Abstract
During carcinogenesis of pancreatic islets in transgenic mice, an angiogenic switch activates the quiescent vasculature. Paradoxically, vascular endothelial growth factor (VEGF) and its receptors are expressed constitutively. Nevertheless, a synthetic inhibitor (SU5416) of VEGF signalling impairs angiogenic switching and tumour growth. Two metalloproteinases, MMP-2/gelatinase-A and MMP-9/gelatinase-B, are upregulated in angiogenic lesions. MMP-9 can render normal islets angiogenic, releasing VEGF. MMP inhibitors reduce angiogenic switching, and tumour number and growth, as does genetic ablation of MMP-9. Absence of MMP-2 does not impair induction of angiogenesis, but retards tumour growth, whereas lack of urokinase has no effect. Our results show that MMP-9 is a component of the angiogenic switch.
Cancers arise through multistep pathways, acquiring necessary capabilities through changes in the programme of the cancer-cell genome, and by recruiting and mobilizing essential accessory cell types1. Among the accessories are capillary endothelial cells, which are activated to produce new blood vessels; this capability for angiogenesis is critical for expansive tumour growth and metastasis2–4.
In one approach to elucidating cancer mechanisms, we have studied a transgenic mouse model (RIP1-Tag2) of multistage carcinogenesis, in which every mouse develops islet tumours of the pancreas by 12–14 weeks of age as a result of expression of the SV40 T antigen (Tag) oncogene in insulin-producing β-cells5. Of the ~400 islets that express this oncogene, only 1–2% develop into adenomas and carcinomas, indicating that rate-limiting changes (steps) may be necessary for manifestation of the tumour stages5. The induction of angiogenesis is a discrete step in this multistage pathway6. Initially, hyperproliferative islets with quiescent vasculature emerge; these nodules (reaching 50% of all islets) have characteristics of in situ carcinoma lesions. Subsequently, a subset (~20%) of hyperproliferative islets switch on angiogenesis, as shown by endothelial sprouting, mitosis, microhaemorrhaging and vascular dilation in vivo, and by their ability to elicit an angiogenic response in vitro6. All tumours similarly show evidence of angiogenesis. The focal nature and statistics of the induction of angiogenesis in small precursor lesions indicate that the angiogenic switch may be governed by a discrete regulatory event.
Although a popular hypothesis for angiogenesis induction involves upregulation of angiogenic genes in response to hypoxia or expression of oncogenes7–9, our analysis of angiogenic genes reveals a surprising conundrum —VEGF and acidic fibroblast growth factor (aFGF) are both constitutively expressed in normal islet β-cells of control mice, and in all stages of the RIP1-Tag2 islet carcinogenesis pathway10; similarly, the two VEGF receptors flk-1 (VEGF-R2) and flt-1 (VEGF-R1) are constitutively expressed in the islet vasculature before and after the angiogenic switch11. Expression of such pro-angiogenic molecules in this neuroendocrine tissue may partly serve to control maintenance and homeostasis of the dense network of fenestrated endocrine capillaries that facilitates glucose homeostasis12. How, then, is angiogenesis facilitated during islet carcinogenesis? Here we show that the switch from vascular quiescence to angiogenesis involves a matrix metalloproteinase (MMP), gelatinase B/MMP-9, which is upregulated in angiogenic islets and tumours, rendering VEGF more available to its receptors. Remarkably, MMP-9 is not expressed in tumour cells, but rather in a small number of cells that are proximal to the vasculature.
Results
Contributions of VEGF signalling
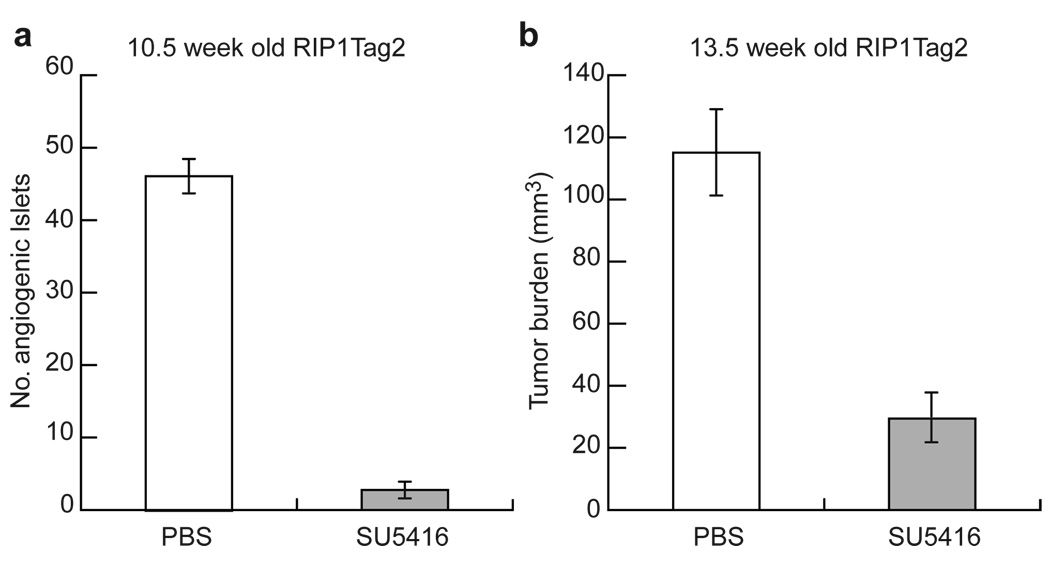
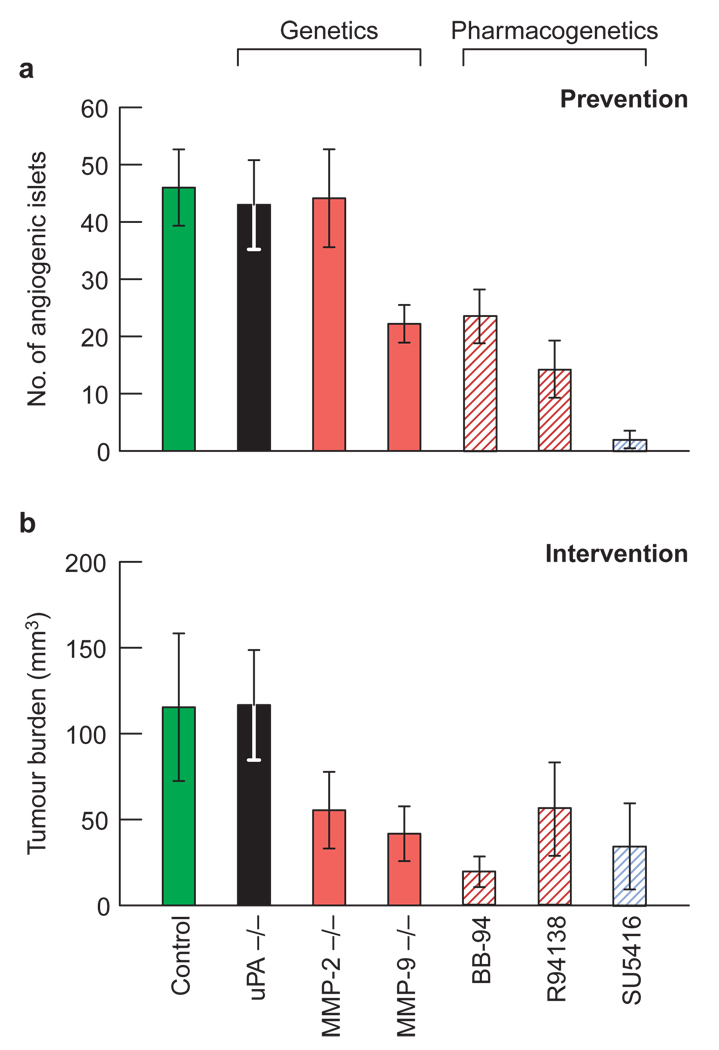
VEGF is known to be a potent inducer of angiogenesis, and yet it is constitutively expressed at similar levels before and after the angiogenic switch in this pathway10,11. Therefore, to determine whether VEGF is involved in the regulation of angiogenesis, we sought to interfere with VEGF signalling pharmacologically, by treating RIP1-Tag2 mice with SU5416, a synthetic small molecule that inhibits VEGF-R2-mediated signal transduction13. We used two distinct therapeutic trial designs that allowed us to assess both the frequency of angiogenic switching and the end-stage tumour burden (Fig. 1). In the ‘prevention trial’, 5-week-old transgenic mice harbouring only hyperplastic, non-angiogenic islets were treated for 5.5 weeks, to the point at which tumours first began to appear in sham-treated animals (Fig. 1a). At the end point (10.5 weeks), 20–25% of hyperplastic nodules (~45 islets) in untreated mice had become angiogenic, thus allowing us to quantify the frequency of the angiogenic switch. In the ‘intervention trial’, we treated RIP1-Tag2 mice, starting at 10 weeks of age, a point when all mice had a few small, highly vascularized, solid tumours. We stopped the trial at 13.5 weeks, when untreated and sham-treated mice had end-stage disease (Fig. 1b). Inhibition of VEGF-R2 signalling prevented activation of the angiogenic switch in 94% of hyperplastic islets (Fig. 1a), reduced the average number of tumours by about 60%, and restricted tumour growth, as shown by a 75% reduction in cumulative tumour volume (Fig. 1b). Thus, VEGF-R2 functionally contributes to tumourigenesis.
