Abstract
Proteins and traces of polysaccharide are the only polymeric colloids consistently transported in the xylem sap of plants. The hypothesis that such proteins could have physical inhibitory effects on xylem water transport was investigated. Ovalbumin, with a molecular weight of 45 kDa and a molecular diameter of 5.4 nm, is an inert, water-soluble protein that is midway along the size range of endogenous xylem sap proteins. Solutions of ovalbumin conjugated to a fluorescent marker and supplied to transpiring shoot explants of tobacco (Nicotiana tabacum L.) and olive (Olea europaea L.) were shown by confocal laser scanning microscopy to accumulate specifically at wall-based pit membranes that connect neighbouring xylem conduits. In addition, pressure-induced perfusion of micro-filtered ovalbumin solutions, at concentrations similar to those of endogenous xylem sap proteins, through the xylem of tobacco stem or olive twig segments resulted in the retention of c. 40% of the ovalbumin and reductions in the axial hydraulic conductance of the xylem. Smaller molecules such as Texas Red 3000 (MW 3 kDa) and Alexafluor 488–cadaverin conjugates (MW 0.64 kDa) did not show similar characteristics. The partial reduction in xylem hydraulic conductance appeared to be related to the accumulation of ovalbumin at xylem pit membranes and the consequent fouling of trans-membrane water-conducting pores with smaller diameters than those of the ovalbumin molecules. Potential implications of these novel findings for whole-plant water relations are considered.
Keywords: Alexafluor 488, CLS microscopy, membrane-fouling, ovalbumin, pit-membranes, plant water, pores, sap protein, xylem conductance
Introduction
In higher terrestrial plants, transpirational water-loss through open stomata in the leaves accompanies CO2 uptake for photosynthesis. Continuous replacement of the water lost by transpiration with soil-derived water is essential for plant function and survival. Under optimal conditions, replacement water is rapidly transported under tension from the soil–root continuum to the transpiring leaves, via the xylem system. However, numerous environmental stress factors, both biotic and abiotic, are known to cause decreases in the axial hydraulic conductance of the xylem and thus, adversely to affect plant functioning (see reviews by McDowell et al., 2008; Brodribb, 2009). This report considers the potential effects of an internal factor, i.e. xylem transported protein.
The xylem-system is a vascular continuum which provides a relatively low resistance, apoplastic pathway for transporting aqueous sap from the roots to the leaves. It comprises interconnected bundles of mature xylem cells. Several mature xylem cells, consisting of lignified secondary-walls and hollow lumens, can be joined together to form finite conduits. The sap that flows through the lumens in these conduits typically contains dissolved nutrient ions, amino acids, hormonal signals, proteins, and traces of carbohydrate (Biles and Abeles, 1991; Davies and Zhang, 1991; Gollan et al., 1992; Satoh et al., 1992; Goodger et al., 2005). The transport of sap between adjacent xylem conduits occurs via numerous pits set into the lignified secondary walls. In angiosperm plants, adjacent pits are separated by pit membranes consisting of primary-wall material. Primary walls are based on cellulose fibre matrices interspersed with up to 800 different types of cell wall proteins (Albenne et al., 2009). The xylem pit membranes are traversed by tortuous pores with mean diameters at around 5 nm, as estimated by perfusion experiments with gold nano particles (Shane et al., 2000; Choat et al., 2003). Pit membranes with far larger pore diameters are also present (Jarbeau et al., 1995). The pit membranes are thought to have lower flow resistances than the thicker secondary cell walls surrounding them and to provide the pathway of least resistance during sap transport from one vessel to another. Nevertheless, they may account for around 50% of overall xylem resistance to flow (see review by Choat et al., 2008).
Proteomic investigations using gel separations and, more recently, tandem mass spectrometry have identified a wide range of proteins, including proteases, protease inhibitors, lipid transfer proteins, peroxidases, and wall metabolizing enzymes that are transported in the xylem-sap of numerous plant species. These sap proteins can range from small polypeptides with molecular weights less than 5 kDa to larger proteins with molecular weights exceeding 90 kDa. The reported total concentrations of sap protein assayed by different methods consistently range between tens and a few hundreds μg ml−1 of sap (Biles and Abeles, 1991; Satoh et al., 1992; Zhu and Zhang, 1997; Bhutz et al., 2004; Alvarez et al., 2006; Djordjevic et al., 2007).
The origins of the apparently ubiquitous sap proteins are unclear. They may be released during the development of mature xylem vessels from immature, live-cell precursors. Moreover, the sequences of most xylem sap proteins reveal predicted N-terminal secretion signals and some may, therefore, be secreted into the xylem from neighbouring tissues. Sap polypeptides that are small enough to be readily transported across cell walls and to move in or out of the xylem, may have signalling roles (Djordjevic et al., 2007; Neumann, 2007, and references therein). Larger proteins in the xylem sap may have roles in cell wall metabolism and defence against microbial invasions (Alvarez et al., 2006; Satoh, 2006).
