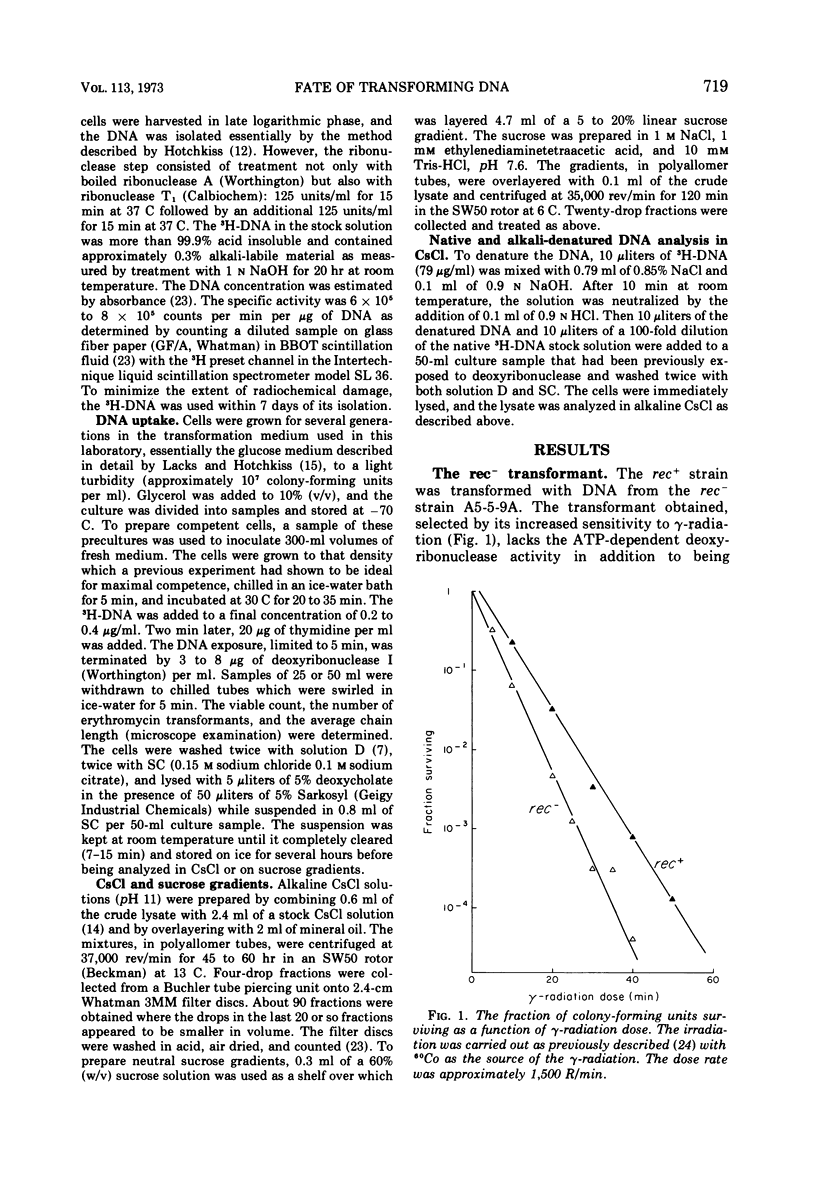
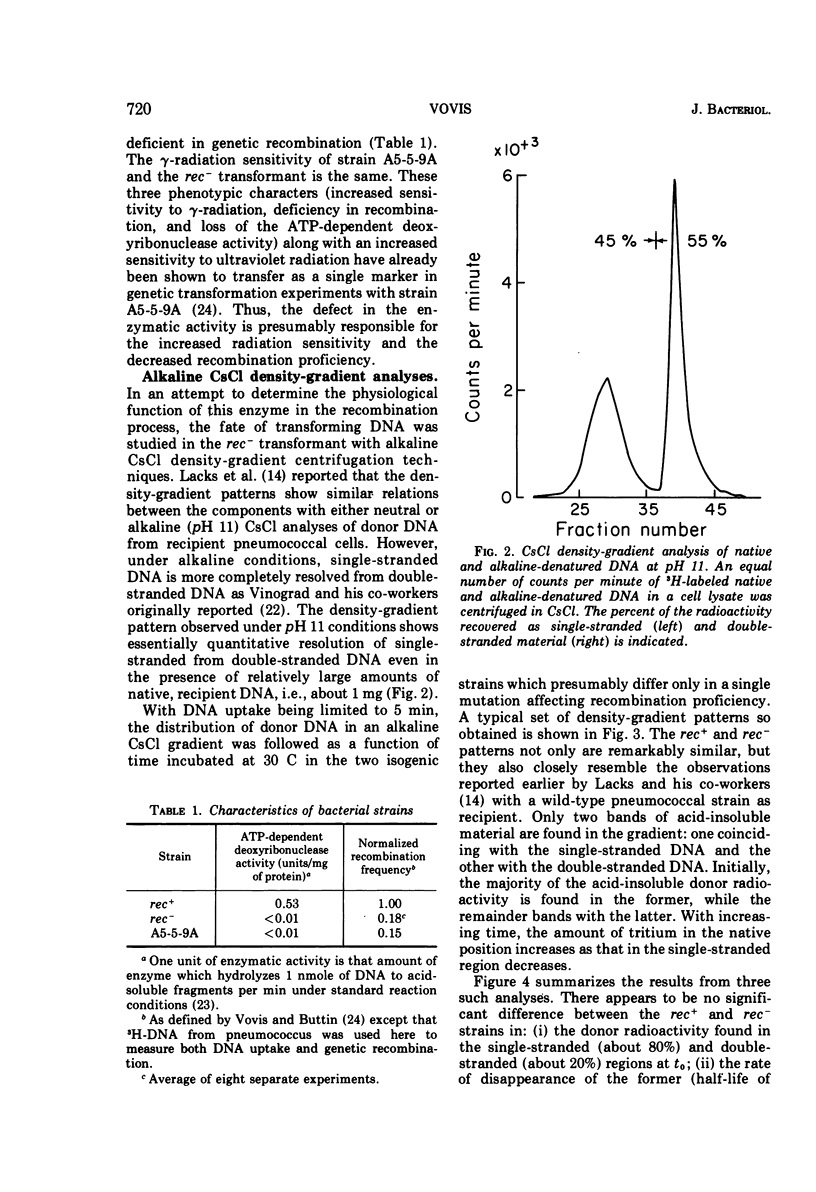
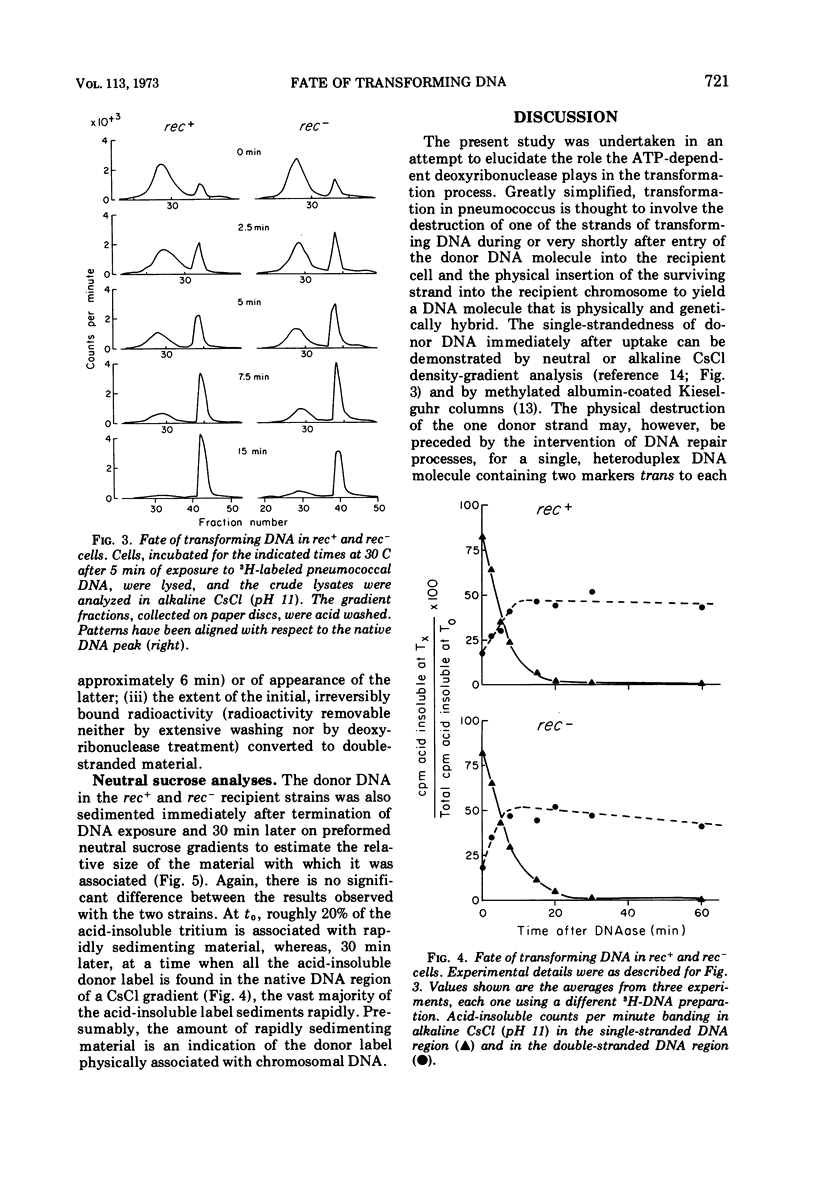
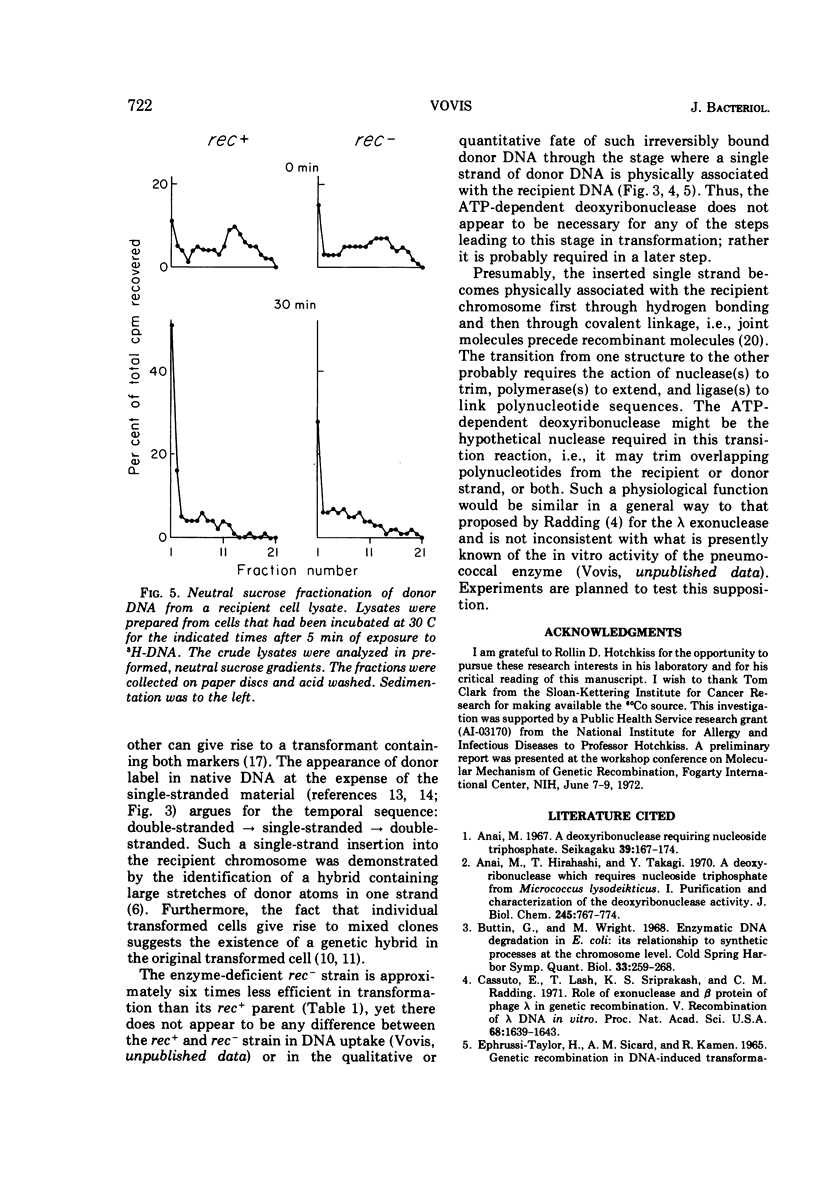
Abstract
The adenosine triphosphate-dependent deoxyribonuclease is required for wild-type levels of deoxyribonucleic acid (DNA) repair and genetic recombination. In an attempt to determine the physiological function of this enzyme in pneumococcal transformation, the fate of transforming DNA was followed in a wild-type strain and in a strain lacking the enzymatic activity. The qualitative and quantitative findings were closely comparable in the two strains through the step of physical association of a single strand of donor DNA with the recipient chromosome. These results are interpreted to mean that the enzyme may be involved in the subsequent hypothetical removal of excess polynucleotide sequences during conversion of the presumed hydrogen-bonded intermediate into a covalently linked recombinant structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anai M., Hirahashi T., Takagi Y. A deoxyribonuclease which requires nucleoside triphosphate from Micrococcus lysodeikticus. I. Purification and characterization of the deoxyribonuclease activity. J Biol Chem. 1970 Feb 25;245(4):767–774. [PubMed] [Google Scholar]
- Anai M. [Deoxyribonuclease requiring nucleoside triphosphate]. Seikagaku. 1967 Mar;39(3):167–174. [PubMed] [Google Scholar]