Figure 1. Inhibition of VEGF activity during carcinogenesis in islet cells.
To test the impact on the angiogenic switch, 5-week-old transgenic mice, whose islets had not yet activated the angiogenic switch, were treated with SU5416, a small synthetic inhibitor of VEGF-R2 signalling, until the first tumours appeared in control mice at 10.5 weeks of age a, To reduce tumour growth, 10-week-old transgenic mice with small solid tumours were treated until the appearance of end-stage disease (13.5 weeks of age, b). Numbers of angiogenic islets (a) and tumour burden (b) in transgenic and control Rip1-Tag2 mice are shown. Values are means ± s.d. (n =7–10).
VEGF–receptor association upon angiogenesis
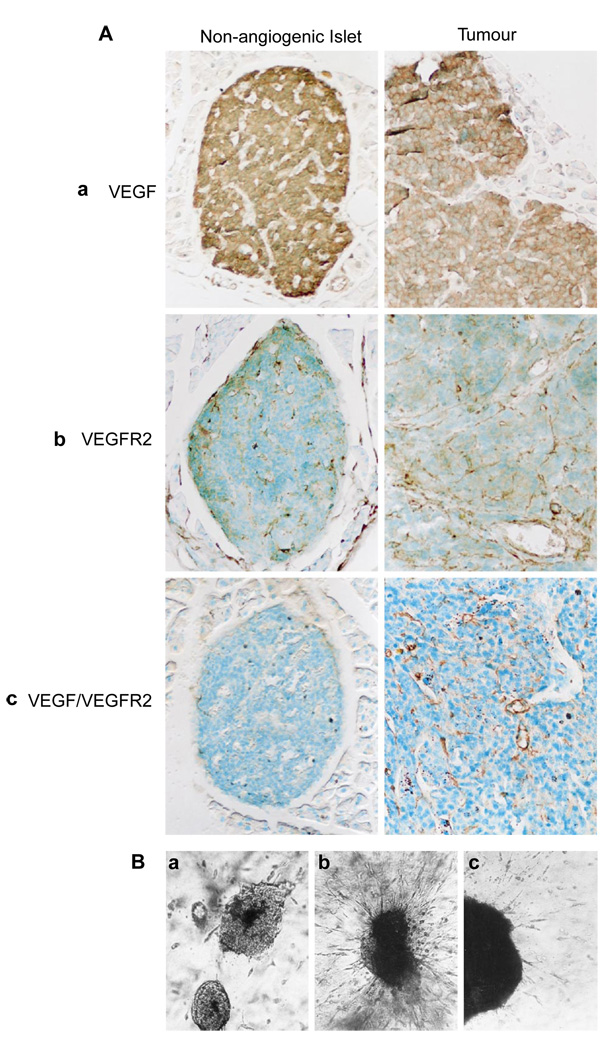
Although VEGF-R signalling has been shown to be important for angiogenic switching and tumour growth, the 120- and 164-amino-acid isoforms of VEGF-A (VEGF) are similarly expressed in the islets of Langerhans before and after the angiogenic switch11. VEGF120 is thought to be freely soluble, whereas VEGF164 can be either soluble or bound to cell surfaces and the extracellular matrix (ECM) as a result of its heparin-binding properties. Whereas immunohistochemical data indicate that VEGF is produced at similar levels in normal islets and throughout carcinogenesis, observations from organ culture indicate that normal islets may secrete lower levels11. These data, along with the finding that the two VEGF receptors are constitutevely expressed in islet endothelium before and after the angiogenic switch11, prompted us to investigate the association of ligand and receptor. We used a monoclonal antibody that specifically recognizes VEGF in complex with its principal signalling receptor, VEGF-R2/flk-1 (ref. 14), as well as antibodies that recognize ligand or receptor individually14. VEGF and VEGF-R2 were expressed in non-angiogenic islets, angiogenic islets and tumours from RIP1-Tag2 mice (Fig. 2A, a and b) and non-transgenic control islets (data not shown), as previously reported11. Surprisingly, despite the presence of VEGF and its receptor in all stages, we only detected the VEGF–VEGF-R2 complex in angiogenic islets and in tumours (Fig. 2A, c). This difference in antibody reactivity is likely to be significant, as the angiogenic stages are characterized by microhaemorrhaging, a hallmark of VEGF-induced angiogenesis15, and an inhibitor of VEGF-R2 signalling is demonstrably effective (Fig. 1).
Figure 2. Changes in VEGF localization and its involvement in angiogenesis.
A, VEGF localizes with its receptor concomitant with the angiogenic switch. Immunolocalization of VEGF, VEGFR-2 and the VEGF–VEGF-R2 complex is shown for hyperplastic, pre-angiogenic islets (left panels) and tumours (right panels). Immunohistochemical analysis was carried out using specific antibodies against VEGF, VEGF-R2 or VEGF–VEGF-R2. VEGF was detected at high levels in β-cells of pre-angiogenic hyperplastic islets and in angiogenic tumours (a). VEGF-R2 is localized on endothelial cells and it antibody therefore visualizes the capillary network in both hyperplastic islets and tumours (b). VEGF–VEGFR2 on endothelial cells was detected only in the angiogenic and tumour stages but not in hyperplastic, non-angiogenic islets (c). B, Inhibition of secreted VEGF impairs the angiogenic response. Non-angiogenic islets (a) and angiogenic islets in the absence (b) or presence (c) of VEGF-neutralizing antibodies were embedded into a three-dimensional collagen gel containing endothelial cells. Angiogenic islets, but not non-angiogenic islets, provoked an angiogenic response. Angiogenesis was delayed and diminished when anti-VEGF antibodies were added.
Further support comes from the starburst endothelial-cell co-culture assay, in which isolated angiogenic islets and tumours, but not normal islets, evoked a radial migration and assembly of endothelial cells into sprouts and tubes (Fig. 2B, a and b). The angiogenic response in this bioassay was severely attenuated when we used an antibody that neutralizes VEGF itself (Fig. 2B, c). Considering that secretion of VEGF from normal islets in culture is comparatively lower than that from cognate angiogenic lesions11, the data collectively indicate that the availability of VEGF may be limited in normal pancreatic islets. Moreover, there is no switch in the isoforms of VEGF-A being produced, which are VEGF120 and VEGF164 in all stages11. We therefore sought to determine whether VEGF is mobilized during the angiogenic switch, focussing on the possible involvement of extracellular proteinases.
