the cell and deposited onto its surface in specific patterns during periods of neuronal migration (260,261,262,263,264,265,266). These astrocyte-based laminin arrays are assembled by the binding of secreted laminin to receptor-bearing integrins that span the astrocyte plasma membrane and then cluster into macromolecular complexes known as focal contacts (267,268). These focal contacts comprise a functional signaling unit that transduces mechanical force through their C-terminal intracellular tail to the actin cytoskeleton and serves as the proximal sensor in a complex signaling cascade that provides contextual information about the immediate environment of the cell. Loss of the actin cytoskeleton in the thyroid hormone-deficient or T3-treated astrocyte prevents focal contact formation and prevents the cells from attaching to laminin-coated surfaces; T4 replacement fully restores focal contact assembly, cell attachment, and growth (269,270). The inability to cluster integrins also prevents the hypothyroid or T3-treated astrocyte from holding on to its newly secreted laminin (221,271,272). As is the case for cell attachment, both T4 and rT3 replacement restore the ability of astrocytes to deposit laminin arrays on their cell surface.
These in vitro findings have an in vivo counterpart in the developing rodent cerebellum where hypothyroidism leads to the loss of laminin-derived migration pathways in the molecular layer during the critical period of granule cell migration from d 8–14 of life (221,271). Interestingly, all of the thyroid hormone influence on laminin deposition observed in cultured astrocytes occurs without any changes in laminin mRNA abundance, laminin protein synthesis, or the rate of laminin secretion (221).
B. Neurons
The loss of the actin cytoskeleton in the neuronal growth cone also directly impacts neuronal migration. Normal brain development requires the developing neuron to migrate over long distances and to project axons along specific pathways toward target cells (221,273,274). Interactions between the actin cytoskeleton and integrins located in the growth cone are essential for interpretation by integrins of extracellular guidance cues (273,274,275,276,277,278). Chemical disruption of the actin cytoskeleton markedly impairs neuronal growth cone pathfinding and motility (278,279,280,281). Work done in cerebellar explants has revealed that thyroid hormone-dependent changes in the actin cytoskeleton modulate neuronal process formation (282) and that the loss of the actin cytoskeleton, due to a lack of thyroid hormone, severely impaired neurite outgrowth and markedly suppressed granule cell migration (282). These developmental defects are not corrected by T3 replacement but can be completely reversed by the addition of either T4 or rT3 to the explants (282). In vivo, a similar consequence of thyroid hormone deficits is observed in the neonatal cerebellum where hypothyroidism results in the loss of laminin-derived migration pathways in the molecular layer just during the critical period of granule cell migration (272). Thus, the available data argue that direct T4-dependent regulation of the actin cytoskeleton in both astrocytes and neurons is likely to modulate the formation and recognition of critical extracellular guidance cues—cues necessary for normal neuronal migration and neuronal process formation—and provides a potential sequence of events that could explain the developmental defects observed in the cretinous brain.
C. The role of TRΔα1 gene in T4-dependent actin polymerization
Analysis of the ability of a family of thyroid hormone analogs to promote actin polymerization revealed that the ligand-binding site of the putative effector molecule differed from that of all other iodothyronine-binding proteins (283). Based on these earlier findings, a unique affinity-labeling molecule was constructed, and a specifically labeled small polypeptide was identified on pull-down assay as the 16-kDa TRΔα1. TRΔα1 and its partner, TRΔα2, are 16- and 26-kDa polypeptides, respectively; are encoded by transcripts originating from an internal transcription start site located in intron 7 of the TRα gene; and are composed of the C-terminal portion of the LBD (284). Neither binds T3, both lack a nuclear localization signal (A/B domain), and both are found in the gut, brain, and lung of the rat (284). Although early work showed that overexpression of TRΔα1 interferes with transcription in vitro (12,284), the absence of a nuclear localization signal and the failure to identify TRΔα1 in the nucleus suggests that this effect may be due to faulty intracellular sorting, a likely artifact of overexpression studies.
Preliminary data suggest that TRΔα1 has all of the properties required of the mediator of T4- and rT3-regulated actin polymerization in the developing cerebellum. Native TRΔα1 is found in the extranuclear compartment of both astrocytes and neurons and shows the ligand affinity and specificity required for the physiological regulation of actin polymerization, i.e., it binds T4 and rT3 with high affinity while failing to bind T3. Structural analysis of the TRα1 LBD shows that the hydrophobic pocket cradling the ligand remains intact, and that Phe405 in helix 12 “locks” the thyroid hormone into the binding site by hydrophobic bonds with the 5′ iodine of T4 and rT3.
Preliminary studies done in astrocytes from two different TRα1 knockout mice (TRα1−/− and TR00) yielded interesting data linking TRΔα1 to TH-dependent regulation of actin polymerization. TR00 animals lack all TRα1-derived gene products (24,285), whereas TRα1−/− express both TRα2 and TRΔα2 (285). Even at supraphysiological levels of T4 and T3, astrocytes from both TR mutants have a poorly developed actin cytoskeleton that is identical in appearance to that in thyroid hormone-deficient cells. Gene replacement with TRΔα1 restored the ability of the actin cytoskeleton to respond to T4 and rT3 in astrocytes from both TR mutants, whereas restoring T3-dependent gene expression with the full-length TRα1 transfactor had no effect on the actin cytoskeleton. Although granule cell migration in the developing cerebellum of TR mutants was reported to be normal (286), closer examination reveals that apoptosis of the migrating granule cells, especially in the cerebellum, is markedly protracted and increased compared with the wild-type mice, illustrating that defects in cell trafficking are present. These initial data will surely spur further work to define the role of the novel TRΔα1 in the nongenomic actions of thyroid hormone and its participation in the developmental program of the brain.
Congenital hypothyroidism is the major preventable cause of mental retardation in the world today, and the molecular events by which thyroid hormone regulates neuronal migration and neuronal process projection in the brain are complex and poorly understood. With the identification of the actin cytoskeleton as a site of thyroid hormone action, we have a new handle on the cellular machinery that participates in this key developmental plan.
V. Concentrations of Thyroid Hormone at Which Molecular Actions of the Hormone Are Measured
A wide range of hormone concentrations have been used in vitro to define genomic and nongenomic actions of iodothyronines. Important genomic actions have been described at 10–100 nm levels of T3 (287,288) that are four or five orders of magnitude above total and free hormone concentrations defined in intact organisms, usually human subjects. In some cases, hormone receptor Kd values are defined in the picomolar range, but studies in the same report that are mediated by the receptor are conducted at the submicromolar level (289). Some genomic actions have been characterized at subnanomolar hormone concentrations (290,291,292).
The nongenomic actions of T4 reviewed here have largely been described at total or free concentrations (10−10 m; Table 1) that approximate physiological free levels of the hormone (10−11 m). Nongenomic effects initially demonstrated at 10−6 m T3 (154) have now been reproduced at lower concentrations, i.e., 10−7 m total and 10−10 m free T4 (214). Using hormone-stripped bovine serum for supplementation of culture medium in studies of nongenomic actions of the hormone in cultured cells, we have shown that addition of physiological 10−7 m T4 yields a directly measured total free T4 of 10−10 m (156) that approximates physiological.
Use of hormone-stripped fetal bovine serum for culture medium supplementation is desirable in cell culture studies in which thyroid hormone is to be added and its effects studied. However, we have recently pointed out that stripping of serum with charcoal or anion-exchange resin will not only remove thyroid hormone and the principal steroids, but also will materially reduce serum content of folate, vitamin B12, magnesium, phosphate, and potassium (293). Thus, this useful product introduces several variables that are not routinely taken into consideration in the design of cell culture experiments.
In interpreting studies carried out in the artificial construct of the culture medium and cultured cells, we have emphasized those that rely on hormone concentrations in the nanomolar range. When the in vitro studies have been extended to the intact organism, we have included the results, although dosing in the intact animal may be subject to criticisms similar to those applied to in vitro experiments.
A. Deiodinases
Extrathyroidal conversion of T4 to T3 by deiodinases in various tissues precedes many actions of thyroid hormone. Discussion of deiodinase activities is beyond the scope of the current review and is recently and extensively presented elsewhere (294,295,296).
B. Thyroid hormone transporters
This review is focused on molecular actions of thyroid hormone. There is substantial information now available about plasma membrane transporters that import the hormone. The description and function of the transporters in anticipation of genomic, mitochondrial and certain cytoplasmic actions of iodothyronines is beyond the scope of the present review. Visser and colleagues (297,298) have recently reviewed the topic of transporters.
Acknowledgments
The authors regret omissions of references dictated by manuscript length limitations. We appreciate the contributions from colleagues and collaborators who participated in the studies conducted by the authors that are described in this review. P.J.D. acknowledges the generous research laboratory endowment established at Ordway by M. Frank and Margaret C. Rudy and the support from the Beltrone Foundation, Candace K. Weir, and Charitable Leadership Foundation Medical Technology Acceleration Program. The authors are grateful for discussions of the manuscript with colleagues, including Faith B. Davis, Alan P. Farwell, Deborah M. Leonard, and H.-Y. Lin. Margaret Wood expertly assisted in the preparation of the manuscript.
Footnotes
The research from S.-Y.C.’s laboratory was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (NIH). Research from J.L.L.’s laboratory was supported in part by NIH.
Disclosure Summary: S.-Y.C. and P.J.D. have nothing to disclose. J.L.L. has received honoraria from The Ordway Institute and has patents with the University of Massachusetts.
