Abstract
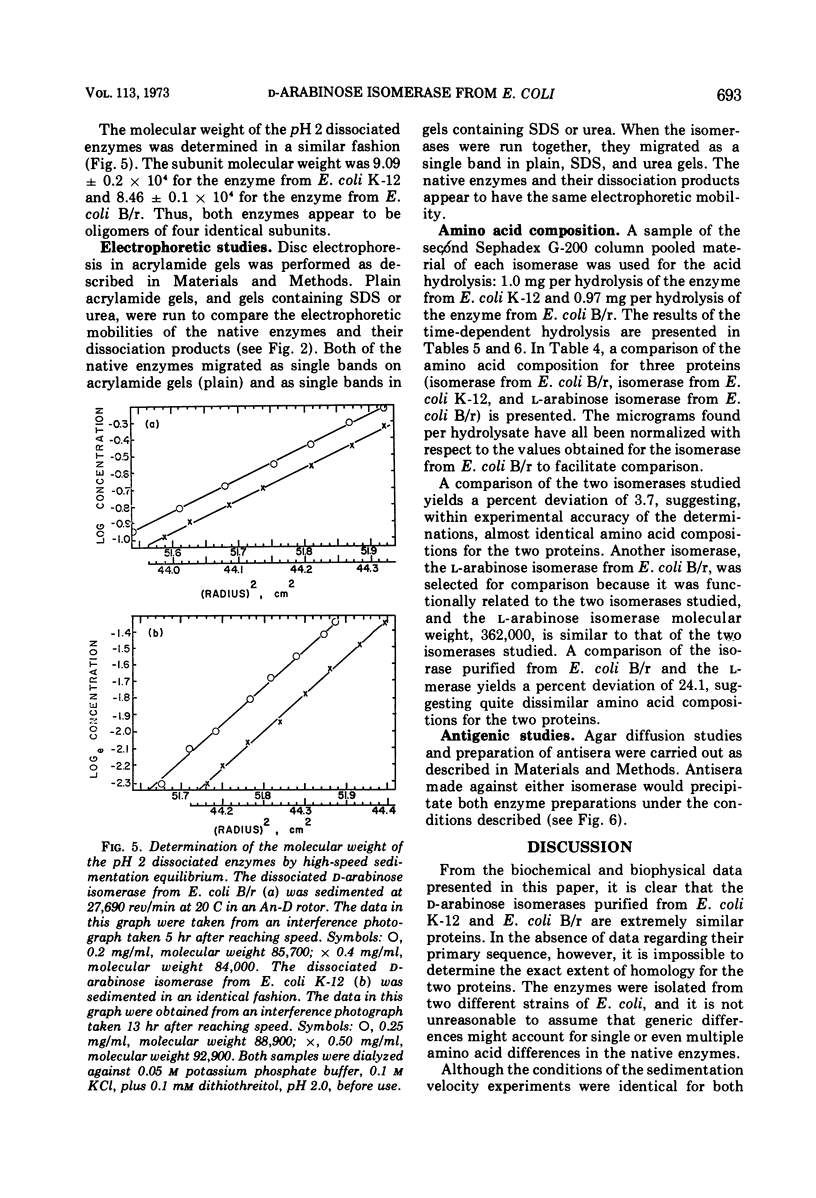
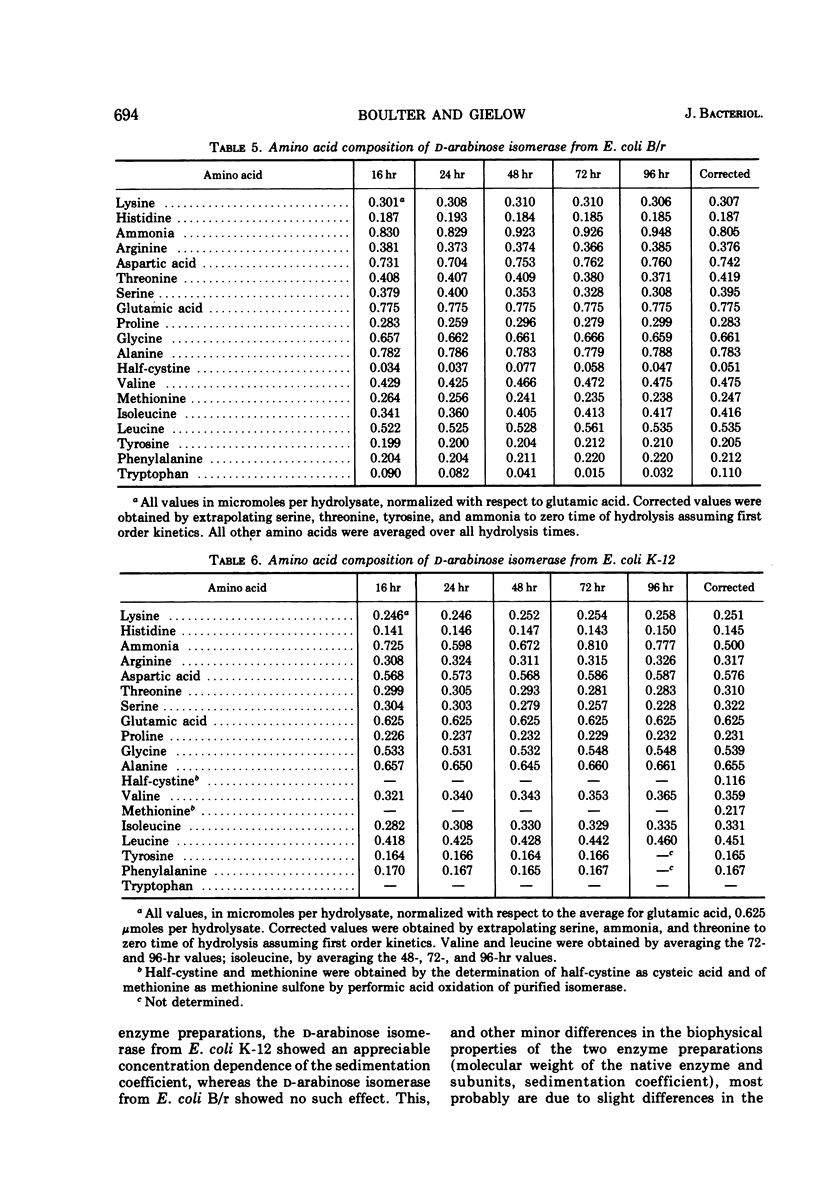
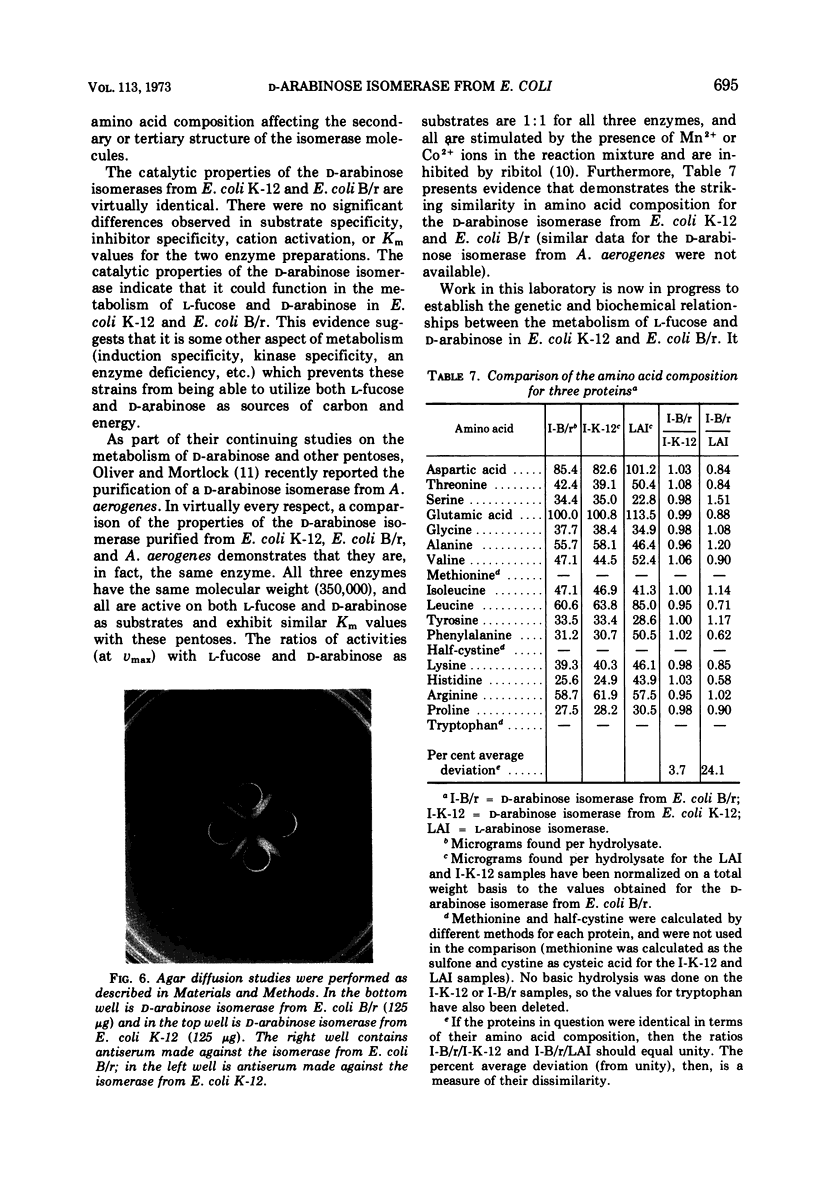
d-Arabinose isomerase (EC 5.3.1.3) has been isolated from l-fucose-induced cultures of Escherichia coli K-12 and d-arabinose-induced cultures of E. coli B/r. Both enzymes were homogeneous in an ultracentrifuge and migrated as single bands upon disc electrophoresis in acrylamide gels. The s20,w was 14.5 × 10−13 sec for the E. coli K-12 enzyme and 14.3 × 10−13 sec for the E. coli B/r enzyme. The molecular weight, determined by high-speed sedimentation equilibrium, was 3.55 ± 0.06 × 105 for the E. coli K-12 enzyme and 3.42 ± 0.04 × 105 for the enzyme isolated from E. coli B/r. Both enzyme preparations were active wth l-fucose or d-arabinose as substrates and showed no activity on any of the other aldopentoses or aldohexoses tested. With the E. coli K-12 enzyme, the Km was 2.8 × 10−1m for d-arabinose and 4.5 × 10−2m for l-fucose; with the E. coli B/r enzyme, the Km was 1.7 × 10−1m for d-arabinose and 4.2 × 10−2m for l-fucose. Both enzymes were inhibited by several of the polyalcohols tested, ribitol, l-arabitol, and dulcitol being the strongest. Both enzymes