It has been found that nano-sized particles of inorganic and organic colloids in the external water source can reduce root hydraulic conductance in a time-, concentration-, and size-dependent manner by accumulating at root cell-wall surfaces and limiting the passage of water through the nano-sized water-conducting pores that traverse the polysaccharide-protein matrices of primary cell walls (Asli and Neumann, 2009; Asli and Neumann, in preparation). This suggested the possibility that nano-sized protein molecules dissolved in plant xylem sap might similarly accumulate at wall-based xylem pit-membranes and reduce their hydraulic conductance.
The specific aims of this investigation were: (i) to use confocal laser scanning microscopy to determine whether ovalbumin, an inert, water-soluble protein with an intermediate molecular weight (45 kDa) and a calculated molecular diameter of 5.4 nm (Baig and Salahuddin, 1978) would accumulate at pit membranes when transported in the xylem of herbaceous and woody angiosperms; and (ii) to quantify any effects of ovalbumin transport through the xylem on axial hydraulic conductance.
Materials and methods
Flow assays
Olive
Leaf free sections of olive (Olea europaea L.) twigs of c. 0.6 cm diameter from well-watered trees in the Technion ecological garden were cut into water at dawn. In the laboratory they were re-cut under water. Both ends were repeatedly rinsed and blotted to remove contaminating debris. The basal end was then inserted into the test solution in a reservoir held inside a pressure bomb. The exposed xylem at the protruding top end was fitted to scale-marked Tygon tubing. The twig sections (20±1 cm length, mean ±SE, n=14) were then flushed with a dilute salt solution (0.1 mM CaCl2 and 0.1 mM KCl) for 30 min at 0.18 MPa. After 30 min, when xylem embolisms had been removed and flow was stable, the perfusate was switched to the same solution containing ovalbumin. Flow was assayed by recording the volume of perfusate entering the scaled tubing at regular intervals.
Tobacco
Leaves and petioles on shoots of 4-week-old, well-irrigated, tobacco plants (Nicotiana tabacum L. cv. Petit Havana) grown in a chamber at 27 °C with 16 h daylight (light irradiance of 200 μE m−2 s−1) in the Department of Horticulture at Florence University were excised in the dark, and the stem–petiole junctions sealed with Parafilm. The stems were then recut under water and repeatedly rinsed and blotted for 10 min to free debris from the cut ends. For flow assays the basal end was connected to a graduated pipette filled with test solutions prepared with ultra-pure water (Sartorius-Stedim arium 611VF) and in turn connected to a nitrogen cylinder with a pressure regulator. Pressures of 0.01 or 0.05 MPa were used to propel solutions through the stem sections (20±1 cm length, mean ±SE, n=6). Care was taken to avoid the accidental introduction of air into the xylem during rapid switches between water and protein solutions.
Ovalbumin perfusion of xylem
Ovalbumin (Grade 11, Sigma) was used as a model protein because it is relatively inert, water soluble and has a molecular weight (45 kDa) that is midway along the range of molecular weights of proteins found in xylem sap. In addition, ovalbumin is available for microscope assays with an attached fluorescent probe (Alexafluor 488, Molecular Probes). Ovalbumin solutions were freshly prepared for each experiment and filtered through 0.20 or 0.22 μm membrane filters before assay of final protein concentrations (Bradford, 1976) and xylem perfusion. SDS 10% polyacrylamide gel electrophoresis (Laemmli, 1970) of the membrane filtered ovalbumin solutions revealed a single Coomassie-blue stained band at ∼45 kDa with traces of dimer at ∼90 kDa.
Ovalbumin solutions at mean concentrations of 96±15 μg ml−1 (n=6, ±SD) for use with tobacco stems, or at 70 and 35 μg ml−1 for use with olive twigs, were perfused through the xylem for up to 60 min.
The percentage retention of protein [100×(protein in) – (protein out)/[protein in]) by tobacco and olive segments was assayed after 5 min of perfusion. In order to evaluate the effects of molecular size, percentage retention of solutions containing 400 μg ml−1 of smaller Texas Red 3000 molecules (MW 3 kDa, Molecular Probes) was similarly estimated by emission spectroscopy, in black microplates (Costar 3915, Corning Inc, USA) using a fluorescence spectrometer (Tecon Infinite M2000 Mamedorf, Switzerland) with excitation at 595 nm and fluorescence emission measured at 625 nm and two dilutions.