- Buttin G., Wright M. Enzymatic DNA degradation in E. coli: its relationship to synthetic processes at the chromosome level. Cold Spring Harb Symp Quant Biol. 1968;33:259–269. doi: 10.1101/sqb.1968.033.01.030. [DOI] [PubMed] [Google Scholar]
- Cassuto E., Lash T., Sriprakash K. S., Radding C. M. Role of exonuclease and beta protein of phage lambda in genetic recombination. V. Recombination of lambda DNA in vitro. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1639–1643. doi: 10.1073/pnas.68.7.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi-Taylor H., Sicard A. M., Kamen R. Genetic Recombination in DNA-Induced Transformation of Pneumococcus. I. the Problem of Relative Efficiency of Transforming Factors. Genetics. 1965 Mar;51(3):455–475. doi: 10.1093/genetics/51.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Initiation of bacterial transformation. Nature. 1957 Jun 29;179(4574):1322–1325. doi: 10.1038/1791322a0. [DOI] [PubMed] [Google Scholar]
- Friedman E. A., Smith H. O. An adenosine triphosphate-dependent deoxyribonuclease from Hemophilus influenzae Rd. I. Purification and properties of the enzyme. J Biol Chem. 1972 May 10;247(9):2846–2853. [PubMed] [Google Scholar]