exhibited a broad plateau of optimal catalytic activity in the alkaline range. Both enzymes were stimulated by the presence of Mn2+ or Co2+ ions, but were strongly inhibited by the presence of Cd2+ ions. Both enzymes were precipitated by antisera prepared against either enzyme preparation. The amino acid composition for both proteins has been determined; a striking similarity has been detected. Both enzymes could be dissociated, by protonation at pH 2 or by dialysis against buffer containing 8 m urea, into subunits that were homogeneous in an ultracentrifuge and migrated as single bands on disc electrophoresis in acrylamide gels containing urea. The molecular weight of the subunit, determined by high-speed sedimentation equilibrium, was 9.09 ± 0.2 × 104 for the enzyme from E. coli K-12 and 8.46 ± 0.1 × 104 for the enzyme from E. coli B/r. On the basis of biophysical studies, both isomerases appear to be oligomeric proteins consisting of four identical subunits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camyre K. P., Mortlock R. P. Growth of Aerobacter aerogenes on D-arabinose and L-xylose. J Bacteriol. 1965 Oct;90(4):1157–1158. doi: 10.1128/jb.90.4.1157-1158.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- GREEN M., COHEN S. S. Enzymatic conversion of L-fucose to L-fuculose. J Biol Chem. 1956 Apr;219(2):557–568. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LeBlanc D. J., Mortlock R. P. Metabolism of D-arabinose: a new pathway in Escherichia coli. J Bacteriol. 1971 Apr;106(1):90–96. doi: 10.1128/jb.106.1.90-96.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Oliver E. J., Mortlock R. P. Competitive inhibition of an L-fucose isomerase activity by dithiothreitol. Biochem Biophys Res Commun. 1969 Jul 7;36(1):24–29. doi: 10.1016/0006-291x(69)90643-3. [DOI] [PubMed] [Google Scholar]
- Oliver E. J., Mortlock R. P. Metabolism of D-arabinose by Aerobacter aerogenes: purification of the isomerase. J Bacteriol. 1971 Oct;108(1):293–299. doi: 10.1128/jb.108.1.293-299.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J. W., Lee N. Purification and properties of an L-arabinose isomerase from Escherichia coli. J Biol Chem. 1968 Aug 25;243(16):4312–4318. [PubMed] [Google Scholar]
- Power J. The L-rhamnose genetic system in Escherichia coli K-12. Genetics. 1967 Mar;55(3):557–568. doi: 10.1093/genetics/55.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]