Activation of matrix metalloproteinases
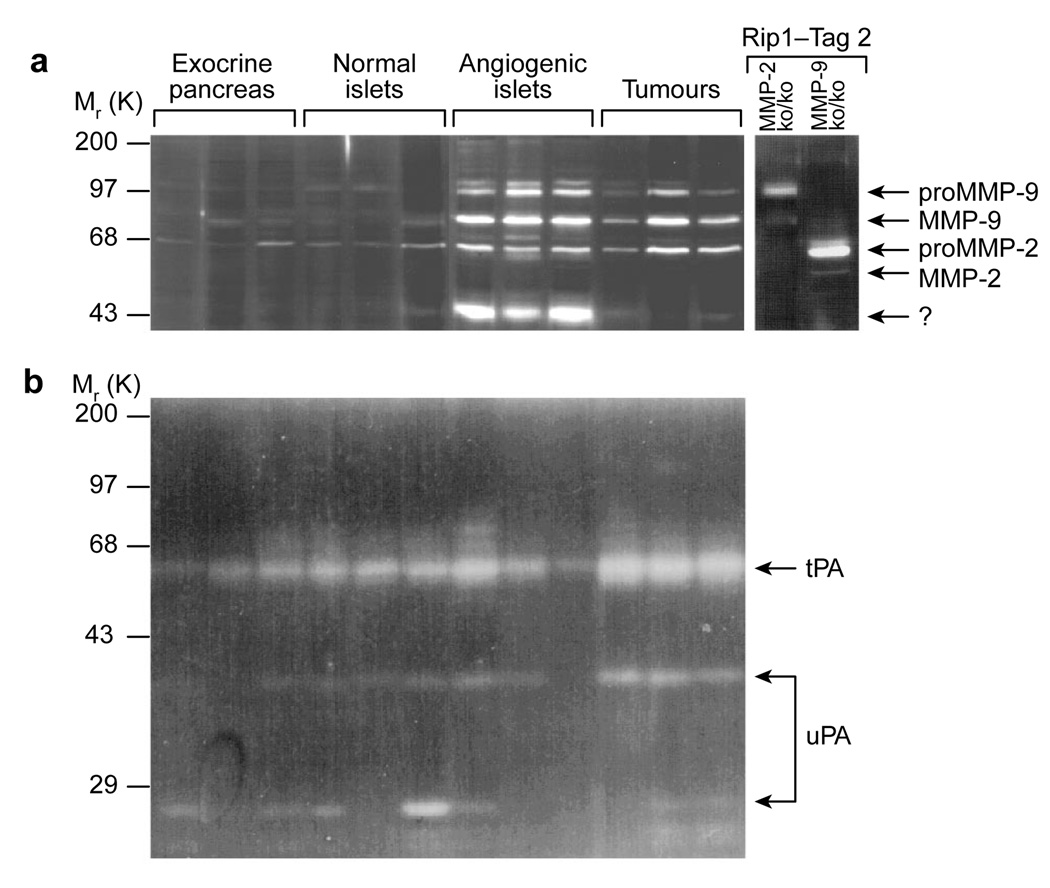
In view of the association of plasminogen activators and MMPs with ECM degradation and neovascularization, we used substrate-gel zymography to assess the expression and activation status of several such enzymes during islet carcinogenesis. MMP-2/gelatinase A (relative molecular masses (Mr) of 72,000 (72K) and 62K) was present in all stages and was upregulated in the angiogenic islet and tumour stages (Fig. 3a). In contrast, MMP-9 (Mr 105K and 95K) was not detectable in normal islets, but was detected in angiogenic islets and tumours (Fig. 3a). The high- and low-Mr forms of urokinase-type (uPA) and tissue-type (tPA) plasminogen activators were present at all stages and their levels exhibited no reproducible change (Fig. 3b). Given that MMP-2 and MMP-9 were both upregulated at the onset of angiogenesis, and both persisted in the tumour stage, we hypothesized that MMP-2 and/or MMP-9 may be functionally involved in the angiogenic switch and in subsequent tumour progression.
Figure 3. Zymography profiles of extracellular proteinase activity during tumorigenesis.
The activity of plasminogen activators and gelatinases in tissue lysates from non-transgenic islets, angiogenic islets, and tumours was revealed by zymography. a, MMP-2 and MMP-9 were upregulated concomitant with the angiogenic switch and persisted in tumours. Both enzymes were found as inactive higher-Mr and active lower-Mr. As controls, gelatinase activity in MMP-2ko/ko and MMP-9ko/ko tumours is shown. b, The serine proteinases urokinase-plasminogen activator (uPA) and tissue-plasminogen activator (tPA) were constitutively present during islet carcinogenesis, before and after the angiogenic switch. Both the inactive high-Mr and the active lower-Mr forms of urokinase are evident.
MMP-9 renders normal islets angiogenic ex vivo
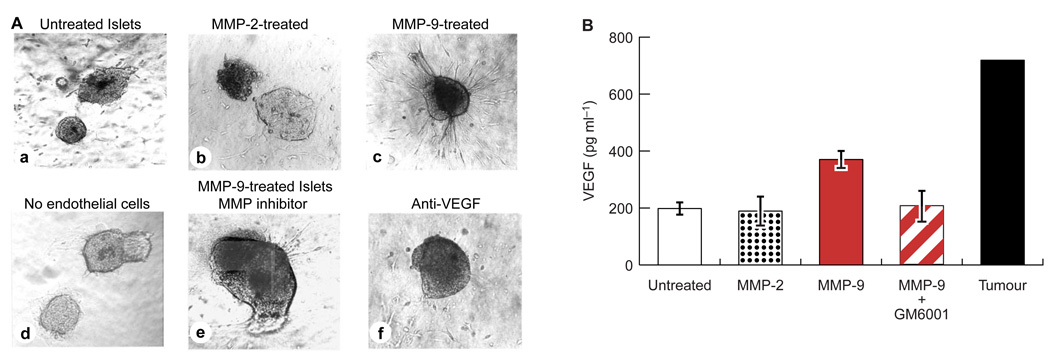
We first assessed the possible interconnection between gelatinase activity and angiogenesis using the starburst endothelial co-culture assay6.We isolated normal, non-transgenic islets from C57/Bl6 mice and pre-incubated them with purified, enzymatically active MMP-2 (0.5–2.0 µg ml−1), MMP-9 (0.5–2.0 µg ml−1), trypsin (2.5 µg ml−1), urokinase (0.2 µg ml−1) or bacterial collagenase P (0.2 µg ml−1). We then washed the treated islets and individually embedded them into collagen gels containing endothelial cells. Control islets and islets treated with MMP-2 (Fig. 4A, a and b), trypsin, urokinase or collagenase P (data not shown) failed to elicit an angiogenic response from the dispersed endothelial cells. In contrast, islets treated with MMP-9 underwent radial alignment, and endothelial cells formed sprouts directed towards the islet (Fig. 4A, c). The starburst was not observed when MMP-9-treated islets were embedded into a collagen matrix that did not contain endothelial cells (Fig. 4A, d), indicating that the observed response involved ingrowth of endothelial cells towards angiogenic islets. Islets treated with MMP-9 in the presence of the metalloproteinase inhibitor (MMP-I) GM6001 did not elicit a subsequent angiogenic response in the starburst assay (Fig. 4A, e). However, when GM6001 was added to the collagen gel after treatment of normal islets with MMP-9, the treated islets still elicited an angiogenic response, demonstrating that enzyme activity was not required during the starburst response (data not shown). Furthermore, MMP-9 protein alone had no direct angiogenic effect on endothelial cells when added to the collagen gel in lieu of islets (data not shown). Thus, active MMP-9 is selectively capable of converting normal, non-angiogenic islets into angiogenic islets.
Figure 4. MMP-9 can activate angiogenesis and release VEGF from normal islets.
A, Effect of treatment with gelatinase on angiogenic activity of non-transgenic normal islets. a, untreated islets; b, MMP-2-treated islets; c, MMP-9-treated islets; d, MMP-9-treated islets in a collagen matrix without endothelial cells; e, MMP-9-treated islets in the presence of the MMP inhibitor GM6001; f, MMP-9-treated islets in the presence of a VEGF-neutralizing antibody. Gelatinases were used at 1 mg ml−1. B, Determination by ELISA of VEGF levels in conditioned media of protease-treated and non-treated islets. Untreated and MMP-2-treated islets release similar, small amounts of VEGF. Treatment with MMP-9 caused a roughly twofold increase in VEGF release; this effect was abolished in the presence of GM6001. Conditioned media of angiogenic islets and tumours served as a positive, but non-quantitative, control.
