Abstract
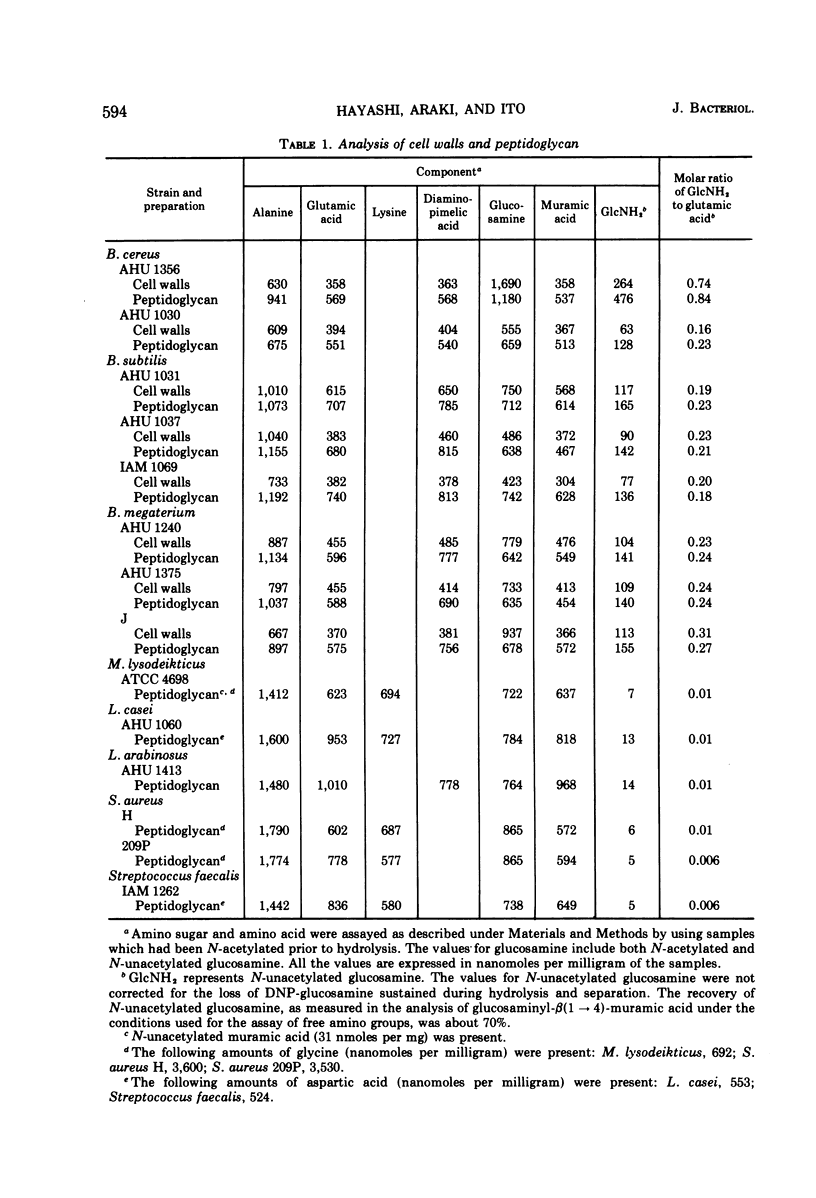
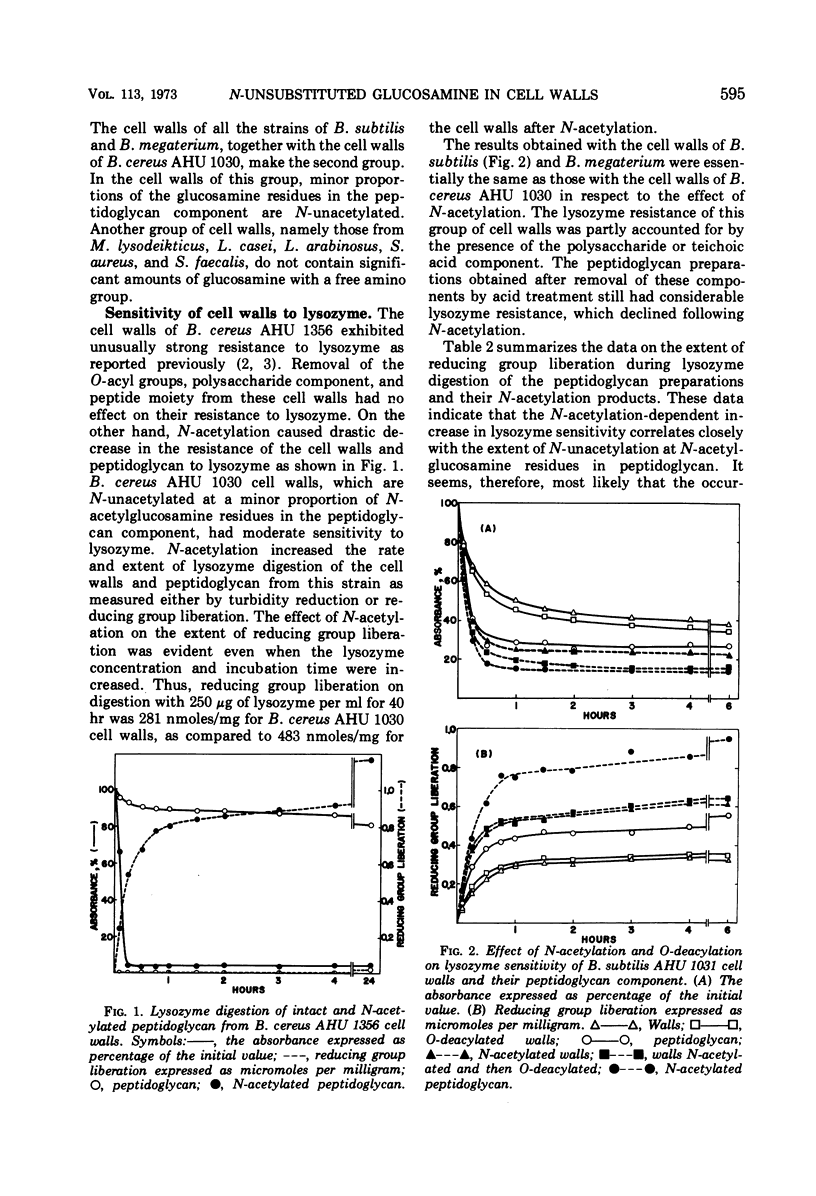
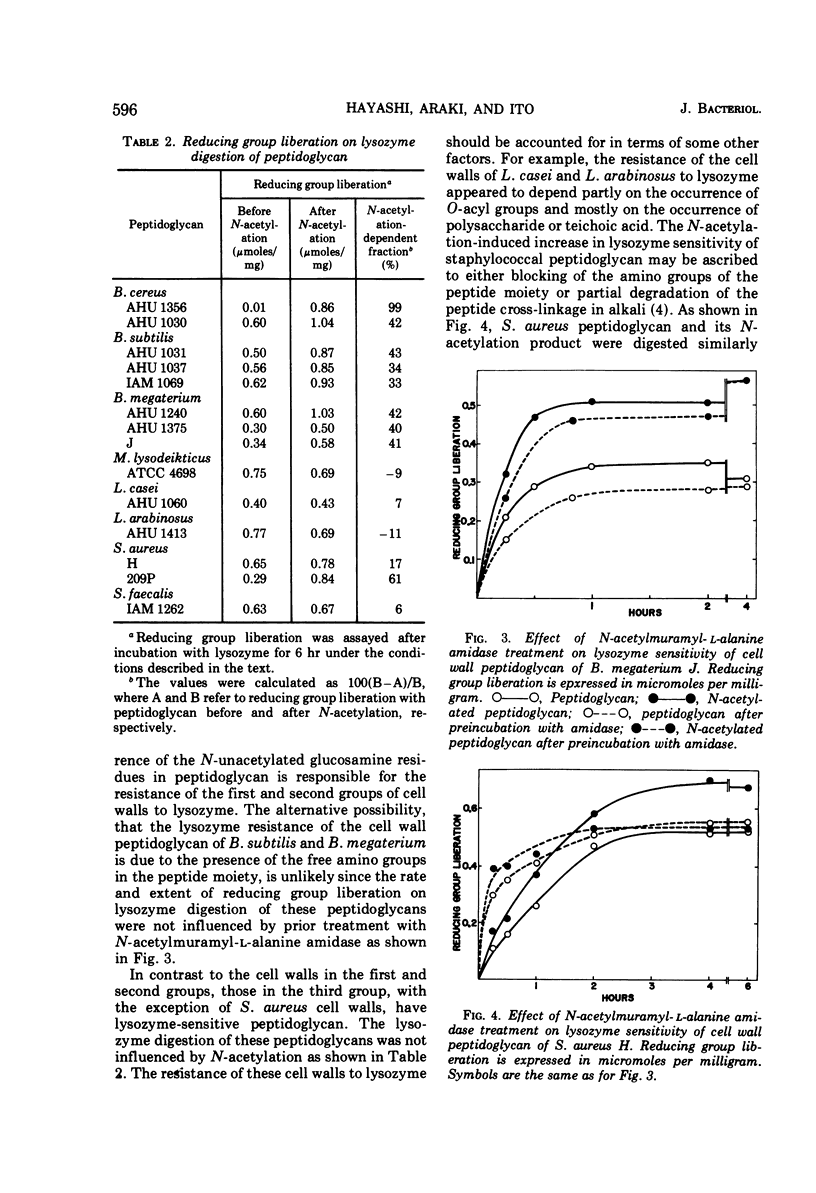
Analysis by dinitrophenylation techniques revealed the occurrence of significant amounts of glucosamine residues with free amino groups in the peptidoglycan component of cell walls isolated from Bacillus cereus, Bacillus subtilis, and Bacillus megaterium. A close correlation was demonstrated between the content of N-unacetylated glucosamine residues in the peptidoglycan component and the resistance of the cell walls to lysozyme. These lysozyme-resistant cell walls and peptidoglycan were converted into a lysozyme-sensitive form by means of N-acetylation with acetic anhydride. Thus, the occurrence of the N-unacetylated glucosamine residues in the peptidoglycan component accounts for the resistance of these cell walls to lysozyme. The N-unacetylated glucosamine residues were not found in a significant amount in the cell walls of Micrococcus lysodeikticus, Staphylococcus aureus, Streptococcus faecalis, Lactobacillus casei, or Lactobacillus arabinosus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Fukuoka S., Oba S., Ito E. Enzymatic deacetylation of N-acetylglucosamine residues in peptidoglycan from Bacillus cereus cell walls. Biochem Biophys Res Commun. 