First Published Online January 5, 2010
Abbreviations: Ang, Angiopoietin; bFGF, basic fibroblast growth factor; bHLH, basic helix loop helix; CAM, chorioallantoic membrane; CoA, coactivator; CoR, corepressor; CTBP, cytosol thyronine-binding protein; DR, direct repeat; DRIP, vitamin D receptor interacting protein; EGF, epidermal growth factor; EGFR, EGF receptor; GFP, green fluorescent protein; HDAC, histone deacetylase; HIF-1α, hypoxia-inducible factor-1α; IFN, interferon; LBD, ligand-binding domain; LXR, liver X receptor; NCoR, nuclear receptor CoR; PA28γ, proteasome activator 28γ; PI3K, phosphatidylinositol 3-kinase; PKM, pyruvate kinase monomer; PPAR, peroxisome proliferator-activated receptor; PTTG, pituitary tumor-transforming gene; RGD, Arg-Gly-Asp; RTH, resistance to thyroid hormone; RXR, retinoid X receptor; SMRT, silencing mediator of retinoid and thyroid hormone receptor; SR, sarcoplasmic reticulum; SRC, steroid hormone receptor CoA; tetrac, tetraiodothyroacetic acid; TR, thyroid hormone receptor; TRAP, TR-associated protein(s); TRE, thyroid hormone response element; Trip, TR-interacting protein; TSH-omas, TSH-secreting pituitary adenomas; UCP, uncoupling protein.
References
- Tata JR, Widnell CC 1966 Ribonucleic acid synthesis during the early action of thyroid hormones. Biochem J 98:604–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer JH, Schwartz HL, Surks MI 1974 Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology 95:897–903 [DOI] [PubMed] [Google Scholar]
- Samuels HH, Tsai JS, Casanova J 1974 Thyroid hormone action: in vitro demonstration of putative receptors in isolated nuclei and soluble nuclear extracts. Science 184:1188–1191 [DOI] [PubMed] [Google Scholar]
- Latham KR, Ring JC, Baxter JD 1976 Solubilized nuclear “receptors” for thyroid hormones. Physical characteristics and binding properties, evidence for multiple forms. J Biol Chem 251:7388–7397 [PubMed] [Google Scholar]
- Apriletti JW, Eberhardt NL, Latham KR, Baxter JD 1981 Affinity chromatography of thyroid hormone receptors. Biospecific elution from support matrices, characterization of the partially purified receptor. J Biol Chem 256:12094–12101 [PubMed] [Google Scholar]
- Apriletti JW, Baxter JD, Lavin TN 1988 Large scale purification of the nuclear thyroid hormone receptor from rat liver and sequence-specific binding of the receptor to DNA. J Biol Chem 263:9409–9417 [PubMed] [Google Scholar]
- Sap J, Muñoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennström B 1986 The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature 324:635–640 [DOI] [PubMed] [Google Scholar]
- Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM 1986 The c-erb-A gene encodes a thyroid hormone receptor. Nature 324:641–646 [DOI] [PubMed] [Google Scholar]
- Cheng SY 2000 Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev Endocr Metab Disord 1:9–18 [DOI] [PubMed] [Google Scholar]
- Williams GR 2000 Cloning and characterization of two novel thyroid hormone receptor β isoforms. Mol Cell Biol 20:8329–8342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi T, Tennyson GE, Nikodem VM 1988 Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc Natl Acad Sci USA 85:5804–5808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateroti M, Gauthier K, Domon-Dell C, Freund JN, Samarut J, Chassande O 2001 Functional interference between thyroid hormone receptor α (TRα) and natural truncated TRΔα isoforms in the control of intestine development. Mol Cell Biol 21:4761–4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM 1988 The steroid and thyroid hormone receptor superfamily. Science 240:889–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro RC, Kushner PJ, Baxter JD 1995 The nuclear hormone receptor gene superfamily. Annu Rev Med 46:443–453 [DOI] [PubMed] [Google Scholar]
- Lonard DM, O'malley BW 2007 Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27:691–700 [DOI] [PubMed] [Google Scholar]
- Wagner RL, Apriletti JW, McGrath ME, West BL, Baxter JD, Fletterick RJ 1995 A structural role for hormone in the thyroid hormone receptor. Nature 378:690–697 [DOI] [PubMed] [Google Scholar]
- Feng W, Ribeiro RC, Wagner RL, Nguyen H, Apriletti JW, Fletterick RJ, Baxter JD, Kushner PJ, West BL 1998 Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science 280:1747–1749 [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Towle HC, Young WS 3rd 1992 Spatial and temporal expression of α- and β-thyroid hormone receptor mRNAs, including the β2-subtype, in the developing mammalian nervous system. J Neurosci 12:2288–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Suzuki H, Zhao L, Willingham MC, Meltzer P, Cheng SY 2003 Mutant thyroid hormone receptor β represses the expression and transcriptional activity of peroxisome proliferator-activated receptor γ during thyroid carcinogenesis. Cancer Res 63:5274–5280 [PubMed] [Google Scholar]
- Jones I, Ng L, Liu H, Forrest D 2007 An intron control region differentially regulates expression of thyroid hormone receptor β2 in the cochlea, pituitary, and cone photoreceptors. Mol Endocrinol 21:1108–1119 [DOI] [PubMed] [Google Scholar]
- Forrest D, Erway LC, Ng L, Altschuler R, Curran T 1996 Thyroid hormone receptor β is essential for development of auditory function. Nature Genet 13:354–357 [DOI] [PubMed] [Google Scholar]
- Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, Wehner JM, Curran T 1996 Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor β: evidence for tissue-specific modulation of receptor function. EMBO J 15:3006–3015 [PMC free article] [PubMed] [Google Scholar]
- Wikström L, Johansson C, Saltó C, Barlow C, Campos Barros A, Baas F, Forrest D, Thorén P, Vennström B 1998 Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor α1. EMBO J 17:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraichard A, Chassande O, Plateroti M, Roux JP, Trouillas J, Dehay C, Legrand C, Gauthier K, Kedinger M, Malaval L, Rousset B, Samarut J 1997 The T3R α gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J 16:4412–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göthe S, Wang Z, Ng L, Kindblom JM, Barros AC, Ohlsson C, Vennström B, Forrest D 1999 Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev 13:1329–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen PM, Feng X, Flamant F, Chen Y, Walker RL, Weiss RE, Chassande O, Samarut J, Refetoff S, Meltzer PS 2003 Effects of ligand and thyroid hormone receptor isoforms on hepatic gene expression profiles of thyroid hormone receptor knockout mice. EMBO Rep 4:581–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Morales A, Gullberg H, Fernandez L, Ståhlberg N, Lee NH, Vennström B, Norstedt G 2002 Patterns of liver gene expression governed by TRβ. Mol Endocrinol 16:1257–1268 [DOI] [PubMed] [Google Scholar]
- Zandieh Doulabi B, Platvoet-Ter Schiphorst M, Kalsbeek A, Fliers E, Bakker O, Wiersinga WM 2004 Biurnal variation in rat liver thyroid hormone receptor (TR)-α messenger ribonucleic acid (mRNA) is dependent on the biological clock in the suprachiasmatic nucleus, whereas diurnal variation of TRβ1 mRNA is modified by food intake. Endocrinology 145:1284–1289 [DOI] [PubMed] [Google Scholar]
- Yen PM 2001 Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- Williams GR, Harney JW, Forman BM, Samuels HH, Brent GA 1991 Oligomeric binding of T3 receptor is required for maximal T3 response. J Biol Chem 266:19636–19644 [PubMed] [Google Scholar]
- Zhang XK, Kahl M 1993 Regulation of retinoid and thyroid hormone action through homodimeric and heterodimeric receptors. TEM 4:156–162 [DOI] [PubMed] [Google Scholar]
- Araki O, Ying H, Furuya F, Zhu X, Cheng SY 2005 Thyroid hormone receptor β mutants: dominant negative regulators of peroxisome proliferator-activated receptor γ action. Proc Natl Acad Sci USA 102:16251–16256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MK, Connolly TM 2008 Nuclear receptors as drug targets in obesity, dyslipidemia and atherosclerosis. Curr Opin Investig Drugs 9:247–255 [PubMed] [Google Scholar]
- Hart CM 2008 The role of PPARγ in pulmonary vascular disease. J Investig Med 56:518–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemenoff RA, Weiser-Evans M, Winn RA 2008 Activation and molecular targets of peroxisome proliferator-activated receptor-γ ligands in lung cancer. PPAR Res 2008:156875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Cohen RN, Yamada M, Markan KR, Monden T, Satoh T, Mori M, Wondisford FE 2006 Cross-talk between thyroid hormone receptor and liver X receptor regulatory pathways is revealed in a thyroid hormone resistance mouse model. J Biol Chem 281:295–302 [DOI] [PubMed] [Google Scholar]
- Lee JW, Ryan F, Swaffield JC, Johnston SA, Moore DD 1995 Interaction of thyroid-hormone receptor with a conserved transcriptional mediator. Nature 374:91–94 [DOI] [PubMed] [Google Scholar]
- Oñate SA, Tsai SY, Tsai MJ, O'Malley BW 1995 Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW 1999 Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW 2002 Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474 [DOI] [PubMed] [Google Scholar]
- Leo C, Chen JD 2000 The SRC family of nuclear receptor coactivators. Gene 245:1–11 [DOI] [PubMed] [Google Scholar]