MMP-9 mobilizes VEGF from normal islets
We next investigated whether VEGF contributes to the angiogenic response elicited from the normal islets by treatment with MMP-9. Indeed, neutralizing anti-VEGF antibodies blocked the angiogenic response when MMP-9-treated islets were embedded into collagen gels containing endothelial cells (Fig. 4A, f). We used an enzyme-linked immunosorbent assay (ELISA) to quantify the amounts of VEGF released into conditioned media of treated and control islets. Untreated, non-transgenic islets and MMP-2-treated islets secreted low levels of VEGF, which is consistent with previous data showing that normal control islets secrete small amounts of the VEGF120 and VEGF164 isoforms11. Levels of VEGF were roughly twofold higher in the medium of MMP-9-treated islets (Fig. 4B). Simultaneous addition of GM6001 and MMP-9 to islets reduced the levels of VEGF released to those of untreated normal islets. The observed twofold increase in VEGF levels was evidently sufficient for an angiogenic response in the starburst assay, as VEGF-blocking antibodies abrogated MMP-9-induced angiogenesis by normal islets (Fig. 4A, f). Notably, a twofold difference in VEGF levels is sufficient to produce a lethal phenotype in mice that are heterozygous for a null mutation in VEGF16,17. The angiogenic response induced by MMP-9 in normal islets was less robust than that produced by genuine angiogenic lesions from the RIP1-Tag2 transgenic mice; so too was the amount of VEGF in conditioned medium. In vitro activation of angiogenesis required diffusion of the proteinase into intact islets in organ culture; it is therefore unsurprising that exogenous addition would be less effective than endogenous MMP-9 in bona fide angiogenic islets and tumours. The observed release of VEGF from normal islets gives rise to a proposed mechanism of action in which MMP-9 mobilizes VEGF from an extracellular reservoir. An alternative is that MMP-9 regulates an inhibitor of angiogenesis18. The VEGF-availability mechanism is supported by fact that treatment of normal islets with the heparinase I–III (5–15 U ml−1), which should release VEGF from heparan sulphate proteoglycans, also rendered the islets angiogenic (data not shown).
MMP-9 is not expressed in tumour cells
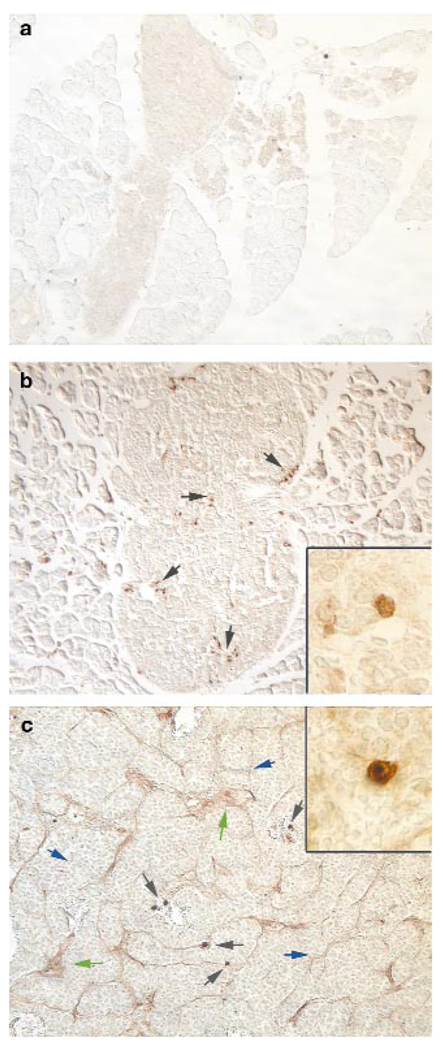
The observations that MMP-9 was induced in angiogenic lesions and was capable of eliciting an angiogenic response in normal islets led us to investigate the cellular source of this proteinase. Interestingly, immunohistochemistry revealed that MMP-9 is not expressed in normal non-transgenic islets (Fig. 5a), or by the oncogene-expressing epithelial cells, but rather by a small number of cells in close apposition to the vasculature (Fig. 5b, c, insets), characteristic of infiltrating, inflammatory cells. In tumours, MMP-9 was also present in the vascular basement membrane and ECM (Fig. 5c), where matrix-associated VEGF is found.
Figure 5. Localization of MMP-9 in angiogenic stages.
Non-angiogenic islets did not exhibit MMP-9-positive cells (a). MMP-9 was expressed by infiltrating cells (black arrows) in angiogenic islets (b) and in tumours (c) and was associated with the basement membrane (blue arrow) and extracellular matrix (green arrow), as visualized by immunohistochemistry. Insets, higher magnification of single MMP-9-expressing cells, proximal to capillaries.
Proteinase inhibitors impair angiogenesis
To determine whether gelatinase activity is functionally significant for carcinogenesis in the RIP1-Tag2 model, we sought to pharmacologically interfere with it by treating mice with MMP-I. We used two compounds: BB-94/Batimastat, a broad spectrum MMP-I19,20, and R94138, an inhibitor that is relatively specific to MMP-9 (ref. 21). We used a similar experimental design to that used for the VEGF inhibitor SU5416, in that we assessed both the frequency of angiogenic switching and the end-stage tumour burden. Each MMP-I prevented activation of the angiogenic switch in many of the hyperplastic islets that would otherwise have switched, with R94138 showing somewhat higher efficacy than BB94 (Fig. 6a). In addition, both inhibitors also reduced the number and growth of tumours by 70–80% (Fig. 6b).
Figure 6. Comparative genetic and pharmacogenetic analyses of the angiogenic switch and tumour growth.
Genetic and pharmacogenetic analyses were carried out to test the impact on the angiogenic switch and tumour growth in proteinase-deficient mice. Rip1-Tag2 mice were either crossed successively with MMP-9, MMP-2 or urokinase gene-knockout mice to produce homozygous knockouts, or treated with BB-94/Batimastat (a broad-spectrum MMP-I) or R94138 (a selective inhibitor of MMP-9). Data for SU5416 from Fig. 1 are reproduced here to allow comparison. For the pharmacological analysis, 5-week-old transgenic mice were treated from 5.0–10.5 weeks of age in a prevention trial (a), and from 10.0–13.5 weeks of age in an intervention trial (b). Homozygous deficient or MMPI-treated Rip1-Tag2 mice were analyzed at two distinct stages of tumour progression and compared with control Rip1-Tag2 mice with regard to the frequency of angiogenic switching (at 10.5 weeks of age, a) and cumulative tumour volume (tumour burden), a measure of relative tumour-growth rates (at 13.5 weeks of age, b). Values are means ± s.d. (n=7–14) and are compared with those of control RIP1-Tag2 mice.
Histological analysis of the angiogenic islets and tumours that formed in the presence of these two MMP-Is revealed no gross morphological differences; angiogenic islets were typical, as were both adenomas and invasive carcinomas. The apoptotic index was increased two- to threefold in treated tumours (data not shown; see ref. 22), which probably explains their stunted growth.
Different functions of MMP-2, MMP-9 and urokinase
Our pharmacogenetic experiments indicated that metalloproteinases are functionally involved in the angiogenic switch and in subsequent tumour growth. However, these inhibitors, although somewhat selective for specific MMPs, actually inhibit several MMPs, as well as related ADAM proteinases. We therefore used a genetic approach, in which we investigated the functional contributions of MMP-2 and MMP-9 to islet tumourigenesis by crossing RIP1-Tag2 mice with mice lacking either MMP-2 or MMP-9.We also generated urokinase-deficient RIP1-Tag2 mice.