Xylem sap assay
Xylem sap protein levels were estimated after collection of sap (Bhutz et al., 2004; Goodger et al., 2005; Neumann, 2007). Briefly, tobacco shoots were excised 2 cm above the soil level with fresh razors. After repeated rinsing and blotting of the remaining cut stump, spontaneous root pressure exudates were collected for 60 min into tubing attached to the stump and analysed for protein content. Xylem sap in year old olive seedlings kindly provided by KKL Nurseries, Golani junction, Israel was similarly obtained except that the water-saturated root media was enclosed in a pressure bomb and a ∼1.5 cm section of the bark was surgically removed from the apical end prior to washing, blotting, and connection of protruding xylem tissue to collection tubing. The pressure (0.18 MPa)-induced exudate was collected at 10 min intervals over 30 min into tygon tubing attached to the cut stump which protruded from the pressure bomb and protein assayed as above. Pooled means of the measurements are presented.
Fluorescence microscopy
Stock solutions of the fluorescent dye Alexafluor 488 conjugated with ovalbumin (MW 46 kDa) were prepared according to the manufacturer's instructions (Molecular Probes). To facilitate subsequent detection in the xylem, stock solutions were diluted to a relatively high concentration of 400 μg ml−1. After centrifugation for 8 min at 1100 g, probe solutions were loaded into the xylem of leafy transpiring explants cut from the apices of olive branches and tobacco stems, via the cut base. Care was taken to minimize light exposure and possible photodegradation of the fluorescent dye during the 30 min of tissue loading. After loading, the surfaces of the basal sections of the treated stem and twig explants were rinsed and a razor was used to cut cross-sections at 1–2 cm from the base. The cross-sections were repeatedly rinsed in water and then imaged by confocal laser scanning microscopy (Leica, TCS SP5) using a ×40 objective and a ×63 oil immersion objective. Excitation of Alexafluor conjugates was at 488 nm and fluorescence emission was detected with a 510–530 nm spectral range. Perfusions of water or solutions of smaller Alexafluor488–cadaverin conjugates (MW 0.64 kDa) at 400 μg ml−1 were used for controls.
Results
The averaged concentrations of endogenous proteins measured in xylem sap exudates were 24±2 μg ml−1 for tobacco and 102±58 μg ml−1 for olive seedlings (means ±SD, n=4). The values were within the range previously reported in the literature for sap collected from other plant species. Effects of similar concentrations of ovalbumin protein on solution flow through tobacco stems or olive twig sections were next assayed.
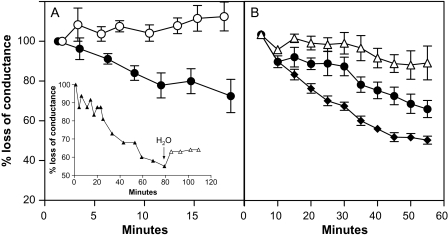
The averages of initial hydraulic conductance values for the pressurized flow of protein-free solutions through tobacco stems and olive twig sections were, respectively, 3.0±1.8 cm3 min−1 MPa−1 (mean ±SD, n=6) and 0.4±0.1 cm3 min−1 MPa−1 (mean ±SD, n=14). Xylem perfusion with ovalbumin protein solutions rapidly reduced conductance in both species as compared with zero protein controls (Fig. 1). Protein-induced flow reduction was observed with or without the additions of 1 mM each of K and Ca ions to the perfused ovalbumin solution (in olive and tobacco, respectively). Thus, the reduction of xylem conductance by ovalbumin did not appear to be dependent on additional ionic interactions with either the protein or the xylem. Protein-induced reductions in xylem conductance could be partly reversed by switching perfusion solutions from ovalbumin to water flowing in the same direction (e.g. Fig 1, inset). This suggested some degree of irreversible interaction between ovalbumin and xylem. The specific accumulation of fluorescent albumin at the xylem pit membranes of both species (see below) supported this conclusion, as did the fact that c. 40% of the ovalbumin in solutions passing through both tobacco and olive sections was retained by the segments (Table 1). Perfused solutions of smaller molecules were not retained. Thus, the levels of smaller Texas Red–dextran 3000 (MW 3 kDa, 400 μg ml−1) in assay solutions entering and leaving tobacco stems were very similar at 19 525±1 382 and 19 148±1 744 microplate fluorescence units, respectively (means ±SD, n=4).
Fig. 1.