- McInerney EM, Rose DW, Flynn SE, Westin S, Mullen TM, Krones A, Inostroza J, Torchia J, Nolte RT, Assa-Munt N, Milburn MV, Glass CK, Rosenfeld MG 1998 Determinants of coactivator LXXLL motif specificity in nuclear receptor transcriptional activation. Genes Dev 12:3357–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson O, Glass CK, Rosenfeld MG 2002 Nuclear receptor coregulators: multiple modes of modification. Trends Endocrinol Metab 13:55–60 [DOI] [PubMed] [Google Scholar]
- Xu J, Li Q 2003 Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol 17:1681–1692 [DOI] [PubMed] [Google Scholar]
- Darimont BD, Wagner RL, Apriletti JW, Stallcup MR, Kushner PJ, Baxter JD, Fletterick RJ, Yamamoto KR 1998 Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev 12:3343–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondell JD, Brunel F, Hisatake K, Roeder RG 1996 Unliganded thyroid hormone receptor α can target TATA-binding protein for transcriptional repression. Mol Cell Biol 16:281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondell JD, Ge H, Roeder RG 1996 Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc Natl Acad Sci USA 93:8329–8333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CX, Ito M, Fondell JD, Fu ZY, Roeder RG 1998 The TRAP220 component of a thyroid hormone receptor-associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc Natl Acad Sci USA [Erratum (1998) 95:14584] 95:7939–7944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachez C, Suldan Z, Ward J, Chang CP, Burakov D, Erdjument-Bromage H, Tempst P, Freedman LP 1998 A novel protein complex that interacts with the vitamin D3 receptor in a ligand-dependent manner and enhances VDR transactivation in a cell-free system. Genes Dev 12:1787–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG 2000 The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14:121–141 [PubMed] [Google Scholar]
- Hörlein AJ, Näär AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass CK 1995 Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377:397–404 [DOI] [PubMed] [Google Scholar]
- Chen JD, Evans RM 1995 A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454–457 [DOI] [PubMed] [Google Scholar]
- Potter GB, Beaudoin 3rd GM, DeRenzo CL, Zarach JM, Chen SH, Thompson CC 2001 The hairless gene mutated in congenital hair loss disorders encodes a novel nuclear receptor corepressor. Genes Dev 15:2687–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel U, Thormeyer D, Altincicek B, Paululat A, Eggert M, Schneider S, Tenbaum SP, Renkawitz R, Baniahmad A 1999 Alien, a highly conserved protein with characteristics of a corepressor for members of the nuclear hormone receptor superfamily. Mol Cell Biol 19:3383–3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillès V, Dauvois S, L'Horset F, Lopez G, Hoare S, Kushner PJ, Parker MG 1995 Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J 14:3741–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LN, Hu X 2004 Receptor interacting protein 140 as a thyroid hormone-dependent, negative co-regulator for the induction of cellular retinoic acid binding protein I gene. Mol Cell Endocrinol 218:39–48 [DOI] [PubMed] [Google Scholar]
- Zamir I, Dawson J, Lavinsky RM, Glass CK, Rosenfeld MG, Lazar MA 1997 Cloning and characterization of a corepressor and potential component of the nuclear hormone receptor repression complex. Proc Natl Acad Sci USA 94:14400–14405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Lazar MA 1999 The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402:93–96 [DOI] [PubMed] [Google Scholar]
- Perissi V, Staszewski LM, McInerney EM, Kurokawa R, Krones A, Rose DW, Lambert MH, Milburn MV, Glass CK, Rosenfeld MG 1999 Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev 13:3198–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HG, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J 2003 Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J 22:1336–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Barak O, Lazar MA 2001 The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol 21:6091–6101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar MA 2003 Nuclear receptor corepressors. Nucl Recept Signal 1:e001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen K, Hermanson O, Onami TM, Gleiberman AS, Lunyak V, McEvilly RJ, Kurokawa R, Kumar V, Liu F, Seto E, Hedrick SM, Mandel G, Glass CK, Rose DW, Rosenfeld MG 2000 Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell 102:753–763 [DOI] [PubMed] [Google Scholar]
- He LZ, Guidez F, Tribioli C, Peruzzi D, Ruthardt M, Zelent A, Pandolfi PP 1998 Distinct interactions of PML-RARα and PLZF-RARα with co-repressors determine differential responses to RA in APL. Nat Genet 18:126–135 [DOI] [PubMed] [Google Scholar]
- Lutterbach B, Westendorf JJ, Linggi B, Patten A, Moniwa M, Davie JR, Huynh KD, Bardwell VJ, Lavinsky RM, Rosenfeld MG, Glass C, Seto E, Hiebert SW 1998 ETO, a target of t(8;21) in acute leukemia, interacts with the N-CoR and mSin3 corepressors. Mol Cell Biol 18:7176–7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoh SM, Chatterjee VK, Privalsky ML 1997 Thyroid hormone resistance syndrome manifests as an aberrant interaction between mutant T3 receptors and transcriptional corepressors. Mol Endocrinol 11:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya F, Guigon CJ, Zhao L, Lu C, Hanover JA, Cheng SY 2007 Nuclear receptor corepressor is a novel regulator of phosphatidylinositol 3-kinase signaling. Mol Cell Biol 27:6116–6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ 2002 D1 in G2. Cell Cycle 1:36–38 [PubMed] [Google Scholar]
- Lin HM, Zhao L, Cheng SY 2002 Cyclin D1 is a ligand-independent co-repressor for thyroid hormone receptors. J Biol Chem 277:28733–28741 [DOI] [PubMed] [Google Scholar]
- Coqueret O 2002 Linking cyclins to transcriptional control. Gene 299:35–55 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Park KS, Kaneshige M, Bhat MK, Zhu Q, Mariash CN, McPhie P, Cheng SY 2000 The orphan nuclear receptor Ear-2 is a negative coregulator for thyroid hormone nuclear receptor function. Mol Cell Biol 20:2604–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A 2008 Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 9:402–412 [DOI] [PubMed] [Google Scholar]
- Yap N, Yu CL, Cheng SY 1996 Modulation of the transcriptional activity of thyroid hormone receptors by the tumor suppressor p53. Proc Natl Acad Sci USA 93:4273–4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera-Hernandez G, Zhan Q, Wong R, Cheng SY 1998 Thyroid hormone receptor is a negative regulator in p53-mediated signaling pathways. DNA Cell Biol 17:743–750 [DOI] [PubMed] [Google Scholar]
- Qi JS, Desai-Yajnik V, Yuan Y, Samuels HH 1997 Constitutive activation of gene expression by thyroid hormone receptor results from reversal of p53-mediated repression. Mol Cell Biol 17:7195–7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat MK, Yu C, Yap N, Zhan Q, Hayashi Y, Seth P, Cheng S 1997 Tumor suppressor p53 is a negative regulator in thyroid hormone receptor signaling pathways. J Biol Chem 272:28989–28993 [DOI] [PubMed] [Google Scholar]
- McGough AM, Staiger CJ, Min JK, Simonetti KD 2003 The gelsolin family of actin regulatory proteins: modular structures, versatile functions. FEBS Lett 552:75–81 [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ 1999 Functions of gelsolin: motility, signaling, apoptosis, cancer. Curr Opin Cell Biol 11:103–108 [DOI] [PubMed] [Google Scholar]
- De Corte V, Bruyneel E, Boucherie C, Mareel M, Vandekerckhove J, Gettemans J 2002 Gelsolin-induced epithelial cell invasion is dependent on Ras-Rac signaling. EMBO J 21:6781–6790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Furuya F, Ying H, Kato Y, Hanover JA, Cheng SY 2007 Gelsolin: a novel thyroid hormone receptor-β interacting protein that modulates tumor progression in a mouse model of follicular thyroid cancer. Endocrinology 148:1306–1312 [DOI] [PubMed] [Google Scholar]
- Yu R, Melmed S 2001 Oncogene activation in pituitary tumors. Brain Pathol 11:328–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Melmed S 1997 Isolation and characterization of a pituitary tumor-transforming gene (PTTG). Mol Endocrinol 11:433–441 [DOI] [PubMed] [Google Scholar]
- Heaney AP, Nelson V, Fernando M, Horwitz G 2001 Transforming events in thyroid tumorigenesis and their association with follicular lesions. J Clin Endocrinol Metab 86:5025–5032 [DOI] [PubMed] [Google Scholar]
- Kim DS, McCabe CJ, Buchanan MA, Watkinson JC 2003 Oncogenes in thyroid cancer. Clin Otolaryngol Allied Sci 28:386–395 [DOI] [PubMed] [Google Scholar]
- Heaney AP, Singson R, McCabe CJ, Nelson V, Nakashima M, Melmed S 2000 Expression of pituitary-tumour transforming gene in colorectal tumours. Lancet 355:716–719 [DOI] [PubMed] [Google Scholar]
- Zhang X, Horwitz GA, Heaney AP, Nakashima M, Prezant TR, Bronstein MD, Melmed S 1999 Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocrinol Metab 84:761–767 [DOI] [PubMed] [Google Scholar]
- Domínguez A, Ramos-Morales F, Romero F, Rios RM, Dreyfus F, Tortolero M, Pintor-Toro JA 1998 hPTTG, a human homologue of rat pttg, is overexpressed in hematopoietic neoplasms. Evidence for a transcriptional activation function of hPTTG. Oncogene 17:2187–2193 [DOI] [PubMed] [Google Scholar]