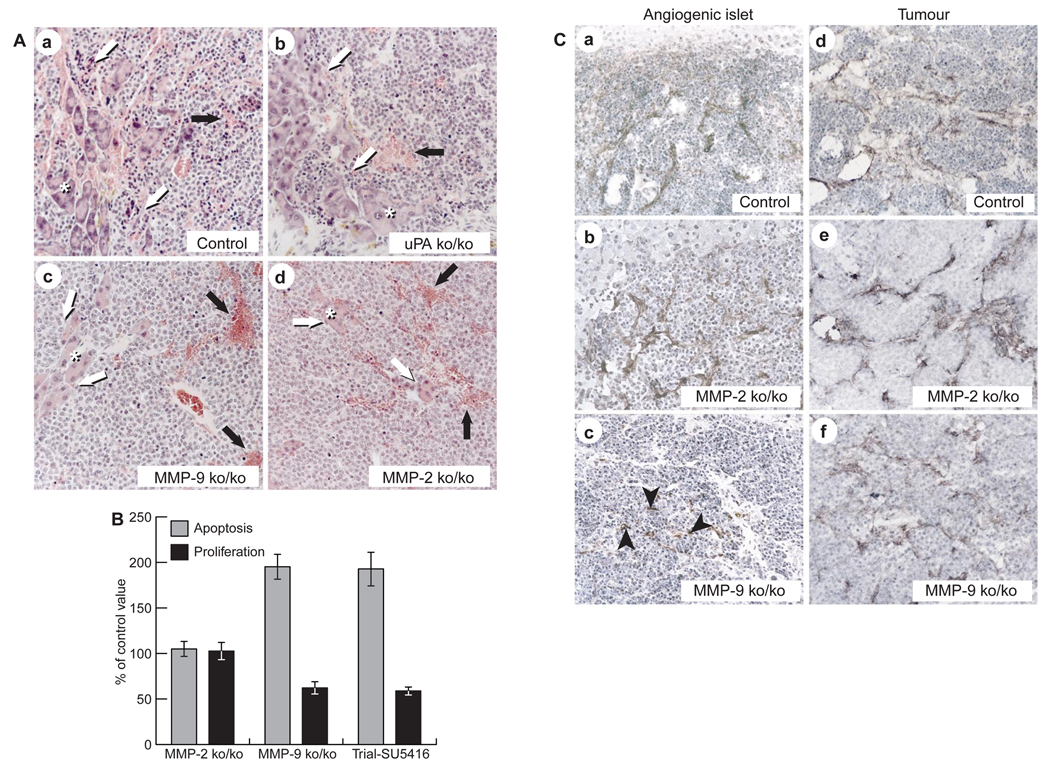
Mice that lack MMP-2, MMP-9 or urokinase are viable and fertile23–26.MMP-9-null mice show developmentally delayed angiogenesis in bone-growth plates but are otherwise asymptomatic23, whereas MMP-2-null mice exhibit delays in wound angiogenesis and transplant-tumour growth24,25. uPA-null mice are asymptomatic26. We examined RIP1-Tag2 mice that were homozygous-null for MMP-9, MMP-2 or urokinase, and compared them with heterozygous littermates and control RIP1-Tag2 animals. We assessed the frequency of angiogenic switching among premalignant islets by 10.5 weeks of age, and the collective tumour volume at end stage (13.5 weeks; Fig. 6). No differences were observed between heterozygous knockout and wild-type littermates, and thus only results for wild-type control animals are shown. MMP-9-deficient RIP1-Tag2 mice developed fewer angiogenic islets (Fig. 6a) relative to control littermates, whereas MMP-2 or urokinase-deficient RIP1-Tag2 mice were comparable to controls (Fig. 6a). RIP1-Tag2 mice that were homozygous-null for either MMP-9 or MMP-2 had significantly reduced tumour burdens (Fig. 6b) in comparison to controls. Interestingly, the reduction was caused by decreased tumour number (~50%) and size in MMP-9-deficient mice, whereas MMP-2 homozygous-null mice developed smaller tumours without an effect on tumour number. Tumour growth in urokinase-deficient RIP1-Tag2 mice was not affected (Fig. 6b). These data show that absence of MMP-9 function reduces the frequency both of angiogenic switching in premalignant lesions and of progression to the tumour stage. Both proteinases contribute to tumour growth, but MMP-2, in contrast to MMP-9, does not affect the frequency of initial angiogenic switching or of progression to solid tumours. Surprisingly, absence of uPA did not affect tumour progression in RIP1-Tag2 mice. Histological analysis revealed no obvious differences in the angiogenic islets or tumours that formed in any of the three proteinase-null backgrounds (Fig. 7A). In each case, tumours were haemorrhagic, and ~50% had progressed into invasive carcinomas; this proportion was similar in wild-type tumours (Fig. 7A).
Figure 7. Characterization of protease-deficient angiogenic islets and tumours.
A, Histopathology of control (a), uPA-ko/ko (b), MMP-9-ko/ko (c) and MMP-2-ko/ko (d) tumours. Asterisks indicate exocrine pancreas. Shadowed arrows point to invasiveness and black arrows to haemorrhage formation. B, Proliferation and apoptotic rates in tumours of MMP-2-ko/ko or MMP-9 ko/ko RIP1-Tag2 mice, or RIP1-Tag2 mice that were treated in an experimental trial with SU5416, as compared to standard RIP1-Tag2 controls. Apoptotic cells were detected by TUNEL staining; proliferating cells were identified by PCNA staining. The significance of results was determined using a non-parametric bootstrap analysis.C, Formation of VEGF–VEGF-R2 complexes in control (a), MMP-2-ko/ko (b) and MMP-9-ko/ko (c) angiogenic islets and in control (d), MMP-2-ko/ko (e) and MMP-9-ko/ko (f) tumours. Black arrowheads in c indicate rare labelled cells.
The observation that inhibition of VEGF by SU5416 produced similar effects to the phenotype seen in the absence of MMP-9 gives rise to the hypothesis that the actions of MMP-9 and VEGF are linked. Thus, in SU5416-treated tumours and those lacking MMP-9, but not MMP-2, rates of proliferation of tumour cells, as measured by PCNA labelling, were reduced to similar extents, and the frequency of apoptosis was increased (Fig. 7B). If MMP-9 and VEGF are functionally interconnected, then angiogenesis that arises in the absence of MMP-9 should not exhibit association of VEGF with its receptors. Alternatively, the few angiogenic islets and tumours that escape the block in the absence of MMP-9 may utilize (and thus reveal) a second mechanism for activation of VEGF. To investigate these possibilities, we assessed the association of VEGF with VEGF-R2 in angiogenic islet progenitors and in solid tumours in MMP-2- and MMP-9-deficient Rip1-Tag2 mice (Fig. 7C). As expected from the unaltered incidence of angiogenic switching, VEGF ligand–receptor complexes were readily detected in the vasculature of MMP-2-deficient angiogenic islets and tumours (Fig. 7C, b and e). In contrast, the vasculature of the few angiogenic islet progenitors that formed in the absence of MMP-9 (Fig. 7C, c) showed little or no VEGF–VEGF-R2 complexes on endothelial cells. These data indicate that an alternative, VEGF-independent, mechanism may induce angiogenesis early in this tumourigenesis pathway when MMP-9 is missing, as is the case when VEGF signalling is inhibited by SU5416. Subsequently, the association of VEGF with VEGF-R2 was observed in tumours in the absence of MMP-9 (Fig. 7C, f), indicating that progression is accompanied by acquisition of alternative means of mobilizing VEGF.
Discussion
We have shown that MMP-9/gelatinase B is a functional component of the angiogenic switch during multistage pancreatic carcinogenesis in RIP1-Tag2 transgenic mice, and we propose that one of its functions is to increase the availability of the angiogenesis inducer VEGF. Several lines of evidence support this conclusion. First, biochemical assays revealed that the activity of MMP-9 is upregulated in angiogenic islets and persists in tumours. Second, the starburst assay of endothelial cells in collagen gels showed that MMP-9, but not MMP-2/gelatinase A or three other proteinases, can induce angiogenic activity in otherwise non-angiogenic normal islets. Third, only MMP-9 among the five proteinases tested increased the release of VEGF from normal islets in vitro. Fourth, antibodies that blocked VEGF impaired the MMP-9 induced angiogenic response in the starburst angiogenesis assay. Fifth, immunohistochemical analysis with a specific antibody against VEGF in complex with its signalling receptor (flk/VEGF-R2) revealed that VEGF is detectably associated with this receptor only in angiogenic islets and tumours, and not in normal or pre-angiogenic islets. Sixth, proteinase inhibitors used in therapeutic trials markedly reduced the frequency of the angiogenic switch and impaired tumour growth; a selective inhibitor of MMP-9 proved most effective at blocking the initial angiogenic switch. Seventh, crosses with gene-knockout mice revealed that only MMP-9 affects the initial angiogenic switch, whereas both MMP-2 and MMP-9 contribute to tumour growth, and urokinase has no apparent function. Eighth, functional interference of VEGF-receptor signalling impaired angiogenic switching and tumour growth, as did removal of MMP-9 function by pharmacological inhibition or gene knockout.