Ovalbumin solution causes partial reductions in xylem flow through tobacco stem and olive twig segments. Filled symbols show flow-reduction expressed as mean percentages of initial (100%) conductance, during perfusion of (A) tobacco stem sections with membrane-filtered solutions containing 96±15 μg ml−1 ovalbumin (means ±SD, n=6), or (B) during perfusion of olive twig sections with filtered ovalbumin solutions at either 70 μg ml−1 (filled diamonds, n=5) or 35 μg ml−1 (filled circles, n=3). Open symbols in each case are for equivalent controls without ovalbumin. Vertical bars indicate ±SE. Segments were approximately 20 cm long and initial conductances were 3.0±1.8 cm3 min−1 MPa−1 (mean ±SD, n=6) for tobacco and 0.4±0.1 cm3 min−1 MPa−1 (mean ±SD, n=14) for olive. Inset (A) shows the kinetics of longer-term flow-reductions induced by ovalbumin in a tobacco stem and limited flow-recovery following transfer (at arrow) from ovalbumin to water flowing in the same direction.
Table 1.
Partial protein retention during xylem perfusion
| Ovalbumin entering | Ovalbumin leaving | Protein retention | |
| (μg ml−1) | (μg ml−1) | (%) | |
| Tobacco | 101±19a | 61±23b | 41 |
| Olive | 57±11a | 32±9b | 44 |
Sections (∼20 cm) of tobacco stem or olive twig were perfused with ovalbumin solutions. Concentrations in the solutions entering or leaving the sections were assayed as in the Materials and methods. Means ±SD, n ≥3, differences in rows significant at P=0.05 using Student's t test.
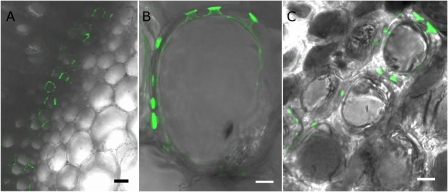
Transpiring, leafy tobacco shoots and olive twigs, i.e. explants, were allowed to take up fluorescent albumin solution via their cut bases and confocal laser scanning microscope images of freshly cut, well-rinsed cross-sections were taken c. 2 cm from the point of probe entry at the base of the explants. Figure 2A shows, at low magnification, the restriction of fluorescent ovalbumin accumulation (green coloration) to some of the xylem within the darker region occupied by the vascular bundle and the comparative absence of accumulation in tobacco pith parenchyma cells adjacent to the vascular bundle. Higher magnification of vascular bundles in both species revealed the specific accumulation of fluorescent ovalbumin inside the xylem vessel pits and, often, at one side of the pit membranes (Fig. 2B, C). There was little or no staining of the xylem secondary walls and no apparent protein accumulation in the xylem lumens. Figure 2C shows the accumulation of fluorescent protein in pits connecting olive xylem cells to several neighbouring xylem cells. Additions of Ca and K ions at 1 mM each and pressurized perfusion of fluorescent ovalbumin solutions instead of transpirational uptake gave similar results (not shown). No pit-specific fluorescence was observable in protein-free controls or when xylem was perfused with solutions of a smaller Alexafluor 488–cadaverin conjugate (MW 0.64 kDa) at equivalent concentrations. It therefore seemed that some of the pit membranes were able to delay or prevent the passage of ovalbumin molecules between adjacent xylem cells and that the resultant local accumulation of protein may have inhibited water transport.
Fig. 2.
Accumulation of fluorescently labelled ovalbumin at pit membranes in xylem of tobacco and olive. Leafy explants of tobacco stem apex and olive twigs were allowed to take up solutions of Alexafluor 488–ovalbumin conjugate by transpiration. Confocal laser scanning microscopy was used to locate any sites of green fluorescence accumulation in rinsed cross-sections taken 1–2 cm from the cut base of the tobacco stems and olive twigs. Specific accumulation of the ovalbumin conjugate can be seen in the walls of tobacco xylem vasculature adjacent to the large pith parenchyma cells (A). At higher magnification, unilateral accumulation at several pit membranes of an individual tobacco xylem cell can be discerned (B). Similar accumulation at pit membranes is observed in the pits connecting to several neighbouring xylem cells in an olive twig cross-section (C). Horizontal bars=50 μm in (A) or 5 μm in (B) and (C).
Discussion
The results raise two important questions. (i) What are the mechanisms involved in the reduction of xylem flow by ovalbumin solution? (ii) Can laboratory findings with a model protein be used to predict the potential effects of endogenous xylem sap proteins in whole plants under natural conditions?