- Yu R, Lu W, Chen J, McCabe CJ, Melmed S 2003 Overexpressed pituitary tumor-transforming gene causes aneuploidy in live human cells. Endocrinology 144:4991–4998 [DOI] [PubMed] [Google Scholar]
- Kim D, Pemberton H, Stratford AL, Buelaert K, Watkinson JC, Lopes V, Franklyn JA, McCabe CJ 2005 Pituitary tumour transforming gene (PTTG) induces genetic instability in thyroid cells. Oncogene 24:4861–4866 [DOI] [PubMed] [Google Scholar]
- Dace A, Zhao L, Park KS, Furuno T, Takamura N, Nakanishi M, West BL, Hanover JA, Cheng S 2000 Hormone binding induces rapid proteasome-mediated degradation of thyroid hormone receptors. Proc Natl Acad Sci USA 97:8985–8990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Furuya F, Zhao L, Araki O, West BL, Hanover JA, Willingham MC, Cheng SY 2006 Aberrant accumulation of PTTG1 induced by a mutated thyroid hormone β receptor inhibits mitotic progression. J Clin Invest 116:2972–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lonard DM, Jung SY, Malovannaya A, Feng Q, Qin J, Tsai SY, Tsai MJ, O'Malley BW 2006 The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGγ proteasome. Cell 124:381–392 [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM 2001 Adhesion signaling: how β-catenin interacts with its partners. Curr Biol 11:R792–R794 [DOI] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N 2002 The promise and perils of Wnt signaling through β-catenin. Science 296:1644–1646 [DOI] [PubMed] [Google Scholar]
- Guigon CJ, Zhao L, Lu C, Willingham MC, Cheng SY 2008 Regulation of β-catenin by a novel nongenomic action of thyroid hormone β receptor. Mol Cell Biol 28:4598–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usala SJ, Bale AE, Gesundheit N, Weinberger C, Lash RW, Wondisford FE, McBride OW, Weintraub BD 1988 Tight linkage between the syndrome of generalized thyroid hormone resistance and the human c-erbA β gene. Mol Endocrinol 2:1217–1220 [DOI] [PubMed] [Google Scholar]
- Sakurai A, Takeda K, Ain K, Ceccarelli P, Nakai A, Seino S, Bell GI, Refetoff S, DeGroot LJ 1989 Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor β. Proc Natl Acad Sci USA 86:8977–8981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refetoff S, Dumitrescu AM 2007 Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab 21:277–305 [DOI] [PubMed] [Google Scholar]
- Cheng SY 2005 Thyroid hormone receptor mutations and disease: beyond thyroid hormone resistance. Trends Endocrinol Metab 16:176–182 [DOI] [PubMed] [Google Scholar]
- Weiss RE, Refetoff S 2000 Resistance to thyroid hormone. Rev Endocr Metab Disord 1:97–108 [DOI] [PubMed] [Google Scholar]
- Yen PM 2003 Molecular basis of resistance to thyroid hormone. Trends Endocrinol Metab 14:327–333 [DOI] [PubMed] [Google Scholar]
- Ono S, Schwartz ID, Mueller OT, Root AW, Usala SJ, Bercu BB 1991 Homozygosity for a dominant negative thyroid hormone receptor gene responsible for generalized resistance to thyroid hormone. J Clin Endocrinol Metab 73:990–994 [DOI] [PubMed] [Google Scholar]
- Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA, Kazlauskaite R, Pankratz DG, Wynshaw-Boris A, Refetoff S, Weintraub B, Willingham MC, Barlow C, Cheng S 2000 Mice with a targeted mutation in the thyroid hormone β receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci USA 97:13209–13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Curty FH, Borges PP, Lee CE, Abel ED, Elmquist JK, Cohen RN, Wondisford FE 2001 An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc Natl Acad Sci USA 98:3998–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y, Zhang XY, Ying H, Kato Y, Willingham MC, Xu J, O'Malley BW, Cheng SY 2003 Modulation by steroid receptor coactivator-1 of target-tissue responsiveness in resistance to thyroid hormone. Endocrinology 144:4144–4153 [DOI] [PubMed] [Google Scholar]
- Siesser WB, Cheng SY, McDonald MP 2005 Hyperactivity, impaired learning on a vigilance task, and a differential response to methylphenidate in the TRβPV knock-in mouse. Psychopharmacology (Berl) 181:653–663 [DOI] [PubMed] [Google Scholar]
- Griffith AJ, Szymko YM, Kaneshige M, Quiñónez RE, Kaneshige K, Heintz KA, Mastroianni MA, Kelley MW, Cheng SY 2002 Knock-in mouse model for resistance to thyroid hormone (RTH): an RTH mutation in the thyroid hormone receptor β gene disrupts cochlear morphogenesis. J Assoc Res Otolaryngol 3:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea PJ, Harvey CB, Suzuki H, Kaneshige M, Kaneshige K, Cheng SY, Williams GR 2003 A thyrotoxic skeletal phenotype of advanced bone formation in mice with resistance to thyroid hormone. Mol Endocrinol 17:1410–1424 [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kaneshige M, Kamiya Y, Kaneshige K, McPhie P, Cheng SY 2002 Differential expression of thyroid hormone receptor isoforms dictates the dominant negative activity of mutant β receptor. Mol Endocrinol 16:2077–2092 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Cheng SY 2003 Compensatory role of thyroid hormone receptor (TR) α1 in resistance to thyroid hormone: study in mice with a targeted mutation in the TR β gene and deficient in TRα1. Mol Endocrinol 17:1647–1655 [DOI] [PubMed] [Google Scholar]
- Kamiya Y, Puzianowska-Kuznicka M, McPhie P, Nauman J, Cheng SY, Nauman A 2002 Expression of mutant thyroid hormone nuclear receptors is associated with human renal clear cell carcinoma. Carcinogenesis 23:25–33 [DOI] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka M, Krystyniak A, Madej A, Cheng SY, Nauman J 2002 Functionally impaired TR mutants are present in thyroid papillary cancer. J Clin Endocrinol Metab 87:1120–1128 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Willingham MC, Cheng SY 2002 Mice with a mutation in the thyroid hormone receptor β gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid 12:963–969 [DOI] [PubMed] [Google Scholar]
- Ying H, Suzuki H, Furumoto H, Walker R, Meltzer P, Willingham MC, Cheng SY 2003 Alterations in genomic profiles during tumor progression in a mouse model of follicular thyroid carcinoma. Carcinogenesis 24:1467–1479 [DOI] [PubMed] [Google Scholar]
- Kroll TG, Sarraf P, Pecciarini L, Chen CJ, Mueller E, Spiegelman BM, Fletcher JA 2000 PAX8-PPAR γ1 fusion oncogene in human thyroid carcinoma. Science 289:1357–1360 [DOI] [PubMed] [Google Scholar]
- Gustafson KS, LiVolsi VA, Furth EE, Pasha TL, Putt ME, Baloch ZW 2003 Peroxisome proliferator-activated receptor γ expression in follicular-patterned thyroid lesions. Caveats for the use of immunohistochemical studies. Am J Clin Pathol 120:175–181 [DOI] [PubMed] [Google Scholar]
- Galusca B, Dumollard JM, Chambonniere ML, Germain N, Prades JM, Péoc'h M, Estour B 2004 Peroxisome proliferator activated receptor γ immunohistochemical expression in human papillary thyroid carcinoma tissues. Possible relationship to lymph node metastasis. Anticancer Res 24:1993–1997 [PubMed] [Google Scholar]
- Marques AR, Espadinha C, Frias MJ, Roque L, Catarino AL, Sobrinho LG, Leite V 2004 Underexpression of peroxisome proliferator-activated receptor (PPAR)γ in PAX8/ PPARγ-negative thyroid tumours. Br J Cancer 91:732–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Ying H, Zhao L, Furuya F, Araki O, Willingham MC, Cheng SY 2006 PPARγ insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-κB signaling pathway. Oncogene 25:2736–2747 [DOI] [PubMed] [Google Scholar]
- Furuya F, Hanover JA, Cheng SY 2006 Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone β receptor. Proc Natl Acad Sci USA 103:1780–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumoto H, Ying H, Chandramouli GV, Zhao L, Walker RL, Meltzer PS, Willingham MC, Cheng SY 2005 An unliganded thyroid hormone β receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol Cell Biol 25:124–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando S, Sarlis NJ, Krishnan J, Feng X, Refetoff S, Zhang MQ, Oldfield EH, Yen PM 2001 Aberrant alternative splicing of thyroid hormone receptor in a TSH-secreting pituitary tumor is a mechanism for hormone resistance. Mol Endocrinol 15:1529–1538 [DOI] [PubMed] [Google Scholar]
- Ando S, Sarlis NJ, Oldfield EH, Yen PM 2001 Somatic mutation of TRβ can cause a defect in negative regulation of TSH in a TSH-secreting pituitary tumor. J Clin Endocrinol Metab 86:5572–5576 [DOI] [PubMed] [Google Scholar]
- Kaneshige M, Suzuki H, Kaneshige K, Cheng J, Wimbrow H, Barlow C, Willingham MC, Cheng S 2001 A targeted dominant negative mutation of the thyroid hormone α1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc Natl Acad Sci USA 98:15095–15100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinnikov A, Nordström K, Thorén P, Kindblom JM, Malin S, Rozell B, Adams M, Rajanayagam O, Pettersson S, Ohlsson C, Chatterjee K, Vennström B 2002 Retardation of post-natal development caused by a negatively acting thyroid hormone receptor α1. EMBO J 21:5079–5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Schultz JJ, Brent GA 2003 A thyroid hormone receptor α gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J Biol Chem 278:38913–38920 [DOI] [PubMed] [Google Scholar]