Notably, angiogenesis is still activated in islets, and solid tumours still form, where MMP-9 is absent or is functionally interfered with, which indicates that alternative regulatory mechanisms exist. Evaluation of angiogenic islets and tumours that developed in the absence of MMP-9 function indicates that the mechanism by which angiogenesis is induced in the previously normal islet vasculature may have aspects that make it distinct from the mechanism that sustains aberrant tumour angiogenesis, as discussed below.
Data from bioassays carried out on normal pancreatic islets and from the use of an antibody specific for VEGF-A complexed to its primary signalling receptor collectively argue that VEGF is sequestered in normal islets, and that it becomes available both in bona fide angiogenic islets concomitant with activation of MMP-9, and in normal islets treated ex vivo with MMP-9. Immunostaining of angiogenic islets that developed in MMP-9 knockout mice showed that VEGF-A was rarely associated with receptor, indicating that a parallel, VEGF-A-independent mechanism can activate the quiescent islet vasculature. As the VEGF-receptor inhibitor SU5416 was more effective than the MMP-9 inhibitor R94138 at blocking the initial switch, it is possible that other VEGF family members may also signal through VEGF-R2, and that these are not sequestered in normal islets in the same way as the constitutively expressed VEGF; transcriptional profiling on microarrays may shed light on these possibilities. Another candidate is aFGF, which has long been known as an inducer of angiogenesis, and which is also expressed in this pathway10 . aFGF has been implicated in angiogenic regulation in other systems27 and, by functional interference, in this one (A. Compagni and G. Christofori, personal communication). However, aFGF is expressed in both normal and angiogenic islets, so it too must somehow be mobilized, perhaps by other enzymes. Indeed, we observed that heparinase I–III can render control islets angiogenic in vitro. Thus, the angiogenic switch may be accomplished by activation of heparin-degrading enzymes27, such as heparanase, which is associated with a variety of tumour types28,29.
The regulation of angiogenesis in the solid tumours that progress from already angiogenic islets weeks after the initial angiogenic switch seems to be qualitatively different. This is shown by the findings that the VEGF-R inhibitor SU5416 had comparable efficacy to the MMP-I (that is, not markedly better), that gene knockouts of MMP-2 and MMP-9 impaired tumour growth to similar extents, and that VEGF was detected immunochemically in association with receptor 2 despite the absence of MMP-9. All these parameters are qualitatively distinct from those in angiogenic islet progenitors (see above), indicating marked differences in the mechanisms by which a normal vasculature is first induced to become angiogenic, and by which an angiogenic vasculature is sustained during tumour growth. In particular, we have no direct biochemical evidence that VEGF is unavailable to its receptor in tumours with or without MMP-9, in contrast to the situation in normal islets. It is therefore possible that the tumour microenvironment (reactive stromal cells, aberrant ECM, etc.) does not have the ability to sequester VEGF, meaning that it is intrinsically available. If so, MMP-2 and MMP-9 must have other functions in tumours, as their absence or functional inhibition impairs tumour growth. Alternatively, a sequestration capability may still exist, but other enzymes activated in tumours may also be able to mobilize VEGF. The similar, incomplete efficacies of the VEGF-R inhibitor SU5416 and the MMP inhibitor BB94 in blocking tumour growth support this latter interpretation, and implicate other angiogenic regulators (such as aFGF; see above) in tumours as well as in angiogenic islets. Irrespective of the biochemical mechanism, the data indicate that initial activation of angiogenesis in premalignant lesions has distinctive qualities to that of full-blown tumour angiogenesis. Notably, a distinction between premalignant and malignant angiogenesis is also evident in squamous carcinogenesis of the skin30.
It is reasonable to consider the potential generality of our results. The data argue clearly that MMP-9 can mobilize VEGF in both a normal tissue and a pre-angiogenic neoplastic derivative (hyperplastic islets) concomitant with induction of angiogenesis. Mobilization of VEGF by MMP-9 is also associated with angiogenesis of the developing bone-growth plate, where knockout of the MMP-9 gene and inhibition of VEGF similarly delay angiogenesis31. However, other developing tissues are not demonstrably affected by loss of MMP-9 function23, indicating either alternative mechanisms or broader redundancy in mobilizing proteinases. We anticipate that MMP-9 itself will prove to be involved in carcinogenesis of some organs, whereas in others the principle of proteolytic mobilization of angiogenic regulatory factors will be vested in different proteinases. In recent work, we have documented the involvement of MMP-9 in a transgenic mouse model of squamous carcinogenesis of the epidermis, where it serves to enhance keratinocyte proliferation and angiogenesis32. Furthermore, a correlation has been reported between prognosis of progression of human colon cancer and levels of MMP-9 in the blood33, indicating that MMP-9 may be involved in this cancer. In other organs, different proteinases may have similar functions. For example, MMP-2 has been implicated in angiogenic switching in subcutaneous transplant models of tumour progression25,34, whereas MT1-MMP, a membrane-associated proteinase, can participate in developmental and pathological angiogenesis35; in none of these cases is it clear whether sequestered VEGF is a target. Moreover, MMP-2 and MMP-9 can activate latent TGF-β, thereby enhancing malignant phenotypes36. Also, given the increasing number of known extracellular proteinases, it is conceivable that they are involved in other types of premalignant angiogenic switching and tumour angiogenesis.
Although urokinase and the gelatinases MMP-2 and MMP-9 have historically been associated with invasive phenotypes in cell-culture models, a surprising result has arisen from histological analysis of RIP1-Tag2 mice lacking MMP-2, MMP-9 or urokinase, as well as of mice treated with inhibitors of MMP family proteins or VEGF-R. None of these losses or attenuations of proteinase function altered the incidence with which adenomas progressed to invasive carcinomas. Although it is possible that a broader redundancy of function underlies this lack of correlation, the data indicate that these proteinases may not be critical determinants of the invasive phenotype, which may instead involve alterations in cell-interaction molecules such as E-cadherin37.
We have presented MMP-9 as a key regulator of angiogenesis in a model of multistage carcinogenesis, and raised many questions and opportunities for future investigation. One important issue is the identification of the cell type that supplies MMP-9 in this pathway; immunohistochemical analysis indicates that it is not the oncogene-expressing cancer cells, but rather rare cells that are associated with the vasculature. In another model, of HPV-16-induced squamous carcinogenesis, we have implicated mast cells and neutrophils as the key suppliers of MMP-9 (refs 30, 32).We predict that a cell derived from bone marrow will prove to be involved in the islet-carcinoma pathway. Another important question relates to the biochemical mechanisms by which VEGF-A is sequestered in normal islet tissue, and whether tumours have a similar capability; this ability is particularly intriguing in light of the fact that the ostensibly diffusible VEFG120 isoform is expressed at similar levels before and after the switch.
The observed constitutive expression and sequestration of VEGF-A in normal pancreatic islets and our demonstration that MMP-9 can selectively render such islets angiogenic, concomitant with increases in VEGF release, presents an interesting opportunity. Perhaps this knowledge will prove to be applicable to the development of islet-transplantation procedures to restore glucose homeostasis in diabetic individuals, in which treatment with MMP-9 ex vivo might facilitate the subsequent connection of transplanted islets to the host vasculature.
Another perspective relates to the differential contributions of MMP-9 and MMP-2 in the RIP1-Tag2 model. In this model, MMP-9 functions in angiogenic switching and tumour growth, whereas MMP-2 functions only in tumour growth. If this difference proves to be a widespread phenomenon, then it may have therapeutic implications. The data (ref. 22 and this study) indicate that specific inhibitors of MMP-9 and VEGF-R may be particularly effective as chemopreventive, and perhaps antimetastatic, agents that target the initial angiogenic switch in quiescent normal vasculature found in premalignant lesions or in normal tissue beds freshly seeded with disseminated tumour cells. MMP inhibitors with broader specificity that target MMP-2 and MMP-9 and others, as well as VEGF inhibitors, may prove effective in treating nascent solid tumours. However, previous results22 indicate that MMP-I (and, by inference, VEGF inhibitors) may not be effective at treating bulky, end-stage disease as single agents, perhaps as a result of the diversification in angiogenic regulation in solid tumours that we have inferred from this investigation.