- Gabor M., Hotchkiss R. D. Manifestation of linear organization in molecules of pneumococcal transforming DNA. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1441–1448. doi: 10.1073/pnas.56.5.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini F., Fox M. S. Genetic heterozygosity in pneumococcal transformation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):429–436. doi: 10.1073/pnas.59.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- RAVIN A. W., IYER V. N. The genetic relationship and phenotypic expression of mutations endowing Pneumococcus with resistance to erythromycin. J Gen Microbiol. 1961 Oct;26:277–301. doi: 10.1099/00221287-26-2-277. [DOI] [PubMed] [Google Scholar]
- ROTHEIM M. B., RAVIN A. W. SITES OF BREAKAGE IN THE DNA MOLECULE AS DETERMINED BY RECOMBINATION ANALYSIS OF STREPTOMYCIN-RESISTANCE MUTATIONS IN PNEUMOCOCCUS. Proc Natl Acad Sci U S A. 1964 Jul;52:30–38. doi: 10.1073/pnas.52.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger M. Evidence for conversion of heteroduplex transforming DNAs to homoduplexes by recipient pneumococcal cells (DNA strand resolution-DNA repair-bacterial transformation-genetic recombination). Proc Natl Acad Sci U S A. 1972 Feb;69(2):466–470. doi: 10.1073/pnas.69.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SICARD A. M. A NEW SYNTHETIC MEDIUM FOR DIPLOCOCCUS PNEUMONIAE, AND ITS USE FOR THE STUDY OF RECIPROCAL TRANSFORMATIONS AT THE AMIA LOCUS. Genetics. 1964 Jul;50:31–44. doi: 10.1093/genetics/50.