- Ying H, Araki O, Furuya F, Kato Y, Cheng SY 2007 Impaired adipogenesis caused by a mutated thyroid hormone α1 receptor. Mol Cell Biol 27:2359–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren M, Alkemade A, Mittag J, Nordström K, Katz A, Rozell B, Westerblad H, Arner A, Vennström B 2007 Hypermetabolism in mice caused by the central action of an unliganded thyroid hormone receptor α1. EMBO J 26:4535–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami T, Jameson JL 1998 Nuclear corepressors enhance the dominant negative activity of mutant receptors that cause resistance to thyroid hormone. Endocrinology 139:640–650 [DOI] [PubMed] [Google Scholar]
- Marimuthu A, Feng W, Tagami T, Nguyen H, Jameson JL, Fletterick RJ, Baxter JD, West BL 2002 TR surfaces and conformations required to bind nuclear receptor corepressor. Mol Endocrinol 16:271–286 [DOI] [PubMed] [Google Scholar]
- Angel RC, Botta JA, Farias RN 1989 High affinity L-triiodothyronine binding to right-side-out and inside-out vesicles from rat and human erythrocyte membranes. J Biol Chem 264:19143–19146 [PubMed] [Google Scholar]
- Davis FB, Cody V, Davis PJ, Borzynski LJ, Blas SD 1983 Stimulation by thyroid hormone analogues of red blood cell Ca2+-ATPase activity in vitro. Correlations between hormone structure and biological activity in a human cell system. J Biol Chem 258:12373–12377 [PubMed] [Google Scholar]
- Kostrouch Z, Felt V, Raska I, Nedvídková J, Holecková E 1987 Binding of [125I]triiodothyronine to human peripheral leukocytes and its internalization. Experientia 43:1117–1118 [DOI] [PubMed] [Google Scholar]
- Blondeau JP 1986 Saturable binding of thyroid hormone to isolated rat hepatocytes. FEBS Lett 204:41–46 [DOI] [PubMed] [Google Scholar]
- Galo MG, Uñates LE, Farías RN 1981 Effect of membrane fatty acid composition on the action of thyroid hormone on (Ca2+ + Mg2+)-adenosine triphosphatase from rat erythrocyte. J Biol Chem 256:7113–7114 [PubMed] [Google Scholar]
- Segal J, Ingbar SH 1979 Stimulation by triiodothyronine of the in vitro uptake of sugars by rat thymocytes. J Clin Invest 63:507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craelius W, Green WL, Harris DR 1990 Acute effects of thyroid hormone on sodium currents in neonatal myocytes. Biosci Rep 10:309–315 [DOI] [PubMed] [Google Scholar]
- Davis FB, Tang HY, Shih A, Keating T, Lansing L, Hercbergs A, Fenstermaker RA, Mousa A, Mousa SA, Davis PJ, Lin HY 2006 Acting via a cell surface receptor, thyroid hormone is a growth factor for glioma cells. Cancer Res 66:7270–7275 [DOI] [PubMed] [Google Scholar]
- Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB, Mousa S, Davis PJ 2005 Integrin αvβ3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146:2864–2871 [DOI] [PubMed] [Google Scholar]
- Plow EF, Haas TA, Zhang L, Loftus J, Smith JW 2000 Ligand binding to integrins. J Biol Chem 275:21785–21788 [DOI] [PubMed] [Google Scholar]
- Tsou R, Isik FF 2001 Integrin activation is required for VEGF and FGF receptor protein presence on human microvascular endothelial cells. Mol Cell Biochem 224:81–89 [DOI] [PubMed] [Google Scholar]
- Shih A, Zhang S, Cao HJ, Tang HY, Davis FB, Davis PJ, Lin HY 2004 Disparate effects of thyroid hormone on actions of epidermal growth factor and transforming growth factor-α are mediated by 3′,5′-cyclic adenosine 5′-monophosphate-dependent protein kinase II. Endocrinology 145:1708–1717 [DOI] [PubMed] [Google Scholar]
- Mousa SA, Bergh JJ, Dier E, Rebbaa A, O'Connor LJ, Yalcin M, Aljada A, Dyskin E, Davis FB, Lin HY, Davis PJ 2008 Tetraiodothyroacetic acid, a small molecule integrin ligand, blocks angiogenesis induced by vascular endothelial growth factor and basic fibroblast growth factor. Angiogenesis 11:183–190 [DOI] [PubMed] [Google Scholar]
- Lin HY, Tang HY, Shih A, Keating T, Cao G, Davis PJ, Davis FB 2007 Thyroid hormone is a MAPK-dependent growth factor for thyroid cancer cells and is anti-apoptotic. Steroids 72:180–187 [DOI] [PubMed] [Google Scholar]
- Tang HY, Lin HY, Zhang S, Davis FB, Davis PJ 2004 Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology 145:3265–3272 [DOI] [PubMed] [Google Scholar]
- Rebbaa A, Chu F, Davis FB, Davis PJ, Mousa SA 2008 Novel function of the thyroid hormone analog tetraiodothyroacetic acid: a cancer chemosensitizing and anti-cancer agent. Angiogenesis 11:269–276 [DOI] [PubMed] [Google Scholar]
- Yalcin M, Lansing L, Bharali D, Bridoux A, Davis FB, Lin HY, Rebbaa A, Davis PJ, Mousa SA, Tetrac and nanoparticulate tetrac arrest growth and inhibit tumor angiogenesis in xenografts of human medullary carcinoma of the thyroid. Program of the 79th Annual Meeting of The American Thyroid Association, Chicago, IL, 2008 (Abstract 110). Thyroid 18(Suppl 1):S46 [Google Scholar]
- Lin HY, Sun M, Tang HY, Lin C, Luidens MK, Mousa SA, Incerpi S, Drusano GL, Davis FB, Davis PJ 2009 L-Thyroxine vs. 3,5,3′-triiodo-L-thyronine and cell proliferation: activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am J Physiol Cell Physiol 296:C980–C991 [DOI] [PubMed] [Google Scholar]
- Moeller LC, Cao X, Dumitrescu AM, Seo H, Refetoff S 2006 Thyroid hormone mediated changes in gene expression can be initiated by cytosolic action of the thyroid hormone receptor β through the phosphatidylinositol 3-kinase pathway. Nucl Recept Signal 4:e020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Mariash CN, Ingbar DH 2004 3,3′,5-Triiodo-L-thyronine up-regulation of Na, K-ATPase activity and cell surface expression in alveolar epithelial cells is Src kinase- and phosphoinositide 3-kinase-dependent. J Biol Chem 279:47589–47600 [DOI] [PubMed] [Google Scholar]
- Storey NM, Gentile S, Ullah H, Russo A, Muessel M, Erxleben C, Armstrong DL 2006 Rapid signaling at the plasma membrane by a nuclear receptor for thyroid hormone. Proc Natl Acad Sci USA 103:5197–5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère A, Lehoux JG, Gallo-Payet N, Bellabarba D 1992 3,5,3′-Triiodothyronine binding sites in synaptosomes from brain of chick embryo. Properties and ontogeny. Brain Res Dev Brain Res 66:221–227 [DOI] [PubMed] [Google Scholar]
- Giguère A, Fortier S, Beaudry C, Gallo-Payet N, Bellabarba D 1996 Effect of thyroid hormone on G proteins in synaptosomes of chick embryo. Endocrinology 137:2558–2564 [DOI] [PubMed] [Google Scholar]
- Baumann CT, Maruvada P, Hager GL, Yen PM 2001 Nuclear cytoplasmic shuttling by thyroid hormone receptors. Multiple protein interactions are required for nuclear retention. J Biol Chem 276:11237–11245 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Hanover JA, Hager GL, Cheng SY 1998 Hormone-induced translocation of thyroid hormone receptors in living cells visualized using a receptor green fluorescent protein chimera. J Biol Chem 273:27058–27063 [DOI] [PubMed] [Google Scholar]
- Davis PJ, Shih A, Lin HY, Martino LJ, Davis FB 2000 Thyroxine promotes association of mitogen-activated protein kinase and nuclear thyroid hormone receptor (TR) and causes serine phosphorylation of TR. J Biol Chem 275:38032–38039 [DOI] [PubMed] [Google Scholar]
- Cao X, Kambe F, Moeller LC, Refetoff S, Seo H 2005 Thyroid hormone induces rapid activation of Akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol Endocrinol 19:102–112 [DOI] [PubMed] [Google Scholar]
- Moeller LC, Dumitrescu AM, Refetoff S 2005 Cytosolic action of thyroid hormone leads to induction of hypoxia-inducible factor-1α glycolytic genes. Mol Endocrinol 19:2955–2963 [DOI] [PubMed] [Google Scholar]
- Lei J, Nowbar S, Mariash CN, Ingbar DH 2003 Thyroid hormone stimulates Na, K-ATPase activity and its plasma membrane insertion in rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 285:L762–L772 [DOI] [PubMed] [Google Scholar]
- Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, Noma K, Ueki K, Nguyen NH, Scanlan TS, Moskowitz MA, Cheng SY, Liao JK 2006 Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci USA 103:14104–14109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ, Leonard JL, Davis FB 2008 Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol 29:211–218 [DOI] [PubMed] [Google Scholar]
- Lei J, Mariash CN, Bhargava M, Wattenberg EV, Ingbar DH 2008 T3 increases Na, K-ATPase activity via a MAPK/ERK1/2-dependent pathway in rat adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 294:L749–L754 [DOI] [PubMed] [Google Scholar]
- Hashizume K, Miyamoto T, Ichikawa K, Yamauchi K, Sakurai A, Ohtsuka H, Kobayashi M, Nishii Y, Yamada T 1989 Evidence for the presence of two active forms of cytosolic 3,5,3′-triiodo-L-thyronine (T3)-binding proteins (CTBP) in rat kidney. Specialized functions of two CTBPs in intracellular T3 translocation. J Biol Chem 264:4864–4871 [PubMed] [Google Scholar]
- Hashizume K, Suzuki S, Ichikawa K, Takeda T 1991 Purification of cytosolic 3,5,3′-triiodo-L-thyronine (T3)-binding protein (CTBP) which regulates nuclear T3 translocation. Biochem Biophys Res Commun 174:1084–1089 [DOI] [PubMed] [Google Scholar]
- Davis PJ, Handwerger BS, Glaser F 1974 Physical properties of a dog liver and kidney cytosol proteins that binds thyroid hormone. J Biol Chem 249:6208–6217 [PubMed] [Google Scholar]
- Vié MP, Blanchet P, Samson M, Francon J, Blondeau JP 1996 High affinity thyroid hormone-binding in human kidney: kinetic characterization and identification by photoaffinity labeling. Endocrinology 137:4563–4570 [DOI] [PubMed] [Google Scholar]