Mechanism of flow reduction by ovalbumin solution
The axial conductance of the xylem is intrinsically dependent on numerous variables such as the number of conduits, their lengths and their diameters. Clearly, the protein perfusion experiments did not affect the number or length of existing conduits. Similarly, perfusion of fluorescent ovalbumin did not result in apparent restrictions of xylem vessel diameter by wall coating or deposition of large protein aggregates in the lumens. The bulk viscosity of the ovalbumin solutions used was also unlikely to be the primary cause of flow reduction since the flow inhibitory effects of solution viscosity would be expected to be nearly immediate while the onset of the observed flow inhibitory effects was gradual. Moreover, subsequent, rinsing of protein-perfused xylem with water did not restore original flow rates, as would be expected if solution viscosity were a key inhibitory factor. A default explanation, indicated by the specific accumulation of Alexa 488–ovalbumin conjugate at xylem pit membranes (Fig. 2) is that the physical accumulation of ovalbumin at pit membranes was related to the reduction of flow.
What caused the protein to accumulate at pit membranes? In view of the inert nature of ovalbumin, selective enzymatic binding to primary cell wall components of the pit membranes seems unlikely. Moreover, the fact that perfusion of the much smaller Alexa 488–cadaverin conjugate (MW 0.64 kDa) did not result in pit membrane accumulation suggests that the accumulation of the ovalbumin conjugate was primarily related to characteristics of the protein part of the molecule rather than the conjugated Alexa 488 probe. In this context it is interesting that relatively small molecules of Texas Red–Dextran 3000 (MW 3 kDa) also showed unhindered passage through the tobacco stems while ovalbumin did not. Van Alfen and Allard Turner (1979) showed that xylem retention and flow reduction could be induced by polymeric dextrans with molecular weights ≥250 kDa while smaller molecules passed through. As with dextrans, xylem-pit-membrane retention of proteins is likely to be size-related.
The much researched mechanisms involved in the fouling of synthetic nano-filtration membranes and associated flow reduction provide a useful comparative source of information. These synthetic membranes are able to remove contaminants such as dissolved proteins or natural organic matter from water sources. Small water molecules pass through the nano-sized pores which traverse the membranes but concentration, size, and time-related concentration polarization combined with electrostatic and Van de Waals interactions result in the physical retention of larger protein molecules at the membrane surfaces. A physical inhibition of membrane hydraulic conductance then occurs as the dissolved proteins with molecular diameters exceeding those of the membrane pores form surface cake layers (reviewed by Goosen et al., 2004).
Similar physical fouling mechanisms can be invoked to explain the interaction between sap proteins and xylem pit membranes: Dissolved ovalbumin molecules, with a calculated molecular diameter of 5.4 nm (Baig and Salahuddin, 1978), or more if the hydration shell is included, would not be expected to traverse pit membrane pores with only 5 nm diameters. Instead they would form cake layers at pit-membrane surfaces, as suggested by confocal microscope images (Fig. 2B, C). The passage of water molecules through the pit membranes would thus be hindered. Ovalbumin molecules would, however, be expected to pass unhindered through the pit membranes with larger pore diameters (≥20 nm) that are also found in the xylem (Jarbeau et al., 1995; Choat et al., 2008; Jansen et al., 2009). This may partly explain the fact that ∼60% of perfused protein passed through the xylem in tobacco and olive segments. An additional explanation is the likelihood of unhindered passage of protein solution through those xylem conduits of >20 cm length that were open at both ends, thereby effectively by-passing the need to traverse pit membranes (Trifilo et al., 2007; Raimondo et al., 2009). The important point here is that ∼ 40% of perfused ovalbumin was retained by both tobacco and olive xylem. In both cases, the ovalbumin was shown to accumulate specifically at xylem pit membranes and, in both cases, hydraulic conductance declined.
The potential whole plant effects of endogenous xylem sap proteins
The finding that physical interactions between perfused ovalbumin solutions and xylem pit membranes are associated with pit accumulation and partial reduction of axial hydraulic conductance in excised segments of both olive and tobacco provides support for the hypothesis that similar interactions could occur between endogenous sap proteins and the xylem pit membranes of intact transpiring plants. The concentrations of ovalbumin used in the pressurized flow experiments (35–120 μg ml−1) were within the concentration ranges of total endogenous protein measured in the xylem sap of tobacco and olive and in sap samples collected from numerous other species by a variety of methods; i.e. from tens to a few hundreds of μg ml−1 (cf. Biles and Abeles, 1991; Satoh et al., 1992; Zhu and Zhang, 1997; Bhutz et al., 2004; Alvarez et al., 2006; Satoh, 2006; Djordjevic et al., 2007; Neumann, 2007). However, xylem sap contains proteins in a range of sizes. The smaller protein molecules, with molecular diameters <5 nm may pass unhindered through pit membrane pores with average diameters of 5 nm and therefore have no fouling effects. Larger proteins with molecular weights equivalent to or greater than that of ovalbumin (45 kDa) and associated molecular diameters equivalent to or greater than 5.4 nm would, however, be expected to accumulate at pit membrane surfaces and foul some of the pit membrane pores. Bhutz et al. (2004) identified several proteins with molecular weights exceeding 50 kDa in xylem sap from broccoli (Brassica oleracea), rape (Brassica napus), pumpkin (Cucurbita maxima), and cucumber (Cucumis sativus). Similarly, a comprehensive study of maize (Zea mays) xylem sap by Alvarez et al. (2006) used 2D gel elecrophoresis and subsequent nano ESI-MS/MS assays to identify 154 different sap proteins. Of these 68 had experimentally determined molecular weights greater than 45 kDa. Thus, many of the so far identified sap proteins are potentially large enough to block xylem pit membrane pores.