1971 Nov 5;45(3):751–758. doi: 10.1016/0006-291x(71)90481-5. [DOI] [PubMed] [Google Scholar]
- Araki Y., Nakatani T., Hayashi H., Ito E. Occurrence of non-N-substituted glucosamine residues in lysozyme-resistant peptidoglycan from Bacillus cereus cell walls. Biochem Biophys Res Commun. 1971 Feb 19;42(4):691–697. doi: 10.1016/0006-291x(71)90543-2. [DOI] [PubMed] [Google Scholar]
- Araki Y., Nakatani T., Nakayama K., Ito E. Occurrence of N-nonsubstituted glucosamine residues in peptidoglycan of lysozyme-resistant cell walls from Bacillus cereus. J Biol Chem. 1972 Oct 10;247(19):6312–6322. [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J., Goundry J. The action of dilute alkali on the glycyl residues of staphylococcal peptidoglycan. Biochem J. 1970 Jan;116(2):313–315. doi: 10.1042/bj1160313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUMFITT W., WARDLAW A. C., PARK J. T. Development of lysozyme-resistance in Micrococcus lysodiekticus and its association with an increased O-acetyl content of the cell wall. Nature. 1958 Jun 28;181(4626):1783–1784. doi: 10.1038/1811783a0. [DOI] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. The chemical composition of the cell wall in some gram-positive bacteria and its possible value as a taxonomic character. J Gen Microbiol. 1956 Jul;14(3):583–600. doi: 10.1099/00221287-14-3-583. [DOI] [PubMed] [Google Scholar]
- HEYMANN H., MANNIELLO J. M., BARKULIS S. S. STRUCTURE OF STREPTOCOCCAL CELL WALLS. 3. CHARACTERIZATION OF AN ALANINE-CONTAINING GLUCOSAMINYLMURAMIC ACID DERIVATIVE LIBERATED BY LYSOZYME FROM STREPTOCOCCAL GLYCOPEPTIDE. J Biol Chem. 1964 Sep;239:2981–2985. [PubMed] [Google Scholar]
- INGRAM V. M., SALTON M. R. The action of fluorodinitrobenzene on bacterial cell walls. Biochim Biophys Acta. 1957 Apr;24(1):9–14. doi: 10.1016/0006-3002(57)90139-7. [DOI] [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Hall E. A. The linkage between the polysaccharide and mucopeptide components of the cell wall of Lactobacillus casei. Biochem J. 1965 Aug;96(2):302–309. doi: 10.1042/bj0960302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- PERKINS H. R. THE ACTION OF HOT FORMAMIDE ON BACTERIAL CELL WALLS. Biochem J. 1965 Jun;95:876–882. doi: 10.1042/bj0950876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]