- Parkison C, Ashizawa K, McPhie P, Lin KH, Cheng SY 1991 The monomer of pyruvate kinase, subtype M1, is both a kinase and a cytosolic thyroid hormone binding protein. Biochem Biophys Res Commun 179:668–674 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hashizume K, Suzuki S, Ichikawa K, Takeda T 1991 A novel, NADPH-dependent cytosolic 3,5,3′-triiodo-L-thyronine-binding protein (CTBP; 5.1 S) in rat liver: a comparison with a 4.7 S NADPH-dependent CTBP. Endocrinology 129:1701–1708 [DOI] [PubMed] [Google Scholar]
- Kato H, Fukuda T, Parkison C, McPhie P, Cheng SY 1989 Cytosolic thyroid hormone-binding protein is a monomer of pyruvate kinase. Proc Natl Acad Sci USA 86:7861–7865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FB, Davis PJ, Blas SD 1983 Role of calmodulin in thyroid hormone stimulation in vitro of human erythrocyte Ca2+-ATPase activity. J Clin Invest 71:579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J, Hardiman J, Ingbar SH 1989 Stimulation of calcium-ATPase activity by 3,5,3′-tri-iodothyronine in rat thymocyte plasma membranes. A possible role in the modulation of cellular calcium concentration. Biochem J 261:749–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence WD, Schoenl M, Davis PJ 1989 Stimulation in vitro of rabbit erythrocyte cytosol phospholipid-dependent protein kinase activity. A novel action of thyroid hormone. J Biol Chem 264:4766–4768 [PubMed] [Google Scholar]
- Nieman LK, Davis FB, Davis PJ, Cunningham EE, Gutman S, Blas SD, Schoenl M 1983 Effect of end-stage renal disease on responsiveness to calmodulin and thyroid hormone of calcium-ATPase in human red blood cells. Kidney Int Suppl 16:S167–S170 [PubMed] [Google Scholar]
- Dube MP, Davis FB, Davis PJ, Schoenl M, Blas SD 1986 Effects of hyperthyroidism and hypothyroidism on human red blood cell Ca2+-ATPase activity. J Clin Endocrinol Metab 62:253–257 [DOI] [PubMed] [Google Scholar]
- Marino F, Guasti L, Cosentino M, De Piazza D, Simoni C, Piantanida E, Cimpanelli M, Klersy C, Bartalena L, Venco A, Lecchini S 2006 Thyroid hormone regulation of cell migration and oxidative metabolism in polymorphonuclear leukocytes: clinical evidence in thyroidectomized subjects on thyroxine replacement therapy. Life Sci 78:1071–1077 [DOI] [PubMed] [Google Scholar]
- Zinman T, Shneyvays V, Tribulova N, Manoach M, Shainberg A 2006 Acute, nongenomic effect of thyroid hormones in preventing calcium overload in newborn rat cardiocytes. J Cell Physiol 207:220–231 [DOI] [PubMed] [Google Scholar]
- Kahaly GJ, Dillmann WH 2005 Thyroid hormone action in the heart. Endocr Rev 26:704–728 [DOI] [PubMed] [Google Scholar]
- He H, Giordano FJ, Hilal-Dandan R, Choi DJ, Rockman HA, McDonough PM, Bluhm WF, Meyer M, Sayen MR, Swanson E, Dillmann WH 1997 Overexpression of the rat sarcoplasmic reticulum Ca2+-ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J Clin Invest 100:380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillmann WH 2002 Cellular action of thyroid hormone on the heart. Thyroid 12:447–452 [DOI] [PubMed] [Google Scholar]
- Mylotte KM, Cody V, Davis PJ, Davis FB, Blas SD, Schoenl M 1985 Milrinone and thyroid hormone stimulate myocardial membrane Ca2+-ATPase activity and share structural homologies. Proc Natl Acad Sci USA 82:7974–7978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM, Despa S 2006 Cardiac myocytes Ca2+ and Na+ regulation in normal and failing hearts. J Pharmacol Sci 100:315–322 [DOI] [PubMed] [Google Scholar]
- Chakrabarti N, Ray AK 2002 Stimulation of Ca2+/Mg2+ activity in adult rat cerebrocortical synaptosomes by 3-5-3′-L-triiodothyronine. Neurosci Res Commun 31:193–201 [Google Scholar]
- Chakrabarti N, Ray AK 2000 Rise of intrasynaptosomal Ca2+ level and activation of nitric oxide synthase in adult rat cerebral cortex pretreated with 3,5,3′-L-triiodothyronine. Neuropsychopharmacology 22:36–41 [DOI] [PubMed] [Google Scholar]
- Sarkar PK, Durga ND, Morris JJ, Martin JV 2006 In vitro thyroid hormone rapidly modulates protein phosphorylation in cerebrocortical synaptosomes from adult rat brain. Neuroscience 137:125–132 [DOI] [PubMed] [Google Scholar]
- Witzmann FA, Arnold RJ, Bai F, Hrncirova P, Kimpel MW, Mechref YS, McBride WJ, Novotny MV, Pedrick NM, Ringham HN, Simon JR 2005 A proteomic survey of rat cerebral cortical synaptosomes. Proteomics 5:2177–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D, Xiao RP 2002 Dual site phospholamban phosphorylation and its physiological relevance in the heart. Trends Cardiovasc Med 12:51–56 [DOI] [PubMed] [Google Scholar]
- Frank KF, Bölck B, Erdmann E, Schwinger RH 2003 Sarcoplasmic reticulum Ca2+-ATPase modulates cardiac contraction and relaxation. Cardiovasc Res 57:20–27 [DOI] [PubMed] [Google Scholar]
- Mattiazzi A, Mundiña-Weilenmann C, Guoxiang C, Vittone L, Kranias E 2005 Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions. Cardiovasc Res 68:366–375 [DOI] [PubMed] [Google Scholar]
- Traaseth NJ, Ha KN, Verardi R, Shi L, Buffy JJ, Masterson LR, Veglia G 2008 Structural and dynamic basis of phospholamban and sarcolipin inhibition of Ca2+-ATPase. Biochemistry 47:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J, Ingbar SH 1981 Studies of the mechanism by which 3,5,3′-triiodothyronine stimulates 2-deoxyglucose uptake in rat thymocytes in vitro. Role of calcium and adenosine 3′:5′-monophsophate. J Clin Invest 68:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J, Ingbar SH 1989 Evidence that an increase in cytoplasmic calcium is the initiating event in certain plasma membrane-mediated responses to 3,5,3′-triiodothyronine in rat thymocytes. Endocrinology 124:1949–1955 [DOI] [PubMed] [Google Scholar]
- Segal J, Ingbar SH 1989 3,5,3′-Triiodothyronine increases cellular adenosine 3′:5′-monophosphate concentration and sugar uptake in rat thymocytes by stimulating adenylate cyclase activity: studies with the adenylate cyclase inhibitor MDL 12330A. Endocrinology 124:2166–2171 [DOI] [PubMed] [Google Scholar]
- Segal J 1989 A rapid, extranuclear effect of 3,5,3′-triiodothyronine on sugar uptake by several tissues in the rat in vivo. Evidence for a physiological role for the thyroid hormone action at the level of the plasma membrane. Endocrinology 124:2755–2764 [DOI] [PubMed] [Google Scholar]
- Bhargava M, Lei J, Mariash CN, Ingbar DH 2007 Thyroid hormone rapidly stimulates alveolar Na, K-ATPase by activation of phosphatidylinositol 3-kinase. Curr Opin Endocrinol Diabetes Obes 14:416–420 [DOI] [PubMed] [Google Scholar]
- Sarkar PK, Ray AK 1998 Specific binding of L-triiodothyronine modulates Na+-K+-ATPase activity in adult rat cerebrocortical synaptosomes. Neuroreport 9:1149–1152 [DOI] [PubMed] [Google Scholar]
- Gick GG, Ismail-Beigi F, Edelman IS 1988 Thyroidal regulation of rat renal and hepatic Na, K-ATPase gene expression. J Biol Chem 263:16610–16618 [PubMed] [Google Scholar]
- Incerpi S, Luly P, De Vito P, Farias RN 1999 Short-term effects of thyroid hormones on the Na/H antiport in L-6 myoblasts: high molecular specificity for 3,3′,5-triiodo-L-thyronine. Endocrinology 140:683–689 [DOI] [PubMed] [Google Scholar]
- D'Arezzo S, Incerpi S, Davis FB, Acconcia F, Marino M, Farias RN, Davis PJ 2004 Rapid nongenomic effects of 3,5,3′-triiodo-L-thyronine on the intracellular pH of L-6 myoblasts are mediated by intracellular calcium mobilization and kinase pathways. Endocrinology 145:5694–5703 [DOI] [PubMed] [Google Scholar]
- Munteanu E, Verdier M, Grandjean-Forestier F, Stenger C, Jayat-Vignoles C, Huet S, Robert J, Ratinaud MH 2006 Mitochondrial localization and activity of P-glycoprotein in doxorubicin-resistant K562 mice. Biochem Pharmacol 71:1162–1174 [DOI] [PubMed] [Google Scholar]
- Davis PJ, Davis FB, Lin HY, Mousa SA, Zhou M, Luidens MK 2009 Translational implications of nongenomic actions of thyroid hormone initiated at its integrin receptor. Am J Physiol Endocrinol Metab 297:E1238–E1246 [DOI] [PubMed] [Google Scholar]
- Davis PJ, Davis FB, Cody V 2005 Membrane receptors mediating thyroid hormone action. Trends Endocrinol Metab 16:429–435 [DOI] [PubMed] [Google Scholar]
- Roepe PD 1992 Analysis of the steady-state and initial rate of doxorubicin efflux from a series of multidrug-resistant cells expressing different levels of P-glycoprotein. Biochemistry 31:12555–125664 [DOI] [PubMed] [Google Scholar]
- Harris DR, Green WL, Craelius W 1991 Acute thyroid hormone promotes slow inactivation of sodium in neonatal cardiac myocytes. Biochim Biophys Acta 1095:175–181 [DOI] [PubMed] [Google Scholar]
- Huang CJ, Geller HM, Green WL, Craelius W 1999 Acute effects of thyroid hormone analogs on sodium currents in neonatal rat myocytes. J Mol Cell Cardiol 31:881–893 [DOI] [PubMed] [Google Scholar]
- Yonkers MA, Ribera AB 2008 Sensory neuron sodium current requires nongenomic actions of thyroid hormone during development. J Neurophysiol 100:2719–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers MA, Ribera AB 2009 Molecular components underlying nongenomic thyroid hormone signaling in embryonic zebrafish neurons. Neural Dev 4:e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachelek SJ, Kowalik TF, Farwell AP, Leonard JL 2000 Myosin V plays an essential role in the thyroid hormone-dependent endocytosis of type II iodothyronine 5′deiodinase. J Biol Chem 275:31701–31707 [DOI] [PubMed] [Google Scholar]