Among several caveats to be considered before extrapolating our findings to whole plants under natural conditions is the fact that axial water flow through the xylem in roots and in stems of intact plants sometimes appears to exceed shoot water requirements under well-watered conditions (Van Alfen and Allard-Turner, 1979; Bramley et al., 2009). In such cases, any occurrence of a partial blockage of stem xylem flow by sap proteins need not necessarily reduce xylem hydraulic conductance and water availability to the leaves below the threshold levels required for optimal leaf functioning. Albeit, other reports indicate that increases in stem resistance can adversely influence leaf water status in whole plants (Sperry et al., 1993; Hubbard et al., 2001). In addition, protein-induced reductions in xylem hydraulic conductance could also occur after passage through the stem, at more apical bottlenecks in petioles and leaf blades (Baum et al., 2000; Brodribb, 2009). Another caveat is that proteomic analyses of xylem sap invariably reveal the presence of proteases. These might act gradually to break down proteins at pit membrane surfaces, thereby lessening any flow-inhibitory effects.
A final consideration is that protein solutions might aggregate into large units which could clog the xylem lumens (Munns et al., 1993; Zhu and Zhang, 1997). However, the ovalbumin solutions used by us were freshly prepared and micro filtered or centrifuged (in the case of Alexa conjugates) to remove protein aggregates before xylem perfusion. Moreover, there was no microscopic evidence of large protein aggregates in the lumens of xylem perfused with fluorescent albumin. Although aggregation of ovalbumin within the xylem, or indeed, of endogenous sap proteins under natural conditions, cannot be completely ruled out, it is noteworthy that such occurrences would further increase the probability of protein induced flow reductions.
A strong argument in support of the hypothesis that pit membrane fouling by sap proteins will also occur under natural conditions is that the responses to xylem perfusion with ovalbumin solution were established within minutes in the laboratory assays. The degree to which endogenous sap proteins may reduce whole plant hydraulic conductance under natural conditions will be influenced by the concentration in the sap of large proteins with molecular diameters >5 nm, the diurnally changing rates of sap flow and, most importantly, the seasonal duration of sap flow (months rather than minutes). Thus, the eventual occurrence of a physical reduction of xylem hydraulic conductance by the endogenous sap proteins appears likely because of the extended time during which sap with dissolved proteins will necessarily flow through the xylem pit membranes.
A confirmatory demonstration of the in planta accumulation of endogenous xylem sap proteins at pit membranes is clearly of interest and future research directions could include (i) The development of methods for assaying time-related increases in the accumulation of endogenous sap proteins at the pit membranes. Albeit, these membranes consist of primary cell wall material and the presence of basal levels of intrinsic cell wall proteins (Albenne et al., 2009) may complicate such measurements. (ii) Investigations of possible correlations between engineered or species related differences in endogenous sap protein concentrations, pit membrane thicknesses (Jansen et al., 2009) and xylem water transport. (iii) Investigations of the possibility that protein fouling is involved in age-related reductions in the hydraulic conductance of leaf blade junctions that were measured, from the base up, over a period of weeks and accompanied sequential onset of leaf senescence in whole plants (Neumann and Stein, 1984; Neumann, 1987). These or other approaches should help to further establish the magnitude of pit membrane fouling caused by sap proteins under natural conditions.
In conclusion, proteins and traces of polysaccharides are the only colloidal polymers consistently transported in the xylem sap of plants. To the best of our knowledge this is the first report to establish the principle that a water-soluble protein midway along the size range of the proteins found naturally in xylem sap, can reduce xylem hydraulic conductance in both herbaceous and woody plants. The reduction of xylem conductance has previously been associated with important whole-plant responses such as leaf growth reduction, stomatal closure, leaf senescence, wilting, limits to maximum tree height (Koch et al., 2004), and drought survival (McDowell et al., 2008).