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUDA Y., STRAUSS B. S. A DEOXYRIBONUCLEASE REACTION REQUIRING NUCLEOSIDE DI- OR TRIPHOSPHATES. Biochemistry. 1964 Nov;3:1678–1684. doi: 10.1021/bi00899a013. [DOI] [PubMed] [Google Scholar]
- Tomizawa J. I. Molecular mechanisms of genetic recombination in bacteriophage: joint molecules and their conversion to recombinant molecules. J Cell Physiol. 1967 Oct;70(2 Suppl):201–213. doi: 10.1002/jcp.1040700414. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., MORRIS J., DAVIDSON N., DOVE W. F., Jr The bouyant behavior of viral and bacterial DNA in alkaline CsCl. Proc Natl Acad Sci U S A. 1963 Jan 15;49:12–17. doi: 10.1073/pnas.49.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vovis G. F., Buttin G. An ATP-dependent deoxyribonuclease from Diplococcus pneumoniae. II. Evidence for its involvement in bacterial recombination. Biochim Biophys Acta. 1970 Nov 12;224(1):42–54. [PubMed] [Google Scholar]
- Vovis G. F., Buttin G. An ATP-dependent deoxyribonuclease from diplococcus pneumoniae. I. Partial purification and some biochemical properties. Biochim Biophys Acta. 1970 Nov 12;224(1):29–41. doi: 10.1016/0005-2787(70)90617-9. [DOI] [PubMed] [Google Scholar]
- Wright M., Buttin G. Les mécanismes de dégradation enzymatique du chromosome bactérien et leur régulation. Bull Soc Chim Biol (Paris) 1969;51(10):1373–1383. [PubMed] [Google Scholar]