- Baqui M, Botero D, Gereben B, Curcio C, Harney JW, Salvatore D, Sorimachi K, Larsen PR, Bianco AC 2003 Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J Biol Chem 278:1206–1211 [DOI] [PubMed] [Google Scholar]
- Smythe E, Ayscough KR 2006 Actin regulation in endocytosis. J Cell Sci 119:4589–4598 [DOI] [PubMed] [Google Scholar]
- Mendelsohn J, Baselga J 2006 Epidermal growth factor receptor targeting in cancer. Semin Oncol 33:369–385 [DOI] [PubMed] [Google Scholar]
- Pietras RJ 2003 Interactions between estrogen and growth factor receptors in human breast cancer and the tumor-associated vasculature. Breast J 9:361–373 [DOI] [PubMed] [Google Scholar]
- Ciardiello F, Tortora G 2008 EGFR antagonists in cancer treatment. N Engl J Med 358:1160–1174 [DOI] [PubMed] [Google Scholar]
- Ren H, Yang BF, Rainov NG 2007 Receptor tyrosine kinases as therapeutic targets in malignant glioma. Rev Recent Clin Trials 2:87–101 [DOI] [PubMed] [Google Scholar]
- Cao HJ, Lin HY, Luidens MK, Davis FB, Davis PJ 2009 Cytoplasm-to-nucleus shuttling of thyroid hormone receptor β1 (TRβ1) is directed from a plasma membrane integrin receptor by thyroid hormone. Endocr Res 34:31–42 [DOI] [PubMed] [Google Scholar]
- Lin HY, Zhang S, West BL, Tang HY, Passaretti T, Davis FB, Davis PJ 2003 Identification of the putative MAP kinase docking site in the thyroid hormone receptor-β1 DNA-binding domain: function consequences of mutations at the docking site. Biochemistry 42:7571–7579 [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen PL, Chen CF, Sharp ZD, Lee WH 1999 Thyroid hormone, T3-dependent phosphorylation and translocation of Trip230 from the Golgi complex to the nucleus. Proc Natl Acad Sci USA 96:4443–4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Martino LJ, Wilcox BD, Davis FB, Gordinier JK, Davis PJ 1998 Potentiation by thyroid hormone of human IFN-γ-induced HLA-DR expression. J Immunol 161:843–849 [PubMed] [Google Scholar]
- Silva CM 2004 Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene 23:8017–8023 [DOI] [PubMed] [Google Scholar]
- Shih A, Lin HY, Davis FB, Davis PJ 2001 Thyroid hormone promotes phosphorylation of p53 by mitogen-activated protein kinase. Biochemistry 40:2870–2878 [DOI] [PubMed] [Google Scholar]
- Hercbergs AA, Goyal LK, Suh JH, Lee S, Reddy CA, Cohen BH, Stevens GH, Reddy SK, Peereboom DM, Elson PJ, Gupta MK, Barnett GH 2003 Propylthiouracil-induced chemical hypothyroidism with high-dose tamoxifen prolongs survival in recurrent high grade glioma: a Phase I/II study. Anticancer Res 23:617–626 [PubMed] [Google Scholar]
- Farwell AP, Dubord-Tomasetti SA, Pietrzykowski AZ, Leonard JL 2006 Dynamic nongenomic actions of thyroid hormone in the developing rat brain. Endocrinology 147:2567–2574 [DOI] [PubMed] [Google Scholar]
- Farwell AP, Lynch RM, Okulicz WC, Comi AM, Leonard JL 1990 The actin cytoskeleton mediates the hormonally regulated translocation of type II iodothyronine 5′deiodinase in astrocytes. J Biol Chem 265:18546–18553 [PubMed] [Google Scholar]
- Mousa SS, Davis FB, Davis PJ, Mousa SA 10 November 2009 Human platelet aggregation and degranulation is enhanced in vitro by L-thyroxine (T4), but not by 3,5,3′-triiodo-L-thyronine (T3), GC-1 or diiodothyropropionic acid (DITPA). Clin Appl Thromb Hemost doi: 1076029609348315v1 [DOI] [PubMed] [Google Scholar]
- El Eter E, Rabee H, Alkayali A, Mousa SA 2007 Role of thyroid hormone analogs in angiogenesis and the development of collaterals in rabbit hind limb ischemia model. J Thromb Haemost 5(Suppl 1):375 [Google Scholar]
- Tomanek RJ, Zimmerman MB, Suvarna PR, Morkin E, Pennock GD, Goldman S 1998 A thyroid hormone analog stimulates angiogenesis in the post-infarcted rat heart. J Mol Cell Cardiol 30:923–932 [DOI] [PubMed] [Google Scholar]
- Schlenker EH, Hora M, Liu Y, Redetzke RA, Morkin E, Gerdes AM 2008 Effects of thyroidectomy, T4, and DITPA on replacement of brain blood vessel density in adult rats. Am J Physiol Regul Integr Comp Physiol 294:R1504–R1509 [DOI] [PubMed] [Google Scholar]
- Kuzman JA, Tang Y, Vogelsang KA, Said S, Anderson BE, Morkin E, Gerdes AM 2007 Thyroid hormone analog, diiodothyropropionic acid (DITPA), exerts beneficial effects on chamber and cellular remodeling in cardiomyopathic hamsters. Can J Physiol Pharmacol 85:311–318 [DOI] [PubMed] [Google Scholar]
- Davis FB, Mousa SA, O'Connor L, Mohamed S, Lin HY, Cao HJ, Davis PJ 2004 Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ Res 94:1500–1506 [DOI] [PubMed] [Google Scholar]
- Mousa SA, O'Connor LJ, Bergh JJ, Davis FB, Scanlan TS, Davis PJ 2005 The proangiogenic action of thyroid hormone analogue GC-1 is initiated at an integrin. J Cardiovasc Pharmacol 46:356–360 [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Yamamura Y, Kau SW, Bevers T, Strom S, Patangan M, Hsu L, Krishnamurthy S, Theriault RL, Hortobagyi GN 2005 Thyroid hormone and breast cancer. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer 103:1122–1128 [DOI] [PubMed] [Google Scholar]
- Gorman CA, Becker DV, Greenspan FS, Levy RP, Oppenheimer JH, Rivlin RS, Robbins J, Vanderlaan WP 1977 Breast cancer and thyroid therapy. Statement by the American Thyroid Association. JAMA 237:1459–1460 [PubMed] [Google Scholar]
- Lin HY, Tang HY, Keating T, Wu YH, Shih A, Hammond D, Sun M, Hercbergs A, Davis FB, Davis PJ 2008 Resveratrol is pro-apoptotic and thyroid hormone is anti-apoptotic in glioma cells: both actions are integrin and ERK mediated. Carcinogenesis 29:62–69 [DOI] [PubMed] [Google Scholar]
- Puymirat J, Etongue-Mayer P, Dussault JH 1995 Thyroid hormones stabilize acetylcholinesterae mRNA in neuron-2A cells that overexpress the β1 thyroid receptor. J Biol Chem 270:30651–30656 [DOI] [PubMed] [Google Scholar]
- Vandenbrouck Y, Janvier B, Loriette C, Bereziat G, Mangeney-Andreani M 1995 Thyroid hormone modulates apolipoprotein-AI gene expression at the post-transcriptional level in Hep G2 cells. Eur J Biochem 231:126–132 [DOI] [PubMed] [Google Scholar]
- Guerra C, Roncero C, Porras A, Fernández M, Benito M 1996 Triiodothyronine induces the transcription of the uncoupling protein gene and stabilizes its mRNA in fetal rat brown adipocyte primary cultures. J Biol Chem 271:2076–2081 [DOI] [PubMed] [Google Scholar]
- Ashizawa K, McPhie P, Lin KH, Cheng SY 1991 An in vitro novel mechanism of regulating the activity of pyruvate kinase M2 by thyroid hormone and fructose 1,6-bisphosphate. Biochemistry 30:7105–7111 [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Cheng SY 1992 Regulation of thyroid hormone receptor-mediated transcription by a cytosol protein. Proc Natl Acad Sci USA 89:9277–9281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper ME, Seifert EL 2008 Thyroid hormone effects on mitochondrial energetics. Thyroid 18:145–156 [DOI] [PubMed] [Google Scholar]
- Kim B 2008 Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid 18:141–144 [DOI] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK 2008 A mitochondrial protein compendium elucidates complex I disease biology. Cell 134:112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BK, Kitami T, Gilbert TJ, Peck D, Ramanathan A, Schreiber SL, Golub TR, Mootha VK 2008 Large-scale chemical dissection of mitochondrial function. Nat Biotechnol 26:343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, Cornwall EJ 2005 The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J 392:353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CY, Parton LE, Ye CP, Krauss S, Shen R, Lin CT, Porco JA Jr, Lowell BB 2006 Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced β cell dysfunction in isolated pancreatic islets. Cell Metab 3:417–427 [DOI] [PubMed] [Google Scholar]
- Ledesma A, de Lacoba MG, Rial E 2002 The mitochondrial uncoupling proteins. Genome Biol 3, Reviews 3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD 2005 The efficiency and plasticity of mitochondrial energy transduction. Biochem Soc Trans 33:897–904 [DOI] [PubMed] [Google Scholar]
- Cadenas S, Echtay KS, Harper JA, Jekabsons MB, Buckingham JA, Grau E, Abuin A, Chapman H, Clapham JC, Brand MD 2002 The basal proton conductance of skeletal muscle mitochondria from transgenic mice overexpressing or lacking uncoupling protein-3. J Biol Chem 277:2773–2778 [DOI] [PubMed] [Google Scholar]
- Couplan E, del Mar Gonzalez-Barroso M, Alves-Guerra MC, Ricquier D, Goubern M, Bouillaud F 2002 No evidence for a basal, retinoic, or superoxide-induced uncoupling activity of the uncoupling protein 2 present in spleen or lung mitochondria. J Biol Chem 277:26268–26275 [DOI] [PubMed] [Google Scholar]
- Pecqueur C, Couplan E, Bouillaud F, Ricquier D 2001 Genetic and physiological analysis of the role of uncoupling proteins in human energy homeostasis. J Mol Med 79:48–56 [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM 1999 Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124 [DOI] [PubMed] [Google Scholar]
- Fisher RP, Lisowsky T, Parisi MA, Clayton DA 1992 DNA wrapping and bending by a mitochondrial high mobility group-like transcriptional activator protein. J Biol Chem 267:3358–3367 [PubMed] [Google Scholar]
- Marin-Garcia J, Ananthakrishnan R, Goldenthal MJ 2000 Heart mitochondrial DNA and enzyme changes during early human development. Mol Cell Biochem 210:47–52 [DOI] [PubMed] [Google Scholar]