Although the magnitude of protein effects on in planta hydraulic conductance still needs to be researched, the potential for physical limitation of xylem flow by endogenous sap proteins should be added to the list of adverse effects on xylem conductance already assigned to nutrient-ion interactions with pit membrane hydrogels (Zwieniecki et al., 2001; Van Ieperen, 2007), xylem embolisms (Tyree and Sperry, 1989), biotic invasion of the xylem (Opalka et al.,1998; Perez-Donoso et al., 2007), and regulated changes in the water conductivity of the aquaporins in plant roots (Lu and Neumann, 1999; Bramley et al., 2009; Ehlert et al., 2009).
Acknowledgments
We thank Dr Luciana Renna for running the protein gels. FMW chair (PMN) and Ente Cassa di Risparmio di Firenze (SM) provided funding.
References
- Albenne C, Canut H, Boudart G, Zhang Y, San Clemente H, Pont-Lezica R, Jamet E. Plant cell wall proteomics: mass spectrometry data, a trove for research on protein structure/function relationships. Molecular Plant. 2009;2:977–989. doi: 10.1093/mp/ssp059. [DOI] [PubMed] [Google Scholar]
- Alvarez S, Goodger JQD, Marsh EL, Chen S, Aslrvatham VS, Schachtman DP. Characterization of maize xylem sap proteome. Journal of Proteome Research. 2006;5:963–972. doi: 10.1021/pr050471q. [DOI] [PubMed] [Google Scholar]
- Asli S, Neumann PM. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant, Cell and Environment. 2009;32:577–584. doi: 10.1111/j.1365-3040.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- Baig MA, Salahuddin A. Occurrence and characterization of stable intermediate state(s) in the unfolding of ovomucoid by guanidine hydrochloride. Biochemical Journal. 1978;171:89–97. doi: 10.1042/bj1710089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S, Tran PM, Silk WK. Effects of salinity on xylem structure and water use in growing leaves of sorghum. New Phytologist. 2000;146:119–127. [Google Scholar]
- Bhutz A, Kolasa A, Arlt K, Walz C, Kehr J. Xylem sap protein composition is conserved among different plant species. Planta. 2004;219:610–618. doi: 10.1007/s00425-004-1259-9. [DOI] [PubMed] [Google Scholar]
- Biles CL, Abeles FB. Xylem sap proteins. Plant Physiology. 1991;96:597–601. doi: 10.1104/pp.96.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ. Xylem hydraulic physiology: the functional backbone of terrestrial plant productivity. Plant Science. 2009;177:245–251. [Google Scholar]
- Bramley H, Turner NC, Turner DW, Tyerman SD. Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiology. 2009;150:348–364. doi: 10.1104/pp.108.134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Ball M, Luly J, Holtum J. Pit membrane porosity and water stress-induced cavitation in four co-existing dry rainforest tree species. Plant Physiology. 2003;131:41–48. doi: 10.1104/pp.014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Cobb AR, Jansen S. Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytologist. 2008;177:608–625. doi: 10.1111/j.1469-8137.2007.02317.x. [DOI] [PubMed] [Google Scholar]
- Davies WJ, Zhang J. Root signals and the regulation of growth and development of plants in drying soil. Annual Reviews of Plant Physiology and Plant Molecular Biology. 1991;42:55–76. [Google Scholar]
- Djordjevic MA, Oakes M, Xue Li D, Hwang CH, Hocart CH, Gresshoff PM. The Glycine max xylem sap and apoplast proteome. Journal of Proteome Research. 2007;6:3771–3779. doi: 10.1021/pr0606833. [DOI] [PubMed] [Google Scholar]
- Ehlert C, Maurel C, Tardieu F, Simonneau T. Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiology. 2009;150:1093–1104. doi: 10.1104/pp.108.131458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan T, Schurr U, Schultze E- D. Stomatal response to drying soil in relation to changes in xylem sap composition of Helianthus annuus. L. The concentrations of cations, anions, amino acids in, and pH of, the xylem sap. Plant, Cell and Environment. 1992;15:453–459. [Google Scholar]
- Goodger JQD, Sharp RE, Marsh EL, Schachtman DP. Relationships between xylem sap constituents and leaf conductance of well-watered and water-stressed maize across three xylem sampling techniques. Journal of Experimental Botany. 2005;56:2389–2400. doi: 10.1093/jxb/eri231. [DOI] [PubMed] [Google Scholar]
- Goosen MFA, Sablani SS, Ai-Hinai H, Ai-Obeidani S, Al-Belushi R, Jackson D. Fouling of reverse osmosis and ultrafiltration membranes: a critical review. Separation Science and Technology. 2004;39:2261–2297. [Google Scholar]
- Hubbard RM, Ryan MG, Stiller V, Sperry JS. Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant, Cell and Environment. 2001;24:113–121. [Google Scholar]