Finally, it will be important to define the alternative triggers for the angiogenic switch that are implicated by the gene knockout and functional inhibition of MMP-9 and of its target VEGF. Perhaps if inhibitors of MMPs or VEGF are combined with inhibitors of the cryptic angiogenic regulators that are implicated by the incomplete efficacy of the former, it will be possible to completely prevent initial angiogenic switching and to shut down sustainable angiogenesis during carcinogenesis.
Methods
Tissue preparation
Normal pancreases and those of 5- and 10–12-week-old RIP1-Tag2 mice were removed. They were then either fixed in 4% paraformaldehyde/PBS overnight, subjected to graded dehydration through 50%, 70%, 95%, 100% ethanol, and xylene, and finally embedded into paraffin (Paraplast), or alternatively they were immersed in 30% sucrose/PBS for ~6 h, embedded in OCT freezing medium and stored at −80 °C(Tissue Tek). Frozen (10-µm thickness) and paraffin (5-µm thickness) sections were used for immunohistochemical analysis. Before use, frozen sections were air-dried and acetone-fixed; paraffin sections were deparaffinized and subjected to graded rehydration. For VEGF and flk-1 staining, paraffin sections were used. Prepared tissue sections were boiled in citric acid for 5 min to unmask antigens. After preblocking in 5% goat serum, 2% BSA, 0.3% Brij A detergent (Biomed Co., Foster City, California), VEGF staining was carried out with a 1:300 dilution of a rabbit anti-mouse VEGF (Ab-1) antibody (Neomarkers, Fremont, California), and flk-1 with a 1:30 dilution of a rabbit antihuman flk-1 antibody14. For visualization of the VEGF–flk-1 complex, paraffin-embedded and frozen tissues were used. Paraffin-embedded sections for VEGF–flk1 were pretreated with a 1:10 dilution of proteinase K (DAKO Corp., Carpinteria, California). Paraffin or frozen sections for VEGF–flk-1 staining were preblocked with an anti-immunoglobulin M antibody and subsequently with preblock buffer as described above. Paraffin sections (Fig. 2) were incubated with a 1:20 dilution of the mouse monoclonal antibody GVM39 (ref. 14). Frozen sections (Fig. 7c) were treated with a 1:50 dilution of directly biotinylated mouse monoclonal antibody GVM39. MMP-9 staining was carried out on paraffin sections pretreated with proteinase K as described above and incubated with a 1:500 dilution of a rabbit anti-mouse MMP-9 antibody38. Apoptotic cells were assessed on paraffin sections by TUNEL staining as described39. Proliferating cells were visualized with a 1:1000 dilution of a mouse monoclonal antibody against proliferating-cell nuclear antigen (PCNA, BioGenex, San Ramon, California) applied to paraffin sections pretreated with proteinase K. All primary antibody reaction products were visualized with respective biotinylated secondary antibodies and were then incubated with the chromophore diaminobenzidine and H2O2 and counterstained with methyl green.
Substrate zymography
Islets and tumours representing distinct histological stages of neoplastic progression were cut out of the pancreas, weighed and then homogenized (1:4 w/v) in lysis buffer containing 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate and 0.1% SDS. Equivalent amounts of soluble extract were analyzed by gelatin zymography40,41 on 10% SDS–polyacrylamide gels co-polymerized with substrate (1 mg ml−1 gelatin or 1 mg ml−1 casein/plasminogen) in sample buffer (10% SDS, 0.25 M Tris–HCl and 0.1% Bromphenol Blue, pH 6.8). After electrophoresis, gels were washed twice for 15 min in 2.5% Triton X-100, incubated for 16 h at 37 °C in 50 mM Tris–HCl and 10 mM CaCl2, pH 7.6, and then stained with 0.5% Coomassie Blue and destained with 50% methanol. The locations of active proteinase bands were determined by the presence of negative staining. Exposure of proenzymes within tissue extracts to SDS during gel-separation procedures led to activation without proteolytic cleavage42; hence the higher-Mr zymogen isoforms were visualized in the zymogram.
Collagen-gel (starburst) angiogenesis bioassay
Non-transgenic islets were isolated from C57Bl/6J mice by retrograde perfusion with collagenase P solution, and then incubated for 24 h at 37 °C in a 10% CO2 balanced air incubator, in DMEM containing 0.5% calf serum and either 0.5–2.0 ng µl−1 active MMP-9 (prepared as described38 or purchased from Calbiochem), 0.5–2.0 ng µl−1 MMP-2 (Calbiochem), 0.2 µg ml−1 urokinase (American Diagnostica, Greenwich, Connecticut), 0.0025% trypsin (Life Technologies, Inc.), 0.2 µg ml−1 collagenase P (Roche), 5–15 U ml−1 heparinase I–III (Sigma) or medium alone. Enzymes were tested for proteolytic activity under experimental conditions. The highest concentration of MMP-2 caused islets to fall apart without eliciting an angiogenic response. Bovine capillary endothelial cells (BCE) were trypsinized and resuspended in DMEM with 10% calf serum. Cells (4 × 105 per ml) were mixed in a 1:1 ratio with a chilled collagen solution (Vitrogen 100, Collagen Corp., Palo Alto, California) in MEM medium containing 0.1% NaHCO3, 2 mM glutamine and 10 mM HEPES. Aliquots (250 µl) of the collagen-cell mixture were pipetted into 48-well tissue-culture plates. Untreated and treated islets were added to each well before the solution was allowed to gel at 37 °C. As controls, 10 µM GM6001 (a gift from R. Galardy, Guilford, Connecticut) or 10 µg ml−1 neutralizing VEGF antibody (Neomarkers) was added to the collagen/endothelial cell/islet mixture. Culture plates were incubated in a 10% CO2, balanced air atmosphere and observed for endothelial cell growth, migration and tube formation.
ELISA for VEGF
One hundred non-transgenic islets, isolated from C57Bl6/J mice, were incubated in 300 µl DMEM containing 0.5% calf serum, with or without 1 ng µl−1 MMP-2 or MMP-9 (Calbiochem). After 24 h incubation in a 10% CO2 balanced air incubator, the conditioned medium was removed, and VEGF levels were assayed by a ‘sandwich’ ELISA kit (MMV00, R&D Systems, San Diego, California), according to the manufacturer’s instructions. Each assay condition was tested in triplicate; results shown are from three to five independent experiments.
Pharmacological intervention
The mice used in these studies were males and females of the RIP1-Tag2 transgenic mouse lineage5, inbred in the C57Bl/6J background. In the prevention trial, animals were treated from 5–10.5 weeks of age; in the intervention trial, mice were treated from 10–13.5 weeks of age. BB-94/Batimastat (British Biotech Pharmaceuticals, Oxford, UK) or R94138 (Sankyo Co. Ltd, Tokyo, Japan) was homogenized in 0.02% Triton and PBS, pH 7.0, and administered as an emulsion. Mice were treated every day with 30 mg kg−1, delivered by intraperitoneal injection. SU5416 (SUGEN Inc.) was provided in a vehicle formulation and 100 mg kg−1 were administered subcutaneously twice a week. Each group comprised five to ten mice; experiments were carried out at least twice. All control animals received intraperitoneal saline injections. All mice were maintained in accord with the University of California, San Francisco, institutional guidelines governing the care of laboratory mice and were euthanized after their respective treatment periods.
Assessment of angiogenic islets and tumour incidence
Angiogenic islets were isolated by retrograde perfusion with collagenase P and counted. Angiogenic islets were identified as those that exhibited a reddish patch or patches (caused by haemorrhaging) in a white nodular background. The visual scoring scheme was confirmed by histology as described43. Tumours were microdissected from freshly excised pancreases. Tumour volumes (in mm3) were measured with calipers, and the formula [volume = 0.52 × (width)2 × length] for approximating the volume of spheroid was applied. Tumour burden per mouse was calculated by accumulating the tumour volumes of every mouse.