- Garstka HL, Fäcke M, Escribano JR, Wiesner RJ 1994 Stoichiometry of mitochondrial transcripts and regulation of gene expression by mitochondrial transcription factor A. Biochem Biophys Res Commun 200:619–626 [DOI] [PubMed] [Google Scholar]
- Wrutniak C, Cassar-Malek I, Marchal S, Rascle A, Heusser S, Keller JM, Fléchon J, Dauça M, Samarut J, Ghysdael J 1995 A 43-kDa protein related to c-Erb A α 1 is located in the mitochondrial matrix of rat liver. J Biol Chem 270:16347–16354 [DOI] [PubMed] [Google Scholar]
- Casas F, Rochard P, Rodier A, Cassar-Malek I, Marchal-Victorion S, Wiesner RJ, Cabello G, Wrutniak C 1999 A variant form of the nuclear triiodothyronine receptor c-ErbAα1 plays a direct role in regulation of mitochondrial RNA synthesis. Mol Cell Biol 19:7913–7924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson ML, Vennström B 1997 Chicken thyroid hormone receptor α requires the N-terminal amino acids for exclusive nuclear localization. FEBS Lett 416:291–296 [DOI] [PubMed] [Google Scholar]
- Saelim N, Holstein D, Chocron ES, Camacho P, Lechleiter JD 2007 Inhibition of apoptotic potency by ligand stimulated thyroid hormone receptors located in mitochondria. Apoptosis 12:1781–1794 [DOI] [PubMed] [Google Scholar]
- Saelim N, John LM, Wu J, Park JS, Bai Y, Camacho P, Lechleiter JD 2004 Nontranscriptional modulation of intracellular Ca2+ signaling by ligand stimulated thyroid hormone receptor. J Cell Biol 167:915–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist-Kaiser CA, Juge-Aubry C, Tranter MP, Ekenbarger DM, Leonard JL 1990 Thyroxine-dependent modulation of actin polymerization in cultured astrocytes. A novel, extranuclear action of thyroid hormone. J Biol Chem 265:5296–5302 [PubMed] [Google Scholar]
- Leonard JL, Farwell AP 1997 Thyroid hormone-regulated actin polymerization in brain. Thyroid 7:147–151 [DOI] [PubMed] [Google Scholar]
- Venstrom KA, Reichardt LF 1993 Extracellular matrix 2: Role of extracellular matrix molecules and their receptors in the nervous system. FASEB J 7:996–1003 [DOI] [PubMed] [Google Scholar]
- Liesi P 1990 Extracellular matrix and neuronal movement. [Review]. Experientia 46:900–907 [DOI] [PubMed] [Google Scholar]
- Liesi P, Silver J 1988 Is astrocyte laminin involved in axon guidance in the mammalian CNS? Develop Biol 130:774–785 [DOI] [PubMed] [Google Scholar]
- Liesi P 1985 Laminin-immunoreactive glia distinguish regenerative adult CNS systems from non-regenerative ones. EMBO J 4:2505–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P 1985 Do neurons in the vertebrate CNS migrate on laminin? EMBO J 4:1163–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Hager G, Dodt HU, Seppälä I, Zieglgänsberger W 1995 Domain-specific antibodies against the B2 chain of laminin inhibit neuronal migration in the neonatal rat cerebellum. J Neurosci Res 40:199–206 [DOI] [PubMed] [Google Scholar]
- Hager G, Dodt HU, Zieglgänsberger W, Liesi P 1995 Novel forms of neuronal migration in the rat cerebellum. J Neurosci Res 40:207–219 [DOI] [PubMed] [Google Scholar]
- Ruoslahti E 1991 Integrins. J Clin Invest 87:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO 1992 Integrin: versatility, modulation, and signaling in cell adhesion. Cell 69:11–25 [DOI] [PubMed] [Google Scholar]
- Farwell AP, Tranter MP, Leonard JL 1995 Thyroxine-dependent regulation of integrin-laminin interactions in astrocytes. Endocrinology 136:3909–3915 [DOI] [PubMed] [Google Scholar]
- Farwell AP, Dubord SA 1996 Thyroid hormone regulates neurite outgrowth and neuronal migration onto laminin. Thyroid 6(Suppl 1):S-6 [Google Scholar]
- Farwell AP, Dubord-Tomasetti SA 1999 Thyroid hormone regulates the extracellular organization of laminin on astrocytes. Endocrinology 140:5014–5021 [DOI] [PubMed] [Google Scholar]
- Farwell AP, Dubord-Tomasetti SA 1999 Thyroid hormone regulates the expression of laminin in the developing rat cerebellum. Endocrinology 140:4221–4227 [DOI] [PubMed] [Google Scholar]
- Dodd J, Jessell TM 1988 Axon guidance and the patterning of neuronal projections in vertebrates. Science 242:692–699 [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS 1996 The molecular biology of axon guidance. Science 274:1123–1133 [DOI] [PubMed] [Google Scholar]
- Smith SJ 1988 Neuronal cytomechanics: the actin-based motility of growth cones. Science 242:708–715 [DOI] [PubMed] [Google Scholar]
- Hatten ME, Mason CA 1990 Mechanisms of glial-guided neuronal migration in vitro and in vivo. Experientia 46:907–916 [DOI] [PubMed] [Google Scholar]
- Hatten ME 1993 The role of migration in central nervous system neuronal development. Curr Opin Neurobiol 3: 38–44 [DOI] [PubMed] [Google Scholar]
- Rivas RJ, Hatten ME 1995 Motility and cytoskeletal organization of migrating cerebellar granule neurons. J Neurosci 15:981–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L, Letourneau PC 1984 Growth of neurites without filapodial or lamellipodial activity in the presence of cytochalasin B. J Cell Biol 99:2041–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D, Toroian-Raymond A 1986 Disoriented pathfinding by pioneer neuron growth cones deprived of filopodia by cytochalasin treatment. Nature 323:712–715 [DOI] [PubMed] [Google Scholar]
- Forscher P, Smith SJ 1988 Actions of cytochalasins on the organization of the actin filaments and microtubules in a neuronal growth cone. J Cell Biol 107:1505–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell AP, Dubord-Tomasetti SA, Pietrzykowski AZ, Stachelek SJ, Leonard JL 2005 Regulation of cerebellar neuronal migration and neurite outgrowth by thyroxine and 3,3′,5′-triiodothyronine. Brain Res Dev Brain Res 154:121–135 [DOI] [PubMed] [Google Scholar]
- Safran M, Farwell AP, Rokos H, Leonard JL 1993 Structural requirements of iodothyronines for the rapid inactivation and internalization of type II iodothyronine 5′-deiodinase in glial cells. J Biol Chem 268:14224–14229 [PubMed] [Google Scholar]
- Chassande O, Fraichard A, Gauthier K, Flamant F, Legrand C, Savatier P, Laudet V, Samarut J 1997 Identification of transcripts initiated from an internal promoter in the c-erbA α locus that encode inhibitors of retinoic acid receptor-α and triiodothyronine receptor activities. Mol Endocrinol 11:1278–1290 [DOI] [PubMed] [Google Scholar]
- Flamant F, Samarut J 2003 Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol Metab 14:85–90 [DOI] [PubMed] [Google Scholar]
- Morte B, Manzano J, Scanlan T, Vennström B, Bernal J 2002 Deletion of the thyroid hormone receptor α 1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc Natl Acad Sci USA 99:3985–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar MA 1990 Sodium butyrate selectively alters thyroid hormone receptor gene expression in GH3 cells. J Biol Chem 265:17474–17477 [PubMed] [Google Scholar]
- Stanley F, Tsai JS, Samuels HH 1986 Stimulation of facilitated [3H]uridine transport by thyroid hormone in GH1 cells. Evidence for regulation by the thyroid hormone nuclear receptor. J Biol Chem 261:9400–9404 [PubMed] [Google Scholar]
- Duncan KG, Jumper MD, Ribeiro RC, Bailey KR, Yen PM, Sugawara A, Patel A, Stern R, Chin WW, Baxter JD, Schwartz DM 1999 Human trabecular meshwork cells as a thyroid hormone target tissue: presence of functional thyroid hormone receptors. Graefes Arch Clin Exp Ophthalmol 237:231–240 [DOI] [PubMed] [Google Scholar]
- Mariash CN, McSwigan CR, Towle HC, Schwartz HL, Oppenheimer JH 1981 Glucose and triiodothyronine both induce malic enzyme in the rat hepatocyte culture: evidence that triiodothyronine multiplies a primary glucose-generated signal. J Clin Invest 68:1485–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Ceccarelli P, Refetoff S, Horwitz AL, Matsui N 1987 Thyroid hormone inhibits fibronectin synthesis by cultured human skin fibroblasts. J Clin Endocrinol Metab 64:334–339 [DOI] [PubMed] [Google Scholar]
- Christoffolete MA, Ribeiro R, Singru P, Fekete C, da Silva WS, Gordon DF, Huang SA, Crescenzi A, Harney JW, Ridgway EC, Larsen PR, Lechan RM, Bianco AC 2006 Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology 147:1735–1743 [DOI] [PubMed] [Google Scholar]
- Cao Z, West C, Norton-Wenzel CS, Rej R, Davis FB, Davis PJ, Rej R 2009 Effects of resin or charcoal treatment on fetal bovine and bovine calf serum. Endocr Res 34:101–108 [DOI] [PubMed] [Google Scholar]
- Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC 2008 Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Germain DL, Galton VA, Hernandez A 2009 Minireview: Defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology 150:1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereben B, Zeöld A, Dentice M, Salvatore D, Bianco AC 2008 Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci 65:570–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer H, Visser TJ 2009 Minireview: pathophysiological importance of thyroid hormone transporters. Endocrinology 150:1078–1083 [DOI] [PubMed] [Google Scholar]
- van der Deure W, Peeters R, Visser T 18 June 2009 Molecular aspects of thyroid hormone transporters, including MCT8, MCT10 and OATPs, and the effects of genetic variation in these transporters. J Mol Endocrinol doi: 10.1677/JME-09-0042 [DOI] [PubMed] [Google Scholar]