- Jansen S, Choat B, Pletsers A. Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. American Journal of Botany. 2009;96:409–419. doi: 10.3732/ajb.0800248. [DOI] [PubMed] [Google Scholar]
- Jarbeau JA, Ewers FW, Davis SD. The mechanism of water-stress-induced embolism in two species of chaparral shrubs. Plant, Cell and Environment. 1995;18:189–196. [Google Scholar]
- Koch GW, Sillett SC, Jennings GM, Davis SD. The limits to tree height. Nature. 2004;428:851–854. doi: 10.1038/nature02417. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural protein during the assembly of head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lu Z, Neumann PM. Water stress inhibits hydraulic conductance and leaf growth in rice seedlings but not transport of water via mercury sensitive water channels in the root. Plant Physiology. 1999;120:143–152. doi: 10.1104/pp.120.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell N, Pockman WT, Allen CD, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- Munns R, Passioura JB, Milborrow BV, James RA, Close TJ. Stored xylem sap from wheat and barley in drying soil contains a transpiration inhibitor with a large molecular size. Plant, Cell and Environment. 1993;16:867–872. [Google Scholar]
- Neumann PM. Sequential leaf senescence and correlatively controlled increases in xylem flow resistance. Plant Physiology. 1987;83:941–944. doi: 10.1104/pp.83.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann PM. Evidence for long-distance xylem transport of signal peptide activity from tomato roots. Journal of Experimental Botany. 2007;58:2217–2223. doi: 10.1093/jxb/erm081. [DOI] [PubMed] [Google Scholar]
- Neumann PM, Stein Z. Relative rates of delivery of xylem solutes to shoot tissues: possible relationship to sequential leaf senescence. Physiologia Plantarum. 1984;62:390–397. [Google Scholar]
- Opalka N, Brugidou C, Bonneau C, Nicole M, Beachy RN, Yeager M, Fauquet C. Movement of rice yellow mottle virus between xylem cells through pit membranes. Proceedings of the National Academy of Sciences, USA. 1998;95:3323–3328. doi: 10.1073/pnas.95.6.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Donoso AG, Greve LC, Walton JH, Shackel KA, Labavitch JM. Xylella fastidiosa infection and ethylene exposure result in xylem and water movement disruption in grapevine shoots. Plant Physiology. 2007;143:1024–1036. doi: 10.1104/pp.106.087023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo F, Trifilò P, Lo Gullo MA, Buffa R, Nardini A, Salleo S. Effects of reduced irradiance on hydraulic architecture and water relations of two olive clones with different growth potentials. Environmental and Experimental Botany. 2009;66:249–256. [Google Scholar]
- Satoh S, Lizuka C, Kikuchi A, Nakamura N, Fujii T. Proteins and carbohydrates in xylem sap from squash root. Plant and Cell Physiology. 1992;33:841–847. [Google Scholar]
- Satoh S. Organic substances in xylem sap delivered to above-ground organs by the roots. Journal of Plant Research. 2006;119:179–187. doi: 10.1007/s10265-005-0257-8. [DOI] [PubMed] [Google Scholar]
- Shane MW, McCully ME, Canny MJ. Architecture of branch–root junctions in maize: structure of the connecting xylem and the porosity of pit membranes. Annals of Botany. 2000;85:613–624. [Google Scholar]
- Sperry JS, Alder NN, Eastlack SE. The effect of reduced hydraulic conductance on stomatal conductance and xylem cavitation. Journal of Experimental Botany. 1993;44:1075–1082. [Google Scholar]
- Trifilò P, Lo Gullo MA, Nardini A, Pernice F, Salleo S. Rootstock effects on xylem conduit dimensions and vulnerability to cavitation of Olea europaea L. Trees. 2007;21:549–556. [Google Scholar]
- Tyree MT, Sperry JS. Vulnerability of xylem to cavitation and embolism. Annual Review of Plant Physiology and Molecular Biology. 1989;40:19–38. [Google Scholar]
- Van Alfen NK, Allard-Turner V. Susceptibility of plants to vascular disruption by macromolecules. Plant Physiology. 1979;63:1072–1075. doi: 10.1104/pp.63.6.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ieperen W. Ion-mediated changes of xylem hydraulic resistance in planta: fact or fiction? Trends in Plant Science. 2007;12:137–142. doi: 10.1016/j.tplants.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Zhang J. Anti-transpiration and anti-growth activities in the xylem sap from plants under different types of soil stress. New Phytologist. 1997;137:657–664. [Google Scholar]
- Zwieniecki MA, Melcher PJ, Holbrook NM. Hydrogel control of xylem hydraulic resistance in plants. Science. 2001;291:1059–1062. doi: 10.1126/science.1057175. [DOI] [PubMed] [Google Scholar]