Generation of RIP1-Tag2, proteinase-null mice
Generation and characterization of MMP-2 (refs 24,25) and MMP-9 (ref. 23) homozygous-null animals has been reported. Urokinase-deficient mice26 were from Jackson Laboratories. MMP-2-ko/ko and urokinase-ko/ko mice were crossed successively with RIP1-Tag2 transgenic mice (~N30 C57BI/6) to generate RIP1-Tag2, MMP-2-ko/ko and -ko/+, and RIP1-Tag2 , urokinase-ko/ko and -ko/+ mice. MMP-9-ko/+ animals were first backcrossed into the C57Bl/6 strain three to four generations before intercrossing with RIP1-Tag2 transgenic mice to generate RIP1-Tag2, MMP-9-ko/ko and -ko/+ animals. Each group contained 10–15 animals.
ACKNOWLEDGEMENTS
We thank British Biotech Pharmaceuticals, Oxford, UK, for BB-94.We also thank E. Soliven, C. Skinner and O. Behrendtsen for excellent technical assistance, A. McMillan for help with statistical analysis, W. Ruth and T. Schoop of Biomed Arts for help with figures, D. Daniel and L. Coussens for valuable discussions, and B. Bowes and J. Folkman for support and encouragement. This work was funded by grants from the National Cancer Institute.
References
- 1.Hanahan D, Weinberg R. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Tumour angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumourigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 4.Bouck N, Stellmach V, Hsu SC. How tumours become angiogenic. Adv. Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D. Heritable formation of pancreatic β-cell tumours in transgenic mice harboring recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 7.Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr. Top. Microbiol. Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- 8.Kerbel RS, Viloria-Petit A, Okada F, Rak J. Establishing a link between oncogenes and tumour angiogenesis. Mol. Med. 1998;4:286–295. [PMC free article] [PubMed] [Google Scholar]
- 9.Faller DV. Endothelial cell responses to hypoxic stress. Clin. Exp. Pharmacol. Physiol. 1999;26:74–84. doi: 10.1046/j.1440-1681.1999.02992.x. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Christofori G, Naik P, Arbeit J. Transgenic mouse models of tumour angiogenesis: the angiogenic switch, its molecular controls, and prospects for preclinical therapeutic models. Eur. J. Cancer. 1996;32A:2386–2393. doi: 10.1016/s0959-8049(96)00401-7. [DOI] [PubMed] [Google Scholar]
- 11.Christofori G, Naik P, Hanahan D. Vascular endothelial growth factor and its receptors, flt-1 and flk-1, are expressed in normal pancreatic islets and throughout islet cell tumourigenesis. Mol. Endocrinol. 1995;9:1760–1770. doi: 10.1210/mend.9.12.8614412. [DOI] [PubMed] [Google Scholar]
- 12.Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997;57:765–772. [PubMed] [Google Scholar]
- 13.Vajkoczy P, et al. Inhibition of tumour growth, angiogenesis, and microcirculation by the novel flk-1 inhibitor SU5416 as assessed by intravital multi-fluorescence videomicroscopy. Neoplasia. 1999;1:31–41. doi: 10.1038/sj.neo.7900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brekken RA, Huang X, King SW, Thorpe PE. Vascular endothelial growth factor as a marker of tumour endothelium. Cancer Res. 1998;58:1952–1959. [PubMed] [Google Scholar]
- 15.Cheng SY, Nagane M, Huang HS, Cavenee WK. Intracerebral tumour-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc. Natl Acad. Sci. USA. 1997;94:12081–12087. doi: 10.1073/pnas.94.22.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrara N, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 17.Carmeliet P, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 18.O’Reilly MS, Widerschain D, Stetler-Stevenson WG, Folkman J, Moses MA. Regulation of angiostatin production by matrix metalloproteinase-2 in a model of concomitant resistance. J. Biol. Chem. 1999;274:29568–29571. doi: 10.1074/jbc.274.41.29568. [DOI] [PubMed] [Google Scholar]
- 19.Talbot DC, Brown PD. Experimental and clinical studies on the use of matrix metalloprotease inhibitors for the treatment of cancer. Eur. J. Cancer. 1996;32A:2528–2533. doi: 10.1016/s0959-8049(96)00398-x. [DOI] [PubMed] [Google Scholar]
- 20.Brown PD. Clinical studies with matrix metalloprotease inhibitors. Acta Pathologica Microbiologica et Immunologica Sacndinavica. 1999;107:174–180. doi: 10.1111/j.1699-0463.1999.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 21.Tamaki K, et al. Synthesis and structure-activity relationships of gelatinase inhibitors derived from matlystatins. Chem. Pharmaceut. Bull. 1995;43:1883–1893. doi: 10.1248/cpb.43.1883. [DOI] [PubMed] [Google Scholar]
- 22.Bergers G, Javaherian K, Lo K-M, Folkman J, Hanahan D. Differential effects of angiogenesis inhibitors on distinct stages of carcinogenesis. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 23.Vu TH, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh T, et al. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloprotease 2)-deficient mice. J. Biol. Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- 25.Itoh T, et al. Reduced angiogenesis and tumour progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 26.Carmeliet P, et al. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nature Gen. 1997;17:439–444. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- 27.Vlodavsky I, et al. Extracellular matrix-resident growth factors and enzymes: possible involvement in tumour metastasis and angiogenesis. Cancer Metastasis Rev. 1990;9:203–226. doi: 10.1007/BF00046361. [DOI] [PubMed] [Google Scholar]
- 28.Hulett MD, et al. Cloning of mammalian heparanase, an important enzyme in tumour invasion and metastasis. Nature Med. 1999;7:803–809. doi: 10.1038/10525. [DOI] [PubMed] [Google Scholar]
- 29.Vlodavsky I, et al. Mammalian heparanase: gene cloning, expression and function in tumour progression and metastasis. Nature Med. 1999;7:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 30.Coussens LM, et al. Inflammatory mast cells upregulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerber H-P, et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 32.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. doi: 10.1016/s0092-8674(00)00139-2. (in the press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zucker S, et al. Measurement of matrix metalloprotease and tissue inhibitors of metalloproteinases in blood and tissues. Clinical and experimental applications. Ann. NY Acad. Sci. 1999;878:212–227. doi: 10.1111/j.1749-6632.1999.tb07687.x. [DOI] [PubMed] [Google Scholar]
- 34.Fang J, et al. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumour model. Proc. Natl Acad. Sci. USA. 2000;97:3884–3889. doi: 10.1073/pnas.97.8.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Z, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl Acad. Sci. USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Q, Stamenkovic I. Cell surface-localized metalloproteinase-9 proteolyically activates TGF-beta and promotes tumour invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 37.Perl AK, et al. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 38.Behrendtsen O, Alexander CM, Werb Z. Metalloproteinases mediate extracellular matrix degradation by cells from mouse blastocyst outgrowths. Development. 1992;114:447–456. doi: 10.1242/dev.114.2.447. [DOI] [PubMed] [Google Scholar]
- 39.Naik P, Karrim J, Hanahan D. The rise and fall of apoptosis during multistage tumourigenesis: downmodulation contributes to progression from angiogenic progenitors. Genes Dev. 1996;10:2105–2116. doi: 10.1101/gad.10.17.2105. [DOI] [PubMed] [Google Scholar]
- 40.Herron GS, Werb Z, Dwyer K, Banda MJ. Secretion of metalloproteinases by stimulated capillary endothelial cells. I. Production of procollagenases and prostromelysin exceeds expression of proteolytic activity. J. Biol. Chem. 1986;261:2810–2813. [PubMed] [Google Scholar]
- 41.Herron GS, Banda MJ, Clark EJ, Gavrilovic J, Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J. Biol. Chem. 1986;261:2814–2818. [PubMed] [Google Scholar]
- 42.Talhouk RS, Chin JR, Unemori EN, Werb Z, Bissell MJ. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991;112:439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parangi S, Dietrich W, Christofori G, Lander ES, Hanahan D. Tumour suppressor loci on mouse chromosomes 9 and 16 are lost at distinct stages of tumourigenesis in a transgenic model of islet cell carcinoma. Cancer Res. 1995;55:6071–6076. [PubMed] [Google Scholar]